Chloride Intracellular Channel Proteins (CLICs) and Malignant Tumor Progression: A Focus on the Preventive Role of CLIC2 in Invasion and Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
1.1. CLIC Family
1.2. Known Structures of CLIC2
1.3. Known Functions of CLIC2
1.4. CLIC2 and Malignancy
1.5. Why CLIC2?
2. Expression of CLIC2 in Human Normal and Cancer Tissues
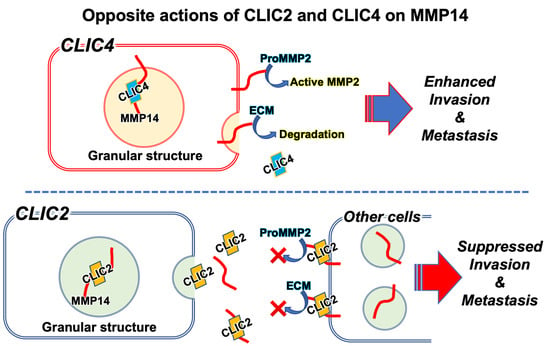
3. Suppressive Effects of CLIC2 on Malignant Cell Invasion and Metastasis
4. Vascular Permeability and CLIC2
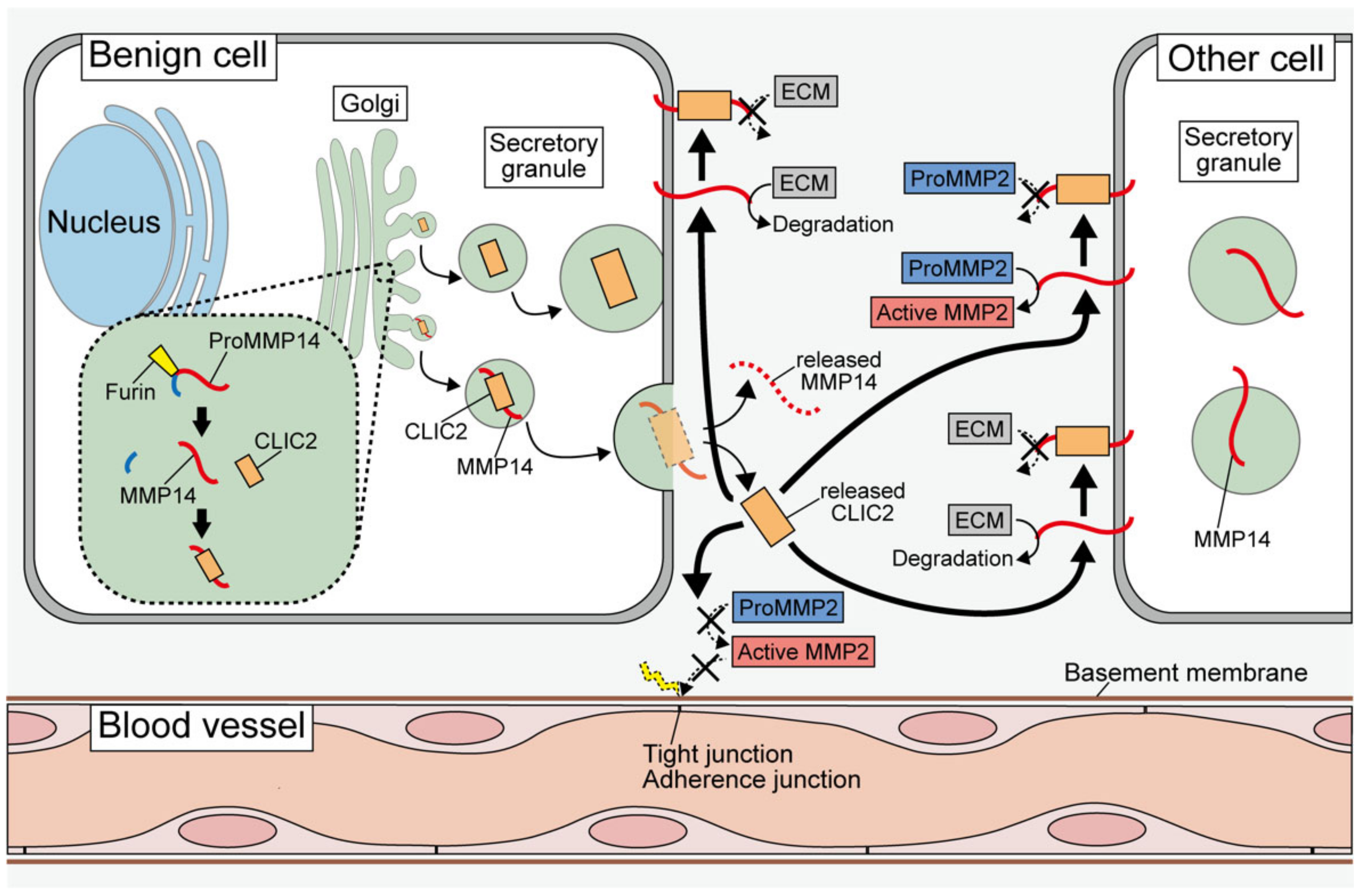
5. Roles of Secreted CLIC2; Relationship with MMPs
6. Intercellular Adhesive Structures and CLIC2
7. Modulation of MMP Activities by CLIC4
8. Conclusions
9. Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Littler, D.R.; Harrop, S.J.; Goodchild, S.C.; Phang, J.M.; Mynott, A.V.; Jiang, L.; Valenzuela, S.M.; Mazzanti, M.; Brown, L.J.; Breit, S.N.; et al. The enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010, 584, 2093–2101. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Z.; Lui, E.Y.; Lam, S.H.; Swaminathan, K. Tilapia and human CLIC2 structures are highly conserved. Biochem. Biophys. Res. Commun. 2018, 495, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Argenzio, E.; Moolenaar, W.H. Emerging biological roles of Cl- intracellular channel proteins. J. Cell Sci. 2016, 129, 4165–4174. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Umakoshi, A.; Yano, H.; Ohsumi, S.; Sumida, Y.; Hayase, E.; Usa, E.; Islam, A.; Choudhury, M.E.; Nishi, Y.; et al. Chloride intracellular channel protein 2 is secreted and inhibits MMP14 activity, while preventing tumor cell invasion and metastasis. Neoplasia 2021, 23, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Ozaki, S.; Umakoshi, A.; Yano, H.; Choudhury, M.E.; Abe, N.; Sumida, Y.; Kuwabara, J.; Uchida, R.; Islam, A.; et al. Chloride intracellular channel protein 2 in cancer and non-cancer human tissues: Relationship with tight junctions. Tissue Barriers 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Ulmasov, B.; Bruno, J.; Gordon, N.; Hartnett, M.E.; Edwards, J.C. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am. J. Pathol. 2009, 174, 1084–1096. [Google Scholar] [CrossRef]
- Berry, K.L.; Bulow, H.E.; Hall, D.H.; Hobert, O. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science 2003, 302, 2134–2137. [Google Scholar] [CrossRef]
- Fernandez-Salas, E.; Sagar, M.; Cheng, C.; Yuspa, S.H.; Weinberg, W.C. p53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J. Biol. Chem. 1999, 274, 36488–36497. [Google Scholar] [CrossRef]
- Berryman, M.; Bretscher, A. Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli. Mol. Biol. Cell 2000, 11, 1509–1521. [Google Scholar] [CrossRef]
- Tang, T.; Lang, X.; Xu, C.; Wang, X.; Gong, T.; Yang, Y.; Cui, J.; Bai, L.; Wang, J.; Jiang, W.; et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 2017, 8, 202. [Google Scholar] [CrossRef]
- Singh, H.; Ashley, R.H. Redox regulation of CLIC1 by cysteine residues associated with the putative channel pore. Biophys. J. 2006, 90, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Tulk, B.M.; Kapadia, S.; Edwards, J.C. CLIC1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. Am. J. Physiol. Cell Physiol. 2022, 282, C1103–C1112. [Google Scholar] [CrossRef] [PubMed]
- Littler, D.R.; Harrop, S.J.; Fairlie, W.D.; Brown, L.J.; Pankhurst, G.J.; Pankhurst, S.; DeMaere, M.Z.; Campbell, T.J.; Bauskin, A.R.; Tonini, R.; et al. The intracellular chloride ion channel protein CLIC1 undergoes a redox-controlled structural transition. J. Biol. Chem. 2004, 279, 9298–9305. [Google Scholar] [CrossRef]
- Peter, B.; Fanucchi, S.; Dirr, H.W. A conserved cationic motif enhances membrane binding and insertion of the chloride intracellular channel protein 1 transmembrane domain. Eur. Biophys. J. 2014, 43, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Salao, K.; Li, H.; Rybicka, J.M.; Yates, R.M.; Luo, X.W.; Shi, X.X.; Kuffner, T.; Tsai, V.W.; Husaini, Y.; et al. Intracellular chloride channel protein CLIC1 regulates macrophage function through modulation of phagosomal acidification. J. Cell Sci. 2012, 125 Pt 22, 5479–5488. [Google Scholar] [CrossRef] [PubMed]
- Salao, K.; Jiang, L.; Li, H.; Tsai, V.W.; Husaini, Y.; Curmi, P.M.; Brown, L.J.; Brown, D.A.; Breit, S.N. CLIC1 regulates dendritic cell antigen processing and presentation by modulating phagosome acidification and proteolysis. Biol. Open 2016, 5, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Al Khamici, H.; Brown, L.J.; Hossain, K.R.; Hudson, A.L.; Sinclair-Burton, A.A.; Ng, J.P.; Daniel, E.L.; Hare, J.E.; Cornell, B.A.; Curmi, P.M.; et al. Members of the chloride intracellular ion channel protein family demonstrate glutaredoxin-like enzymatic activity. PLoS ONE 2015, 10, e115699. [Google Scholar] [CrossRef]
- Board, P.G.; Coggan, M.; Watson, S.; Gage, P.W.; Dulhunty, A.F. CLIC-2 modulates cardiac ryanodine receptor Ca2+ release channels. Int. J. Biochem. Cell Biol. 2004, 36 Pt 18, 1599–1612. [Google Scholar] [CrossRef]
- Cromer, B.A.; Gorman, M.A.; Hansen, G.; Adams, J.J.; Coggan, M.; Littler, D.R.; Brown, L.J.; Mazzanti, M.; Breit, S.N.; Curmi, P.M.; et al. Structure of the Janus protein human CLIC2. J. Mol. Biol. 2007, 374, 719–731. [Google Scholar] [CrossRef]
- Qian, Z.; Okuhara, D.; Abe, M.K.; Rosner, M.R. Molecular cloning and characterization of a mitogen-activated protein kinase-associated intracellular chloride channel. J. Biol. Chem. 1999, 274, 1621–1627. [Google Scholar] [CrossRef]
- Dozynkiewicz, M.A.; Jamieson, N.B.; Macpherson, I.; Grindlay, J.; van den Berghe, P.V.; von Thun, A.; Morton, J.P.; Gourley, C.; Timpson, P.; Nixon, C.; et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev. Cell 2012, 22, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, I.R.; Rainero, E.; Mitchell, L.E.; van den Berghe, P.V.; Speirs, C.; Dozynkiewicz, M.A.; Chaudhary, S.; Kalna, G.; Edwards, J.; Timpson, P.; et al. CLIC3 controls recycling of late endosomal MT1-MMP and dictates invasion and metastasis in breast cancer. J. Cell Sci. 2014, 127, 3893–3901. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Fernaud, J.R.; Ruengeler, E.; Casazza, A.; Neilson, L.J.; Pulleine, E.; Santi, A.; Ismail, S.; Lilla, S.; Dhayade, S.; MacPherson, I.R.; et al. Secreted CLIC3 drives cancer progression through its glutathione-dependent oxidoreductase activity. Nat. Commun. 2017, 8, 14206. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.R.; Westwood, P.K.; Boyd, A.; Ashley, R.H. Rat brain p64H1, expression of a new member of the p64 chloride channel protein family in endoplasmic reticulum. J. Biol. Chem. 1997, 272, 23880–23886. [Google Scholar] [CrossRef]
- Singh, H.; Ashley, R.H. CLIC4 (p64H1) and its putative transmembrane domain form poorly selective, redox-regulated ion channels. Mol. Membr. Biol. 2007, 24, 41–52. [Google Scholar] [CrossRef]
- Shukla, A.; Edwards, R.; Yang, Y.; Hahn, A.; Folkers, K.; Ding, J.; Padmakumar, V.C.; Cataisson, C.; Suh, K.S.; Yuspa, S.H. CLIC4 regulates TGF-beta-dependent myofibroblast differentiation to produce a cancer stroma. Oncogene 2014, 33, 842–850. [Google Scholar] [CrossRef]
- Fernandez-Salas, E.; Suh, K.S.; Speransky, V.V.; Bowers, W.L.; Levy, J.M.; Adams, T.; Pathak, K.R.; Edwards, L.E.; Hayes, D.D.; Cheng, C.; et al. mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol. Cell. Biol. 2002, 22, 3610–3620. [Google Scholar] [CrossRef]
- Tung, J.J.; Hobert, O.; Berryman, M.; Kitajewski, J. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis 2009, 12, 209–220. [Google Scholar] [CrossRef]
- Hsu, K.S.; Otsu, W.; Li, Y.; Wang, H.C.; Chen, S.; Tsang, S.H.; Chuang, J.Z.; Sung, C.H. CLIC4 regulates late endosomal trafficking and matrix degradation activity of MMP14 at focal adhesions in RPE cells. Sci Rep. 2019, 9, 12247. [Google Scholar] [CrossRef]
- Salles, F.T.; Andrade, L.R.; Tanda, S.; Grati, M.; Plona, K.L.; Gagnon, L.H.; Johnson, K.R.; Kachar, B.; Berryman, M.A. CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI. Cytoskeleton 2014, 71, 61–78. [Google Scholar] [CrossRef]
- Ferofontov, A.; Strulovich, R.; Marom, M.; Giladi, M.; Haitin, Y. Inherent flexibility of CLIC6 revealed by crystallographic and solution studies. Sci. Rep. 2018, 8, 6882. [Google Scholar] [CrossRef] [PubMed]
- Griffon, N.; Jeanneteau, F.; Prieur, F.; Diaz, J.; Sokoloff, P. CLIC6, a member of the intracellular chloride channel family, interacts with dopamine D(2)-like receptors. Mol. Brain Res. 2003, 117, 47–57. [Google Scholar] [CrossRef]
- Friedli, M.; Guipponi, M.; Bertrand, S.; Bertrand, D.; Neerman-Arbez, M.; Scott, H.S.; Antonarakis, S.E.; Reymond, A. Identification of a novel member of the CLIC family, CLIC6, mapping to 21q22.12. Gene 2003, 320, 31–40. [Google Scholar] [CrossRef]
- Littler, D.R.; Assaad, N.N.; Harrop, S.J.; Brown, L.J.; Pankhurst, G.J.; Luciani, P.; Aguilar, M.I.; Mazzanti, M.; Berryman, M.A.; Breit, S.N.; et al. Crystal structure of the soluble form of the redox-regulated chloride ion channel protein CLIC4. FEBS J. 2005, 272, 4996–5007. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, C.; Gallant, E.M.; Board, P.G.; Dulhunty, A.F. Redox potential and the response of cardiac ryanodine receptors to CLIC-2, a member of the glutathione S-transferase structural family. Antioxid. Redox. Signal. 2008, 10, 1675–1686. [Google Scholar] [CrossRef]
- Dulhunty, A.; Gage, P.; Curtis, S.; Chelvanayagam, G.; Board, P. The glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J. Biol. Chem. 2001, 276, 3319–3323. [Google Scholar] [CrossRef] [PubMed]
- Molina-Navarro, M.M.; Rosello-Lleti, E.; Ortega, A.; Tarazon, E.; Otero, M.; Martinez-Dolz, L.; Lago, F.; Gonzalez-Juanatey, J.R.; Espana, F.; Garcia-Pavia, P.; et al. Differential gene expression of cardiac ion channels in human dilated cardiomyopathy. PLoS ONE 2013, 8, e79792. [Google Scholar] [CrossRef] [PubMed]
- Pierchala, B.A.; Munoz, M.R.; Tsui, C.C. Proteomic analysis of the slit diaphragm complex: CLIC5 is a protein critical for podocyte morphology and function. Kidney Int. 2010, 78, 868–882. [Google Scholar] [CrossRef]
- Rickhag, M.; Wieloch, T.; Gido, G.; Elmer, E.; Krogh, M.; Murray, J.; Lohr, S.; Bitter, H.; Chin, D.J.; von Schack, D.; et al. Comprehensive regional and temporal gene expression profiling of the rat brain during the first 24 h after experimental stroke identifies dynamic ischemia-induced gene expression patterns, and reveals a biphasic activation of genes in surviving tissue. J. Neurochem. 2006, 96, 14–29. [Google Scholar] [CrossRef]
- Suh, K.S.; Mutoh, M.; Mutoh, T.; Li, L.; Ryscavage, A.; Crutchley, J.M.; Dumont, R.A.; Cheng, C.; Yuspa, S.H. CLIC4 mediates and is required for Ca2+-induced keratinocyte differentiation. J. Cell Sci. 2007, 120 Pt 15, 2631–2640. [Google Scholar] [CrossRef]
- Shukla, A.; Malik, M.; Cataisson, C.; Ho, Y.; Friesen, T.; Suh, K.S.; Yuspa, S.H. TGF-beta signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat. Cell Biol. 2009, 11, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Gururaja Rao, S.; Patel, N.J.; Singh, H. Intracellular Chloride Channels: Novel Biomarkers in Diseases. Front. Physiol. 2020, 11, 96. [Google Scholar] [CrossRef]
- Ponnalagu, D.; Rao, S.G.; Farber, J.; Xin, W.; Hussain, A.T.; Shah, K.; Tanda, S.; Berryman, M.A.; Edwards, J.C.; Singh, H. Data supporting characterization of CLIC1, CLIC4, CLIC5 and DmCLIC antibodies and localization of CLICs in endoplasmic reticulum of cardiomyocytes. Data Brief. 2016, 7, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.C.; Kahl, C.R. Chloride channels of intracellular membranes. FEBS Lett. 2010, 584, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Gururaja Rao, S.; Ponnalagu, D.; Patel, N.J.; Singh, H. Three Decades of Chloride Intracellular Channel Proteins: From Organelle to Organ Physiology. Curr. Protoc. Pharmacol. 2018, 80, 11–21. [Google Scholar] [CrossRef]
- Singh, H. Two decades with dimorphic Chloride Intracellular Channels (CLICs). FEBS Lett. 2010, 584, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, P.C.; Hong, J.H. Chloride Channels and Transporters: Roles beyond Classical Cellular Homeostatic pH or Ion Balance in Cancers. Cancers 2022, 14, 856. [Google Scholar] [CrossRef]
- Takano, K.; Liu, D.; Tarpey, P.; Gallant, E.; Lam, A.; Witham, S.; Alexov, E.; Chaubey, A.; Stevenson, R.E.; Schwartz, C.E.; et al. An X-linked channelopathy with cardiomegaly due to a CLIC2 mutation enhancing ryanodine receptor channel activity. Hum. Mol. Genet. 2012, 21, 4497–4507. [Google Scholar] [CrossRef]
- Witham, S.; Takano, K.; Schwartz, C.; Alexov, E. A missense mutation in CLIC2 associated with intellectual disability is predicted by in silico modeling to affect protein stability and dynamics. Proteins 2011, 79, 2444–2454. [Google Scholar] [CrossRef]
- Richardson, S.J.; Steele, G.A.; Gallant, E.M.; Lam, A.; Schwartz, C.E.; Board, P.G.; Casarotto, M.G.; Beard, N.A.; Dulhunty, A.F. Association of FK506 binding proteins with RyR channels-effect of CLIC2 binding on sub-conductance opening and FKBP binding. J. Cell Sci. 2017, 130, 3588–3600. [Google Scholar] [CrossRef]
- Meng, X.; Wang, G.; Viero, C.; Wang, Q.; Mi, W.; Su, X.D.; Wagenknecht, T.; Williams, A.J.; Liu, Z.; Yin, C.C. CLIC2-RyR1 interaction and structural characterization by cryo-electron microscopy. J. Mol. Biol. 2009, 387, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Mutoh, M.; Gerdes, M.; Crutchley, J.M.; Mutoh, T.; Edwards, L.E.; Dumont, R.A.; Sodha, P.; Cheng, C.; Glick, A.; et al. Antisense suppression of the chloride intracellular channel family induces apoptosis, enhances tumor necrosis factor α-induced apoptosis, and inhibits tumor growth. Cancer Res. 2005, 65, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.T.; Wong, C.H.; Chan, S.L. Embedding of Genes Using Cancer Gene Expression Data: Biological Relevance and Potential Application on Biomarker Discovery. Front Genet. 2018, 9, 682. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Z.; Dong, M.; Wu, D.; Liao, S.; Li, X. Chloride intracellular channel protein 2: Prognostic marker and correlation with PD-1/PD-L1 in breast cancer. Aging 2020, 12, 17305–17327. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, K.T.S.; Mandal, S.; Singh, S.; Gundimeda, S.; Jolly, M.K.; Pandey, A.; Sharma, J. In Silico Analysis of Ion Channels and Their Correlation with Epithelial to Mesenchymal Transition in Breast Cancer. Cancers 2022, 14, 1444. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, J.; Umakoshi, A.; Abe, N.; Sumida, Y.; Ohsumi, S.; Usa, E.; Taguchi, K.; Choudhury, M.E.; Yano, H.; Matsumoto, S.; et al. Truncated CD200 stimulates tumor immunity leading to fewer lung metastases in a novel Wistar rat metastasis model. Biochem. Biophys. Res. Commun. 2018, 496, 542–548. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yano, H.; Umakoshi, A.; Matsumoto, S.; Mise, A.; Funahashi, Y.; Ueno, Y.; Kamei, Y.; Takada, Y.; Kumon, Y.; et al. A Truncated form of CD200 (CD200S) Expressed on Glioma Cells Prolonged Survival in a Rat Glioma Model by Induction of a Dendritic Cell-Like Phenotype in Tumor-Associated Macrophages. Neoplasia 2016, 18, 229–241. [Google Scholar] [CrossRef]
- Martin, T.A.; Jiang, W.G. Loss of tight junction barrier function and its role in cancer metastasis. Biochim. Biophys. Acta 2009, 1788, 872–891. [Google Scholar] [CrossRef]
- Martin, T.A.; Mason, M.D.; Jiang, W.G. HGF and the regulation of tight junctions in human prostate cancer cells. Oncol. Rep. 2014, 32, 213–224. [Google Scholar] [CrossRef]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Tanaka, J.; Takahashi, H.; Kohno, S.; Ohue, S.; Umakoshi, A.; Gotoh, K.; Ohnishi, T. Blood vessels expressing CD90 in human and rat brain tumors. Neuropathology 2016, 36, 168–180. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, G.; Wang, Y.; Jin, H.; Yang, S.; Liu, C. The potential involvement of E-cadherin and beta-catenins in meningioma. PLoS ONE 2010, 5, e11231. [Google Scholar] [CrossRef] [PubMed]
- Galaup, A.; Cazes, A.; Le Jan, S.; Philippe, J.; Connault, E.; Le Coz, E.; Mekid, H.; Mir, L.M.; Opolon, P.; Corvol, P.; et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc. Natl. Acad. Sci. USA 2006, 103, 18721–18726. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Padhan, N.; Sjostrom, E.O.; Roche, F.P.; Testini, C.; Honkura, N.; Sainz-Jaspeado, M.; Gordon, E.; Bentley, K.; Philippides, A.; et al. VEGFR2 pY949 signalling regulates adherens junction integrity and metastatic spread. Nat. Commun. 2016, 7, 11017. [Google Scholar] [CrossRef] [PubMed]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef]
- Lecat, S.; Matthes, H.W.; Pepperkok, R.; Simpson, J.C.; Galzi, J.L. A Fluorescent Live Imaging Screening Assay Based on Translocation Criteria Identifies Novel Cytoplasmic Proteins Implicated in G Protein-coupled Receptor Signaling Pathways. Mol. Cell. Proteom. 2015, 14, 1385–1399. [Google Scholar] [CrossRef]
- Liu, J.; Jin, X.; Liu, K.J.; Liu, W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J. Neurosci. 2012, 32, 3044–3057. [Google Scholar] [CrossRef]
- Romanic, A.M.; White, R.F.; Arleth, A.J.; Ohlstein, E.H.; Barone, F.C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: Inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 1998, 29, 1020–1030. [Google Scholar] [CrossRef]
- Miyamori, H.; Takino, T.; Kobayashi, Y.; Tokai, H.; Itoh, Y.; Seiki, M.; Sato, H. Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J. Biol. Chem. 2001, 276, 28204–28211. [Google Scholar] [CrossRef]
- Mao, Y.; Kleinjan, M.L.; Jilishitz, I.; Swaminathan, B.; Obinata, H.; Komarova, Y.A.; Bayless, K.J.; Hla, T.; Kitajewski, J.K. CLIC1 and CLIC4 mediate endothelial S1P receptor signaling to facilitate Rac1 and RhoA activity and function. Sci. Signal. 2021, 14, eabc0425. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.J.; Kitajewski, J. Chloride intracellular channel 1 functions in endothelial cell growth and migration. J. Angiogenes Res. 2010, 2, 23. [Google Scholar] [CrossRef]
- Lucitti, J.L.; Tarte, N.J.; Faber, J.E. Chloride intracellular channel 4 is required for maturation of the cerebral collateral circulation. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1141–H1150. [Google Scholar] [CrossRef] [PubMed]
- Rønnov-Jessen, L.; Villadsen, R.; Edwards, J.C.; Petersen, O.W. Differential Expression of a Chloride Intracellular Channel Gene, CLIC4, in Transforming Growth Factor-β1-Mediated Conversion of Fibroblasts to Myofibroblasts. Am. J. Pathol. 2002, 161, 471–480. [Google Scholar] [CrossRef]
- Shiomi, T.; Lemaitre, V.; D’Armiento, J.; Okada, Y. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol. Int. 2010, 60, 477–496. [Google Scholar] [CrossRef]
- Hu, J.; Van den Steen, P.E.; Sang, Q.X.; Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007, 6, 480–498. [Google Scholar] [CrossRef]
- Nagase, H.; Woessner, J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef]
| CLICs | Distribution | Ion Channel | Biological Function | References |
|---|---|---|---|---|
| CLIC1 | Various organs | Poorly selective anion channels | Participates in inflammatory processes Activation of MYC signaling Enzymatic activity Redox regulation | [11,12,13,14,15,16,17] |
| CLIC2 | Blood vessels. heart, liver | Anion channels | Modulation of ryanodine receptor Inhibition of MMP14 activity | [4,5,13,18,19] |
| CLIC3 | Muscles, heart, lung, kidney | Component of anion channel, regulator of channel | Endosomal trafficking Promote invasive behavior | [20,21,22,23] |
| CLIC4 | Various organs | Poorly selective ion channels | Enhance tumor invasiveness Enhance TGF-β signaling Induce apoptosis Involved in angiogenesis Stimulation of MMP14 activity | [24,25,26,27,28,29] |
| CLIC5 | Kidney, heart, lung, colon | Poorly selective ion channels | Actin cytoskeleton-dependent membrane remodeling | [9,25,30] |
| CLIC6 | Soluble and membrane fractions | Unknown | Interact with dopamine receptors | [31,32,33] |
| Cancer | Breast | Ovarian | Lung | Gastric | Liver | Pancreatic |
|---|---|---|---|---|---|---|
| CLIC1 | detrimental | detrimental | n.s. | ameliorative | detrimental | detrimental |
| CLIC2 | ameliorative | detrimental | ameliorative | ameliorative | ameliorative | n.s. |
| CLIC3 | detrimental | detrimental | n.s. | detrimental | n.s. | detrimental |
| CLIC4 | n.s. | detrimental | detrimental | detrimental | n.s. | detrimental |
| CLIC5 | ameliorative | detrimental | ameliorative | ameliorative | n.s. | n.s. |
| CLIC6 | ameliorative | ameliorative | ameliorative | ameliorative | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozaki, S.; Mikami, K.; Kunieda, T.; Tanaka, J. Chloride Intracellular Channel Proteins (CLICs) and Malignant Tumor Progression: A Focus on the Preventive Role of CLIC2 in Invasion and Metastasis. Cancers 2022, 14, 4890. https://doi.org/10.3390/cancers14194890
Ozaki S, Mikami K, Kunieda T, Tanaka J. Chloride Intracellular Channel Proteins (CLICs) and Malignant Tumor Progression: A Focus on the Preventive Role of CLIC2 in Invasion and Metastasis. Cancers. 2022; 14(19):4890. https://doi.org/10.3390/cancers14194890
Chicago/Turabian StyleOzaki, Saya, Kanta Mikami, Takeharu Kunieda, and Junya Tanaka. 2022. "Chloride Intracellular Channel Proteins (CLICs) and Malignant Tumor Progression: A Focus on the Preventive Role of CLIC2 in Invasion and Metastasis" Cancers 14, no. 19: 4890. https://doi.org/10.3390/cancers14194890
APA StyleOzaki, S., Mikami, K., Kunieda, T., & Tanaka, J. (2022). Chloride Intracellular Channel Proteins (CLICs) and Malignant Tumor Progression: A Focus on the Preventive Role of CLIC2 in Invasion and Metastasis. Cancers, 14(19), 4890. https://doi.org/10.3390/cancers14194890