Simple Summary
Immuno-Oncology (IO) based combinations are now the standard of care for frontline advanced clear cell renal cell carcinoma. Despite the unprecedented durable efficacy of such combinations, many patients do not respond to IO or will eventually develop resistance. This review summarizes the ongoing efforts in drug development in clear cell renal cell carcinoma focusing on novel targets.
Abstract
The dual immune checkpoint blockade targeting CTLA-4 and PD-1 (ipilimumab/nivolumab) or the IO combinations targeting PD-1 and anti-VEGF TKIs (pembrolizumab/axitinib, nivolumab/cabozantinib, pembrolizumab/lenvatinib) have demonstrated an overall survival benefit in advanced clear cell renal cell carcinoma (ccRCC). Despite this significant improvement in clinical outcomes in the frontline setting from IO/IO or the IO/TKI combinations, there is a subset of patients of advanced ccRCC that do not respond to such combinations or will lose the initial efficacy and have disease progression. Therefore, a remarkable unmet need exists to develop new therapeutics to improve outcomes. With an enhanced understanding of ccRCC biology and its interaction with the tumor microenvironment, several new therapies are under development targeting ccRCC metabolism, cytokine-signaling, alternative immune checkpoint proteins, and novel biological pathways. In addition, microbiome products enhancing IO response, antibody–drug conjugates, and targeted radionuclides are also being investigated. This review summarizes selected emerging agents that are under development in ccRCC.
1. Introduction
The treatment landscape for ccRCC has evolved tremendously with the advent of immune checkpoint inhibitors (ICIs). The current standard first-line treatment for advanced ccRCC includes an immuno-oncology (IO) combination. The dual immune checkpoint blockade of ipilimumab plus nivolumab targeting cytotoxic T-lymphocytes-associated protein 4 (CTLA-4) and programmed-cell death protein 1 (PD-1), respectively, was the first and the only IO/IO combination approved by the Food and Drug Administration (FDA) based on the CheckMate 214 [1]. The other IO combination strategy involves an ICI targeting anti-PD-1 or its ligand (anti-PD-L1) plus an anti-VEGF tyrosine kinase inhibitor (TKI). There are four FDA approved combinations that belong to the IO/TKI category: pembrolizumab/axitinib [2] (KEYNOTE 426), avelumab/axitinib [3] (JAVELIN Renal 101), nivolumab/cabozantinib [4] (CheckMate 9ER), and pembrolizumab/lenvatinib [5] (CLEAR). The IO combinations brought improved overall survival benefits and unprecedented durable response in metastatic ccRCC. However, a significant subset of patients still has primary resistance to IO combinations or develop acquired resistance and eventually succumb to their disease. Therefore, there is an unmet need to develop new treatment options to improve the outcomes in ccRCC. This review will discuss how an understanding of ccRCC biology shapes current drug development in this disease and introduce emerging novel therapeutic agents that are currently under investigation.
2. Metabolic Inhibitors
2.1. Hypoxia-Inducible Factor (HIF) Inhibitor
Inactivating alterations in von Hippel-Lindau (VHL) tumor suppressor gene occurs in the majority of ccRCC tumors [6]. VHL regulates transcription factors hypoxia-inducible factor-1A (HIF-1A) and HIF-2A through ubiquitin-proteosome degradation [7]. In a hypoxic environment, the levels of HIF-1A and HIF-2A increase which upregulates erythropoietin expression and activates angiogenesis and VEGF pathways [7]. In pathogenic VHL variants, the HIF-mediated pathways are constitutively activated.
The understanding of the VHL/HIF pathway led to the development of the first-in-class HIF-2A inhibitor, belzutifan [8]. In a phase II trial consisting of RCC patients associated with VHL germline mutations (VHL disease), belzutifan showed an objective response rate of 49% (95% CI: 36–62) among 30 out of 61 patients. Following the positive results of this study, the U.S. Food and Drug Administration (FDA) approved belzutifan in patients with VHL disease who require treatment for RCC, central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors not requiring immediate surgery [9].
In sporadic ccRCC, it is estimated that >90% of patients have a loss of VHL heterozygosity, and inactivating VHL mutations are found in 50–65% of cases [10] (reviewed in reference 10). The phase I LITESPARK-001 study investigated belzutifan monotherapy in solid tumors, including a cohort of pretreated ccRCC (n = 55, median number of prior therapies: 3). The overall response rate (ORR) was 25% and the median progression-free survival (mPFS) was 14.5 months [11]. In an updated analysis after a medium follow-up >3 years, the medium duration of response was not reached [12]. Belzutifan plus cabozantinib is being evaluated in the treatment-naïve (cohort 1) and previously treated advanced ccRCC (cohort 2). The preliminary results of cohort 2 showed an ORR of 22% (9 partial responses among 41 patients) and a disease control rate of 92.7%. Of note, 53% had prior first-line therapy and 45% had prior second-line therapy. The mPFS was 16.8 months and the duration of response was not reached [13]. Most common treatment-related adverse event (TRAE) included anemia (76%; grade 3: 11.3%), fatigue (68%; grade 3: 11.3%), hand-foot syndrome (52.8%), diarrhea (45%), and hypertension (43%; grade 3: 22.4%). There was no grade 4 TRAEs or treatment-related death.
Ongoing trials are investigating belzutifan in the first or later lines of therapy for patients with advanced ccRCC as monotherapy or in various combinations (Table 1). Of note, a phase III randomized-controlled study investigating belzutifan versus everolimus in the second/third line setting in ccRCC (NCT04195750) has completed enrollment. For first-line treatment, a phase III triplet study investigating the combination of belzutifan with pembrolizumab/lenvatinib or quavonlimab (anti-CTLA4) with pembrolizumab/lenvatinib vs. pembrolizumab/lenvatinb [14] (NCT04736706) is ongoing. Belzutifan is also being investigated in combination with pembrolizumab (versus pembrolizumab monotherapy) in the adjuvant setting after nephrectomy [15] (NCT05239728).

Table 1.
Ongoing clinical trials of HIF inhibitors.
Other HIF-2A targeted agents are under development. ARO-HIF2 is a synthetic double-stranded RNA interference (RNAi) that is given by intravenous infusion. The initial results of the ongoing phase I ARO-HIF21001 (NCT04169711) demonstrated decreased HIF-2A mRNA expression after treatment, and the safety profile was favorable. Disease control was observed in 7 out of 23 patients (30%) [16]. Another oral HIF-2A small molecule inhibitor, NKT2152, is being investigated in a phase 1/2 dose-escalation/expansion study (NCT05119335).
2.2. Glutaminase Inhibitor
Glutamine has been reported to be a vital nutrient source in many cancer cells, including ccRCC [17,18,19,20]. The glutamine transporters, which transport glutamine from the blood into the cells, have higher expression in ccRCC [21] compared to normal renal cells. The constitutively activated HIF pathway in ccRCC also shifts the cancer cells to utilize more glutamine than glucose to fuel the tricarboxylic acid (TCA) cycle [18,21]. Glutamine is imported into mitochondria and is converted to glutamate by the enzyme glutaminase [22] in the TCA cycle. Telaglenastat (CB-839) is a first-in-class glutaminase inhibitor and has shown antitumor activity and favorable safety profile in early phase studies in ccRCC as monotherapy [23] or in combinations with everolimus or cabozantinib [24]. The phase II double-blinded placebo-controlled ENTRATA trial (NCT03163667) compared telaglenastat plus everolimus vs. everolimus in a cohort of heavily treated ccRCC (median of three prior lines of systemic therapy) [25]: telaglenastat plus everolimus showed an improved mPFS (3.8 months vs. 1.9 months, HR: 0.64, 95% CI: 0.34–1.2, p-value: 0.079) which met the trial primary endpoint (one-sided alpha < 0.2). However, in another similarly designed phase II CANTANA trial [26] (NCT03428217), telaglenastat plus cabozantinib failed to show improved efficacy compared to cabozantinib alone (mPFS: 9.2 months vs. 9.3 months, p-value: 0.65). The contrasting results of these trials emphasize the need to better understand the involved biological mechanisms and pathways to select partnering agents with telaglenastat in ccRCC. Further correlative data from both trials hopefully can bring more insights to guide future trial design [25,26] using glutaminase inhibitors.
2.3. Adenosine Inhibition
The accumulation of adenosine and its interaction with the adenosine 2A receptors (A2AR) on immune cells in the tumor microenvironment (TME) has been shown to restrict the anti-tumor activity of cytotoxic T cells and natural killer (NK) cells [27,28,29]. In addition, adenosine also augments the immunosuppressive activity of regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC) [29,30,31]. Targeting the adenosine-mediated pathway can potentially reverse the resistance to immune checkpoint inhibitors in preclinical studies [32,33]. Ciforadenant (CPI-444) is a small molecule that competes with adenosine to bind the A2AR and inhibits the signaling of the pathway [34]. In a first-in-human phase I study, ciforadenant was given as a monotherapy or in combination with anti-PD-L1 atezolizumab in an advanced RCC cohort of 68 patients, of which 72% were resistant or refractory to prior anti-PD1/PD-L1 therapy [35]. The safety profile was acceptable in both the ciforadenant monotherapy and combination arms. Partial response was observed in 1 of 33 (3%) patients treated with ciforadenant monotherapy and 4 of 35 (11%) patients treated with ciforadenant/atezolizumab. The disease control rate at 6 months was 39% in the combination vs. 17% in the monotherapy in this patient population with a median of three prior lines of therapy. The survival endpoints also favored the combination (mPFS: 5.8 months vs. 4.1 months; OS at 25 months: 90% vs. 69%). The efficacy suggested that the combination had higher anti-tumor activity than the ciforadenant monotherapy, although this trial was not designed to compare the two arms. A correlative study revealed the efficacy of ciforadenant was associated with CD8+ T-cell infiltration and diversification of the T-cell receptor repertoire; this trial also proposed an adenosine-related gene signature as a predictive biomarker [35]. There is a planned phase II trial of ciforadenant in combination with ipilimumab and nivolumab in the first-line treatment of advanced RCC through the Kidney Cancer Research Consortium [36].
Blocking the formation of adenosine is another potential mechanism to inhibit adenosine-mediated immunosuppression in the TME. Adenosine triphosphate (ATP) serves as the energy source in cells. In the extracellular space, ATP is also a signaling molecule [37]. Extracellularly, ATP is metabolized to adenosine monophosphate and then adenosine through CD39 and CD73, respectively [38]. CD39 and CD73 are both ectonucleotidases expressed on the cell membrane [37]. Preclinical research suggested that CD73 inhibition could enhance the antitumor activity of ICIs [39]. BMS-986179 is an anti-CD73 antibody and was investigated as a monotherapy or in combination with nivolumab in a phase I/IIa study consisting of pre-treated advanced solid tumor patients. Among 59 patients treated with BMS-986179 with/without nivolumab, 7 patients had a partial response, and 10 patients had stable disease. The combination safety profile was similar to nivolumab monotherapy [40].
3. Cytokines
3.1. Interleukin-2
A small subset of advanced RCC patients treated with high-dose (HD) interleukin-2 (IL-2) can achieve complete response and have durable long-term remission [41]. However, the overall response rate is low, and the significant muti-organ adverse events, including cardiac toxicities of HD IL-2, require inpatient delivery and close monitoring. Therefore, the use of HD IL-2 is limited at experienced centers. The interest in HD IL-2 has reemerged in the ICI era. A single institution trial combined HD IL-2 with pembrolizumab in advanced RCC patients without prior PD-1/PD-L1 therapy showed a promising response among 19 of 25 patients (79%) [42]. There are ongoing trials investigating HD IL-2 with anti-PD-1 antibodies in the frontline setting or after disease progression on PD-1/PD-L1 antibodies (Table 2).

Table 2.
Ongoing clinical trials targeting cytokines and new immune checkpoints.
Efforts have been made to improve the anti-tumor activity of IL-2-based therapy and to reduce the associated toxicities. Nemvaleukin alfa (ALKS-4230) is a fusion protein of permuted IL-2 to the extracellular domain of IL-2 receptor α (IL-2Rα). It is designed to simulate the intermediate but not the high-affinity IL-2R, leading the IL-2 fusion protein to preferentially stimulate effector T cells [43] and not stimulate Tregs [43]. There is an ongoing phase I/II study assessing nemvaleukin alfa monotherapy or in combination with pembrolizumab in second-line solid tumors (ARTISTRY-1, NCT02799095). Part B of the protocol included an advanced RCC-focused cohort: 4 of 21 evaluable patients had an objective response and 11 had a stable disease when nemvaleukin was given as monotherapy [44]. Part C of this trial is investigating nemvaleukin with pembrolizumab in solid tumors. The preliminary results showed an ORR of 16.1% (22/137) and a disease control rate of 59.9% in this heavily pretreated solid tumor cohort (1 to 9 prior lines of therapy, including ICI) [45].
Bempegaldesleukin (NKTR-214) is a modified pegylated (PEG) IL-2R agonist which preferentially binds to IL-2Rβ over IL-2Rα [46]. Compared to unmodified conventional IL-2, bempegaldesleukin leads to more CD8+ T cell and NK cell activation/expansion and less Treg expansion and is, thus, hypothesized to have increased anti-tumor activity and reduced toxicities [46]. The FDA granted breakthrough therapy designation to bempegaldesleukin in August 2019 and several phase III clinical trials were opened in multiple tumor types. In March 2022, the first analysis of the phase III PIVOT IO-001 trial comparing bempegaldesleukin/nivolumab vs. nivolumab in untreated metastatic melanoma showed no improved clinical benefit of this combination [47]. In April 2022, negative findings of this combination were also reported in the phase III PIVOT-09 trial in advanced ccRCC (comparator: TKI monotherapy) and the phase II PIVOT-10 trial in cisplatin-ineligible urothelial cancer. Future development of bempegaldesleukin in combination with nivolumab has been halted [48]. Perhaps the PEG modification to decrease Treg activity from bempegaldesleukin ultimately also decreased the needed CD8 T cell effector function leading to ultimately negative trials across several tumor types.
3.2. Interleukin-15
Interleukin 15 (IL-15) has similar immunostimulatory properties as IL-2 on NK and T effector cells aiding in activation and expansion but has less interaction with Tregs [49]. The half-life of human IL-15 is short (<1 h), limiting the clinical implications [50]. The IL-15 superagonist, SOT101 (formerly SO-C101), is a fusion molecule of human IL-15 that is linked to IL-15Rα [49] and has increased stability and activity delivered subcutaneously [50,51]. A phase I study assessing SOT101 as monotherapy and in combination with pembrolizumab in solid tumors, including ccRCC, is ongoing (NCT04234113) (Table 2).
3.3. Interleukin-27 Inhibitor
IL-27 consists of two subunits: IL27p28 and Epstein-Barr virus-induced gene 3 (EBI3). IL-27 binds to its receptor, which is composed of glycoprotein 130 (gp130) and IL-27RA. IL-27 has an immunoregulatory effect. It can upregulate the inhibitory immune checkpoint receptors and downregulate proinflammatory cytokines [52,53]. The Cancer Genome Atlas (TCGA) dataset revealed higher transcript expression of EBI3, IL-27RA, and IL27p28 in ccRCC tumors compared to normal kidney tissue. In addition, higher transcript expression of EBI3, IL27p28, and IL-27RA was associated with worse overall survival in ccRCC [54].
Inhibiting IL-27-mediated signaling theoretically can augment antitumor activity. SRF388 is a first-in-class anti-IL-27p28 antibody that blocks the interaction between IL-27 and IL-27RA [54]. SRF388 is being investigated in a phase I dose-escalation and expansion study, including a cohort of ccRCC. SRF388 showed a favorable safety profile as monotherapy or in combination with pembrolizumab. The preliminary results revealed encouraging monotherapy activity of SRF388 in the ccRCC (of the 10 evaluable ccRCC patients, one had confirmed partial response) and met the criteria to expand the cohort for further investigation [55] (NCT04374877) (Table 2).
4. Targeting New Immune Checkpoints Proteins
4.1. LAG3 Inhibitor
Lymphocyte-activation gene 3 (LAG3) is an inhibitory receptor that is expressed on activated T cells, Tregs, and NK cells [56]. LAG3 binds to its major ligand, the major histocompatibility complex-II (MHC-II), on the antigen-presenting cells, which prevents its interaction with the T cell receptor. This leads to the inhibition of T-cell receptor signaling and downregulates T-cell proliferation and cytokine release [57,58]. LAG-3 and PD-L1 are often found to be co-expressed on tumor-infiltrating T cells, and dual blockade of LAG-3 and PD-1 has shown synergistic anti-tumor efficacy in preclinical studies [59,60].
Relatlimab is the first-in-class anti-LAG-3 antibody. In a randomized double-blinded phase III study in frontline advanced melanoma (RELATIVITY-047), the combination of relatlimab with nivolumab showed a superior PFS compared to nivolumab monotherapy (10.1 months vs. 4.6 months, HR: 0.75, 95% CI: 0.62–0.92, p-value: 0.006) [61] and subsequently received FDA approval [62]. The overall survival data has not yet matured. An ongoing randomized phase II trial investigating several nivolumab-based combinations vs. ipilimumab/nivolumab in advanced RCC includes one arm of relatlimab with nivolumab (NCT02996110) (Table 2). Relatlimab/nivolumab is also being investigated in the neoadjuvant setting of ccRCC in a phase II trial (NCT05148546) (Table 2).
Ieramilimab (LAG525) is another LAG-3 inhibitor under investigation. In a completed phase I/II study of Ieramilimab with or without the anti-PD-1 spartalizumab (PDR001) in 255 advanced solid tumor patients, including RCC, ieramilimab was well tolerated and showed modest antitumor activity in the combination arm (n = 121, 3 had a complete response, 10 had a partial response, and 35 had stable disease) including patients pre-treated with anti-PD-1/PD-L1 [63].
4.2. TIGIT Inhibitor
T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT) is a receptor of the Ig family and is expressed on activated CD8+ or CD4+ T cells, Tregs, and NK cells [64]. There are three ligands for TIGIT expressed on antigen-presenting cells and cancer cells: CD155, CD112, and CD113. Compared to CD112 and CD113, TIGIT has the highest affinity with CD155. The interaction of TIGIT and its ligands causes altered functions of the antigen-presenting cells and decreased cytokine release leading to reduced T cell activation [65]. TIGIT has been reported to be expressed on exhausted CD8+ T cells in RCC [66]. Preclinical studies have demonstrated antitumor activities with the combination of TIGIT blockade and anti-PD-1/anti-PD-L1 inhibition [67,68].
Tiragolumab is an anti-TIGIT monoclonal antibody. Early phase studies showed favorable tolerability of Tiragolumab monotherapy and in combination with atezolizumab. In a randomized phase II placebo-controlled study of PD-L1 positive non-small cell lung cancer in the frontline setting, the combination of tiragolumab/atezolizumab showed improved mPFS (5.4 months vs. 3.6 months, HR: 0.57, 95% CI: 0.37–0.90, p-value: 0.015) and objective response rate (31% vs. 16%, p-value: 0.03) compared to placebo/atezolizumab [69]. A phase II study investigating tiragolumab with atezolizumab in advanced solid tumors, including RCC, is ongoing (NCT03977467) (Table 2).
4.3. ILT2/ILT4 Inhibitors
Ig-like transcript 2 (ILT2) and 4 (ILT4) are immune-inhibitory receptors that are expressed in the NK cells and T cells [70]. The human leukocyte antigen-G (HLA-G) is the ligand for ILT2/ILT4 that has increased expression in most cancers, including 50% of RCC [71]. HLA-G has been reported to be associated with inflammation and poor prognosis [72].
NGM707 is a dual antagonist for ILT2/ILT4. A preclinical study has shown that NGM707 stimulates the activation of myeloid/lymphoid cells and reprograms suppressive myeloid cells [73]. There is an ongoing phase I/II dose escalation/expansion study of NGM707 with/without pembrolizumab in solid tumors, including RCC (NCT04913337) (Table 2).
4.4. ILT3 Inhibitor
Myeloid-derived suppressor cells (MDSCs) have been shown to impair antitumor activity and promote immunosuppression leading to a pro-tumorigenic environment [74]. Ig-like transcript 3 (ILT3, also called LILRB4) is a receptor expressed on MDSC shown to promote immunosuppressive function [75]. A phase I/Ib study of ILT3 inhibitor (NGM831) with/without pembrolizumab has been launched in advanced solid tumors (NCT05215574) (Table 2).
4.5. TREM2 Inhibitor
Tumor-associated macrophages (TAMs) are immunosuppressive cells in the TME and have been shown to contribute to T-cell dysfunction/exhaustion, resistance to anti-PD1/PDL1 therapy, and associated with worse clinical outcomes [76,77].
TREM2 is a transmembrane protein that is highly expressed on TAMs [78]. Compared to normal renal tissue, TREM2-expressing macrophages were found to be higher in abundance in the ccRCC tumors. In addition, high TREM-2 expressing macrophages were associated with a higher risk of recurrence [79]. PY314 is a monoclonal antibody against TREM2 and has shown efficacy as monotherapy and in combination with an anti-PD1 agent in the preclinical study [80]. A phase Ia/Ib investigating PY314 with/without pembrolizumab in advanced solid tumors is ongoing (NCT04691375) (Table 2).
4.6. OX40 Agonist
Full T cell activation requires both T cell receptor signaling and activation of its co-stimulatory receptors [81]. OX40 (CD134) is a co-stimulatory molecule that is mainly expressed on activated T cells [81]. The binding of OX40 with its ligand (OX40L), which is primarily expressed on antigen-presenting cells, promotes T cell expansion and prolongs their survival [82]. Preclinical studies have revealed synergistic antitumor activity combining anti-PD-1 and OX40 agonists [83,84].
A phase I dose-escalation study investigating OX40 agonist PF-04518600 in solid tumors showed a favorable toxicity profile and preliminary anti-tumor activity [85]. However, in a subsequent phase II study in advanced ccRCC after progression of PD-1/PD-L1 inhibition, axitinib with PF-04518600 did not improve clinical outcomes compared to axitinib alone (median PFS: 13.1 vs. 8.5 months, HR: 0.85, 95% CI: 0.45–1.60, p-value: 0.61) [86]. In another phase I/IIa study investigating OX40 agonist BMS-986178 in solid tumors including RCC, BMS-986178 was well tolerated either as monotherapy or in combination with nivolumab and/or ipilimumab but did not show clear efficacy [87].
INBRX-106 is a novel hexavalent OX40 agonist under development. Preclinical studies showed it outperformed bivalent antibodies in costimulatory capacity and anti-tumor activity [88]. INBRX-106 is being investigated in a phase I dose-escalation study with/without pembrolizumab in advanced solid tumors (NCT04198766) (Table 2).
5. Novel Mechanisms
5.1. Batiraxcept (AVB-S6-500)
AXL is a member of the TAM (Tyro3, Axl, Mer) receptor tyrosine kinase family, which has a high affinity for its ligand growth arrest-specific protein 6 (Gas6) [89]. AXL is highly expressed in many cancer cells. The Gas6/AXL signaling promotes cellular invasion/migration and has been shown to be associated with treatment resistance and poor clinical outcomes [90,91,92]. In ccRCC, the constitutively activated HIF pathway leads to increased expression of AXL [93], and the Gas6/AXL pathway has been associated with treatment resistance of anti-VEGF TKIs [94]. In addition, Gas6/AXL also fosters immunosuppressive TME by promoting the infiltrations of macrophages, MDSCs, and monocytes and decreasing the infiltrations of T effector cells [95].
Batiraxcept (AVB-S6-500) is a first-in-class fusion protein consisting of the extracellular domain of the AXL receptor fused to a human immunoglobulin G1 Fc domain [96]. Batiraxcept has a 200-fold higher affinity to Gas6 than the AXL receptor [97] and can effectively neutralize the Gas6 protein and inhibit AXL signaling. In a preclinical study of the ovarian cancer mouse model, batiraxcept improved tumor response to chemotherapy and PARP inhibitor [98]. Batiraxcept is being investigated in a phase Ib/II trial in ccRCC in combination with cabozantinib in 2nd line therapy, in combination with cabozantinib/nivolumab in frontline treatment, and lastly as monotherapy in the refractory setting (NCT04300140) (Table 3). The preliminary results of the phase Ib portion of the trial showed favorable safety/tolerability in combination with cabozantinib (no dose-limiting toxicity observed) [99,100] and ORR of 46% (12 partial responses out of 26 patients) [101]. The trial is enrolling the expansion cohorts in this and other line settings [100].

Table 3.
Ongoing clinical trials targeting novel mechanisms.
5.2. Adavosertib (AZD1775)
The cell cycle consists of 4 phases: G1, S, G2, and M, and the cyclin-dependent kinases (CDK) are responsible for the regulation of the cell cycle and DNA replication [102]. In response to DNA damage, the WEE1 kinase, which is a serine-threonine kinase [103], suppresses the CDK1/2 activity by phosphorylation. This results in cell cycle pause at the intra-S or G2/M checkpoint, allowing DNA damage repair to occur prior to mitosis and avoiding aberrant DNA-induced apoptosis [104]. This process maintains the integrity of the cell’s genome [105]. Inhibition of WEE1 increases the activity of CDK1, which results in cells passing through the G2-M checkpoint unregulated and subsequently leads to the accumulation of unrepaired DNA damage and cell deaths [106]. Inhibition of WEE1 also increases the activity of CDK2, which results in aberrant DNA replication [107]. WEE1 inhibition with adavosertib (AZD1775) has been shown to increase the sensitivity of chemotherapy and radiotherapy in preclinical and early phase studies [103]. Of note, in a preclinical study in SETD2-deficient cancers, WEE1 inhibition with adavosertib was shown to have antitumor activity in vitro and in vivo [108]. In fact, ccRCC is the most common SETD2-inactivated cancers [109]. It has been observed that 10% of primary ccRCC tumors and 30% of metastatic ccRCC tumors have SETD2 mutations, suggesting its role in driving tumor progression [6,110]. A phase II trial investigating adavosertib in SETD-2 deficient cancers, including a cohort for advanced ccRCC, is ongoing (NCT03284385) (Table 3).
5.3. DS-6000a
The successful development of HER-2 targeted antibody-drug conjugate (ADC) trastuzumab deruxtecan (T-DXd) in advanced breast cancer [111] has led to the further investigation of DXd-based ADC in ccRCC. DS-6000a is a DXd-based ADC that targets the human cadherin 6 (CDH6), which is a transmembrane protein overexpressed in ovarian cancer and RCC [112]. The DXd-based ADC is internalized after binding to its target cells, and the linker is degraded intracellularly by lysosomal enzymes to release deruxtecan which causes DNA damage and apoptotic cell death. Because of the bystander effect, ADC not only kills the cells it binds to but also has anti-tumor activity against neighboring cells. DS-6000a is currently being investigated in a phase I trial that includes an expansion cohort of ccRCC (NCT04707248) (Table 3). The interim results showed acceptable tolerability and early signs of antitumor activity (2 had a partial response and 9 had stable disease among 15 evaluable patients) in heavily pretreated RCC [113].
6. Tyrosine Kinase Inhibitors
6.1. Sitravatinib
Sitravatinib is a multi-targeted tyrosine kinase inhibitor targeting TAM (Tyro3, Axl, Mer) receptors, VEGFR, c-MET, and c-KIT. Preclinical data showed that sitravatinib could reduce immune-suppressive myeloid cells in the TME and shift the TME to a more proinflammatory phenotype [114]. In a phase I/II study, sitravatinib was combined with nivolumab in advanced immunotherapy-naïve ccRCC that progressed after 1 or 2 prior anti-angiogenic regimens (n = 42). After a median follow-up of 18.7 months, the sitravatinib/nivolumab combination demonstrated an ORR of 35.7% and a median progression-free survival of 11.7 months [115]. Sitravatinib/nivolumab was also evaluated in a phase II neoadjuvant study in ccRCC patients who underwent curative nephrectomy [116]. Sitravatinib was given for the first two weeks and nivolumab was added after. The total treatment was 6–8 weeks prior to nephrectomy. There were 17 patients evaluable for efficacy and 2 patients had partial response [116].
Sitravatinib/nivolumab is currently being investigated in a single-arm phase II study of advanced ccRCC after the progression of prior immunotherapy (NCT04904302) (Table 3). Another ongoing phase I study investigates sitravatinib/ipilimumab/nivolumab in treatment-naive advanced ccRCC [117] (NCT04518046) (Table 3).
6.2. XL092
XL092 is a novel oral tyrosine kinase inhibitor that targets VEGFR, AXL, MER, and MET kinases similar to cabozantinib with a shorter half-life. There are two ongoing phase I studies investigating XL092 as monotherapy or in combination as doublets or triplets when combined with other IO agents (NCT03845166; NCT05176483) (Table 3). Each of these studies includes an expansion cohort focusing on advanced ccRCC [118,119].
7. Live Microbiome Product
CBM588
Several studies have shown that the composition of the gut microbiome modulates the treatment response of ICIs in several cancers [120,121,122]. There are many species proposed to have such an effect, including Bifidobacterium spp. In a preclinical study, oral administration of Bifidobacterium improves the anti-tumor activity of the anti-PD-L1 antibody against melanoma [123]. In a retrospective study consisting of nivolumab or ipilimumab/nivolumab-treated mRCC, patients’ gut microbiome enriched in Bifidobacterium spp. was associated with improved clinical response. The early studies have led to the development of a phase I trial investigating CBM588 given with ipilimumab/nivolumab in first-line mRCC [124]. CBM588 is an orally administered live Clostridium butyricum product that has been shown to increase the abundance of gut Bifidobacterium spp. [125]. In this study, patients with International Metastatic RCC Database Consortium (IMDC) intermediate and poor risk were randomized to ipilimumab/nivolumab with (n = 19; poor risk: 11%) or without (n = 10; poor risk 30%) CBM588. After a medium follow-up of 12.2 months, patients in the CBM588/ipilimumab/nivolumab arm had superior mPFS (12.7 vs. 2.5 months, HR: 0.15, 95% CI: 0.05–0.47, p-value < 0.001). Among responders, patients who received CBM588 had an interval increase in gut Bifidobacterium spp. from baseline to week 12 in stool samples; such an increase was not observed among non-responders. The results should be interpreted with caution given the small sample size [124] and future studies are warranted to confirm this finding. There is an ongoing phase I trial investigating CBM588 with an IO/TKI combination of nivolumab/cabozantinib in ccRCC (NCT05122546) (Table 3).
8. Targeted Radionuclide Therapy
8.1. Lu-177-Girentuximab
The successful developments of Lutetium-177 (Lu-177) dotatate and Lu-177 prostate-specific membrane antigen (PSMA)-617 in advanced neuroendocrine tumor [126] and castration-resistant prostate cancer [127], respectively, have brought targeted radionuclide therapy to daily oncology practice. The selective binding of the radioligand to the specific marker expressed by a given tumor is the key concept to maximizing therapeutic efficacy and minimizing toxicities of the radionuclide [128]. In ccRCC, a potential tumor target is carbonic anhydrase IX (CAIX), which is ubiquitously expressed in ccRCC (both primary and metastatic tumors) but not in normal kidney tissue [129]. Girentuximab is a monoclonal antibody that targets CAIX [130]. Lu-177-girentuximab was previously investigated in a small single-arm phase II study [131] consisting of progressive ccRCC before the ICI era. Among the 14 enrolled patients, eight patients had stable disease after the first cycle and one had a partial response. Grade 3–4 myelotoxicities were observed in most patients. Six patients received the second cycle and five patients had continued stable disease. None of the patients received further treatment due to prolonged thrombocytopenia [131]. Currently, Lu-177-girentuximab is being investigated as a combination with nivolumab in previously treated ccRCC (NCT05239533) (Table 3). Of note, the eligibility criteria require at least one evaluable metastatic lesion on zirconium-89 (89Zr)-girentuximab PET/CT [132]. The diagnostic value of 89Zr-girentuximab PET/CT in ccRCC is also being evaluated in a phase III trial (NCT03849118).
8.2. Lu-177-EB-PSMA-617
PSMA is a transmembrane glycoprotein encoded by the folate hydrolase 1 gene (FOLH1) and is highly expressed in prostate adenocarcinoma [133]. However, PSMA is a misnomer and it is not specific to prostate cancer. In fact, PSMA is also expressed in the neovasculature of many solid tumors [134]. As a result of upregulated HIF and VEGF pathways, ccRCC is characterized by substantial neovasculature with high expression of PSMA. Several studies have investigated the diagnostic utility of PSMA PET/CT in kidney cancer [134,135]. The current data suggest that PSMA PET/CT may aid in detecting metastatic RCC lesions but has a limited role in staging the primary tumors, given the uptake of PSMA by normal renal parenchyma. Given the success of Lu-177-PSMA-617 in prostate cancer, using the same rationale targeting PSMA in non-prostate tumors may be feasible. There is an ongoing trial using Lu-177-EB-PSMA-617, which conjugates a truncated Evans blue (EB) molecule to PSMA-617, to investigate its use in detection and therapeutic benefits in RCC [136] (NCT05170555) (Table 3).
9. Novel Trial Design
9.1. Triplet Studies
While IO/IO and IO/TKI doublets have become the standard frontline treatments for advanced ccRCC, the field has continued to evolve and investigate the efficacy of triplet combinations. COSMIC-313(NCT03937219) (Table 4) is a phase III randomized placebo-controlled trial comparing the triplet regimen of cabozantinib/ipilimumab/nivolumab vs. ipilimumab/nivolumab in IMDC intermediate and high-risk advanced ccRCC. This is the first study to use a standard of care IO combination as the control. Cabozantinib has been shown to have an immunomodulatory effect [137] and the rationale of this triplet is to increase the clinical response of ipilimumab/nivolumab. The results of COSMIC 313 were presented at the European Society for Medical Oncology (ESMO) Congress 2022 meeting. The triplet of cabozantinib/ipilimumab/nivolumab showed superior PFS over ipilimumab/nivolumab (mPFS: not reached vs. 11.3 months; HR: 0.73, 95% CI: 0.57–0.94, p-value: 0.013) and met the trial primary endpoint. The secondary endpoint of OS is not mature yet. The ORR was numerically higher in the triplet (43%, 95% CI: 37.2–49.2) than ipilimumab/nivolumab (36%, 95% CI: 30.1–41.8). Grade 3/4 TRAEs were 73% in the triplet versus 41% in the control. Of note, 45% of patients in the triplet arm had treatment discontinuation, while it was 24% in the control [138].

Table 4.
Ongoing clinical trials using triplets or novel trial designs.
Other triplets under investigation include the previously mentioned phase III study combining the IO/TKI backbone of pembrolizumab/lenvatinib with belzutifan or the anti-CTLA4 agent quavonlimab (NCT04736706) (Table 4). Moreover, a phase 1b/2 triplet umbrella study includes the same triplets and also additional combinations of favezelimab (anti-LAG-3) with pembrolizumab/lenvatinib, and vibostolimab (anti-TIGIT) with pembrolizumab/belzutifan (NCT 04626479) (Table 4).
9.2. Adaptive Trial Design-PDIGREE
In contrast to triplet combinations upfront, the PDIGREE trial (NCT03793166) (Table 4) uses an adaptive trial design that intensifies treatment sequentially based on the first scan obtained after completing ipilimumab/nivolumab induction [139]. At the 3-month response assessment, patients with progressive disease are assigned to cabozantinib 60 mg daily. Patients with complete responses will be assigned to nivolumab maintenance. Patients with partial response or stable disease are randomized to the cabozantinib (40 mg daily)/nivolumab vs. nivolumab monotherapy. The PDIGREE trial will help identify the timing and patient subsets that will benefit from adding TKI to the standard nivolumab maintenance after ipilimumab/nivolumab induction.
9.3. Biomarker Driven Design- OPTIC RCC
The current standard first-line treatment for advanced ccRCC involves either an IO/TKI or IO/IO combination. However, there is no level-1 evidence to guide the treating physicians to choose one over the other category. A correlative study of the phase III IMmotion 151 study used the RNA-sequencing from 823 ccRCC tumors and characterized them into seven biologically distinct clusters. Based on the biology of the clusters and their response to immunotherapy, the phase II OPtimal Treatment by Invoking biologic Clusters in Renal Cell Carcinoma (OPTIC RCC) trial (NCT05361720) (Table 4) adopts a biomarker-driven design to assign protocol treatment. The hypothesis is that gene expression clusters will characterize a patient’s tumor biology to select frontline treatment with either cabozantinib/nivolumab or the IO/IO combination of ipilimumab/nivolumab. The primary endpoint is ORR and will compare efficacy to historical control in unselected patients.
10. Conclusions
While IO-based combinations have dramatically improved the care of patients with metastatic ccRCC, there are still patients who do not respond to frontline IO-based combinations or, after initial benefit, ultimately develop progressive disease on this treatment. Thus, a remarkable unmet need remains to develop novel treatment options in this field. This review article summarized novel and encouraging therapeutics that are currently in development (Figure 1).
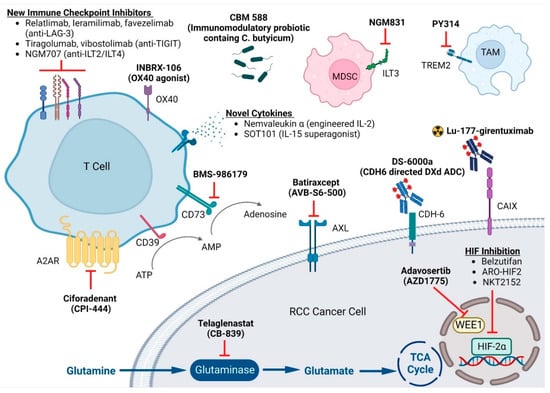
Figure 1.
Emerging Targets in Clear Cell Renal Cell Carcinoma. This figure is created with BioRender.com. LAG-3: Lymphocyte-activation gene 3; TIGIT: T cell immunoreceptor with Ig and ITIM domains; ILT: Ig-like transcript; MDSC: Myeloid-derived suppressor cell; TAM: tumor-associated macrophage; A2AR: adenosine 2A receptors; ATP: adenosine triphosphate; AMP: adenosine monophosphate; CDH6: human cadherin 6; CAIX: carbonic anhydrase IX; TCA: tricarboxylic acid; HIF: hypoxia-inducible factor.
With an improved understanding of the ccRCC biology, tumor metabolism, and tumor microenvironment, we foresee the treatment paradigm in ccRCC would enter an era that adopts biomarkers to select treatment options for this heterogenous disease in the near future.
Author Contributions
Y.-W.C. and K.E.B.: writing—original draft preparation. B.I.R.: writing—editing and review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Y.W.C.: has received consulting fee for Deloitte, and holds Amgen stock (an immediate family member). B.I.R.: has received research funding for his institution from Pfizer, Roche/Genentech, Bristol-Myers Squibb, Merck, AstraZeneca/MedImmune, Incyte, Arrowhead Pharmaceuticals, Taris, Seattle Genetics, ImmunoMedics, Surface Oncology, Dragonfly Therapeutics, Aravive, Exelixis. Consulting fee from Pfizer, Merck, Synthrox, Bristol-Myers Squibb, AVEO, Surface Oncology, 3D Medicines, Corvus Pharmaceuticals, Aravive, Arrowhead Pharmaceuticals, Shionogi, GlaxoSmithKline. Travel support from Pfizer, Bristol-Myers Squibb, Merck. Leadership to MJH Life Sciences. Stock: PTC Therapeutics. K.E.B.: has received research funding for her institution from Bristol-Myers Squibb, Merck Sharp & Dohme. Consulting fees from Aravive, Aveo, Exelixis, Bristol-Myers Squibb, Sanofi, Astellas, Seattle Genetics and Alpine Immune Sciences.
References
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013, 499, 43–49. [CrossRef]
- Kaelin, W.G., Jr. The von Hippel-Lindau tumor suppressor gene and kidney cancer. Clin. Cancer Res. 2004, 10, 6290s–6295s. [Google Scholar] [CrossRef]
- Courtney, K.D.; Infante, J.R.; Lam, E.T.; Figlin, R.A.; Rini, B.I.; Brugarolas, J.; Zojwalla, N.J.; Lowe, A.M.; Wang, K.; Wallace, E.M.; et al. Phase I Dose-Escalation Trial of PT2385, a First-in-Class Hypoxia-Inducible Factor-2α Antagonist in Patients with Previously Treated Advanced Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2018, 36, 867–874. [Google Scholar] [CrossRef]
- Fallah, J.; Brave, M.H.; Weinstock, C.; Mehta, G.U.; Bradford, D.; Gittleman, H.; Bloomquist, E.W.; Charlab, R.; Hamed, S.S.; Miller, C.P.; et al. FDA Approval Summary: Belzutifan for von Hippel-Lindau disease associated tumors. Clin. Cancer Res. 2022. [Google Scholar] [CrossRef]
- Dizman, N.; Philip, E.J.; Pal, S.K. Genomic profiling in renal cell carcinoma. Nat. Rev. Nephrol. 2020, 16, 435–451. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Bauer, T.M.; Papadopoulos, K.P.; Plimack, E.R.; Merchan, J.R.; McDermott, D.F.; Michaelson, M.D.; Appleman, L.J.; Thamake, S.; Perini, R.F.; et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: A phase 1 trial and biomarker analysis. Nat. Med. 2021, 27, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Bauer, T.M.; Papadopoulos, K.; Plimack, E.R.; Merchan, J.R.; McDermott, D.F.; Michaelson, M.D.; Appleman, L.J.; Roy, A.; Liu, Y.; et al. Phase 1 LITESPARK-001 (MK-6482-001) study of belzutifan in advanced solid tumors: Update of the clear cell renal cell carcinoma (ccRCC) cohort with more than 3 years of total follow-up. J. Clin. Oncol. 2022, 40, 4509. [Google Scholar] [CrossRef]
- Toni, K.C.; Bauer, T.M.; McDermott, D.F.; Arrowsmith, E.; Roy, A.; Perini, R.F.; Vickery, D.; Tykodi, S. Phase 2 study of the oral hypoxia-inducible factor 2α (HIF-2α) inhibitor MK-6482 in combination with cabozantinib in patients with advanced clear cell renal cell carcinoma (ccRCC). J. Clin. Oncol. 2021, 39, 272. [Google Scholar]
- Choueiri, T.K.; Plimack, E.R.; Powles, T.; Voss, M.H.; Gurney, H.; Silverman, R.K.; Perini, R.F.; Rodriguez-Lopez, K.; Rini, B.I. Phase 3 study of first-line treatment with pembrolizumab + belzutifan + lenvatinib or pembrolizumab/quavonlimab + lenvatinib versus pembrolizumab + lenvatinib for advanced renal cell carcinoma (RCC). J. Clin. Oncol. 2022, 40. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Bedke, J.; Karam, J.A.; McKay, R.R.; Motzer, R.J.; Pal, S.K.; Suárez, C.; Uzzo, R.; Liu, H.; Burgents, J.; et al. LITESPARK-022: A phase 3 study of pembrolizumab + belzutifan as adjuvant treatment of clear cell renal cell carcinoma (ccRCC). J. Clin. Oncol. 2022, 40, TPS4602. [Google Scholar] [CrossRef]
- Brugarolas, J.; Beckermann, K.; Rini, B.I.; Vogelzang, N.J.; Lam, E.T.; Hamilton, J.C.; Schluep, T.; Yi, M.; Wong, S.; Gamelin, E.; et al. Initial results from the phase 1 study of ARO-HIF2 to silence HIF2-alpha in patients with advanced ccRCC (AROHIF21001). J. Clin. Oncol. 2022, 40, 339. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Reznik, E.; Lee, C.H.; Creighton, C.J.; Brannon, A.R.; Luna, A.; Aksoy, B.A.; Liu, E.M.; Shen, R.; Lee, W.; et al. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 2016, 29, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, P.A.; Yang, J.; Metelo, A.M.; Pérez-Carro, R.; Baker, R.; Wang, Z.; Arreola, A.; Rathmell, W.K.; Olumi, A.; López-Larrubia, P.; et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab. 2013, 17, 372–385. [Google Scholar] [CrossRef]
- Wettersten, H.I.; Hakimi, A.A.; Morin, D.; Bianchi, C.; Johnstone, M.E.; Donohoe, D.R.; Trott, J.F.; Aboud, O.A.; Stirdivant, S.; Neri, B.; et al. Grade-Dependent Metabolic Reprogramming in Kidney Cancer Revealed by Combined Proteomics and Metabolomics Analysis. Cancer Res. 2015, 75, 2541–2552. [Google Scholar] [CrossRef]
- Reinfeld, B.I.; Madden, M.Z.; Wolf, M.M.; Chytil, A.; Bader, J.E.; Patterson, A.R.; Sugiura, A.; Cohen, A.S.; Ali, A.; Do, B.T.; et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 2021, 593, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Hoerner, C.R.; Miao, S.Y.; Hsieh, J.J.; Fan, A.C. Targeting Metabolic Pathways in Kidney Cancer: Rationale and Therapeutic Opportunities. Cancer J. 2020, 26, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Telli, M.; Munster, P.; Voss, M.H.; Infante, J.R.; DeMichele, A.; Dunphy, M.; Le, M.H.; Molineaux, C.; Orford, K.; et al. A Phase I Dose-Escalation and Expansion Study of Telaglenastat in Patients with Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2021, 27, 4994–5003. [Google Scholar] [CrossRef] [PubMed]
- Emberley, E.; Pan, A.; Chen, J.; Dang, R.; Gross, M.; Huang, T.; Li, W.; MacKinnon, A.; Singh, D.; Sotirovska, N.; et al. The glutaminase inhibitor telaglenastat enhances the antitumor activity of signal transduction inhibitors everolimus and cabozantinib in models of renal cell carcinoma. PLoS ONE 2021, 16, e0259241. [Google Scholar] [CrossRef]
- Motzer, R.J.; Lee, C.H.; Emamekhoo, H.; Matrana, M.; Percent, I.; Hsieh, J.J.; Hussain, A.; Vaishampayan, U.N.; Graham, R.; Liu, S.; et al. LBA54-ENTRATA: Randomized, double-blind, phase II study of telaglenastat (tela; CB-839) + everolimus (E) vs placebo (pbo) + E in patients (pts) with advanced/metastatic renal cell carcinoma (mRCC). Ann. Oncol. 2019, 30, v889–v890. [Google Scholar] [CrossRef]
- Tannir, N.M.; Agarwal, N.; Porta, C.; Lawrence, N.J.; Motzer, R.J.; Lee, R.J.; Jain, R.K.; Davis, N.B.; Appleman, L.J.; Goodman, O.B.; et al. CANTATA: Primary analysis of a global, randomized, placebo (Pbo)-controlled, double-blind trial of telaglenastat (CB-839) + cabozantinib versus Pbo + cabozantinib in advanced/metastatic renal cell carcinoma (mRCC) patients (pts) who progressed on immune checkpoint inhibitor (ICI) or anti-angiogenic therapies. J. Clin. Oncol. 2021, 39, 4501. [Google Scholar]
- Cekic, C.; Linden, J. Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Cancer Res. 2014, 74, 7239–7249. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Ngiow, S.F.; Gao, Y.; Patch, A.M.; Barkauskas, D.S.; Messaoudene, M.; Lin, G.; Coudert, J.D.; Stannard, K.A.; Zitvogel, L.; et al. A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer Res. 2018, 78, 1003–1016. [Google Scholar] [CrossRef]
- Morello, S.; Pinto, A.; Blandizzi, C.; Antonioli, L. Myeloid cells in the tumor microenvironment: Role of adenosine. Oncoimmunology 2016, 5, e1108515. [Google Scholar] [CrossRef]
- Zarek, P.E.; Huang, C.T.; Lutz, E.R.; Kowalski, J.; Horton, M.R.; Linden, J.; Drake, C.G.; Powell, J.D. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 2008, 111, 251–259. [Google Scholar] [CrossRef]
- Mandapathil, M.; Hilldorfer, B.; Szczepanski, M.J.; Czystowska, M.; Szajnik, M.; Ren, J.; Lang, S.; Jackson, E.K.; Gorelik, E.; Whiteside, T.L. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J. Biol. Chem. 2010, 285, 7176–7186. [Google Scholar] [CrossRef] [PubMed]
- Beavis, P.A.; Milenkovski, N.; Henderson, M.A.; John, L.B.; Allard, B.; Loi, S.; Kershaw, M.H.; Stagg, J.; Darcy, P.K. Adenosine Receptor 2A Blockade Increases the Efficacy of Anti-PD-1 through Enhanced Antitumor T-cell Responses. Cancer Immunol. Res. 2015, 3, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Maj, T.; Wang, W.; Crespo, J.; Zhang, H.; Wang, W.; Wei, S.; Zhao, L.; Vatan, L.; Shao, I.; Szeliga, W.; et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 2017, 18, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Ho, P.Y.; Hotson, A.; Hill, C.; Piccione, E.C.; Hsieh, J.; Liu, L.; Buggy, J.J.; McCaffery, I.; Miller, R.A. A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti-PD-(L)1 and Anti-CTLA-4 in Preclinical Models. Cancer Immunol. Res. 2018, 6, 1136–1149. [Google Scholar] [CrossRef]
- Fong, L.; Hotson, A.; Powderly, J.D.; Sznol, M.; Heist, R.S.; Choueiri, T.K.; George, S.; Hughes, B.G.M.; Hellmann, M.D.; Shepard, D.R.; et al. Adenosine 2A Receptor Blockade as an Immunotherapy for Treatment-Refractory Renal Cell Cancer. Cancer Discov. 2020, 10, 40–53. [Google Scholar] [CrossRef]
- CORVUS Pharmaceutical. Pipeline. Available online: https://www.corvuspharma.com/our-science/our-pipeline/ (accessed on 8 May 2022).
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Allard, B.; Pommey, S.; Smyth, M.J.; Stagg, J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin. Cancer Res. 2013, 19, 5626–5635. [Google Scholar] [CrossRef]
- Siu, L.L.; Burris, H.; Le, D.T.; Hollebecque, A.; Steeghs, N.; Delord, J.; Hilton, J.; Barnhart, B.; Sega, E.; Sanghavi, K.; et al. Abstract CT180: Preliminary phase 1 profile of BMS-986179, an anti-CD73 antibody, in combination with nivolumab in patients with advanced solid tumors. Cancer Res. 2018, 78, CT180. [Google Scholar] [CrossRef]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef]
- Chatzkel, J.A.; Swank, J.; Ludlow, S.; Lombardi, K.; Croft, C.; Artigas, Y.; Rodriguez, Y.; Terraciano, T.; Hart, S.; Rembisz, J.; et al. Overall responses with coordinated pembrolizumab and high dose IL-2 (5-in-a-row schedule) for therapy of metastatic clear cell renal cancer: A single center, single arm trial. J. Clin. Oncol. 2019, 37, 657. [Google Scholar] [CrossRef]
- Lopes, J.E.; Fisher, J.L.; Flick, H.L.; Wang, C.; Sun, L.; Ernstoff, M.S.; Alvarez, J.C.; Losey, H.C. ALKS 4230: A novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000673. [Google Scholar] [CrossRef] [PubMed]
- Calvo, E.; Boni, V.; Chaudhry, A.; Debruyne, P.R.; Rosen, S.D.; Wang, Y.; Sun, L.; Desai, M.; Dalal, R.P.; Du, Y.; et al. Nemvaleukin alfa in patients with advanced renal cell carcinoma: ARTISTRY-1. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 330. [Google Scholar] [CrossRef]
- Vaishampayan, U.N.; Tomczak, P.; Muzaffar, J.; Winer, I.; Rosen, S.; Hoimes, C.; Chauhan, A.; Spreafico, A.; Lewis, K.D.; Bruno, D.S.; et al. Nemvaleukin alfa monotherapy and in combination with pembrolizumab in patients (pts) with advanced solid tumors: ARTISTRY-1. J. Clin. Oncol. 2022, 40, 2500. [Google Scholar] [CrossRef]
- Rolig, A.S.; Rose, D.C.; McGee, G.H.; Rubas, W.; Kivimäe, S.; Redmond, W.L. Combining bempegaldesleukin (CD122-preferential IL-2 pathway agonist) and NKTR-262 (TLR7/8 agonist) improves systemic antitumor CD8(+) T cell cytotoxicity over BEMPEG+RT. J. Immunother. Cancer 2022, 10, e004218. [Google Scholar] [CrossRef]
- Bristol Myers Squibb. Bristol Myers Squibb and Nektar Announce Update on Phase 3 PIVOT IO-001 Trial Evaluating Bempegaldesleukin (BEMPEG) in Combination with Opdivo (Nivolumab) in Previously Untreated Unresectable or Metastatic Melanoma. Available online: https://news.bms.com/news/corporate-financial/2022/Bristol-Myers-Squibb-and-Nektar-Announce-Update-on-Phase-3-PIVOT-IO-001-Trial-Evaluating-Bempegaldesleukin-BEMPEG-in-Combination-with-Opdivo-nivolumab-in-Previously-Untreated-Unresectable-or-Metastatic-Melanoma/default.aspx (accessed on 8 May 2022).
- Bristol Myers Squibb. Nektar and Bristol Myers Squibb Announce Update on Clinical Development Program for Bempegaldesleukin (BEMPEG) in Combination with Opdivo (Nivolumab). Available online: https://news.bms.com/news/corporate-financial/2022/Nektar-and-Bristol-Myers-Squibb-Announce-Update-on-Clinical-Development-Program-for-Bempegaldesleukin-BEMPEG-in-Combination-with-Opdivo-nivolumab/default.aspx (accessed on 8 May 2022).
- Desbois, M.; Béal, C.; Charrier, M.; Besse, B.; Meurice, G.; Cagnard, N.; Jacques, Y.; Béchard, D.; Cassard, L.; Chaput, N. IL-15 superagonist RLI has potent immunostimulatory properties on NK cells: Implications for antimetastatic treatment. J. Immunother. Cancer 2020, 8, e000632. [Google Scholar] [CrossRef]
- Rubinstein, M.P.; Kovar, M.; Purton, J.F.; Cho, J.H.; Boyman, O.; Surh, C.D.; Sprent, J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. Proc. Natl. Acad. Sci. USA 2006, 103, 9166–9171. [Google Scholar] [CrossRef]
- Stoklasek, T.A.; Schluns, K.S.; Lefrançois, L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 2006, 177, 6072–6080. [Google Scholar] [CrossRef]
- Chihara, N.; Madi, A.; Kondo, T.; Zhang, H.; Acharya, N.; Singer, M.; Nyman, J.; Marjanovic, N.D.; Kowalczyk, M.S.; Wang, C.; et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 2018, 558, 454–459. [Google Scholar] [CrossRef]
- DeLong, J.H.; O’Hara Hall, A.; Rausch, M.; Moodley, D.; Perry, J.; Park, J.; Phan, A.T.; Beiting, D.P.; Kedl, R.M.; Hill, J.A.; et al. IL-27 and TCR Stimulation Promote T Cell Expression of Multiple Inhibitory Receptors. ImmunoHorizons 2019, 3, 13–25. [Google Scholar] [CrossRef]
- Rausch, M.; Hua, J.; Moodley, D.; White, K.F.; Walsh, K.H.; Miller, C.E.; Tan, G.; Lee, B.H.; Cousineau, I.; Lattouf, J.B.; et al. Abstract 4550: Increased IL-27 is associated with poor prognosis in renal cell carcinoma and supports use of SRF388, a first-in-class IL-27p28 blocking antibody, to counteract IL-27-mediated immunosuppression in this setting. Cancer Res. 2020, 80, 4550. [Google Scholar] [CrossRef]
- Naing, A.; Mantia, C.; Morgensztern, D.; Kim, T.; Li, D.; Kang, Y.; Marron, T.; Tripathi, A.; George, S.; Rini, B.; et al. First-in-human study of SRF388, a first-in-class IL-27 targeting antibody, as monotherapy and in combination with pembrolizumab in patients with advanced solid tumors. J. Clin. Oncol. 2022, 40, 2501. [Google Scholar] [CrossRef]
- Ruffo, E.; Wu, R.C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Lymphocyte-activation gene 3 (LAG3): The next immune checkpoint receptor. Semin. Immunol. 2019, 42, 101305. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.V.; Drake, C.G. LAG-3 in Cancer Immunotherapy. Curr. Top. Microbiol. Immunol. 2011, 344, 269–278. [Google Scholar] [CrossRef]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef]
- Woo, S.R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- FDA. FDA Approves Opdualag for Unresectable or Metastatic Melanoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-opdualag-unresectable-or-metastatic-melanoma (accessed on 26 May 2022).
- Schöffski, P.; Tan, D.S.W.; Martín, M.; Ochoa-de-Olza, M.; Sarantopoulos, J.; Carvajal, R.D.; Kyi, C.; Esaki, T.; Prawira, A.; Akerley, W.; et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Harjunpää, H.; Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.S.; Prokhnevska, N.; Master, V.A.; Sanda, M.G.; Carlisle, J.W.; Bilen, M.A.; Cardenas, M.; Wilkinson, S.; Lake, R.; Sowalsky, A.G.; et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 2019, 576, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.H.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 impair tumor antigen-specific CD8⁺ T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022. [Google Scholar] [CrossRef]
- Shiroishi, M.; Tsumoto, K.; Amano, K.; Shirakihara, Y.; Colonna, M.; Braud, V.M.; Allan, D.S.; Makadzange, A.; Rowland-Jones, S.; Willcox, B.; et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc. Natl. Acad. Sci. USA 2003, 100, 8856–8861. [Google Scholar] [CrossRef] [PubMed]
- Dunker, K.; Schlaf, G.; Bukur, J.; Altermann, W.W.; Handke, D.; Seliger, B. Expression and regulation of non-classical HLA-G in renal cell carcinoma. Tissue Antigens 2008, 72, 137–148. [Google Scholar] [CrossRef]
- Rouas-Freiss, N.; LeMaoult, J.; Verine, J.; Tronik-Le Roux, D.; Culine, S.; Hennequin, C.; Desgrandchamps, F.; Carosella, E.D. Intratumor heterogeneity of immune checkpoints in primary renal cell cancer: Focus on HLA-G/ILT2/ILT4. Oncoimmunology 2017, 6, e1342023. [Google Scholar] [CrossRef]
- Mondal, K.; Song, C.; Tian, J.; Ho, C.; Rivera, L.B.; Huang, J.; Chen, P.; Crawley, S.; Lin, V.Y.; Sitrin, J.; et al. Abstract LB156: Preclinical evaluation of NGM707, a novel anti-ILT2/anti-ILT4 dual antagonist monoclonal antibody. Cancer Res. 2021, 81, LB156. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Singh, L.; Muise, E.S.; Bhattacharya, A.; Grein, J.; Javaid, S.; Stivers, P.; Zhang, J.; Qu, Y.; Joyce-Shaikh, B.; Loboda, A.; et al. ILT3 (LILRB4) Promotes the Immunosuppressive Function of Tumor-Educated Human Monocytic Myeloid-Derived Suppressor Cells. Mol. Cancer Res. 2021, 19, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Ji, Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front. Immunol. 2022, 13, 874589. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Choi, S.H. Tumor-associated macrophages in cancer: Recent advancements in cancer nanoimmunotherapies. J. Exp. Clin. Cancer Res. 2022, 41, 68. [Google Scholar] [CrossRef] [PubMed]
- Katzenelenbogen, Y.; Sheban, F.; Yalin, A.; Yofe, I.; Svetlichnyy, D.; Jaitin, D.A.; Bornstein, C.; Moshe, A.; Keren-Shaul, H.; Cohen, M.; et al. Coupled scRNA-Seq and Intracellular Protein Activity Reveal an Immunosuppressive Role of TREM2 in Cancer. Cell 2020, 182, 872–885.e819. [Google Scholar] [CrossRef]
- Obradovic, A.; Chowdhury, N.; Haake, S.M.; Ager, C.; Wang, V.; Vlahos, L.; Guo, X.V.; Aggen, D.H.; Rathmell, W.K.; Jonasch, E.; et al. Single-cell protein activity analysis identifies recurrence-associated renal tumor macrophages. Cell 2021, 184, 2988–3005.e2916. [Google Scholar] [CrossRef]
- Binnewies, M.; Abushawish, M.; Lee, T.; Le, T.; Pollack, J.L.; Lu, E.; Chen, A.; Mehta, R.; Jahchan, N.; Huang, V.; et al. Abstract C104: Therapeutic targeting of TREM2+ tumor-associated macrophages as a means of overcoming checkpoint inhibitor resistance. Mol. Cancer Ther. 2019, 18, C104. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Linch, S.N.; McNamara, M.J.; Redmond, W.L. OX40 Agonists and Combination Immunotherapy: Putting the Pedal to the Metal. Front. Oncol. 2015, 5, 34. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Cheng, D.; Xia, Z.; Luan, M.; Zhang, S. PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS ONE 2014, 9, e89350. [Google Scholar] [CrossRef]
- Lao, J.; Cao, C.; Niu, X.; Deng, S.; Ming, S.; Liang, S.; Shang, Y.; Yuan, Y.; Shi, X.; Liang, Z.; et al. OX40 enhances T cell immune response to PD-1 blockade therapy in non-small cell lung cancer. Int. Immunopharmacol. 2022, 108, 108813. [Google Scholar] [CrossRef]
- Diab, A.; Hamid, O.; Thompson, J.A.; Ros, W.; Eskens, F.; Doi, T.; Hu-Lieskovan, S.; Klempner, S.J.; Ganguly, B.; Fleener, C.; et al. A Phase I, Open-Label, Dose-Escalation Study of the OX40 Agonist Ivuxolimab in Patients with Locally Advanced or Metastatic Cancers. Clin. Cancer Res. 2022, 28, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Parikh, R.; Tsao-Wei, D.; Groshen, S.; Li, M.; Appleman, L.; Tagawa, S.; Nanus, D.M.; Molina, A.; Kefauver, C.; et al. Phase II randomized double blind trial of axitinib (Axi) +/− PF-04518600, an OX40 antibody (PFOX) after PD1/PDL1 antibody (IO) therapy (Tx) in metastatic renal cell carcinoma (mRCC). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 4529. [Google Scholar] [CrossRef]
- Gutierrez, M.; Moreno, V.; Heinhuis, K.M.; Olszanski, A.J.; Spreafico, A.; Ong, M.; Chu, Q.; Carvajal, R.D.; Trigo, J.; Ochoa de Olza, M.; et al. OX40 Agonist BMS-986178 Alone or in Combination With Nivolumab and/or Ipilimumab in Patients With Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Rowell, E.; Kinkead, H.; Torretti, E.; Becklund, B.; Sulzmaier, F.; Crago, W.; Jones, K.; Timmer, J.; Deveraux, Q.; Eckelman, B.; et al. 856 INBRX-106: A novel hexavalent anti-OX40 agonist for the treatment of solid tumors. J. ImmunoTherapy Cancer 2021, 9, A897. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, Y.; Wei, X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef]
- Shieh, Y.S.; Lai, C.Y.; Kao, Y.R.; Shiah, S.G.; Chu, Y.W.; Lee, H.S.; Wu, C.W. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia 2005, 7, 1058–1064. [Google Scholar] [CrossRef]
- Gjerdrum, C.; Tiron, C.; Høiby, T.; Stefansson, I.; Haugen, H.; Sandal, T.; Collett, K.; Li, S.; McCormack, E.; Gjertsen, B.T.; et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl. Acad. Sci. USA 2010, 107, 1124–1129. [Google Scholar] [CrossRef]
- Gustafsson, A.; Martuszewska, D.; Johansson, M.; Ekman, C.; Hafizi, S.; Ljungberg, B.; Dahlbäck, B. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin. Cancer Res. 2009, 15, 4742–4749. [Google Scholar] [CrossRef]
- Rankin, E.B.; Fuh, K.C.; Castellini, L.; Viswanathan, K.; Finger, E.C.; Diep, A.N.; LaGory, E.L.; Kariolis, M.S.; Chan, A.; Lindgren, D.; et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc. Natl. Acad. Sci. USA 2014, 111, 13373–13378. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, X.D.; Sun, M.; Zhang, X.; German, P.; Bai, S.; Ding, Z.; Tannir, N.; Wood, C.G.; Matin, S.F.; et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016, 35, 2687–2697. [Google Scholar] [CrossRef]
- Tanaka, M.; Siemann, D.W. Gas6/Axl Signaling Pathway in the Tumor Immune Microenvironment. Cancers 2020, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, L.; Dodds, M.; Prohaska, D.; Moss, A.; Giaccia, A.; Tabibiazar, R.; McIntyre, G. Target-Mediated Drug Disposition Pharmacokinetic/Pharmacodynamic Model-Informed Dose Selection for the First-in-Human Study of AVB-S6-500. Clin. Transl. Sci. 2020, 13, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Kariolis, M.S.; Miao, Y.R.; Diep, A.; Nash, S.E.; Olcina, M.M.; Jiang, D.; Jones, D.S., 2nd; Kapur, S.; Mathews, I.I.; Koong, A.C.; et al. Inhibition of the GAS6/AXL pathway augments the efficacy of chemotherapies. J. Clin. Investig. 2017, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Mullen, M.M.; Lomonosova, E.; Toboni, M.D.; Oplt, A.; Cybulla, E.; Blachut, B.; Zhao, P.; Noia, H.; Wilke, D.; Rankin, E.B.; et al. GAS6/AXL Inhibition Enhances Ovarian Cancer Sensitivity to Chemotherapy and PARP Inhibition through Increased DNA Damage and Enhanced Replication Stress. Mol. Cancer Res. 2022, 20, 265–279. [Google Scholar] [CrossRef]
- Beckermann, K.; Vogelzang, N.; Shifeng, M.; Ornstein, M.; Shah, N.; Hammers, H.; Campbell, M.; Gao, X.; McDermott, D.; Anderson, R.; et al. 424 A phase 1b/2 randomized study of AVB-S6–500 in combination with cabozantinib versus cabozantinib alone in patients with advanced clear cell renal cell carcinoma who have received front-line treatment. J. ImmunoTherapy Cancer 2021, 9, A454. [Google Scholar] [CrossRef]
- Beckermann, K.; Shah, N.; Vogelzang, N.; Mao, S.; Ornstein, M.; Hammers, H.; Gao, X.; McDermott, D.F.; Haas, N.; Yan, H.; et al. A phase 1b/2 study of batiraxcept (AVB-S6-500) in combination with cabozantinib, cabozantinib and nivolumab, and as monotherapy in patients with advanced or metastatic clear cell renal cell carcinoma (NCT04300140). J. Clin. Oncol. 2022, 40, TPS4599. [Google Scholar] [CrossRef]
- Shah, N.; Beckermann, K.; Vogelzang, N.J.; Mao, S.; Ornstein, M.; Hammers, H.J.; Gao, X.; McDermott, D.F.; Haas, N.; Yan, H.; et al. A phase 1b/2 study of batiraxcept (AVB-S6-500) in combination with cabozantinib in patients with advanced or metastatic clear cell renal cell (ccRCC) carcinoma who have received front-line treatment (NCT04300140). J. Clin. Oncol. 2022, 40, 4511. [Google Scholar] [CrossRef]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Kong, A.; Mehanna, H. WEE1 Inhibitor: Clinical Development. Curr. Oncol. Rep. 2021, 23, 107. [Google Scholar] [CrossRef]
- Garcia, T.B.; Fosmire, S.P.; Porter, C.C. Increased activity of both CDK1 and CDK2 is necessary for the combinatorial activity of WEE1 inhibition and cytarabine. Leuk. Res. 2018, 64, 30–33. [Google Scholar] [CrossRef]
- Beck, H.; Nähse, V.; Larsen, M.S.; Groth, P.; Clancy, T.; Lees, M.; Jørgensen, M.; Helleday, T.; Syljuåsen, R.G.; Sørensen, C.S. Regulators of cyclin-dependent kinases are crucial for maintaining genome integrity in S phase. J. Cell Biol. 2010, 188, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, T.N.; Qian, C.; Sugitani, N.; Osmanbeyoglu, H.U.; Bakkenist, C.J. WEE1 kinase inhibitor AZD1775 induces CDK1 kinase-dependent origin firing in unperturbed G1- and S-phase cells. Proc. Natl. Acad. Sci. USA 2019, 116, 23891–23893. [Google Scholar] [CrossRef] [PubMed]
- Geenen, J.J.J.; Schellens, J.H.M. Molecular Pathways: Targeting the Protein Kinase Wee1 in Cancer. Clin. Cancer Res. 2017, 23, 4540–4544. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.X.; Markkanen, E.; Jiang, Y.; Sarkar, S.; Woodcock, M.; Orlando, G.; Mavrommati, I.; Pai, C.C.; Zalmas, L.P.; Drobnitzky, N.; et al. Inhibiting WEE1 Selectively Kills Histone H3K36me3-Deficient Cancers by dNTP Starvation. Cancer Cell 2015, 28, 557–568. [Google Scholar] [CrossRef]
- Li, J.; Duns, G.; Westers, H.; Sijmons, R.; van den Berg, A.; Kok, K. SETD2: An epigenetic modifier with tumor suppressor functionality. Oncotarget 2016, 7, 50719–50734. [Google Scholar] [CrossRef]
- Gerlinger, M.; Horswell, S.; Larkin, J.; Rowan, A.J.; Salm, M.P.; Varela, I.; Fisher, R.; McGranahan, N.; Matthews, N.; Santos, C.R.; et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet. 2014, 46, 225–233. [Google Scholar] [CrossRef]
- Lebert, J.; Lilly, E.J. Developments in the Management of Metastatic HER2-Positive Breast Cancer: A Review. Curr. Oncol. 2022, 29, 2539–2549. [Google Scholar] [CrossRef]
- Bartolomé, R.A.; Robles, J.; Martin-Regalado, Á.; Pintado-Berninches, L.; Burdiel, M.; Jaén, M.; Aizpurúa, C.; Imbaud, J.I.; Casal, J.I. CDH6-activated αIIbβ3 crosstalks with α2β1 to trigger cellular adhesion and invasion in metastatic ovarian and renal cancers. Mol. Oncol. 2021, 15, 1849–1865. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Jauhari, S.; Moore, K.N.; Rini, B.I.; McLeod, R.; Lin, J.; Izumi, N.; Gopal Kundu, M.; Myobatake, Y.; Laadem, A.; et al. Phase I, two-part, multicenter, first-in-human (FIH) study of DS-6000a in subjects with advanced renal cell carcinoma (RCC) and ovarian tumors (OVC). J. Clin. Oncol. 2022, 40, 3002. [Google Scholar] [CrossRef]
- Du, W.; Huang, H.; Sorrelle, N.; Brekken, R.A. Sitravatinib potentiates immune checkpoint blockade in refractory cancer models. JCI Insight 2018, 3, e124184. [Google Scholar] [CrossRef]
- Msaouel, P.; Goswami, S.; Thall, P.F.; Wang, X.; Yuan, Y.; Jonasch, E.; Gao, J.; Campbell, M.T.; Shah, A.Y.; Corn, P.G.; et al. A phase 1-2 trial of sitravatinib and nivolumab in clear cell renal cell carcinoma following progression on antiangiogenic therapy. Sci. Transl. Med. 2022, 14, eabm6420. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.A.; Msaouel, P.; Matin, S.F.; Campbell, M.T.; Zurita, A.; Shah, A.Y.; Wistuba, I.I.; Haymaker, C.L.; Marmonti, E.; Duose, D.Y.; et al. A phase II study of sitravatinib (Sitra) in combination with nivolumab (Nivo) in patients (Pts) undergoing nephrectomy for locally-advanced clear cell renal cell carcinoma (accRCC). J. Clin. Oncol. 2021, 39, 312. [Google Scholar] [CrossRef]
- Msaouel, P.; Gao, J.; Yuan, Y.; Siefker-Radtke, A.O.; Jonasch, E.; Goswami, S.; Zurita, A.; Campbell, M.T.; Shah, A.Y.; Corn, P.G.; et al. Phase I/IB trial of sitravatinib (Sitra) + nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with advanced clear cell renal cell carcinoma (accRCC) or other solid malignancies. J. Clin. Oncol. 2021, 39. [Google Scholar] [CrossRef]
- Sharma, M.; Subbiah, V.; Shapiro, G.; Pal, S.; Agarwal, N.; Wentzel, K.; Hazra, S.; Vora, R.; Waldes, J.; Cha, E.; et al. A phase 1 first-in-human study of XL092 administered alone or in combination with immune checkpoint inhibitors (ICIs) in patients (pts) with inoperable locally advanced or metastatic solid tumors: Description of genitourinary (GU) expansion cohorts. J. Clin. Oncol. 2022, 40. [Google Scholar] [CrossRef]
- Choueiri, T.K.; McGregor, B.; Shah, N.; Bajaj, A.; Chahoud, J.; O’Neil, B.; Michalski, J.; Garmezy, B.; Jin, L.; Oliver, J.; et al. A phase 1b study (STELLAR-002) of XL092 administered in combination with nivolumab (NIVO) with or without ipilimumab (IPI) or bempegaldesleukin (BEMPEG) in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2022, 40, TPS4600. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Sciences 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Sciences 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Salgia, N.J.; Bergerot, P.G.; Maia, M.C.; Dizman, N.; Hsu, J.; Gillece, J.D.; Folkerts, M.; Reining, L.; Trent, J.; Highlander, S.K.; et al. Stool Microbiome Profiling of Patients with Metastatic Renal Cell Carcinoma Receiving Anti-PD-1 Immune Checkpoint Inhibitors. Eur. Urol. 2020, 78, 498–502. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Sciences 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat. Med. 2022, 28, 704–712. [Google Scholar] [CrossRef]
- Hagihara, M.; Kuroki, Y.; Ariyoshi, T.; Higashi, S.; Fukuda, K.; Yamashita, R.; Matsumoto, A.; Mori, T.; Mimura, K.; Yamaguchi, N.; et al. Clostridium butyricum Modulates the Microbiome to Protect Intestinal Barrier Function in Mice with Antibiotic-Induced Dysbiosis. iScience 2020, 23, 100772. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Stillebroer, A.B.; Mulders, P.F.; Boerman, O.C.; Oyen, W.J.; Oosterwijk, E. Carbonic anhydrase IX in renal cell carcinoma: Implications for prognosis, diagnosis, and therapy. Eur. Urol. 2010, 58, 75–83. [Google Scholar] [CrossRef]
- Chamie, K.; Donin, N.M.; Klöpfer, P.; Bevan, P.; Fall, B.; Wilhelm, O.; Störkel, S.; Said, J.; Gambla, M.; Hawkins, R.E.; et al. Adjuvant Weekly Girentuximab Following Nephrectomy for High-Risk Renal Cell Carcinoma: The ARISER Randomized Clinical Trial. JAMA Oncol. 2017, 3, 913–920. [Google Scholar] [CrossRef]
- Muselaers, C.H.; Boers-Sonderen, M.J.; van Oostenbrugge, T.J.; Boerman, O.C.; Desar, I.M.; Stillebroer, A.B.; Mulder, S.F.; van Herpen, C.M.; Langenhuijsen, J.F.; Oosterwijk, E.; et al. Phase 2 Study of Lutetium 177-Labeled Anti-Carbonic Anhydrase IX Monoclonal Antibody Girentuximab in Patients with Advanced Renal Cell Carcinoma. Eur. Urol. 2016, 69, 767–770. [Google Scholar] [CrossRef]
- van Oostenbrugge, T.; Mulders, P. Targeted PET/CT imaging for clear cell renal cell carcinoma with radiolabeled antibodies: Recent developments using girentuximab. Curr. Opin. Urol. 2021, 31, 249–254. [Google Scholar] [CrossRef]
- Donin, N.M.; Reiter, R.E. Why Targeting PSMA Is a Game Changer in the Management of Prostate Cancer. J. Nucl. Med. 2018, 59, 177–182. [Google Scholar] [CrossRef]
- Urso, L.; Castello, A.; Rocca, G.C.; Lancia, F.; Panareo, S.; Cittanti, C.; Uccelli, L.; Florimonte, L.; Castellani, M.; Ippolito, C.; et al. Role of PSMA-ligands imaging in Renal Cell Carcinoma management: Current status and future perspectives. J. Cancer Res. Clin. Oncol. 2022, 148, 1299–1311. [Google Scholar] [CrossRef]
- Siva, S.; Udovicich, C.; Tran, B.; Zargar, H.; Murphy, D.G.; Hofman, M.S. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat. Rev. Urol. 2020, 17, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Liu, Q.; Sui, H.; Wang, R.; Jacobson, O.; Fan, X.; Zhu, Z.; Chen, X. (177)Lu-EB-PSMA Radioligand Therapy with Escalating Doses in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Bergerot, P.; Lamb, P.; Wang, E.; Pal, S.K. Cabozantinib in Combination with Immunotherapy for Advanced Renal Cell Carcinoma and Urothelial Carcinoma: Rationale and Clinical Evidence. Mol Cancer 2019, 18, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- TK, C.; Powles, T.; Albiges, L.; Burotto, M.; Szczylik, C.; Zurawski, B.; Riuz, E.; Maruzzo, M.; Suarez Zaizar, A.; Fein, L.; et al. LBA8-Phase III study of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in previously untreated advanced renal cell carcinoma (aRCC) of IMDC intermediate or poor risk (COSMIC-313). Ann. Oncol. 2022, 33, S808–S869. [Google Scholar]
- Zhang, T.; Ballman, K.V.; Choudhury, A.D.; Chen, R.C.; Watt, C.; Wen, Y.; Shergill, A.; Zemla, T.J.; Emamekhoo, H.; Vaishampayan, U.N.; et al. PDIGREE: An adaptive phase III trial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).