Preclinical Efficacy and Toxicology Evaluation of RAC1 Inhibitor 1A-116 in Human Glioblastoma Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Genomic Database Analysis
2.2. Cell Lines
2.3. Cell Proliferation Assay
2.4. 3D Spheroid Cell Culture Growth
2.5. Cell Cycle Analysis
2.6. In Silico Prediction Tools: 1A-116 Pharmacokinetics
2.7. Genotyping LN229 Cell Line: Clinically Relevant Genes
2.8. Acute Toxicology and Non-Clinical Safety Studies
2.9. Glioblastoma Orthotopic Xenografts in Nude Mice
3. Results
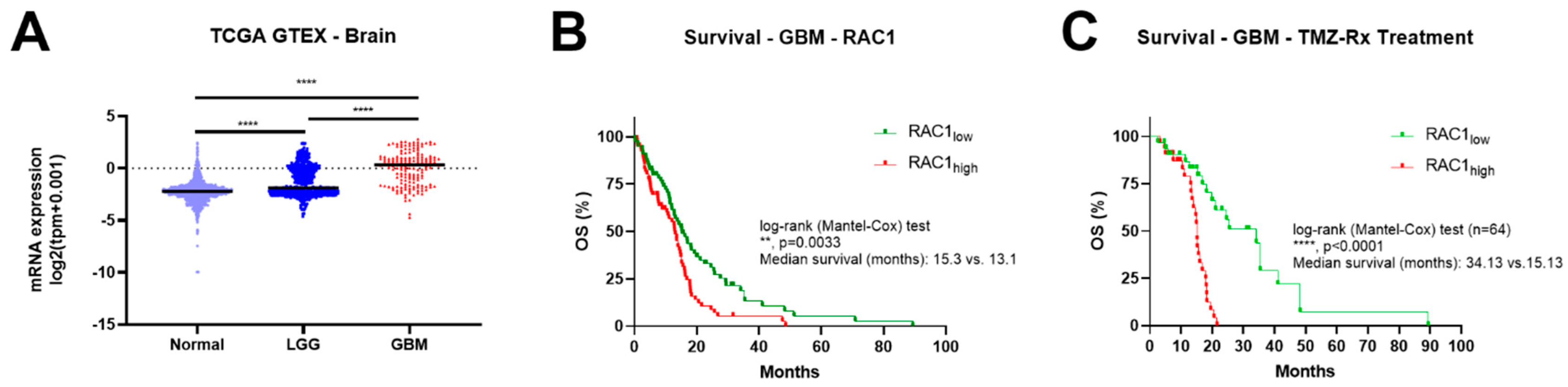
3.1. Rac1 Expression Correlates with Brain Tumor Grade and Poor Patient Outcome
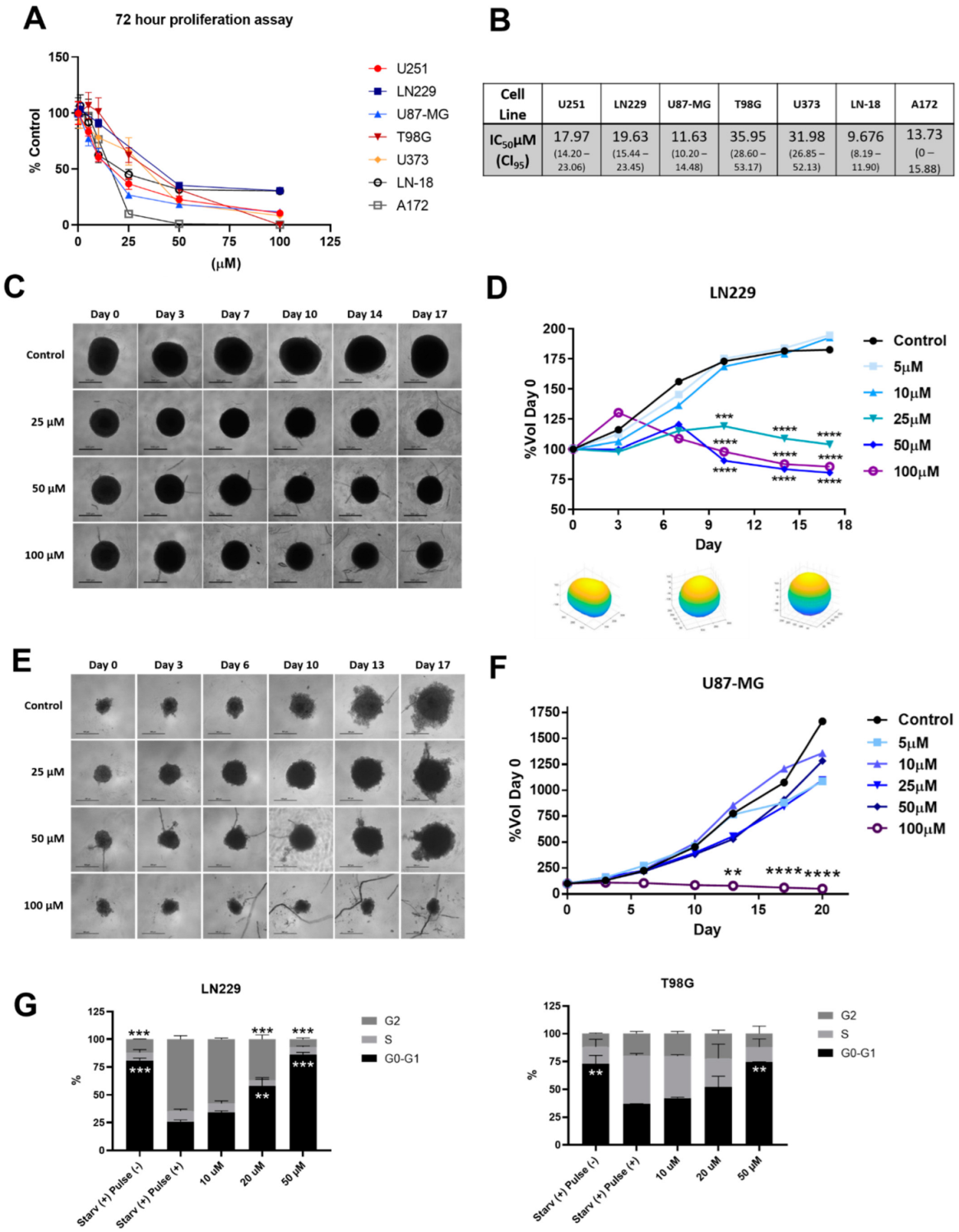
3.2. 1A-116 Rac1 Inhibitor Suppresses 2D and 3D Proliferation and Inhibits Cell Cycle Progression of Glioma Cells
3.3. 1A-116 Is Predicted to Penetrate the Blood–Brain Barrier and Present a Favorable Metabolic Fate
3.4. 1A-116 Shows a Favorable Toxicity Profile
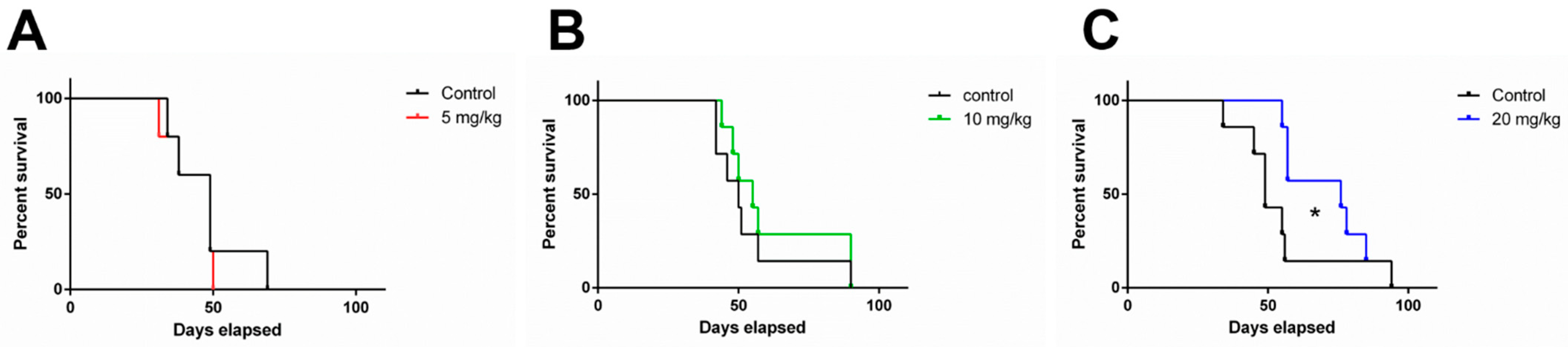
3.5. 1A-116 Elicits In Vivo Antitumor Activity in an Orthotopic Human Glioblastoma IDH-Wildtype Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwig, K.; Kornblum, H.I. Molecular markers in glioma. J. Neurooncol. 2017, 134, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Barthel, L.; Hadamitzky, M.; Dammann, P.; Schedlowski, M.; Sure, U.; Thakur, B.K.; Hetze, S. Glioma: Molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 2022, 41, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Cardama, G.A.; González, N.; Maggio, J.; Menna, P.L.; Gomez, D.E. Rho GTPases as therapeutic targets in cancer (Review). Int. J. Oncol. 2017, 51, 1025–1034. [Google Scholar] [CrossRef]
- Cardama, G.A.; Alonso, D.F.; González, N.; Maggio, J.; Gomez, D.E.; Rolfo, C.; Menna, P.L. Relevance of small GTPase Rac1 pathway in drug and radio-resistance mechanisms: Opportunities in cancer therapeutics. Crit. Rev. Oncol. Hematol. 2018, 124, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Hyun, K.H.; Kim, R.K.; Lee, H.; Lim, E.J.; Chung, H.Y.; An, S.; Park, M.J.; Suh, Y.; Kim, M.J.; et al. The small GTPase Rac1 is involved in the maintenance of stemness and malignancies in glioma stem-like cells. FEBS Lett. 2011, 585, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Oyang, L.; Rao, S.; Han, Y.; Luo, X.; Yi, P.; Lin, J.; Xia, L.; Hu, J.; Tan, S.; et al. Rac1, A Potential Target for Tumor Therapy. Front. Oncol. 2011, 11, 674426. [Google Scholar] [CrossRef] [PubMed]
- Cardama, G.A.; Comin, M.J.; Hornos, L.; Gonzalez, N.; Defelipe, L.; Turjanski, A.G.; Alonso, D.F.; Gomez, D.E.; Menna, P.L. Preclinical development of novel Rac1-GEF signaling inhibitors using a rational design approach in highly aggressive breast cancer cell lines. Anticancer Agents Med. Chem. 2014, 14, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Echeverria, E.; Lenicov, F.R.; Cardama, G.; Gonzalez, N.; Davio, C.; Fernández, N.; Menna, P.L. Pharmacological Rac1 inhibitors with selective apoptotic activity in human acute leukemic cell lines. Oncotarget 2017, 8, 98509–98523. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Cardama, G.A.; Chinestrad, P.; Robles-Valero, J.; Rodríguez-Fdez, S.; Lorenzo-Martín, L.F.; Bustelo, X.R.; Lorenzano Menna, P.; Gomez, D.E. Computational and in vitro Pharmacodynamics Characterization of 1A-116 Rac1 Inhibitor: Relevance of Trp56 in Its Biological Activity. Front. Cell Dev. Biol. 2020, 8, 240. [Google Scholar] [CrossRef]
- Huang, S.; Deng, W.; Wang, P.; Yan, Y.; Xie, C.; Cao, X.; Chen, M.; Zhang, C.; Shi, D.; Dong, Y.; et al. Fermitin family member 2 promotes melanoma progression by enhancing the binding of p-alpha-Pix to Rac1 to activate the MAPK pathway. Oncogene 2021, 40, 5626–5638. [Google Scholar] [CrossRef] [PubMed]
- Hemsing, A.L.; Rye, K.P.; Hatfield, K.J.; Reikvam, H. NPM1-Mutated Patient-Derived AML Cells Are More Vulnerable to Rac1 Inhibition. Biomedicines 2022, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Cardama, G.A.; Gonzalez, N.; Ciarlantini, M.; Donadío, L.G.; Comin, M.J.; Alonso, D.F.; Menna, P.L.; Gomez, D.E. Proapoptotic and antiinvasive activity of Rac1 small molecule inhibitors on malignant glioma cells. OncoTargets Ther. 2014, 7, 2021–2033. [Google Scholar]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Xu, J.; Galvanetto, N.; Nie, J.; Yang, Y.; Torre, V. Rac1 Promotes Cell Motility by Controlling Cell Mechanics in Human Glioblastoma. Cancers 2020, 12, 1667. [Google Scholar] [CrossRef]
- Jarzynka, M.J.; Hu, B.; Hui, K.M.; Bar-Joseph, I.; Gu, W.; Hirose, T.; Haney, L.B.; Ravichandran, K.S.; Nishikawa, R.; Cheng, S.Y. ELMO1 and Dock180, a bipartite Rac1 guanine nucleotide exchange factor, promote human glioma cell invasion. Cancer Res. 2007, 67, 7203–7211. [Google Scholar] [CrossRef]
- Ilina, E.I.; Armento, A.; Sanchez, L.G.; Reichlmeir, M.; Braun, Y.; Penski, C.; Capper, D.; Sahm, F.; Jennewein, L.; Harter, P.N.; et al. Effects of soluble CPE on glioma cell migration are associated with mTOR activation and enhanced glucose flux. Oncotarget 2017, 8, 67567–67591. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Bach, C.T.; Shum, M.S.; O’Neill, G.M. NEDD9 regulates 3D migratory activity independent of the Rac1 morphology switch in glioma and neuroblastoma. Mol. Cancer Res. 2014, 12, 264–273. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Levatic, J.; Curak, J.; Kralj, M.; Smuc, T.; Osmak, M.; Supek, F. Accurate models for P-gp drug recognition induced from a cancer cell line cytotoxicity screen. J. Med. Chem. 2013, 56, 5691–5708. [Google Scholar] [CrossRef] [PubMed]
- Matlock, M.K.; Hughes, T.B.; Swamidass, S.J. XenoSite server: A web-available site of metabolism prediction tool. Bioinformatics 2015, 31, 1136–1137. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.F.; Gennaro, V.M.; Bastone, L.C.; Godoy, A.R.; Torruella, M.; Perez, G.R. Molecular biomarkers and integrated pathological diagnosis in the reclassification of gliomas. Mol. Clin. Oncol. 2021, 15, 150. [Google Scholar] [CrossRef]
- Dixon, W.J. Staircase bioassay: The up-and-down method. Neurosci. Biobehav. Rev. 1991, 15, 47–50. [Google Scholar] [CrossRef]
- Bruce, R.D. An up-and-down procedure for acute toxicity testing. Fundam. Appl. Toxicol. 1985, 5, 151–157. [Google Scholar] [CrossRef]
- Fallacara, A.L.; Zamperini, C.; Podolski-Renić, A.; Dinić, J.; Stanković, T.; Stepanović, M.; Mancini, A.; Rango, E.; Iovenitti, G.; Molinari, A.; et al. A New Strategy for Glioblastoma Treatment: In Vitro and In Vivo Preclinical Characterization of Si306, a Pyrazolo[3,4-d]Pyrimidine Dual Src/P-Glycoprotein Inhibitor. Cancers 2019, 11, 848. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, L.; He, Y.; Xu, X.; Li, K.; Ma, Y.; Gao, Y.; Wei, D.; Wei, L. Knockdown of RAC1 and VASP gene expression inhibits breast cancer cell migration. Oncol. Lett. 2018, 16, 2151–2160. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Eichler, M.; Jahnke, H.G.; Krinke, D.; Müller, A.; Schmidt, S.; Azendorf, R.; Robitzki, A.A. A novel 96-well multielectrode array based impedimetric monitoring platform for comparative drug efficacy analysis on 2D and 3D brain tumor cultures. Biosens. Bioelectron. 2015, 67, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Caragher, S.; Chalmers, A.J.; Gomez-Roman, N. Glioblastoma’s Next Top Model: Novel Culture Systems for Brain Cancer Radiotherapy Research. Cancers 2019, 11, 44. [Google Scholar] [CrossRef]
- Alfonso, J.C.; Talkenberger, K.; Seifert, M.; Klink, B.; Hawkins-Daarud, A.; Swanson, K.R.; Hatzikirou, H.; Deutsch, A. The biology and mathematical modelling of glioma invasion: A review. J. R. Soc. Interface 2017, 14, 20170490. [Google Scholar] [CrossRef]
- Han, J.H.; Yoon, J.S.; Chang, D.Y.; Cho, K.G.; Lim, J.; Kim, S.S.; Suh-Kim, H. CXCR4-STAT3 Axis Plays a Role in Tumor Cell Infiltration in an Orthotopic Mouse Glioblastoma Model. Mol. Cells 2020, 43, 539–550. [Google Scholar]
- Neervannan, S. Preclinical formulations for discovery and toxicology: Physicochemical challenges. Expert Opin. Drug Metab. Toxicol. 2006, 2, 715–731. [Google Scholar] [CrossRef]
- Guishard, A.F.; Yakisich, J.S.; Azad, N.; Iyer, A.K.V. Translational gap in ongoing clinical trials for glioma. J. Clin. Neurosci. 2018, 47, 28–42. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Van Meir, E.; Sawamura, Y.; Diserens, A.C.; Hamou, M.F.; de Tribolet, N. Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res. 1990, 50, 6683–6688. [Google Scholar]

| Parameter | Control Animals (n = 5) | Treated Animals | |
|---|---|---|---|
| 31.2 mg/kg | 68 mg/kg | ||
| HCT (%) | 46.51 ± 4.47 | 42.2 ± 0.83 | 40.0 ± 2.44 |
| RBC (×106/µL) | 9.84 ± 0.72 | 9.46 ± 1.71 | 10.09 ± 0.61 |
| HGB (g/dL) | 12.02 ± 0.76 | 11.8 ± 0.26 | 13.47 ± 0.68 |
| WBC (×103/µL) | 6.106 ± 1.316 | 6.251 ± 1.310 | 7.030 ± 0.718 |
| NEU (×103/µL) | 1.864 ± 0.413 | 1.753 ± 0.0371 | 2.022 ± 0.256 |
| EOS (×103/µL) | 0.065 ± 0.039 | 0.081 ± 0.061 | 0.088 ± 0.038 |
| BAS (×103/µL) | 0 | 0 | 0 |
| LYM (×103/µL) | 3.967 ± 0.881 | 4.176 ± 0.806 | 4.711 ± 0.927 |
| MNO (×103/µL) | 0.208 ± 0.143 | 0.257 ± 0.224 | 0.208 ± 47.65 |
| ALT (UI/L) | 41.20 ± 5.67 | 42.00 ± 14.94 | 39.00 ± 5.35 |
| AST (UI/L) | 123.60 ± 14.22 | 131.00 ± 19.33 | 149.75 ± 16.05 |
| GGT (UI/L) | 5.20 ± 1.31 | 4.60 ± 0.54 | 6.00 ± 1.00 |
| AMY (UI/L) | 376.00 ± 56.46 | 389.8 ± 73.27 | 333.0 ± 14.98 |
| LN229 | |
|---|---|
| Mutations in IDH1 and IDH2 | |
| variant R132H—IDH1 | Not detectable |
| variant R132C—IDH1 | Not detectable |
| variant R172K—IDH2 | Not detectable |
| variant R172M—IDH2 | Not detectable |
| Methylation status of MGMT promoter | |
| High (r = 099) | |
| Gene Amplification/Deletion | |
| PDGFRA | Normal |
| EGFR | Ampl (r = 1.5) |
| EGFR vIII presence | Not Detectable |
| CDKN2A | Del (r = 0.0) |
| PTEN | Normal |
| CDK4 | Ampl (r = 1.47) |
| MIR26A2 | Ampl (r = 1.38) |
| MDM2 | Normal |
| NFKBIA | Normal |
| TP53 | Normal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardama, G.A.; Maggio, J.; Valdez Capuccino, L.; Gonzalez, N.; Matiller, V.; Ortega, H.H.; Perez, G.R.; Demarco, I.A.; Spitzer, E.; Gomez, D.E.; et al. Preclinical Efficacy and Toxicology Evaluation of RAC1 Inhibitor 1A-116 in Human Glioblastoma Models. Cancers 2022, 14, 4810. https://doi.org/10.3390/cancers14194810
Cardama GA, Maggio J, Valdez Capuccino L, Gonzalez N, Matiller V, Ortega HH, Perez GR, Demarco IA, Spitzer E, Gomez DE, et al. Preclinical Efficacy and Toxicology Evaluation of RAC1 Inhibitor 1A-116 in Human Glioblastoma Models. Cancers. 2022; 14(19):4810. https://doi.org/10.3390/cancers14194810
Chicago/Turabian StyleCardama, Georgina A., Julian Maggio, Lucas Valdez Capuccino, Nazareno Gonzalez, Valentina Matiller, Hugo H. Ortega, German R. Perez, Ignacio A. Demarco, Eduardo Spitzer, Daniel E. Gomez, and et al. 2022. "Preclinical Efficacy and Toxicology Evaluation of RAC1 Inhibitor 1A-116 in Human Glioblastoma Models" Cancers 14, no. 19: 4810. https://doi.org/10.3390/cancers14194810
APA StyleCardama, G. A., Maggio, J., Valdez Capuccino, L., Gonzalez, N., Matiller, V., Ortega, H. H., Perez, G. R., Demarco, I. A., Spitzer, E., Gomez, D. E., Lorenzano Menna, P., & Alonso, D. F. (2022). Preclinical Efficacy and Toxicology Evaluation of RAC1 Inhibitor 1A-116 in Human Glioblastoma Models. Cancers, 14(19), 4810. https://doi.org/10.3390/cancers14194810






