Differences and Similarities in the Pattern of Early Metabolic and Morphologic Response after Induction Chemo-Immunotherapy versus Induction Chemotherapy Alone in Locally Advanced Squamous Cell Head and Neck Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatments (Figure 1)
2.2. CT/MRI Response Evaluation
2.3. Metabolic Response Evaluation
2.4. Clinical and Pathological Response Evaluation

2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Change in Tumor Diameter after IC versus ICIT
3.3. Metabolic Response Rates after IC versus ICIT
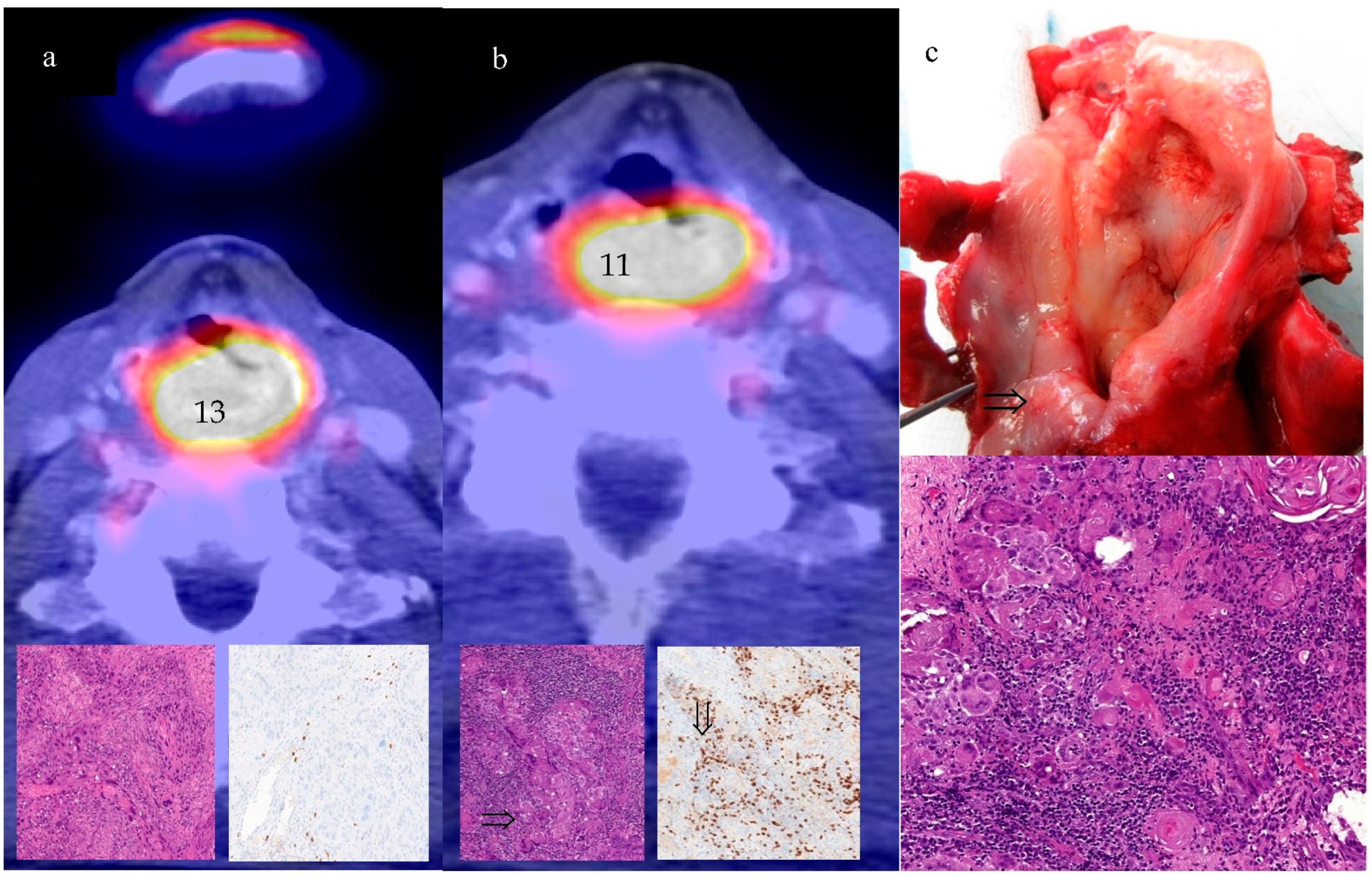
3.4. Clinical and Pathologic Response to ICIT versus IC
3.5. Differences in Imaging Variables between CR and non-CR after ICIT versus IC
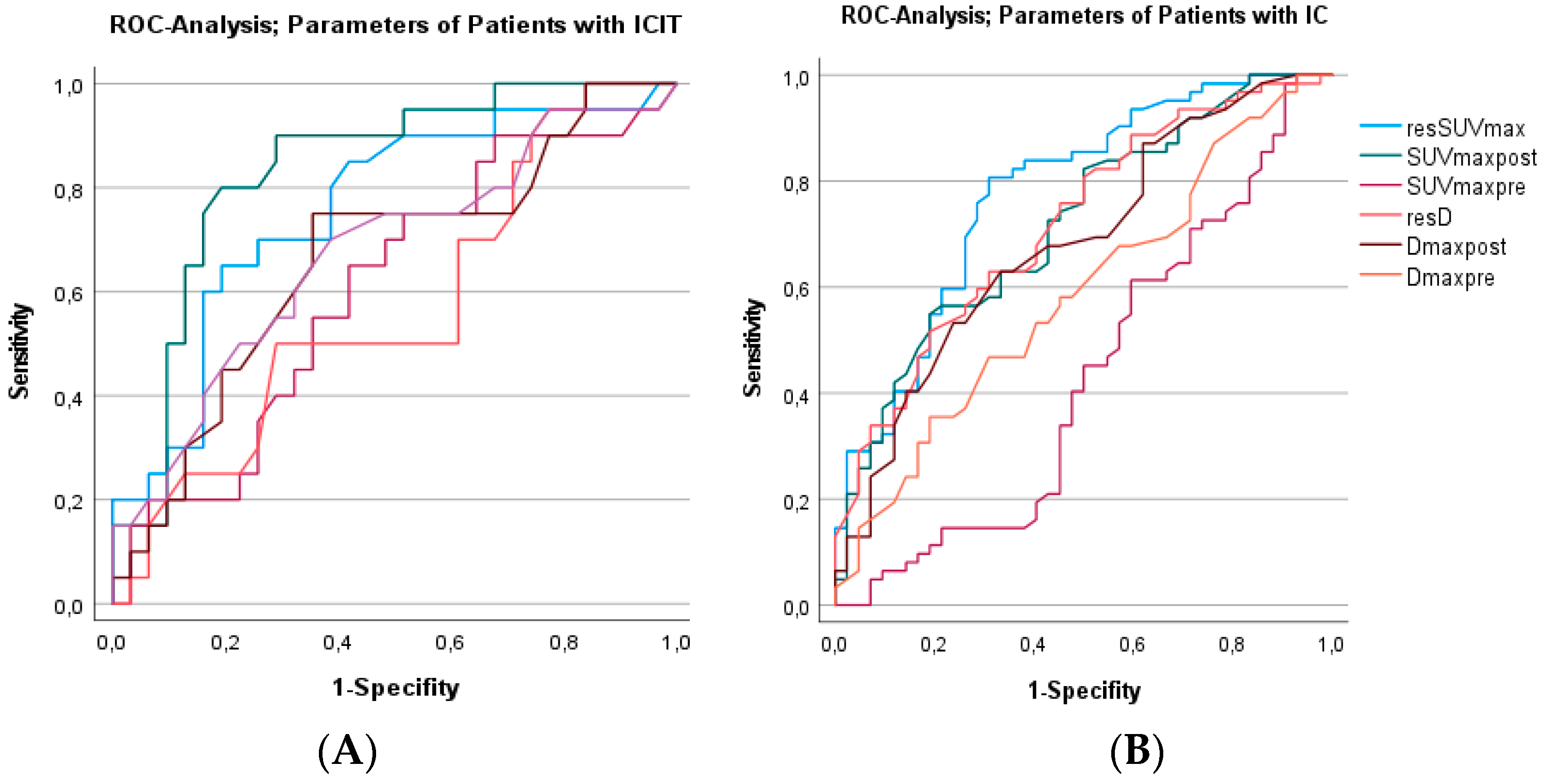
3.6. The Accuracy of Predicting CR to IC and ICIT Using Several Established Metabolic Values Based on resSUVmax
3.7. Metabolic Nonresponse and Frequency of Unexpected CR (Discrepant Response)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutchings, M.; Loft, A.; Hansen, M.; Pedersen, L.M.; Buhl, T.; Jurlander, J.; Buus, S.; Keiding, S.; D’Amore, F.; Boesen, A.M.; et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 2006, 107, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Radford, J.; Illidge, T.; Counsell, N.; Hancock, B.; Pettengell, R.; Johnson, P.; Wimperis, J.; Culligan, D.; Popova, B.; Smith, P.; et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Ott, K.; Krause, B.J.; Weber, W.A.; Becker, K.; Stein, H.J.; Lorenzen, S.; Schuster, T.; Wieder, H.; Herrmann, K.; et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol. 2007, 8, 797–805. [Google Scholar] [CrossRef]
- Kikuchi, M.; Nakamoto, Y.; Shinohara, S.; Fujiwara, K.; Yamazaki, H.; Kanazawa, Y.; Kurihara, R.; Kishimoto, I.; Harada, H.; Naito, Y. Early evaluation of neoadjuvant chemotherapy response using FDG-PET/CT predicts survival prognosis in patients with head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2013, 18, 402–410. [Google Scholar] [CrossRef]
- Wichmann, G.; Krüger, A.; Boehm, A.; Kolb, M.; Hofer, M.; Fischer, M.; Müller, S.; Purz, S.; Stumpp, P.; Sabri, O.; et al. Induction chemotherapy followed by radiotherapy for larynx preservation in advanced laryngeal and hypopharyngeal cancer: Outcome prediction after one cycle induction chemotherapy by a score based on clinical evaluation, computed tomography-based volumetry and 18F-FDG-PET/CT. Eur. J. Cancer 2017, 72, 144–155. [Google Scholar] [PubMed]
- Semrau, S.; Haderlein, M.; Schmidt, D.; Lell, M.; Wolf, W.; Waldfahrer, F.; Uder, M.; Iro, H.; Kuwert, T.; Fietkau, R. Single-cycle induction chemotherapy followed by chemoradiotherapy or surgery in patients with head and neck cancer: What are the best predictors of remission and prognosis? Cancer 2015, 121, 1214–1222. [Google Scholar] [CrossRef]
- Breheret, M.; Lubgan, D.; Haderlein, M.; Hecht, M.; Traxdorf, M.; Schmidt, D.; Müller, S.; Kitzsteiner, C.; Kuwert, T.; Iro, H.; et al. Single-cycle induction chemotherapy before chemoradiotherapy or surgery in functionally inoperable head and neck squamous cell carcinoma: 10-year results. Eur. Arch. Otorhinolaryngol. 2020, 277, 245–254. [Google Scholar] [CrossRef]
- Urba, S.; Wolf, G.; Eisbruch, A.; Worden, F.; Lee, J.; Bradford, C.; Teknos, T.; Chepeha, D.; Prince, M.; Hogikyan, N.; et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: A new treatment paradigm. J. Clin. Oncol. 2006, 24, 593–598. [Google Scholar] [CrossRef]
- Semrau, S.; Schmidt, D.; Hecht, M.; Haderlein, M.; Kitzsteiner, C.; Müller, S.; Traxdorf, M.; Agaimy, A.; Iro, H.; Kuwert, T.; et al. Classification of three prognostically different groups of head and neck cancer patients based on their metabolic response to induction chemotherapy (IC-1). Oral. Oncol. 2020, 100, 104479. [Google Scholar] [CrossRef]
- Geoffrois, L.; Martin, L.; De Raucourt, D.; Sun, X.S.; Tao, Y.; Maingon, P.; Buffet, J.; Pointreau, Y.; Sire, C.; Tuchais, C.; et al. Induction Chemotherapy Followed by Cetuximab Radiotherapy Is Not Superior to Concurrent Chemoradiotherapy for Head and Neck Carcinomas: Results of the GORTEC 2007-02 Phase III Randomized Trial. J. Clin. Oncol. 2018, 36, 3077–3083. [Google Scholar] [CrossRef]
- Weykamp, F.; Seidensaal, K.; Rieken, S.; Green, K.; Mende, S.; Zaoui, K.; Freier, K.; Adeberg, S.; Debus, J.; Welte, S.E. Age-dependent hemato- and nephrotoxicity in patients with head and neck cancer receiving chemoradiotherapy with weekly cisplatin. Strahlenther Onkol. 2020, 196, 515–521. [Google Scholar] [CrossRef]
- Mogadas, S.; Busch, C.J.; Pflug, C.; Hanken, H.; Krüll, A.; Petersen, C.; Tribius, S. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020, 196, 522–529. [Google Scholar] [CrossRef]
- Hecht, M.; Gostian, A.O.; Eckstein, M.; Rutzner, S.; von der Grün, J.; Illmer, T.; Hautmann, M.G.; Klautke, G.; Laban, S.; Brunner, T.; et al. Safety and efficacy of single cycle induction treatment with cisplatin/docetaxel/ durvalumab/tremelimumab in locally advanced HNSCC: First results of CheckRad-CD8. J. Immunother. Cancer 2020, 8, e001378. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Hartwich, J.; Eckstein, M.; Schmidt, D.; Gostian, A.O.; Müller, S.; Rutzner, S.; Gaipl, U.S.; von der Grün, J.; Illmer, T.; et al. F18-FDG PET/CT imaging early predicts pathologic complete response to induction chemoimmunotherapy of locally advanced head and neck cancer: Preliminary single-center analysis of the checkrad-cd8 trial. Ann. Nucl. Med. 2022, 36, 623–633. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; De Castro Carpeño, J.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.S.; Yi, J.S.; Lee, J.H.; Choi, S.H.; Nam, S.Y.; Cho, K.J.; Lee, S.W.; Kim, S.B.; Roh, J.L. Evaluation of 18F-FDG PET/CT and CT/MRI with histopathologic correlation in patients undergoing salvage surgery for head and neck squamous cell carcinoma. Ann. Surg. Oncol. 2011, 18, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef]
- Aide, N.; Hicks, R.J.; Le Tourneau, C.; Lheureux, S.; Fanti, S.; Lopci, E. FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Nucl. Med. Mol. Imaging. 2019, 46, 238–250. [Google Scholar] [CrossRef]
- Beer, L.; Hochmair, M.; Haug, A.R.; Schwabel, B.; Kifjak, D.; Wadsak, W.; Fuereder, T.; Fabikan, H.; Fazekas, A.; Schwab, S.; et al. Comparison of RECIST, iRECIST, and PERCIST for the Evaluation of Response to PD-1/PD-L1 Blockade Therapy in Patients With Non-Small Cell Lung Cancer. Clin. Nucl. Med. 2019, 44, 535–543. [Google Scholar] [CrossRef]
- Humbert, O.; Cadour, N.; Paquet, M.; Schiappa, R.; Poudenx, M.; Chardin, D.; Borchiellini, D.; Benisvy, D.; Ouvrier, M.J.; Zwarthoed, C.; et al. 18FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: Frequency and clinical significance of atypical evolutive patterns. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1158–1167. [Google Scholar] [CrossRef]
- Carter, B.W.; Bhosale, P.R.; Yang, W.T. Immunotherapy and the role of imaging. Cancer 2018, 124, 2906–2922. [Google Scholar] [CrossRef] [PubMed]
- Aide, N.; De Pontdeville, M.; Lopci, E. Evaluating response to immunotherapy with 18F-FDG PET/CT: Where do we stand? Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1019–1021. [Google Scholar] [CrossRef] [PubMed]
- Humbert, O.; Chardin, D. Dissociated Response in Metastatic Cancer: An Atypical Pattern Brought Into the Spotlight With Immunotherapy. Front. Oncol. 2020, 18, 566297. [Google Scholar] [CrossRef]
- Echarti, A.; Hecht, M.; Büttner-Herold, M.; Haderlein, M.; Hartmann, A.; Fietkau, R.; Distel, L. CD8+ and Regulatory T cells Differentiate Tumor Immune Phenotypes and Predict Survival in Locally Advanced Head and Neck Cancer. Cancers 2019, 11, 1398. [Google Scholar] [CrossRef]
- Tozuka, T.; Kitazono, S.; Sakamoto, H.; Yoshida, H.; Amino, Y.; Uematsu, S.; Yoshizawa, T.; Hasegawa, T.; Uchibori, K.; Yanagitani, N.; et al. Dissociated responses at initial computed tomography evaluation is a good prognostic factor in non-small cell lung cancer patients treated with anti-programmed cell death-1/ligand 1 inhibitors. BMC Cancer 2020, 20, 207. [Google Scholar] [CrossRef]
- Schwenck, J.; Schörg, B.; Fiz, F.; Sonanini, D.; Forschner, A.; Eigentler, T.; Weide, B.; Martella, M.; Gonzalez-Menendez, I.; Campi, C.; et al. Cancer immunotherapy is accompanied by distinct metabolic patterns in primary and secondary lymphoid organs observed by non-invasive in vivo18F-FDG-PET. Theranostics 2020, 10, 925–937. [Google Scholar] [CrossRef]
- Nobashi, T.; Baratto, L.; Reddy, S.A.; Srinivas, S.; Toriihara, A.; Hatami, N.; Yohannan, T.K.; Mittra, E. Predicting Response to Immunotherapy by Evaluating Tumors, Lymphoid Cell-Rich Organs, and Immune-Related Adverse Events Using FDG-PET/CT. Clin. Nucl. Med. 2019, 44, e272–e279. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Larribère, L.; Kopp-Schneider, A.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Can benign lymphoid tissue changes in 18F-FDG PET/CT predict response to immunotherapy in metastatic melanoma? Cancer Immunol. Immunother. 2019, 68, 297–303. [Google Scholar] [CrossRef]
| IC Patients | ICIT Patients | p-Value | |||
|---|---|---|---|---|---|
| (n) | (%) | (n) | (%) | (Chi2, * Fisher’s exact and ** independent samples t-test) | |
| Patients | 104 | 53 | |||
| Male | 84 | 80.8 | 45 | 84.9 | p = 0.66 * |
| Female | 20 | 19.2 | 8 | 15.1 | |
| Median age (range) | 58 (35–78) | 61 (38–78) | p = 0.27 ** | ||
| T stage | p < 0.001 | ||||
| 1 | 1 | 0.9 | 2 | 3.8 | |
| 2 | 35 | 33.7 | 7 | 13.2 | |
| 3 | 42 | 40.4 | 10 | 18.9 | |
| 4 | 26 | 25.0 | 34 | 64.2 | |
| N stage | p = 0.803 | ||||
| 0 | 32 | 30.7 | 14 | 26.4 | |
| 1 | 17 | 16.3 | 10 | 18.9 | |
| 2a | 1 | 1.0 | 2 | 3.8 | |
| 2b | 26 | 25.0 | 13 | 24.5 | |
| 2c | 27 | 26.0 | 14 | 26.4 | |
| 3 | 1 | 1.0 | 0 | 0 | |
| UICC Stage (7th Edition) | p = 0.025 | ||||
| 2 | 12 | 11.5 | 0 | 0 | |
| 3 | 23 | 22.1 | 10 | 18.9 | |
| 4 | 69 | 66.4 | 43 | 81.1 | |
| Grade | p = 0.016 | ||||
| 1 | 4 | 3.8 | 0 | 0 | |
| 2 | 49 | 47.2 | 8 | 15.1 | |
| 3 | 46 | 44.2 | 24 | 45.3 | |
| Missing, HPV-positive OPSCC | 5 | 4.8 | 21 | 39.6 | |
| HPV-associated OPSCC | p < 0.001 * | ||||
| No | 99 | 95.2 | 32 | 60.4 | |
| Yes | 5 | 4.8 | 21 | 39.6 | |
| Localization | p < 0.001 | ||||
| Oral cavity/oropharynx | 18 | 17.3 | 33 | 62.3 | |
| Hypopharynx | 42 | 40.4 | 11 | 20.8 | |
| Larynx | 44 | 42.3 | 9 | 17.0 | |
| Complete Response (CR) | Noncomplete Response (non-CR) | p-Value: CR versus non-CR to ICIT | p-Value: CR versus non-CR to IC | |||
|---|---|---|---|---|---|---|
| ICIT (n = 31/32) | IC (n = 42) | ICIT (n = 20/21) | IC (n = 62) | |||
| SUVmax pre | 12.7 ± 7.7 | 17.5 ± 11.0 | 15.9 ± 8.1 | 15.7 ± 8.0 | p = 0.213 | p = 0.24 |
| p = 0.021 | p = 0.901 | |||||
| SUVmax post | 4.8 ± 4.1 | 4.6 ± 4.3 | 10.6 ± 5.6 | 7.5 ± 7.2 | p < 0.001 | p < 0.001 |
| p = 0.828 | p = 0.063 | |||||
| Residual SUVmax (%) | 41 ± 28 | 28 ± 20 | 76 ± 40 | 53 ± 33 | p = 0.02 | p = 0.00 |
| p = 0.025 | p = 0.032 | |||||
| Dmax pre (mm) | 28 ± 11 | 20 ± 8 | 33 ± 15 | 22 ± 10 | p = 0.038 | p = 0.13 |
| p = 0.003 | p < 0.001 | |||||
| Dmax post (mm) | 18 ± 14 | 13 ± 7 | 27 ± 16 | 18 ± 9 | p = 0.056 | p = 0.002 |
| p = 0.063 | p = 0.023 | |||||
| Residual Dmax (%) | 72 ± 34 | 70 ± 19 | 68 ± 24 | 81 ± 17 | p = 0.47 | p < 0.001 |
| p = 0.97 | p = 0.218 | |||||
| PET Response and Clinical or Pathological Response | ||||
|---|---|---|---|---|
| Sensitivity | Specifity | NPV | PPV | |
| resSUVmax ≤ 75% (EORTC for metabolic response) | ||||
| ICIT | 50% | 72% | 66% | 72% |
| IC | 26% | 92% | 94% | 47% |
| resSUVmax ≤ 40% (Semrau 2015, 2021) for metabolic response) | ||||
| ICIT | 90% | 48% | 42% | 88% |
| IC | 74% | 71% | 79% | 65% |
| resSUVmax ≤ 50% + SUVmaxpost < 6 (Beck 2022 for metabolic response) | ||||
| ICIT | 95% | 45% | 52% | 93% |
| IC | 71% | 59% | 72% | 58% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, M.; Semrau, S.; Haderlein, M.; Gostian, A.-O.; Hartwich, J.; Müller, S.; Kallies, A.; Geppert, C.-I.; Schonath, M.; Putz, F.; et al. Differences and Similarities in the Pattern of Early Metabolic and Morphologic Response after Induction Chemo-Immunotherapy versus Induction Chemotherapy Alone in Locally Advanced Squamous Cell Head and Neck Cancer. Cancers 2022, 14, 4811. https://doi.org/10.3390/cancers14194811
Beck M, Semrau S, Haderlein M, Gostian A-O, Hartwich J, Müller S, Kallies A, Geppert C-I, Schonath M, Putz F, et al. Differences and Similarities in the Pattern of Early Metabolic and Morphologic Response after Induction Chemo-Immunotherapy versus Induction Chemotherapy Alone in Locally Advanced Squamous Cell Head and Neck Cancer. Cancers. 2022; 14(19):4811. https://doi.org/10.3390/cancers14194811
Chicago/Turabian StyleBeck, Michael, Sabine Semrau, Marlen Haderlein, Antoniu-Oreste Gostian, Julius Hartwich, Sarina Müller, Annett Kallies, Carol-Immanuel Geppert, Miriam Schonath, Florian Putz, and et al. 2022. "Differences and Similarities in the Pattern of Early Metabolic and Morphologic Response after Induction Chemo-Immunotherapy versus Induction Chemotherapy Alone in Locally Advanced Squamous Cell Head and Neck Cancer" Cancers 14, no. 19: 4811. https://doi.org/10.3390/cancers14194811
APA StyleBeck, M., Semrau, S., Haderlein, M., Gostian, A.-O., Hartwich, J., Müller, S., Kallies, A., Geppert, C.-I., Schonath, M., Putz, F., Gaipl, U., Frey, B., Saake, M., Iro, H., Uder, M., Hartmann, A., Kuwert, T., Fietkau, R., Eckstein, M., & Hecht, M. (2022). Differences and Similarities in the Pattern of Early Metabolic and Morphologic Response after Induction Chemo-Immunotherapy versus Induction Chemotherapy Alone in Locally Advanced Squamous Cell Head and Neck Cancer. Cancers, 14(19), 4811. https://doi.org/10.3390/cancers14194811












