Vulvar High-Grade Squamous Intraepithelial Lesions Treated with Imiquimod: Can Persistence of Human Papillomavirus Predict Recurrence?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Vulvar Biopsy
2.3. Imiquimod Treatment
2.4. Follow-Up
2.5. Clinical Response
2.6. HPV Determination
2.7. Outcomes
2.8. Data Analysis
2.9. Ethical Aspects
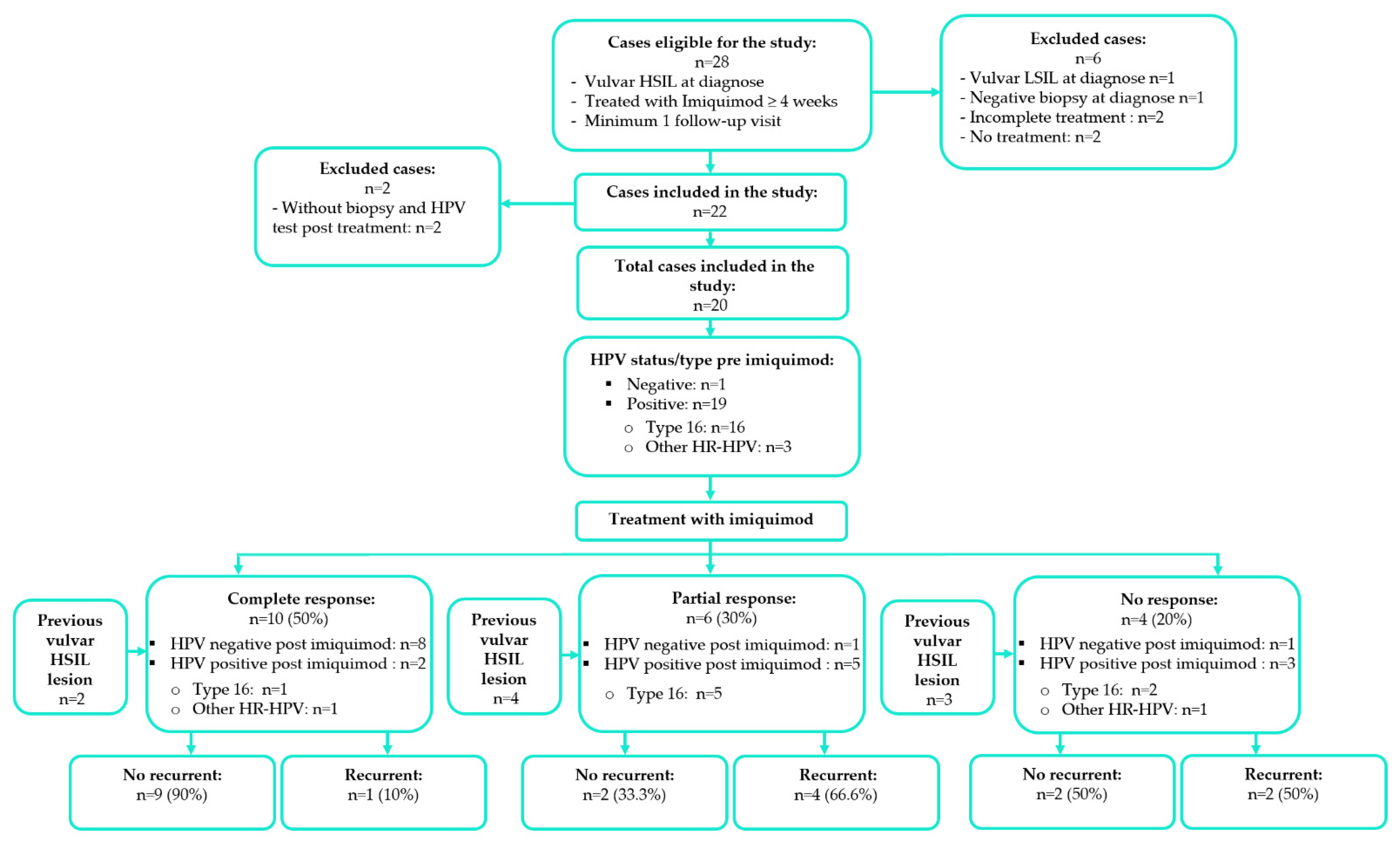
3. Results
3.1. Study Cohort
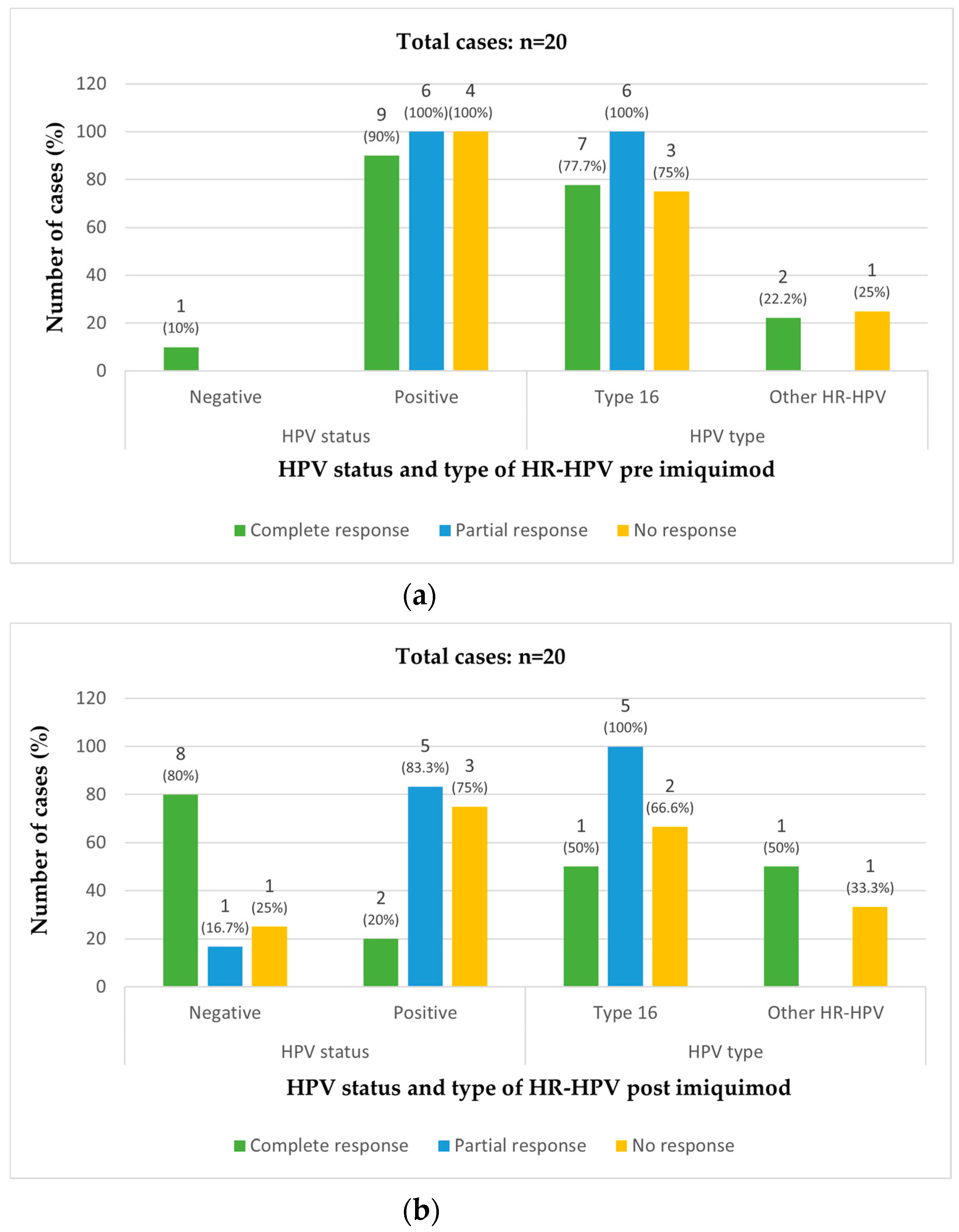
3.2. Treatment Success and Factors Associated with Response to Imiquimod
3.3. Partial Response of Vulvar HSIL to Imiquimod
3.4. No Response of Vulvar HSIL to Imiquimod
3.5. Recurrence of Vulvar HSIL and Factors Associated with Recurrence
4. Discussion
4.1. Response to Imiquimod
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECSVD | European College for the Study of Vulval Disease |
| EFC | European Federation for Colposcopy |
| ESGO | European Society of Gynaecological Oncology |
| FFPE | Formalin fixed paraffin Embedded |
| HSIL | High-grade squamous intraepithelial lesion |
| ISSVD | International Society for the Study of Vulvovaginal Disease |
| VIN | Vulvar intraepithelial neoplasia |
References
- Jones, R.; Rowan, D.; Stewart, A.W. Vulvar intraepithelial neoplasia aspects of the Natural History and Outcome in 405 Women. Obs. Gynecol. 2005, 106, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Hoang Lien NPark, K.J.; Soslow, R.A.; Murali, R. Squamous precursor lesions of the vulva: Current classification and diagnostic challenges. Pathology 2016, 48, 291–302. [Google Scholar]
- De Sanjosé, S.; Alemany, L.; Ordi, J.; Tous, S.; Alejo, M.; Bigby, S.M.; Joura, E.A.; Maldonado, P.; Laco, J.; Bravo, I.G.; et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur. J. Cancer 2013, 49, 3450–3461. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, M.; Carton, I.; Brousse, S.; Lavoué, V.; Body, G.; Levêque, J.; Nyangoh-Timoh, K. Vulvar intraepithelial neoplasia: Classification, epidemiology, diagnosis, and management. J. Gynecol. Obs. Hum. Reprod. 2020, 49, 101801. [Google Scholar] [CrossRef] [PubMed]
- The American College of Obstetricians and Gynecologists; Committee Opinion Number 675. Management of Vulvar Intraepithelial Neoplasia. Obs. Gynecol. 2016, 128, 178–182. [Google Scholar] [CrossRef]
- Wallbillich, J.J.; Rhodes, H.E.; Milbourne, A.M.; Munsell, M.F.; Frumovitz, M.; Brown, J.; Trimble, C.L.; Schmeler, K.M. Vulvar intraepithelial neoplasia (VIN 2/3): Comparing clinical outcomes and evaluating risk factors for recurrence. Gynecol. Oncol. 2012, 127, 312–315. [Google Scholar] [CrossRef]
- Preti, M.; Joura, E.; Vieira-Baptista, P.; Van Beurden, M.; Bevilacqua, F.; Bleeker, M.C.G.; Bornstein, J.; Carcopino, X.; Chargari, C.; Cruickshank, M.E.; et al. The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD) and the European Federation for Colposcopy (EFC) Consensus Statem. J. Low Genit. Tract. Dis. 2022, 27, 229–244. [Google Scholar] [CrossRef]
- Iavazzo, C.; Pitsouni, E.; Athanasiou, S.; Falagas, M.E. Imiquimod for treatment of vulvar and vaginal intraepithelial neoplasia. Int. J. Gynecol. Obs. 2008, 101, 3–10. [Google Scholar] [CrossRef] [PubMed]
- De Witte, C.J.; Van De Sande, A.J.M.; Van Beekhuizen, H.J.; Koeneman, M.M.; Kruse, A.J.; Gerestein, C.G. Imiquimod in cervical, vaginal and vulvar intraepithelial neoplasia: A review. Gynecol. Oncol. 2015, 139, 377–384. [Google Scholar] [CrossRef]
- Van Seters Manon van Beurden, M.; ten Kate, F.J.W.; Beckmann, I.; Ewing, P.; Eijkemans, M.J.C.; Kagie, M.J.; Meijer, C.J.M.; Aaronson, N.K.; Kleinjan, A.; Heijmans-Antonissen, C. Treatment of Vulvar Intraepithelial Neoplasia with topical Imiquimod. N. Engl. J. Med. 2008, 358, 1465–1473. [Google Scholar] [CrossRef]
- Terlou, A.; Van Seters, M.; Kleinjan, A.; Heijmans-Antonissen, C.; Santegoets, L.A.; Beckmann, I.; Van Beurden, M.; Helmerhorst, T.J.; Blok, L.J. Imiquimod-induced clearance of HPV is associated with normalization of immune cell counts in usual type vulvar intraepithelial neoplasia. Int. J. Cancer 2010, 127, 2831–2840. [Google Scholar] [CrossRef] [PubMed]
- Westermann, C.; Fischer, A.; Clad, A. Treatment of vulvar intraepithelial neoplasia with topical 5% imiquimod cream. Int. J. Gynecol. Obs. 2013, 120, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Terlou, A.; van Seters, M.; Ewing, P.C.; Aaronson, N.K.; Gundy, C.M.; Heijmans-Antonissen, C.; Quint, W.G.; Blok, L.J.; van Beurden, M.; Helmerhorst, T.J. Treatment of vulvar intraepithelial neoplasia with topical imiquimod: Seven years median follow-up of a randomized clinical trial. Gynecol. Oncol. 2011, 121, 157–162. [Google Scholar] [CrossRef]
- Mathiesen, O.; Buus, S.K.; Cramers, M. Topical imiquimod can reverse vulvar intraepithelial neoplasia: A randomised, double-blinded study. Gynecol. Oncol. 2007, 107, 219–222. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Female Genital Tumours. 2020. Available online: https://tumourclassification.iarc.who.int/chapters/34 (accessed on 20 July 2022).
- Mena, M.; Lloveras, B.; Tous, S.; Bogers, J.; Maffini, F.; Gangane, N.; Kumar, R.V.; Somanathan, T.; Lucas, E.; Anantharaman, D.; et al. Development and validation of a protocol for optimizing the use of paraffin blocks in molecular epidemiological studies: The example from the HPV-AHEAD study. PLoS ONE 2017, 12, e0184520. [Google Scholar] [CrossRef] [PubMed]
- Larsson, G.L.; Carlsson, J.; Karlsson, M.G.; Helenius, G. Evaluation of HPV genotyping assays for archival clinical samples. J. Mol. Diagn. 2015, 17, 293–301. [Google Scholar] [CrossRef]
- Trutnovsky, G.; Reich, O.; A Joura, E.; Holter, M.; Ciresa-König, A.; Widschwendter, A.; Schauer, C.; Bogner, G.; Jan, Z.; Boandl, A.; et al. Topical imiquimod versus surgery for vulvar intraepithelial neoplasia: A multicentre, randomised, phase 3, non-inferiority trial. Lancet 2022, 399, 1790–1798. [Google Scholar] [CrossRef]
- Frega, A.; Sesti, F.; Sopracordevole, F.; Biamonti, A.; Scirpa, P.; Milazzo, G.N.; Catalano, A.; Assorgi, C.; Lombardi, D.; Gentile, M.; et al. Imiquimod 5% cream versus cold knife excision for treatment of VIN 2/3: A five-year follow-up. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 936–940. [Google Scholar] [PubMed]
- Paternotte, J.; Hebert, T.; Ouldamer, L.; Marret, H.; Body, G. Traitement des néoplasies vulvaires intra-épithéliales par imiquimod. Gynecol. Obs. Fertil. 2015, 43, 528–532. [Google Scholar] [CrossRef]
- Lawrie, T.A.; Nordin, A.; Chakrabarti, M. Medical and Surgical Treatments for Usual-Type Vulvar Intraepithelial Neoplasia. JAMA Oncol. 2017, 2, 1647. [Google Scholar] [CrossRef] [PubMed]
- Fatalska, A.; Rusetska, N.; Bakuła-zalewska, E.; Kowalik, A.; Zięba, S.; Wroblewska, A.; Zalewski, K.; Goryca, K.; Domański, D.; Kowalewska, M. Inflammatory proteins HMGA2 and PRTN3 as drivers of vulvar squamous cell carcinoma progression. Cancers 2021, 13, 27. [Google Scholar] [CrossRef]
- Brickman, C.; Palefsky, J.M. Human Papillomavirus in the HIV-Infected Host: Epidemiology and Pathogenesis in the Antiretroviral Era. Curr. HIV/AIDS Rep. 2015, 12, 6–15. [Google Scholar] [CrossRef]
- Song, D.; Li, H.; Li, H.; Dai, J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer (Review). Oncol. Lett. 2015, 10, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Rakislova, N.; Saco, A.; Sierra, A.; Del Pino, M.; Ordi, J. Role of Human Papillomavirus in Vulvar Cancer. Adv. Anat. Pathol. 2017, 24, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Massad, L.S.; Xie, X.; Darragh, T.; Minkoff, H.; Levine, A.M.; Watts, D.H.; Wright, R.L.; D’Souza, G.; Colie, C.; Strickler, H.D.; et al. Genital warts and vulvar intraepithelial neoplasia: Natural history and effects of treatment and human immunodeficiency virus infection. Obs. Gynecol. 2012, 118, 831–839. [Google Scholar] [CrossRef]
- Hinten, F.; Hilbrands, L.B.; Meeuwis, K.A.P.; IntHout, J.; Quint, W.G.V.; Hoitsma, A.J.; Massuger, L.F.A.G.; Melchers, W.J.G.; De Hullu, J.A. Reactivation of Latent HPV Infections After Renal Transplantation. Am. J. Transpl. 2017, 17, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Meeuwis, K.; Melchers, W.; Bouten, H.; van de Kerkhof, P.; Hinten, F.; Quint, W.; Massuger, L.; Hoitsma, A.; van Rossum, M.; de Hullu, J. Anogenital malignancies in women after renal transplantation over 40 years in a single center. Transplantation 2012, 93, 914–922. [Google Scholar] [CrossRef]
- Reinholdt, K.; Thomsen, L.T.; Dehlendorff, C.; Larsen, H.K.; Sørensen, S.S.; Haedersdal, M.; Kjaer, S.K. Human papillomavirus-related anogenital premalignancies and cancer in renal transplant recipients: A Danish nationwide, registry-based cohort study. Int. J. Cancer 2020, 146, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Grulich, A.E.; van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef]
- Fernández-Montolí, M.E.; Tous, S.; Medina, G.; Castellarnau, M.; García-Tejedor, A.; de Sanjosé, S. Long-term predictors of residual or recurrent cervical intraepithelial neoplasia 2–3 after treatment with a large loop excision of the transformation zone: A retrospective study. BJOG Int. J. Obs. Gynaecol. 2019, 127, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; E Redman, C.W.; Verdoodt, F.; Kyrgiou, M.; Tzafetas, M.; Ghaem-Maghami, S.; Petry, K.-U.; Leeson, S.; Bergeron, C.; Nieminen, P.; et al. Incomplete excision of cervical precancer as a predictor of treatment failure: A systematic review and meta-analysis. Lancet Oncol. 2017, 18, 1665–1679. [Google Scholar] [CrossRef]
- Satmary, W.; Holschneider, C.H.; Brunette, L.L.; Natarajan, S. Vulvar intraepithelial neoplasia: Risk factors for recurrence. Gynecol. Oncol. 2018, 148, 126–131. [Google Scholar] [CrossRef]
- Faber, M.T.; Sand, F.L.; Albieri, V.; Norrild, B.; Kjær, S.K.; Verdoodt, F. Prevalence and type distribution of human papillomavirus in squamous cell carcinoma and intraepithelial neoplasia of the vulva. Int. J. Cancer 2017, 141, 1161–1169. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016, 2, 1–20. [Google Scholar] [CrossRef]
- Van Seters, M.; Van Beurden, M.; De Craen, A.J.M. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol. Oncol. 2005, 97, 645–651. [Google Scholar] [CrossRef]
- van der Meijden, W.I.; Boffa, M.J.; ter Harmsel, W.A.; Kirtschig, G.; Lewis, F.M.; Moyal-Barracco, M.; Tiplica, G.S.; Sherrard, J. 2016 European guideline for the management of vulval conditions. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 925–941. [Google Scholar] [CrossRef]
- Hurt, C.N.; Jones, S.E.; Madden, T.; Fiander, A.; Nordin, A.J.; Naik, R.; Powell, N.; Carucci, M.; Tristram, A. Recurrence of vulval intraepithelial neoplasia following treatment with cidofovir or imiquimod: Results from a multicentre, randomised, phase II trial (RT3VIN). BJOG Int. J. Obs. Gynaecol. 2018, 125, 1171–1177. [Google Scholar] [CrossRef]
- Vici, P.; Pizzuti, L.; Mariani, L.; Zampa, G.; Santini, D.; Di Lauro, L.; Gamucci, T.; Natoli, C.; Marchetti, P.; Barba, M.; et al. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: Hope or reality from clinical studies. Expert Rev. Vaccines 2016, 15, 1327–1336. [Google Scholar] [CrossRef]
- Kenter, G.G.; Welters, M.J.P.; Valentijn, A.R.P.M.; Lowik, M.J.G.; Berends-van der Meer, D.M.A.; Vloon, A.P.G.; Essahsah, F.; Fathers, L.M.; Offringa, R.; Drijfhout, J.W.; et al. Vaccination against HPV-16 Oncoproteins for Vulvar Intraepithelial Neoplasia. N. Engl. J. Med. 2009, 361, 1838–1847. [Google Scholar] [CrossRef]
- Bhuyan, P.K. Immunotherapy: The Missing piece of the Vulvar HSIL Management Puzzle. 2020, 124. Available online: www.HPVworld.com (accessed on 20 July 2020).
| Number of Patients n = 20 n (%) * | No response n = 4 n (%) ** | Partial Response n = 6 n (%) ** | Complete Response n = 10 n (%) ** | p-Value *** | |
|---|---|---|---|---|---|
| Patient Characteristics | |||||
| Follow-Up Time (Months) **** | 0.944 ***** | ||||
| Median (Min–Max) | 37.3 (1–89.1) | 42 (1–64.4) | 38.2 (17.1–75.9) | 37.3 (6.2–89.1) | |
| IQR | 24.3–64.2 | 16.1–58.6 | 26.8–69.7 | 21.9–64 | |
| Age (Years) | 0.294 ***** | ||||
| Median (Min–Max) | 50 (27–87) | 53.5 (52–81) | 41 (27–70) | 47 (28–87) | |
| Age (Categorized at 60 Years) | 1.000 | ||||
| <60 | 16 (80.0) | 3 (18.7) | 5 (31.3) | 8 (50.0) | |
| ≥60 | 4 (20.0) | 1 (25.0) | 1 (25.0) | 2 (50.0) | |
| Smokers | 0.687 | ||||
| No | 7 (35.0) | 1 (14.3) | 2 (28.6) | 4 (57.1) | |
| Yes | 11 (55.0) | 3 (27.2) | 4 (36.4) | 4 (36.4) | |
| Unknown | 2 (10.0) | 0 (0.0) | 0 (0.0) | 2 (100.0) | |
| HIV Status | 0.111 | ||||
| No | 18 (90.0) | 4 (22.2) | 4 (22.2) | 10 (55.6) | |
| Yes | 2 (10.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | |
| Immunosuppression | 0.021 | ||||
| No | 17 (85.0) | 4 (23.5) | 3 (17.6) | 10 (58.8) | |
| Yes | 3 (15.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | |
| Previous Vulvar HSIL Lesion | 0.086 | ||||
| No | 11 (55.0) | 1 (9.1) | 2 (18.2) | 8 (72.7) | |
| Yes | 9 (45.0) | 3 (33.3) | 4 (44.5) | 2 (22.2) | |
| Previous Vulvar HSIL Surgery | 0.060 | ||||
| No | 13 (65.0) | 2 (15.4) | 2 (15.4) | 9 (69.2) | |
| Yes | 7 (35.0) | 2 (28.6) | 4 (57.1) | 1 (14.3) | |
| Total | 20 (100.0) | 4 (20.0) | 6 (30.0) | 10 (50.0) | |
| Number of Patients n = 20 n (%) *1 | No Response n = 4 n (%) ** | Partial Response n = 6 n (%) ** | Complete Response n = 10 n (%) ** | p-Value *** | |
|---|---|---|---|---|---|
| Lesion Characteristics Pre Imiquimod | |||||
| HPV Status | 1.000 | ||||
| Negative | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Positive | 19 (95.0) | 4 (21.0) | 6 (31.6) | 9 (47.4) | |
| HPV Type | 0.777 | ||||
| Negative | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Type 16 | 16 (80.0) | 3 (18.7) | 6 (37.5) | 7 (43.8) | |
| Other HR-HPV types | 3 (15.0) | 1 (33.3) | 0 (0.0) | 2 (66.7) | |
| Type of Lesion | 0.897 | ||||
| Unifocal | 5 (25.0) | 1 (20.0) | 2 (40.0) | 2 (40.0) | |
| Isolated multifocal | 6 (30.0) | 2 (33.3) | 1 (16.7) | 3 (50.0) | |
| Multifocal | 9 (45.0) | 1 (11.1) | 3 (33.3) | 5 (55.6) | |
| Maximum Diameter of Lesion (mm) | 0.143 **** | ||||
| Median (Min-Max) | 20 (5–60) | 11.5 (7–20) | 22 (15–30) | 21 (5–60) | |
| Lesion Characteristics Post-Imiquimod | |||||
| HPV Status | 0.030 | ||||
| Negative | 10 (50.0) | 1 (10.0) | 1 (10.0) | 8 (80.0) | |
| Positive | 10 (50.0) | 3 (30.0) | 5 (50.0) | 2 (20.0) | |
| HPV Type | 0.012 | ||||
| Negative | 10 (50.0) | 1 (10.0) | 1 (10.0) | 8 (80.0) | |
| Type 16 | 8 (40.0) | 2 (25.0) | 5 (62.5) | 1 (12.5) | |
| Other HR-HPV types | 2 (10.0) | 1 (50.0) | 0 (0.0) | 1 (50.0) | |
| Histology Result | 0.000 | ||||
| Vulvar epithelium without pathology | 9 (45.0) | 0 (0.0) | 0 (0.0) | 9 (100.0) | |
| Vulvar LSIL | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Vulvar HSIL | 9 (45.0) | 3 (33.3) | 6 (66.7) | 0 (0.0) | |
| Squamous carcinoma of the vulva | 1 (5.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | |
| Type of Lesion | 0.329 | ||||
| No lesion | 3 (15.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) | |
| Unifocal | 7 (35.0) | 1 (14.3) | 2 (28.6) | 4 (57.1) | |
| Isolated multifocal | 7 (35.0) | 1 (14.3) | 3 (42.9) | 3 (42.9) | |
| Multifocal | 3 (15.0) | 2 (66.7) | 1 (33.3) | 0 (0.0) | |
| Maximum Diameter of Lesion (mm) | 0.015 **** | ||||
| Median (Min-Max) | 10 (0–80) | 10 (10–80) | 8.5 (3–20) | 0 (0–0) | |
| Recurrent Vulvar HSIL ***** | 0.060 | ||||
| No | 13 (65.0) | 2 (15.4) | 2 (15.4) | 9 (69.2) | |
| Yes | 7 (35.0) | 2 (28.6) | 4 (57.1) | 1 (14.3) | |
| Total | 20 (100.0) | 4 (20.0) | 6 (30.0) | 10 (50.0) | |
| Type of Response: n (%) | Follow Up Time (Months) * | Biopsy Time (Months) ** | Time of Diagnosis of Recurrent Vulvar HSIL (Months) *** | Duration of Treatment (Weeks) |
|---|---|---|---|---|
| Median (Min–Max) | Median (Min–Max) | Median (Min–Max) | Median (Min–Max) | |
| Complete response: 10 (50.0) | 37.3 (6.2–89.1) | 4.7 (1.0–20.9) | 19.7 **** | 13.0 (4.0–20) |
| Partial response: 6 (30.0) | 38.2 (17.1–75.9) | 5.8 (3.6–37.5) | 16.2 (3.2–32.7) | 16.5 (15.0–32.0) |
| No response: 4 (20.0) | 42.0 (1.0–64.4) | 3.3 (1.1–11.1) | 22.1 (12.0–32.2) ***** | 9.0 (4.0–15.0) |
| Total: 20 (100.0) | 37.3 (1.0–89.1) | 4.9 (1.0–37.5) | 19.7 (3.2–32.7) | 14.5 (4.0–32.0) |
| Number of Patients n = 20 n (%) * | No Response n = 4 n (%) ** | Partial Response n = 6 n (%) ** | Complete Response n = 10 n (%) ** | p-Value *** | |
|---|---|---|---|---|---|
| Imiquimod Side effects and Withdrawal | |||||
| Imiquimod Side Effect_Signs | 0.940 | ||||
| No | 8 (40.0) | 1 (12.5) | 3 (37.5) | 4 (50.0) | |
| Erythema | 6 (30.0) | 2 (33.3) | 2 (33.3) | 2 (33.3) | |
| Erosion | 2 (10.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | |
| Depigmentation | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Other | 3 (15.0) | 1 (33.3) | 0 (0.0) | 2 (66.7) | |
| Imiquimod Side Effect_Symptoms | 0.227 | ||||
| No | 9 (45.0) | 2 (22.2) | 2 (22.2) | 5 (55.6) | |
| Pain | 2 (10.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | |
| Pain, stinging | 2 (10.0) | 1 (50.0) | 0 (0.0) | 1 (50.0) | |
| Burning | 2 (10.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | |
| Other | 5 (25.0) | 0 (0.0) | 1 (20.0) | 4 (80.0) | |
| Reduction of Doses | 1.000 | ||||
| No | 18 (90.0) | 4 (22.2) | 5 (27.8) | 9 (50.0) | |
| Yes | 2 (10.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | |
| Cessation of Treatment | 0.629 | ||||
| No | 5 (25.0) | 1 (20.0) | 3 (60.0) | 1 (20.0) | |
| Yes, temporary | 10 (50.0) | 2 (20.0) | 2 (20.0) | 6 (60.0) | |
| Yes, definitely | 5 (25.0) | 1 (20.0) | 1 (20.0) | 3 (60.0) | |
| Total | 20 (100.0) | 4 (20.0) | 6 (30.0) | 10 (50.0) | |
| Additional Treatment * | Number of Patients n = 20 n (%) ** | No Response n = 4 n (%) *** | Partial Response n = 6 n (%) *** | Complete Response n = 10 n (%) *** | p-Value **** | ||
|---|---|---|---|---|---|---|---|
| Patient Characteristics | |||||||
| Immunosuppression | No | No | 10 (50.0) | 1 (10.0) | - | 9 (90.0) | 0.004 |
| Yes | 7 (35.0) | 3 (42.9) | 3 (42.9) | 1 (14.3) | |||
| Yes | No | - | - | - | - | - | |
| Yes | 3 (15.0) | - | 3 (100.0) | - | |||
| Previous vulvar HSIL lesion | No | No | 9 (45.0) | 1 (11.1) | - | 8 (88.9) | 0.018 |
| Yes | 2 (10.0) | - | 2 (100.0) | - | |||
| Yes | No | 1 (5.0) | - | - | 1 (100.0) | 0.222 | |
| Yes | 8 (40.0) | 3 (37.5) | 4 (50.0) | 1 (12.5) | |||
| Previous vulvar HSIL surgery | No | No | 9 (45.0) | 1 (11.1) | - | 8 (88.9) | 0.052 |
| Yes | 4 (20.0) | 1 (25.0) | 2 (50.0) | 1 (25.0) | |||
| Yes | No | 1 (5.0) | - | - | 1 (100.0) | 0.143 | |
| Yes | 6 (30.0) | 2 (33.3) | 4 (66.7) | - | |||
| Lesion Characteristics Post Imiquimod | |||||||
| HPV status | Negative | No | 8 (40.0) | 1 (12.5) | - | 7 (87.5) | 0.378 |
| Yes | 2 (10.0) | - | 1 (50.0) | 1 (50.0) | |||
| Positive | No | 2 (10.0) | - | - | 2 (100.0) | 0.022 | |
| Yes | 8 (40.0) | 3 (37.5) | 5 (62.5) | - | |||
| HPV types | Negative | No | 8 (40.0) | 1 (12.5) | - | 7 (87.5) | 0.378 |
| Yes | 2 (10.0) | - | 1 (50.0) | 1 (50.0) | |||
| Type 16 | No | 1 (5.0) | - | - | 1 (100.0) | 0.125 | |
| Yes | 7 (35.0) | 2 (28.6) | 5 (71.4) | - | |||
| Other HR-HPV | No | 1 (50.0) | - | - | 1 (100.0) | 1.000 | |
| Yes | 1 (50.0) | 1 (100.0) | - | - | |||
| Recurrent vulvar HSIL ***** | No | No | 10 (50.0) | 1 (10.0) | - | 10 (90.0) | 0.014 |
| Yes | 3 (15.0) | 1 (33.3) | 2 (66.7) | - | |||
| Yes | No | - | - | - | - | - | |
| Yes | 7 (35.0) | 2 (28.6) | 4 (57.1) | 1 (14.3) | |||
| Additional Treatment * | Number of Patients n = 20 n (%) ** | No Recurrent Vulvar HSIL n = 13 n (%) *** | Recurrent Vulvar HSIL n = 7 n (%) ***1 | p-Value **** | ||
|---|---|---|---|---|---|---|
| Patient Characteristics | ||||||
| Immunosuppression | No | No | 10 (50.0) | 10 (100.0) | - | 0.015 |
| Yes | 7 (35.0) | 3 (42.9) | 4 (57.1) | |||
| Yes | No | - | - | - | - | |
| Yes | 3 (15.0) | - | 3 (100.0) | |||
| Previous vulvar HSIL lesion | No | No | 9 (45.0) | 9 (100.0) | - | 0.182 |
| Yes | 2 (10.0) | 1 (50.0) | 1 (50.0) | |||
| Yes | No | 1 (5.0) | 1 (100.0) | - | 0.333 | |
| Yes | 8 (40.0) | 2 (25.0) | 6 (75.0) | |||
| Previous vulvar HSIL surgery | No | No | 9 (45.0) | 9 (100.0) | - | 0.077 |
| Yes | 4 (20.0) | 2 (50.0) | 2 (50.0) | |||
| Yes | No | 1 (5.0) | 1 (100.0) | - | 0.286 | |
| Yes | 6 (30.0) | 1 (16.7) | 5 (83.3) | |||
| Lesion Characteristics Post Imiquimod | ||||||
| HPV status | Negative | No | 8 (40.0) | 8 (100.0) | - | 0.200 |
| Yes | 2 (10.0) | 1 (50.0) | 1 (50.0) | |||
| Positive | No | 2 (10.0) | 2 (100.0) | - | 0.133 | |
| Yes | 8 (40.0) | 2 (25.0) | 6 (75.0) | |||
| HPV type | Negative | No | 8 (40.0) | 8 (100.0) | - | 0.200 |
| Yes | 2 (10.0) | 1 (50.0) | 1 (50.0) | |||
| Type 16 | No | 1 (5.0) | 1 (100.0) | - | 0.250 | |
| Yes | 7 (35.0) | 1 (14.3) | 6 (85.7) | |||
| Other HR-HPV | No | 1 (5.0) | 1 (100.0) | - | - | |
| Yes | 1 (5.0) | 1 (100.0) | - | |||
| Recurrent Vulvar HSIL n (%) | |||||
|---|---|---|---|---|---|
| No n = 13 (65.0) | Yes n = 7 (35.0) * | Total n = 20 (100.0) ** | |||
| HPV Pre-Imiquimod | HPV status | Negative | 1 (7.7) | - | 1 (5.0) |
| Positive | 12 (92.3) | 7 (100.0) | 19 (95.0) | ||
| Type of HR-HPV | Type 16 | 9 (75.0) | 7 (100.0) | 16 (84.2) | |
| Other HR-HPV | 3 (25.0) | - | 3 (15.7) | ||
| HPV Post-Imiquimod | HPV status | Negative | 9 (69.2) | 1 (14.3) | 10 (50.0) |
| Positive | 4 (30.7) | 6 (85.7) | 10 (50.0) | ||
| Type of HR-HPV | Type 16 | 2 (50.0) | 6 (100.0) | 8 (80.0) | |
| Other HR-HPV | 2 (50.0) | - | 2 (20.0) | ||
| Number of Patients n = 20 n (%) *1 | No Recurrent Vulvar HSIL n = 13 n (%) ** | Recurrent Vulvar HSIL n = 7 n (%) **2 | p-Value *** | |
|---|---|---|---|---|
| Patient Characteristics | ||||
| Immunosuppression | 0.031 | |||
| No | 17 (85.0) | 13 (76.5) | 4 (23.5) | |
| Yes | 3 (15.0) | 0 (0.0) | 3 (100.0) | |
| Previous Vulvar HSIL Lesion | 0.017 | |||
| No | 11 (55.0) | 10 (90.9) | 1 (9.1) | |
| Yes | 9 (45.0) | 3 (33.3) | 6 (66.7) | |
| Previous Vulvar HSIL Surgery | 0.022 | |||
| No | 13 (65.0) | 11 (84.6) | 2 (15.4) | |
| Yes | 7 (35.0) | 2 (28.6) | 5 (71.4) | |
| Lesion Characteristics Post Imiquimod | ||||
| HPV Status | 0.057 **** | |||
| Negative | 10 (50.0) | 9 (90.0) | 1 (10.0) | |
| Positive | 10 (50.0) | 4 (40.0) | 6 (60.0) | |
| HPV Type | 0.010 | |||
| Negative | 10 (50.0) | 9 (90.0) | 1 (10.0) | |
| Type 16 | 8 (40.0) | 2 (25.0) | 6 (75.0) | |
| Other HR-HPV types | 2 (10.0) | 2 (100.0) | 0 (0.0) | |
| Histology Result | 0.028 | |||
| Vulvar epithelium without pathology | 9 (45.0) | 8 (88.9) | 1 (11.1) | |
| Vulvar LSIL | 1 (5.0) | 1 (100.0) | 0 (0.0) | |
| Vulvar HSIL | 9 (45.0) | 3 (33.3) | 6 (66.7) | |
| Squamous carcinoma of the vulva | 1 (5.0) | 1 (100.0) | 0 (0.0) | |
| Total | 20 (100.0) | 13 (65.0) | 7 (35.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Montolí, M.-E.; Heydari, F.; Lavecchia, F.; Pavón, M.-Â.; Guerra, E.; Matias-Guiu, X.; Marti, M.-D.; Tous, S. Vulvar High-Grade Squamous Intraepithelial Lesions Treated with Imiquimod: Can Persistence of Human Papillomavirus Predict Recurrence? Cancers 2022, 14, 4808. https://doi.org/10.3390/cancers14194808
Fernández-Montolí M-E, Heydari F, Lavecchia F, Pavón M-Â, Guerra E, Matias-Guiu X, Marti M-D, Tous S. Vulvar High-Grade Squamous Intraepithelial Lesions Treated with Imiquimod: Can Persistence of Human Papillomavirus Predict Recurrence? Cancers. 2022; 14(19):4808. https://doi.org/10.3390/cancers14194808
Chicago/Turabian StyleFernández-Montolí, Maria-Eulalia, Fatima Heydari, Fabrizia Lavecchia, Miquel-Ângel Pavón, Esther Guerra, Xavier Matias-Guiu, Maria-Dolores Marti, and Sara Tous. 2022. "Vulvar High-Grade Squamous Intraepithelial Lesions Treated with Imiquimod: Can Persistence of Human Papillomavirus Predict Recurrence?" Cancers 14, no. 19: 4808. https://doi.org/10.3390/cancers14194808
APA StyleFernández-Montolí, M.-E., Heydari, F., Lavecchia, F., Pavón, M.-Â., Guerra, E., Matias-Guiu, X., Marti, M.-D., & Tous, S. (2022). Vulvar High-Grade Squamous Intraepithelial Lesions Treated with Imiquimod: Can Persistence of Human Papillomavirus Predict Recurrence? Cancers, 14(19), 4808. https://doi.org/10.3390/cancers14194808






