Verification of a Novel Minimally Invasive Device for the Isolation of Rare Circulating Tumor Cells (CTC) in Cancer Patients’ Blood
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Flow Optimized Geometry
2.2. Flow System
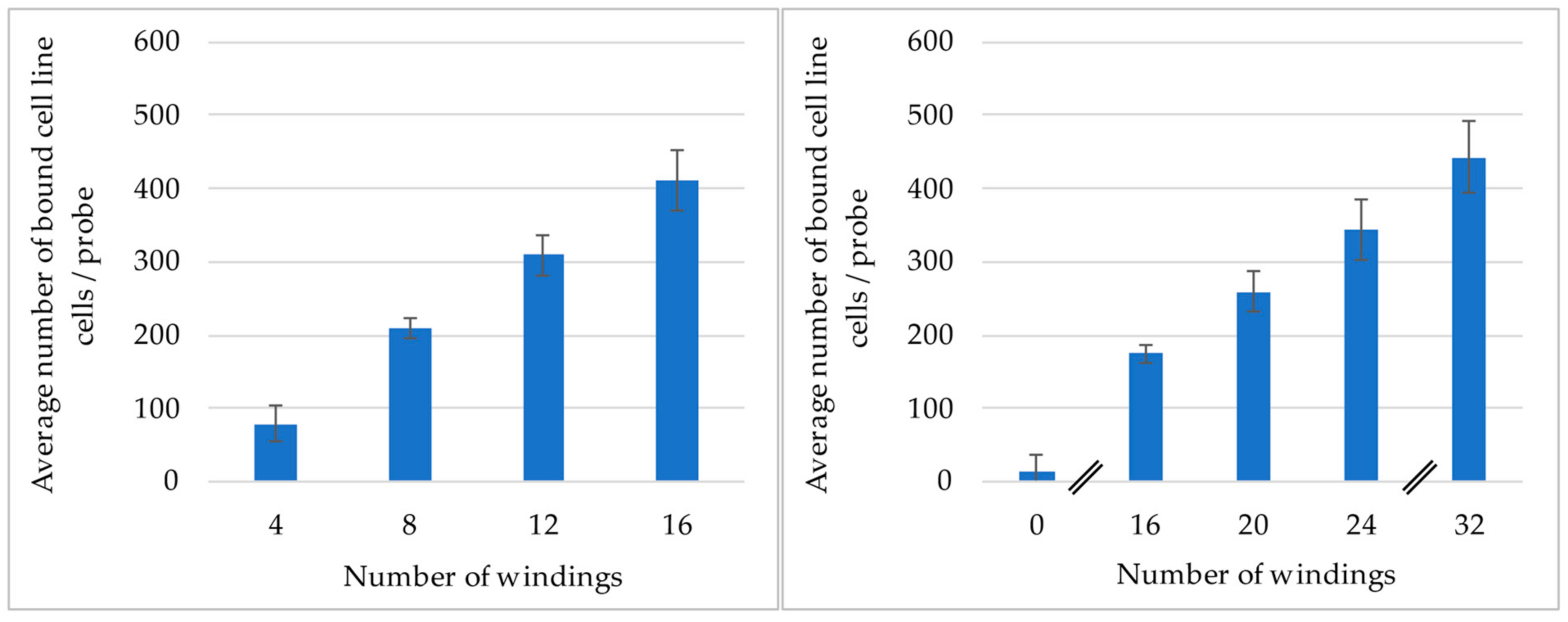
2.3. Experiments to Determine the Influence of the Number of Windings of the Probe
2.4. Staining Protocol
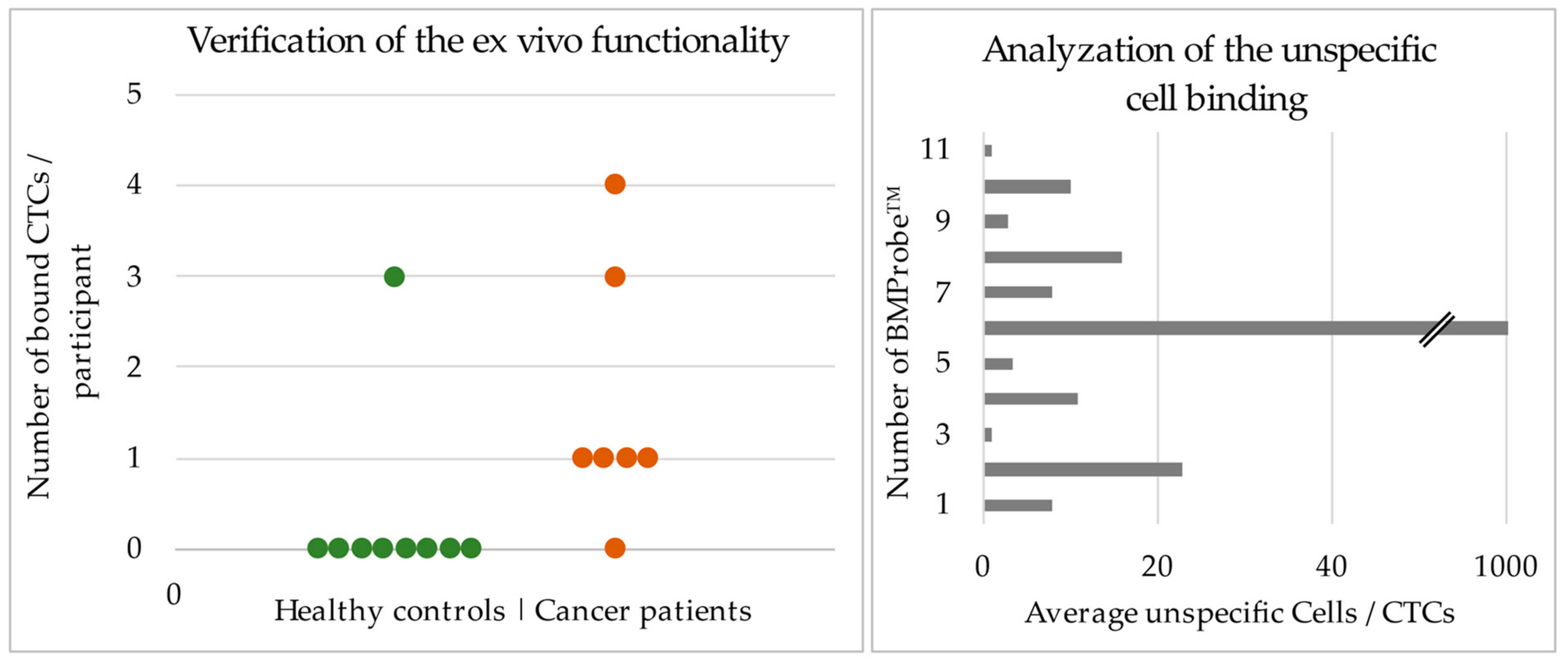
2.5. Validating the Functionality with Blood from Lung Cancer Patients
3. Results
3.1. Analysis of the Influence the Number of Windings Has on the Binding Efficiency
3.2. Results of the Ex Vivo Experiments to Validate the Functionality of the BMProbe™
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turetta, M.; Bulfoni, M.; Brisotto, G.; Fasola, G.; Zanello, A.; Biscontin, E.; Mariuzzi, L.; Steffan, A.; Di Loreto, C.; Cesselli, D.; et al. Assessment of the Mutational Status of NSCLC Using Hypermetabolic Circulating Tumor Cells. Cancers 2018, 10, 270. [Google Scholar] [CrossRef]
- Marchetti, A.; Del Grammastro, M.; Felicioni, L.; Malatesta, S.; Filice, G.; Centi, I.; De Pas, T.; Santoro, A.; Chella, A.; Brandes, A.A.; et al. Assessment of EGFR Mutations in Circulating Tumor Cell Preparations from NSCLC Patients by Next Generation Sequencing: Toward a Real-Time Liquid Biopsy for Treatment. PLoS ONE 2014, 9, e103883. [Google Scholar] [CrossRef]
- Markou, A.; Tzanikou, E.; Lianidou, E. The Potential of Liquid Biopsy in the Management of Cancer Patients. Semin. Cancer Biol. 2022, 84, 69–79. [Google Scholar] [CrossRef]
- Lozano, R.; Lorente, D.; Aragon, I.M.; Romero-Laorden, N.; Nombela, P.; Mateo, J.; Reid, A.H.M.; Cendón, Y.; Bianchini, D.; Llacer, C.; et al. Value of Early Circulating Tumor Cells Dynamics to Estimate Docetaxel Benefit in Metastatic Castration-Resistant Prostate Cancer (MCRPC) Patients. Cancers 2021, 13, 2334. [Google Scholar] [CrossRef]
- Jesus Magbanua, M.M.; Savenkov, O.; Asmus, E.J.; Ballman, K.V.; Scott, J.H.; Park, J.W.; Dickler, M.; Partridge, A.; Carey, L.; Winer, E.; et al. Clinical Significance of Circulating Tumor Cells in Hormone Receptor-Positive Metastatic Breast Cancer Patients Who Received Letrozole with or without Bevacizumab HHS Public Access. Clin. Cancer Res. 2020, 26, 4911–4920. [Google Scholar] [CrossRef]
- Hendricks, A.; Brandt, B.; Geisen, R.; Dall, K.; Röder, C.; Schafmayer, C.; Becker, T.; Hinz, S.; Sebens, S. Isolation and Enumeration of CTC in Colorectal Cancer Patients: Introduction of a Novel Cell Imaging Approach and Comparison to Cellular and Molecular Detection Techniques. Cancers 2020, 12, 2643. [Google Scholar] [CrossRef]
- Tay, R.Y.; Fernández-Gutiérrez, F.; Foy, V.; Burns, K.; Pierce, J.; Morris, K.; Priest, L.; Tugwood, J.; Ashcroft, L.; Lindsay, C.R.; et al. Prognostic Value of Circulating Tumour Cells in Limited-Stage Small-Cell Lung Cancer: Analysis of the Concurrent Once-Daily versus Twice-Daily Radiotherapy (CONVERT) Randomised Controlled Trial. Ann. Oncol. 2019, 30, 1114–1120. [Google Scholar] [CrossRef]
- Smit, D.J.; Pantel, K.; Jücker, M. Circulating Tumor Cells as a Promising Target for Individualized Drug Susceptibility Tests in Cancer Therapy. Biochem. Pharmacol. 2021, 188, 114589. [Google Scholar] [CrossRef]
- Hosseini, H.; Obradović, M.; Hoffmann, M.; Harper, K.L.; Sosa, M.S.; Werner-Klein, M.; Nanduri, L.K.; Werno, C.; Ehrl, C.; Maneck, M.; et al. Early Dissemination Seeds Metastasis in Breast Cancer. Nature 2016, 540, 552–558. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Kokuryo, T.; Yokoyama, Y.; Ebata, T.; Ochiai, Y.; Nagino, M. Premalignant Pancreatic Cells Seed Stealth Metastasis in Distant Organs in Mice. Oncogene 2021, 40, 2273–2284. [Google Scholar] [CrossRef]
- Neumann, M.H.D.; Bender, S.; Krahn, T.; Schlange, T. CtDNA and CTCs in Liquid Biopsy—Current Status and Where We Need to Progress. Comput. Struct. Biotechnol. J. 2018, 16, 190–195. [Google Scholar] [CrossRef]
- Chemi, F.; Mohan, S.; Guevara, T.; Clipson, A.; Rothwell, D.G.; Dive, C. Early Dissemination of Circulating Tumor Cells: Biological and Clinical Insights. Front. Oncol. 2021, 11, 672195. [Google Scholar] [CrossRef]
- Alvarez Cubero, M.J.; Lorente, J.A.; Robles-Fernandez, I.; Rodriguez-Martinez, A.; Puche, J.L.; Serrano, M.J. Circulating Tumor Cells: Markers and Methodologies for Enrichment and Detection. Methods Mol. Biol. 2017, 1634, 283–303. [Google Scholar] [CrossRef]
- Coumans, F.; van Dalum, G.; Terstappen, L.W.M.M. CTC Technologies and Tools. Cytom. Part A 2018, 93, 1197–1201. [Google Scholar] [CrossRef]
- Ashworth, T.R. A Case of Cancer in Which Cells Similar to Those in the Tumours Were Seen in the Blood after Death. Med. J. Aust. 1869, 14, 146–147. [Google Scholar]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating Tumor Cells: Biology and Clinical Significance. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Giret, T.M.; Cote, R.J.; Alix-Panabieres, C.; González Hernández, Á. Cancers Circulating Tumor Cells from Enumeration to Analysis: Current Challenges and Future Opportunities. Cancers 2021, 13, 2723. [Google Scholar] [CrossRef]
- Vermesh, O.; Aalipour, A.; Ge, T.J.; Saenz, Y.; Guo, Y.; Alam, I.S.; Park, S.M.; Adelson, C.N.; Mitsutake, Y.; Vilches-Moure, J.; et al. An Intravascular Magnetic Wire for the High-Throughput Retrieval of Circulating Tumour Cells in Vivo. Nat. Biomed. Eng. 2018, 2, 696–705. [Google Scholar] [CrossRef]
- Saucedo-Zeni, N.; Mewes, S.; Niestroj, R.; Gasiorowski, L.; Murawa, D.; Nowaczyk, P.; Tomasi, T.; Weber, E.; Dworacki, G.; Morgenthaler, N.G.; et al. A Novel Method for the in Vivo Isolation of Circulating Tumor Cells from Peripheral Blood of Cancer Patients Using a Functionalized and Structured Medical Wire. Int. J. Oncol. 2012, 41, 1241. [Google Scholar] [CrossRef]
- Cieślikowski, W.A.; Budna-Tukan, J.; Świerczewska, M.; Ida, A.; Hrab, M.; Jankowiak, A.; Mazel, M.; Nowicki, M.; Milecki, P.; Pantel, K.; et al. Circulating Tumor Cells as a Marker of Disseminated Disease in Patients with Newly Diagnosed High-Risk Prostate Cancer. Cancers 2020, 12, 160. [Google Scholar] [CrossRef]
- Dizdar, L.; Fluegen, G.; van Dalum, G.; Honisch, E.; Neves, R.P.; Niederacher, D.; Neubauer, H.; Fehm, T.; Rehders, A.; Krieg, A.; et al. Detection of Circulating Tumor Cells in Colorectal Cancer Patients Using the GILUPI CellCollector: Results from a Prospective, Single-Center Study. Mol. Oncol. 2019, 13, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Hehnen, F.; Wendt, G.; Schaller, J.; Geus, P.; Villwock, J.; Kertzscher, U.; Goubergrits, L. Investigation of the Attachment of Circulating Endothelial Cells to a Cell Probe: Combined Experimental and Numerical Study. Adv. Eng. Mater. 2021, 24, 2101317. [Google Scholar] [CrossRef]
- Hofmann, O.; Voirin, G.; Niedermann, P.; Manz, A. Three-Dimensional Microfluidic Confinement for Efficient Sample Delivery to Biosensor Surfaces. Application to Immunoassays on Planar Optical Waveguides. Anal. Chem. 2002, 74, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Spivack, D.E.; Kelly, P.; Gaughan, J.P.; Van Bemmelen, P.S. Mapping of Superficial Extremity Veins: Normal Diameters and Trends in a Vascular Patient-Population. Ultrasound Med. Biol. 2012, 38, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Nakajima, Y.; Nakano, T.; Okuhira, M.; Kasashima, A.; Hayashi, R.; Yamashita, M.; Urai, T.; Nakatani, T. Safety of Venipuncture Sites at the Cubital Fossa as Assessed by Ultrasonography. J. Patient Saf. 2020, 16, 98. [Google Scholar] [CrossRef]
- Rossi, G.; Mu, Z.; Rademaker, A.W.; Austin, L.K.; Strickland, K.S.; Costa, R.L.B.; Nagy, R.J.; Zagonel, V.; Taxter, T.J.; Behdad, A.; et al. Cell-Free DNA and Circulating Tumor Cells: Comprehensive Liquid Biopsy Analysis in Advanced Breast Cancer. Clin. Cancer Res. 2018, 24, 560–568. [Google Scholar] [CrossRef]
- Goldkorn, A.; Ely, B.; Quinn, D.I.; Tangen, C.M.; Fink, L.M.; Xu, T.; Twardowski, P.; Van Veldhuizen, P.J.; Agarwal, N.; Carducci, M.A.; et al. Circulating Tumor Cell Counts Are Prognostic of Overall Survival in SWOG S0421: A Phase III Trial of Docetaxel with or without Atrasentan for Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2014, 32, 1136–1142. [Google Scholar] [CrossRef]
- Cohen, S.J.; Punt, C.J.A.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.A.; Mitchell, E.; Miller, M.C.; et al. Prognostic Significance of Circulating Tumor Cells in Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2009, 20, 1223–1229. [Google Scholar] [CrossRef]
- De Wit, S.; Van Dalum, G.; Lenferink, A.T.M.; Tibbe, A.G.J.; Hiltermann, T.J.N.; Groen, H.J.M.; Van Rijn, C.J.M.; Terstappen, L.W.M.M. The Detection of EpCAM+ and EpCAM- Circulating Tumor Cells. Sci. Rep. 2015, 5, 12270. [Google Scholar] [CrossRef]
- O’Shannessy, D.J.; Yu, G.; Smale, R.; Fu, Y.S.; Singhal, S.; Thiel, R.P.; Somers, E.B.; Vachani, A. Folate Receptor Alpha Expression in Lung Cancer: Diagnostic and Prognostic Significance. Oncotarget 2012, 3, 414–425. [Google Scholar] [CrossRef]
- Nunez, M.I.; Behrens, C.; Woods, D.M.; Lin, H.; Suraokar, M.; Kadara, H.; Hofstetter, W.; Kalhor, N.; Lee, J.J.; Franklin, W.; et al. High Expression of Folate Receptor Alpha in Lung Cancer Correlates with Adenocarcinoma Histology and EGFR [Corrected] Mutation. J. Thorac. Oncol. 2012, 7, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, F.; Li, X.; Yang, G.; Zhang, L.; Ren, S.; Zhao, C.; Deng, Q.; Li, W.; Gao, G.; et al. Folate Receptor-Positive Circulating Tumor Cell Detected by LT-PCR-Based Method as a Diagnostic Biomarker for Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate Receptor Expression in Carcinomas and Normal Tissues Determined by a Quantitative Radioligand Binding Assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, L.; Wang, X.; Fan, W.H.; Qin, Y.; Lin, X.; Xie, Z.; Liu, M.; Ouyang, M.; Li, S.; et al. Evaluation of Cell Surface Vimentin Positive Circulating Tumor Cells as a Diagnostic Biomarker for Lung Cancer. Front. Oncol. 2021, 11, 672687. [Google Scholar] [CrossRef]
- Gerratana, L.; Davis, A.A.; Polano, M.; Zhang, Q.; Shah, A.N.; Lin, C.; Basile, D.; Toffoli, G.; Wehbe, F.; Puglisi, F.; et al. Understanding the Organ Tropism of Metastatic Breast Cancer through the Combination of Liquid Biopsy Tools. Eur. J. Cancer 2021, 143, 147–157. [Google Scholar] [CrossRef]
- Tamminga, M.; De Wit, S.; Schuuring, E.; Timens, W.; Terstappen, L.W.M.M.; Hiltermann, T.J.N.; Groen, H.J.M. Circulating Tumor Cells in Lung Cancer Are Prognostic and Predictive for Worse Tumor Response in Both Targeted- and Chemotherapy. Transl. Lung Cancer Res. 2019, 8, 854–861. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Abdulmawjood, B.; Roma-Rodrigues, C.; Fernandes, A.R.; Baptista, P.V. Liquid Biopsies in Myeloid Malignancies. Cancer Drug Resist. 2019, 2, 1044–1061. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, Z.; Wang, H.; Zhang, H.; Duan, G.; Zhang, X. Role of Circulating Tumor Cells in Diagnosis of Lung Cancer: A Systematic Review and Meta-Analysis. J. Int. Med. Res. 2021, 49, 0300060521994926. [Google Scholar] [CrossRef]
- Ried, K.; Tamanna, T.; Matthews, S.; Eng, P.; Sali, A. New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers. Front. Oncol. 2020, 10, 582. [Google Scholar] [CrossRef]
- Stevens, M.; Nanou, A.; Terstappen, L.W.M.M.; Driemel, C.; Stoecklein, N.H.; Coumans, F.A.W. StarDist Image Segmentation Improves Circulating Tumor Cell Detection. Cancers 2022, 14, 2916. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lu, Q.; Lang, J.; Yu, H.; Peng, C.; Bing, P.; Li, S.; Zhou, Q.; Liang, Y.; Tian, G. A New Method for CTC Images Recognition Based on Machine Learning. Front. Bioeng. Biotechnol. 2020, 8, 897. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geus, P.F.; Hehnen, F.; Krakowski, S.; Lücke, K.; Hoon, D.S.B.; Frost, N.; Kertzscher, U.; Wendt, G. Verification of a Novel Minimally Invasive Device for the Isolation of Rare Circulating Tumor Cells (CTC) in Cancer Patients’ Blood. Cancers 2022, 14, 4753. https://doi.org/10.3390/cancers14194753
Geus PF, Hehnen F, Krakowski S, Lücke K, Hoon DSB, Frost N, Kertzscher U, Wendt G. Verification of a Novel Minimally Invasive Device for the Isolation of Rare Circulating Tumor Cells (CTC) in Cancer Patients’ Blood. Cancers. 2022; 14(19):4753. https://doi.org/10.3390/cancers14194753
Chicago/Turabian StyleGeus, Paul Friedrich, Felix Hehnen, Sophia Krakowski, Klaus Lücke, Dave S. B. Hoon, Nikolaj Frost, Ulrich Kertzscher, and Gabi Wendt. 2022. "Verification of a Novel Minimally Invasive Device for the Isolation of Rare Circulating Tumor Cells (CTC) in Cancer Patients’ Blood" Cancers 14, no. 19: 4753. https://doi.org/10.3390/cancers14194753
APA StyleGeus, P. F., Hehnen, F., Krakowski, S., Lücke, K., Hoon, D. S. B., Frost, N., Kertzscher, U., & Wendt, G. (2022). Verification of a Novel Minimally Invasive Device for the Isolation of Rare Circulating Tumor Cells (CTC) in Cancer Patients’ Blood. Cancers, 14(19), 4753. https://doi.org/10.3390/cancers14194753





