The Differential Paracrine Role of the Endothelium in Prostate Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
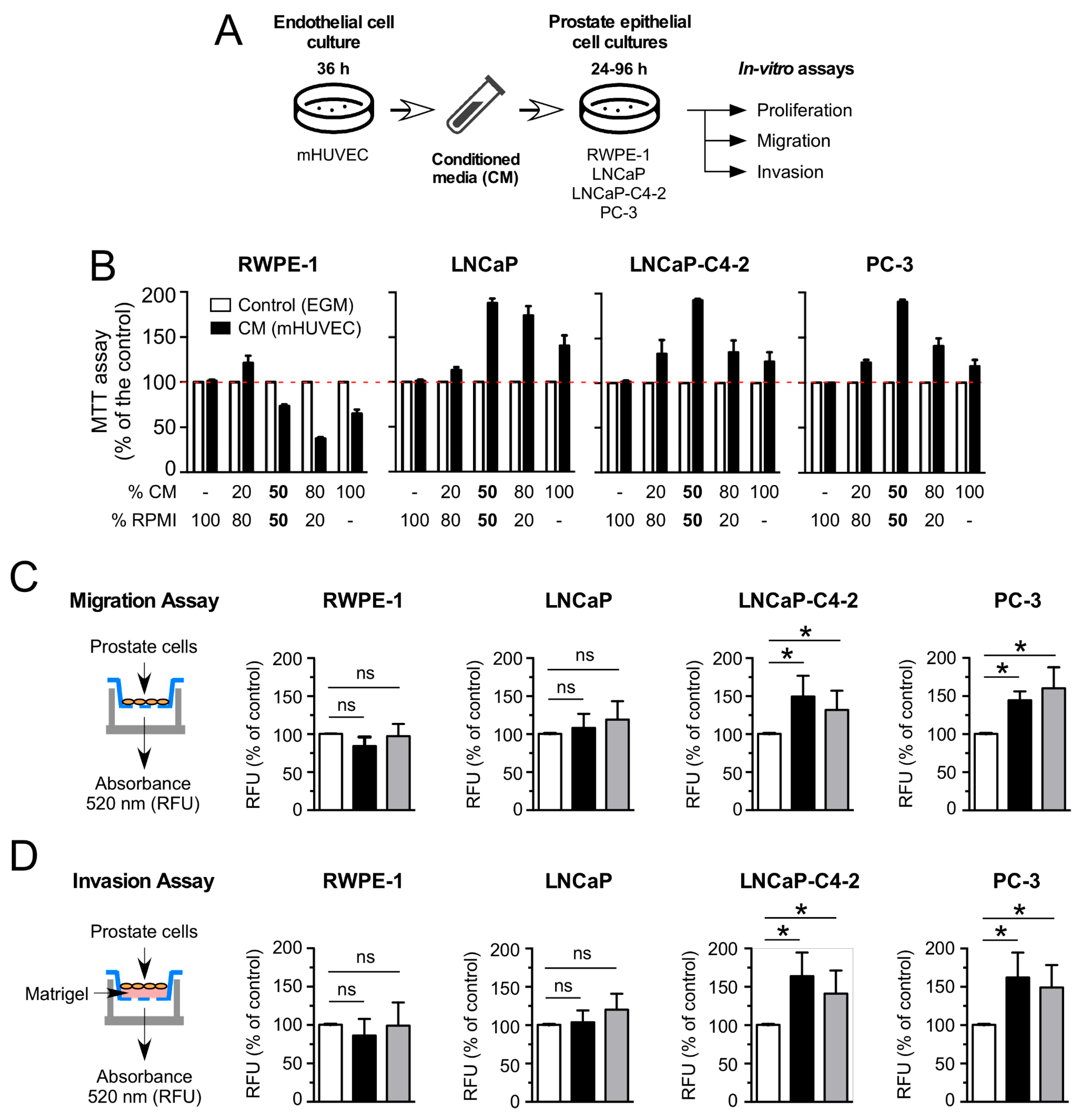
2.1. Cell Cultures
2.2. Conditioned Media
2.3. Animals Models
2.4. Cell Proliferation Assays
2.5. Transwell Migration and Invasion Assays
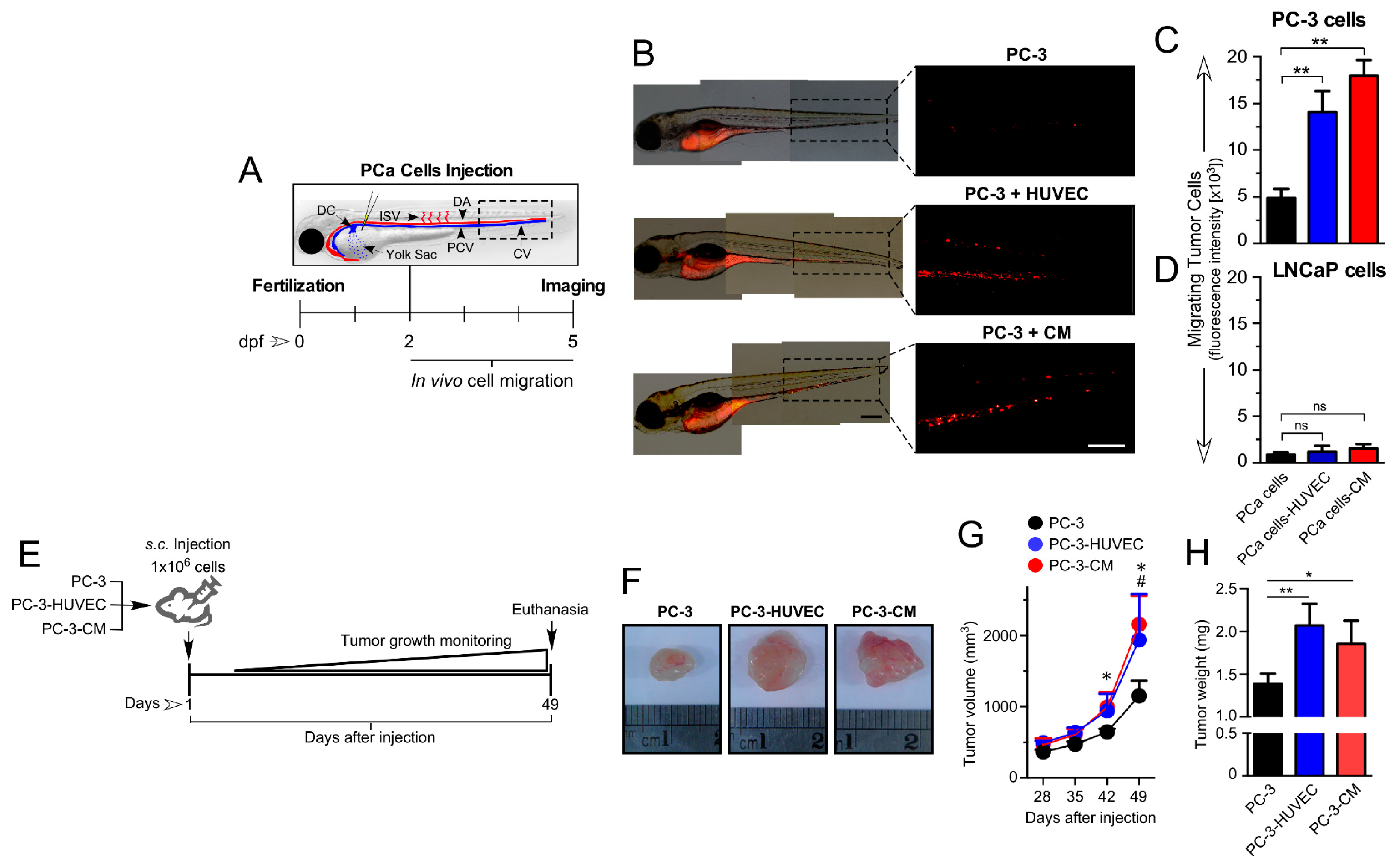
2.6. Zebrafish Xenograft Model
2.7. Cell Line-Derived Xenograft Model
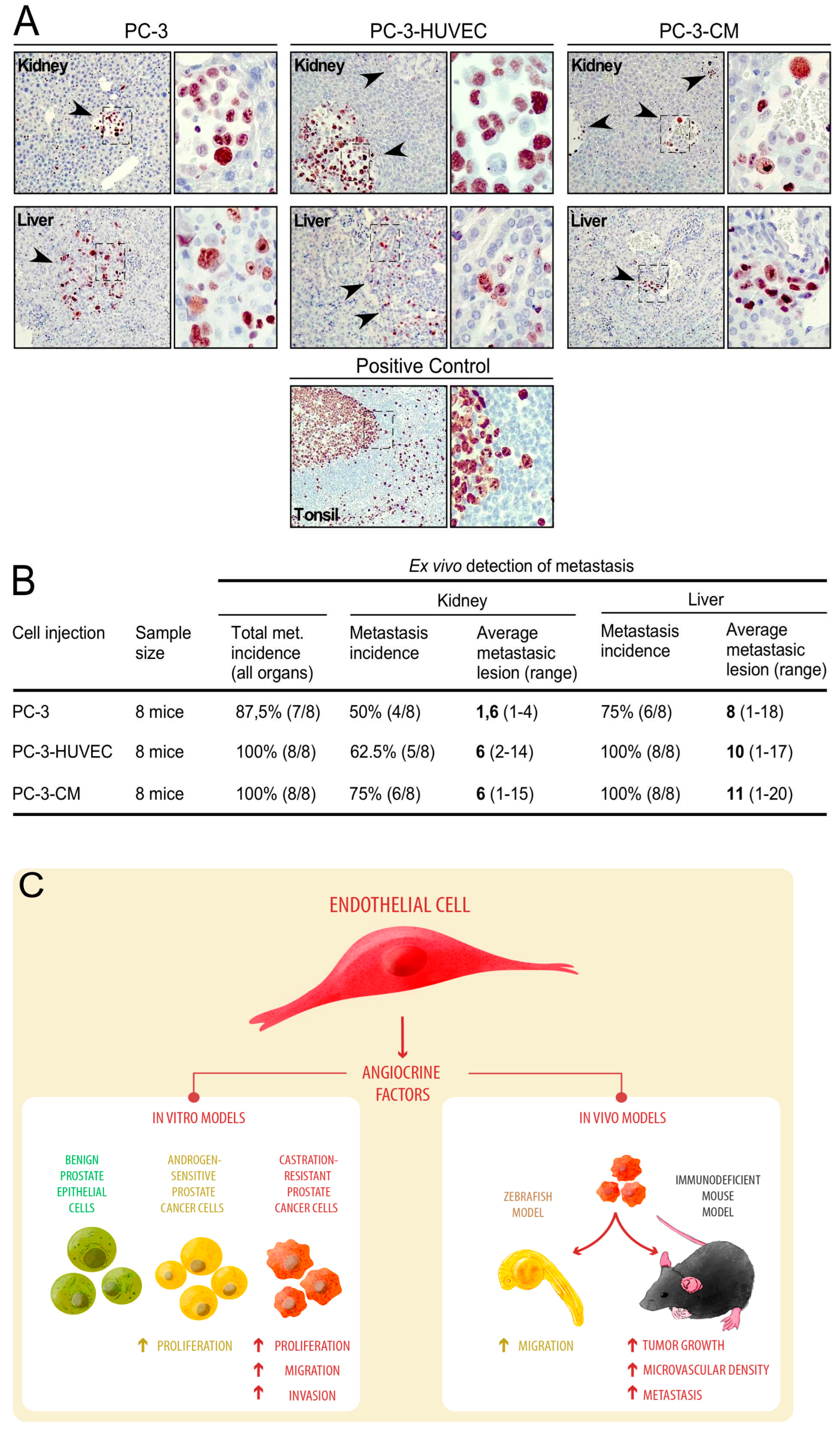
2.8. Immunohistochemistry
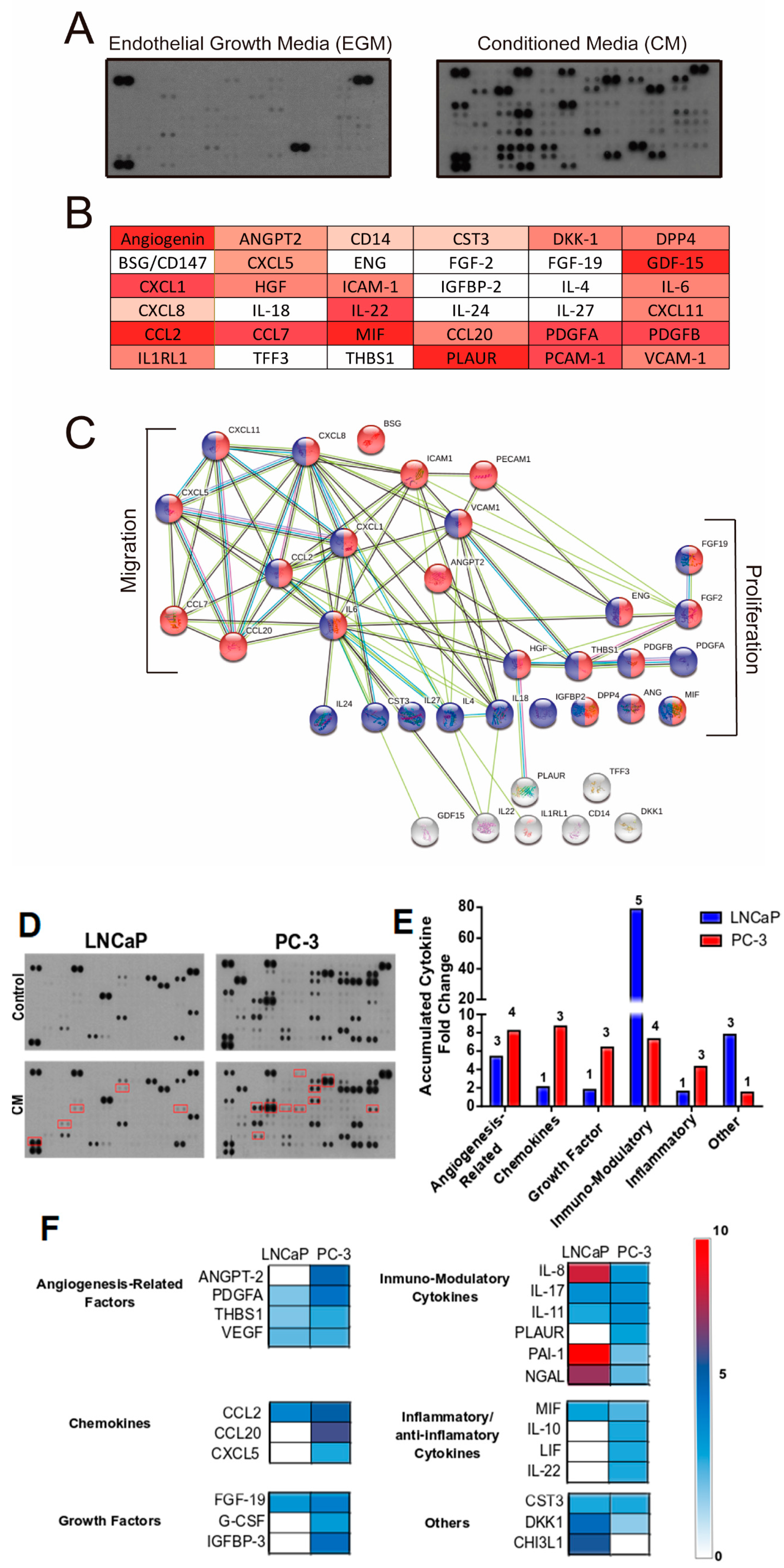
2.9. Human Cytokine Array
2.10. Expression Analysis
2.11. Statistical Analysis
3. Results
3.1. HUVEC-CM Increases Proliferation, Migration and Invasion in Aggressive PCa Cell Lines
3.2. HUVEC-Mediated Paracrine/Angiocrine Effects Enhance Migration and Growth of Tumors In Vivo
3.3. HUVEC-CM Increases PCa Cell Proliferation, Microvascular Density and VEGF-A Expression in PC-3 Cell-Line-Derived Xenograft Tumors
3.4. Human Endothelial Cells Enhance Metastasis of PC-3 Cell-Line-Derived Xenograft Tumors
3.5. Proteomic Analysis of The Primary Signals/Factors Involved in Intercellular Communication from HUVEC-CM
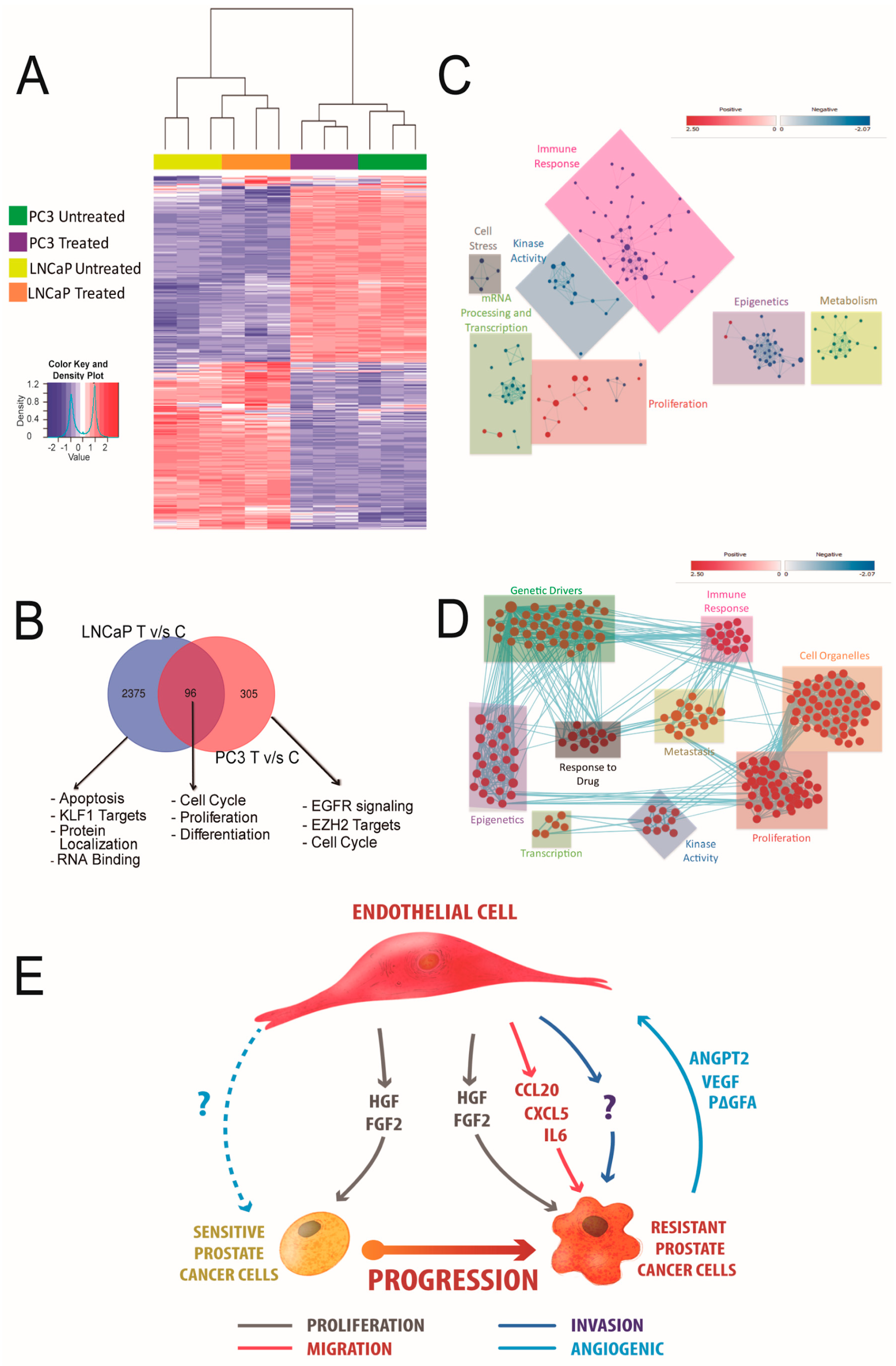
3.6. HUVEC-CM Induces Differential Changes in Gene Expression That Determine Aggressiveness in PCa Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Janoff, D.M.; Peterson, C.; Mongoue-Tchokote, S.; Peters, L.; Beer, T.M.; Wersinger, E.M.; Mori, M.; Garzotto, M. Clinical outcomes of androgen deprivation as the sole therapy for localized and locally advanced prostate cancer. BJU Int. 2005, 96, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Lissbrant, I.F.; Stattin, P.; Damber, J.E.; Bergh, A. Vascular density is a predictor of cancer-specific survival in prostatic carcinoma. Prostate 1997, 33, 38–45. [Google Scholar] [CrossRef]
- Weidner, N.; Carroll, P.R.; Flax, J.; Blumenfeld, W.; Folkman, J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am. J. Pathol. 1993, 143, 401–409. [Google Scholar]
- Kobayashi, H.; Kosaka, T.; Mikami, S.; Miyazaki, Y.; Matsumoto, K.; Kikuchi, E.; Miyajima, A.; Kameyama, K.; Sato, Y.; Oya, M. Vasohibin-1 as a novel microenvironmental biomarker for patient risk reclassification in low-risk prostate cancer. Oncotarget 2018, 9, 10203–10210. [Google Scholar] [CrossRef][Green Version]
- Miyata, Y.; Mitsunari, K.; Asai, A.; Takehara, K.; Mochizuki, Y.; Sakai, H. Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy. Prostate 2015, 75, 84–91. [Google Scholar] [CrossRef]
- Yang, M.; Zu, K.; Mucci, L.A.; Rider, J.R.; Fiorentino, M.; Clinton, S.K.; Loda, M.; Stampfer, M.J.; Giovannucci, E. Vascular morphology differentiates prostate cancer mortality risk among men with higher Gleason grade. Cancer Causes Control CCC 2016, 27, 1043–1047. [Google Scholar] [CrossRef]
- Bilusic, M.; Wong, Y.N. Anti-angiogenesis in prostate cancer: Knocked down but not out. Asian J. Androl. 2014, 16, 372–377. [Google Scholar] [CrossRef]
- Cereda, V.; Formica, V.; Roselli, M. Issues and promises of bevacizumab in prostate cancer treatment. Expert Opin. Biol. Ther. 2018, 18, 707–717. [Google Scholar] [CrossRef]
- Butler, J.M.; Kobayashi, H.; Rafii, S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer 2010, 10, 138–146. [Google Scholar] [CrossRef]
- Franses, J.W.; Drosu, N.C.; Gibson, W.J.; Chitalia, V.C.; Edelman, E.R. Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int. J. Cancer 2013, 133, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Franses, J.W.; Edelman, E.R. The evolution of endothelial regulatory paradigms in cancer biology and vascular repair. Cancer Res. 2011, 71, 7339–7344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galan-Moya, E.M.; Le Guelte, A.; Lima Fernandes, E.; Thirant, C.; Dwyer, J.; Bidere, N.; Couraud, P.O.; Scott, M.G.; Junier, M.P.; Chneiweiss, H.; et al. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO Rep. 2011, 12, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Dong, Z.; Vodopyanov, D.; Imai, A.; Helman, J.I.; Prince, M.E.; Wicha, M.S.; Nor, J.E. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010, 70, 9969–9978. [Google Scholar] [CrossRef]
- Ou, J.; Peng, Y.; Deng, J.; Miao, H.; Zhou, J.; Zha, L.; Zhou, R.; Yu, L.; Shi, H.; Liang, H. Endothelial cell-derived fibronectin extra domain A promotes colorectal cancer metastasis via inducing epithelial-mesenchymal transition. Carcinogenesis 2014, 35, 1661–1670. [Google Scholar] [CrossRef]
- Wang, Y.H.; Dong, Y.Y.; Wang, W.M.; Xie, X.Y.; Wang, Z.M.; Chen, R.X.; Chen, J.; Gao, D.M.; Cui, J.F.; Ren, Z.G. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-kappaB pathways induced by paracrine cytokines. J. Exp. Clin. Cancer Res. 2013, 32, 51. [Google Scholar] [CrossRef]
- Weidner, N. Tumour vascularity and proliferation: Clear evidence of a close relationship. J. Pathol. 1999, 189, 297–299. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Z.; Lauxen, I.S.; Filho, M.S.; Nor, J.E. Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer Res. 2014, 74, 2869–2881. [Google Scholar] [CrossRef]
- Ghiabi, P.; Jiang, J.; Pasquier, J.; Maleki, M.; Abu-Kaoud, N.; Rafii, S.; Rafii, A. Endothelial cells provide a notch-dependent pro-tumoral niche for enhancing breast cancer survival, stemness and pro-metastatic properties. PLoS ONE 2014, 9, e112424. [Google Scholar] [CrossRef]
- Poulos, M.G.; Gars, E.J.; Gutkin, M.C.; Kloss, C.C.; Ginsberg, M.; Scandura, J.M.; Rafii, S.; Butler, J.M. Activation of the vascular niche supports leukemic progression and resistance to chemotherapy. Exp. Hematol. 2014, 42, 976–986. [Google Scholar] [CrossRef]
- Wang, X.; Lee, S.O.; Xia, S.; Jiang, Q.; Luo, J.; Li, L.; Yeh, S.; Chang, C. Endothelial cells enhance prostate cancer metastasis via IL-6-->androgen receptor-->TGF-beta-->MMP-9 signals. Mol. Cancer 2013, 12, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Is tissue mass regulated by vascular endothelial cells? Prostate as the first evidence. Endocrinology 1998, 139, 441–442. [Google Scholar] [CrossRef]
- Pedrosa, A.R.; Trindade, A.; Carvalho, C.; Graca, J.; Carvalho, S.; Peleteiro, M.C.; Adams, R.H.; Duarte, A. Endothelial Jagged1 promotes solid tumor growth through both pro-angiogenic and angiocrine functions. Oncotarget 2015, 6, 24404–24423. [Google Scholar] [CrossRef] [PubMed]
- Torres-Estay, V.; Carreno, D.V.; Fuenzalida, P.; Watts, A.; San Francisco, I.F.; Montecinos, V.P.; Sotomayor, P.C.; Ebos, J.; Smith, G.J.; Godoy, A.S. Androgens modulate male-derived endothelial cell homeostasis using androgen receptor-dependent and receptor-independent mechanisms. Angiogenesis 2017, 20, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Franses, J.W.; Baker, A.B.; Chitalia, V.C.; Edelman, E.R. Stromal endothelial cells directly influence cancer progression. Sci. Transl. Med. 2011, 3, 66ra65. [Google Scholar] [CrossRef]
- Gialeli, C.; Viola, M.; Barbouri, D.; Kletsas, D.; Passi, A.; Karamanos, N.K. Dynamic interplay between breast cancer cells and normal endothelium mediates the expression of matrix macromolecules, proteasome activity and functional properties of endothelial cells. Biochim. Biophys Acta 2014, 1840, 2549–2559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Godoy, A.; Kawinski, E.; Li, Y.; Oka, D.; Alexiev, B.; Azzouni, F.; Titus, M.A.; Mohler, J.L. 5alpha-reductase type 3 expression in human benign and malignant tissues: A comparative analysis during prostate cancer progression. Prostate 2011, 71, 1033–1046. [Google Scholar] [CrossRef]
- Godoy, A.; Montecinos, V.P.; Gray, D.R.; Sotomayor, P.; Yau, J.M.; Vethanayagam, R.R.; Singh, S.; Mohler, J.L.; Smith, G.J. Androgen Deprivation Induces Rapid Involution and Recovery of Human Prostate Vasculature. Am. J. Physiol. 2011, 300, E263–E275. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.; Watts, A.; Sotomayor, P.; Montecinos, V.P.; Huss, W.J.; Onate, S.A.; Smith, G.J. Androgen receptor is causally involved in the homeostasis of the human prostate endothelial cell. Endocrinology 2008, 149, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Stachon, A.; Schluter, T.; Koller, M.; Weisser, H.; Krieg, M. Primary culture of microvascular endothelial cells from human benign prostatic hyperplasia. Prostate 2001, 48, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bhattacharya, R.; Ye, X.; Fan, F.; Boulbes, D.R.; Xia, L.; Ellis, L.M. Endothelial cells activate the cancer stem cell-associated NANOGP8 pathway in colorectal cancer cells in a paracrine fashion. Mol. Oncol. 2017, 11, 1023–1034. [Google Scholar] [CrossRef]
- Buchanan, C.F.; Szot, C.S.; Wilson, T.D.; Akman, S.; Metheny-Barlow, L.J.; Robertson, J.L.; Freeman, J.W.; Rylander, M.N. Cross-talk between endothelial and breast cancer cells regulates reciprocal expression of angiogenic factors in vitro. J. Cell. Biochem. 2012, 113, 1142–1151. [Google Scholar] [CrossRef]
- Tung, M.C.; Hsieh, S.C.; Yang, S.F.; Cheng, C.W.; Tsai, R.T.; Wang, S.C.; Huang, M.H.; Hsieh, Y.H. Knockdown of lipocalin-2 suppresses the growth and invasion of prostate cancer cells. Prostate 2013, 73, 1281–1290. [Google Scholar] [CrossRef]
- Araki, S.; Omori, Y.; Lyn, D.; Singh, R.K.; Meinbach, D.M.; Sandman, Y.; Lokeshwar, V.B.; Lokeshwar, B.L. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007, 67, 6854–6862. [Google Scholar] [CrossRef]
- Beider, K.; Abraham, M.; Begin, M.; Wald, H.; Weiss, I.D.; Wald, O.; Pikarsky, E.; Abramovitch, R.; Zeira, E.; Galun, E.; et al. Interaction between CXCR4 and CCL20 pathways regulates tumor growth. PLoS ONE 2009, 4, e5125. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, W.; Li, M.; Shao, M.; Wang, J.; Sui, H.; Yu, H.; Shao, W.; Gui, S.; Li, J.; et al. High C-X-C motif chemokine 5 expression is associated with malignant phenotypes of prostate cancer cells via autocrine and paracrine pathways. Int. J. Oncol. 2018, 53, 358–370. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Estay, V.; Mastri, M.; Rosario, S.; Fuenzalida, P.; Echeverría, C.E.; Flores, E.; Watts, A.; Cerda-Infante, J.; Montecinos, V.P.; Sotomayor, P.C.; et al. The Differential Paracrine Role of the Endothelium in Prostate Cancer Cells. Cancers 2022, 14, 4750. https://doi.org/10.3390/cancers14194750
Torres-Estay V, Mastri M, Rosario S, Fuenzalida P, Echeverría CE, Flores E, Watts A, Cerda-Infante J, Montecinos VP, Sotomayor PC, et al. The Differential Paracrine Role of the Endothelium in Prostate Cancer Cells. Cancers. 2022; 14(19):4750. https://doi.org/10.3390/cancers14194750
Chicago/Turabian StyleTorres-Estay, Verónica, Michalis Mastri, Spencer Rosario, Patricia Fuenzalida, Carolina E. Echeverría, Emilia Flores, Anica Watts, Javier Cerda-Infante, Viviana P. Montecinos, Paula C. Sotomayor, and et al. 2022. "The Differential Paracrine Role of the Endothelium in Prostate Cancer Cells" Cancers 14, no. 19: 4750. https://doi.org/10.3390/cancers14194750
APA StyleTorres-Estay, V., Mastri, M., Rosario, S., Fuenzalida, P., Echeverría, C. E., Flores, E., Watts, A., Cerda-Infante, J., Montecinos, V. P., Sotomayor, P. C., Amigo, J., Escudero, C., Nualart, F., Ebos, J. M. L., Smiraglia, D. J., & Godoy, A. S. (2022). The Differential Paracrine Role of the Endothelium in Prostate Cancer Cells. Cancers, 14(19), 4750. https://doi.org/10.3390/cancers14194750







