Simple Summary
Uterine or endometrial cancer is one of the most common types of cancer among the female population. Different alterations of molecules are related to many types of cancer. Some molecules called ion channels have been described as involved in the development of cancer, including endometrial cancer. We review the scientific evidence about the involvement of the ion channels in endometrial cancer and how some treatments can be developed with these molecules as a target. Even though they are involved in the progression of endometrial cancer, since they are present throughout the whole body, some possible treatments based on these could be studied.
Abstract
Uterine or endometrial cancer (EC) is the sixth most common neoplasia among women worldwide. Cancer can originate from a myriad of causes, and increasing evidence suggests that ion channels (IC) play an important role in the process of carcinogenesis, taking part in many pathways such as self-sufficiency in growth signals, proliferation, evasion of programmed cell death (apoptosis), angiogenesis, cell differentiation, migration, adhesion, and metastasis. Hormones and growth factors are well-known to be involved in the development and/or progression of many cancers and can also regulate some ion channels and pumps. Since the endometrium is responsive and regulated by these factors, the ICs could make an important contribution to the development and progression of endometrial cancer. In this review, we explore what is beyond (ion) flow regulation by investigating the role of the main families of ICs in EC, including as possible targets for EC treatment.
1. Introduction
Cancer is considered the main public health problem and the second leading cause of global mortality [1]. In 2020, there were estimated to be more than 19 million new cases (including non-melanoma skin cancer) and 10 million deaths from cancer. In the female population, gynecological cancers are common, and uterine cancer is the sixth most common cancer among women worldwide. Approximately 417,000 new cases and 97,000 deaths caused by uterine cancer were diagnosed in 2020 [2].
Cell proliferation, migration, apoptosis, and differentiation are involved in cancer initiation and progression, and it is well-recognized that ion channels and transporters have a central role in regulating these processes.
For this review, a vast search was performed in the principal sources of biomedical literature to find studies involving the function and/or expression of ion channels in endometrial cancer. To give an overview of the topic, we also searched for complementary information about the general characteristics of each ion channel family. To perform the search about the role of IC in EC, the MeSH terms (((“Membrane Transport Proteins”[Mesh]) AND “Ion Channels”[Mesh]) AND “Endometrial Neoplasms”[Mesh]) were selected. In-cited literature was used as a search source to find and include other papers not shown in the initial search.
1.1. Endometrial Cancer
Most uterine cancers are usually referred to as endometrial cancer (EC), originating from the inner lining of the uterine cavity (endometrium) [3]. Based on the histological characteristics, stages, and hormone receptor expression, EC is classified into two types: endometrioid endometrial carcinoma (EEC; Type 1) and non-EEC subtype (NEEC; Type 2). EEC corresponds to more than 80% of the cases and is generally estrogen-dependent, and NEEC develops independently of estrogen [3]. The stages of endometrial cancer vary from I to IV according to the International Federation of Gynecology and Obstetrics (FIGO) [4] and the American Joint Committee on Cancer TNM staging system [5]. Higher stages correspond to a higher grade and have higher chances of cancer spreading throughout the body [6].
Carcinogenesis includes the support of proliferative signaling, the avoidance of growth suppressors, the resistance to cell death allowing for replicative immortality, and metastasis. The presence of genomic instability and mutations, inflammation, and reprogramming of energy metabolism are considered emerging hallmarks and enabling characteristics [7]. The abnormal proliferation of the endometrial glands increases in the gland/stroma ratio when compared to the endometrium of the proliferative phase of the cycle. The majority of endometrioid neoplastic lesions appear to evolve from endometrial hyperplasia (EH) without atypia to hyperplasia with atypia lesions (AEH) until well-differentiated EC [8,9]. It is believed that most ECs occur because of stimulation of the endometrium by unopposed estrogens. Endogenous or exogenous estrogen is not balanced simultaneously by progestogen, increasing the risk of inducing mitotic activity of the endometrial cells [10].
The endometrium can receive “unopposed” estrogenic stimulation by several routes or mechanisms: (I) iatrogenic (for example, hormone replacement with estrogens only); (II) production of estrogens by functional tumors (for example, granulosa cell tumor); (III) perimenopause, which leads to high levels of follicle-stimulating hormone (FSH), a decreased ovarian reserve, and frequent anovulatory cycles; (IV) obesity, which leads to insulin resistance, increased insulin levels, decreased levels of sex hormone-binding globulin (SHBG), and aromatization of androgens into estrogens; (V) polycystic ovary syndrome, which develops with hyperinsulinemia, an increased luteinizing hormone (LH)/FSH ratio, hyperandrogenemia, and anovulatory cycles [9]. Conversely, NEECs (Type 2) tend to be estrogen-independent, often associated with endometrial atrophy in postmenopausal women rather than with EH as in EEC (Type 1). Type 2 ECs are clinically more aggressive and are linked with a poorer clinical prognosis [11].
The main known risk factors for developing endometrial cancer are metabolic syndrome, use of oral contraceptives, and null parity [12,13,14]. The incidence of EC is steadily increasing, mainly as a result of raised rates of obesity and population aging [15]. Surgery (hysterectomy with bilateral salpingo-oophorectomy) is still the most frequent treatment for EC used in clinical practice [3]. Radiation and chemotherapy as adjuvant treatments may be recommended depending on the tumor degree differentiation. For those patients with metastatic disease or who wish to preserve their fertility, hormonal therapy (aromatase inhibitors, progestins, and LH-releasing hormone agonists) is an alternative treatment [16].
A favorable prognosis is found in most patients newly diagnosed with EC, with five-year relative survival rates of 81% [17]. However, it is expected that 15 to 20% of patients diagnosed early have recurrences or metastases after surgery [18]. It is estimated that radiotherapy and/or chemotherapy after surgical treatment benefit only 10 to 15% of patients [19]. Patients with recurrent EC or who have metastases have a poor prognosis, with survival rates of less than one year [20]. The success rate of hormonal treatments is also limited to the individual response profile as well as the positive expression of estrogen receptors by tumor cells [21].
Therapeutic resistance leads to highly harmful side effects for patients as it limits the use of medications and the way they are administered [22]. The identification of new biomarkers and therapeutic targets for EC is essential to broaden therapeutic approaches and increase the overall survival of patients. Increasing evidence points to ion transport mechanisms as an adjuvant in the carcinogenesis process, also offering novel therapeutic possibilities [23].
1.2. Ion Channels
Physiological processes such as the pH balance, volume, and cell cycle regulation, immune responses, secretion, muscle contraction, and electrical signals (nerves, muscles, and synapses) are mediated by the movement of ions between intracellular and extracellular fluid [24]. Ion channels and transporters (ICTs) are transmembrane proteins that strictly control the movement of ions across the cell membrane while maintaining the ionic gradients of cells. In this way, ICT assists in the selectively permeable nature of the cell membrane, functioning as gateways for charged ions that cannot diffuse freely through the lipid membrane barriers [25].
Ion channels are fast translocators and have pores that allow specific ions to cross the membrane in favor of an electrochemical gradient. Some channels, such as those dependent on electrical voltage, can detect the electrical potential opening or closing in response to the magnitude of the membrane potential. Ion channels can also be controlled by extracellular (neurotransmitter) and intracellular (second messenger) chemical signals or they can respond to mechanical and thermal stimuli. In contrast, transporters (also called ion pumps and exchangers) slow translocations, and the movement of ions occurs actively against the concentration gradient using energy, typically in the form of adenosine triphosphate (ATP) [24,25].
Rising evidence suggests that ion channels also play an important role in the process of carcinogenesis. Although cancer is not cataloged as a channelopathy, channels and ion pumps contribute to the progression of cancer by playing important roles in self-sufficiency in growth signals, proliferation, evasion of programmed cell death (apoptosis), angiogenesis, cell differentiation, migration, adhesion, and metastasis. [26,27,28]. Growth factors and hormones are well-known to be involved in the development and/or progression of many cancers and also can regulate some ion channels and pumps, contributing to carcinogenesis [29].
Given they are critically important in carcinogenesis, ICs may represent promising therapeutic targets, potentially combined with chemotherapy, immunotherapy, or other molecules that affect essential processes in tumor cells, such as oxidative stress and metabolic pathways [30]. The role of ion channels and transporters as potential therapeutic targets is one of the most innovative approaches to anticancer treatment [30]. However, the oncologic therapeutic strategy must be specific to a given ionic channel or pump to damage the targeted cell without causing toxic effects in other tissues expressing the same channels [27,29].
Channels are usually classified by the ion to which they are selective. They can be subdivided according to functional properties, such as a mechanism of regulation (Ca+2-activated) or a biophysical characteristic (inward rectifier). However, the receptor-operated channels (selective for either cations or anions) and novel transient receptor potential (TRP) channels (which discriminate poorly between monovalent and divalent cations) do not adhere to these simple rules [31].
1.2.1. Potassium Channels
The potassium (K+) channels are a complex family of ion channels. They can be divided into four classes: (I) voltage-gated potassium channels (VGKC), (II) calcium-activated potassium channels (KCa), (III) inward rectifying potassium channels (Kir), and (IV) two-pore domain potassium channels (K2P) [26]. The K+ levels play an important role in membrane potential control, determination, and duration of the action potential, modulation of hormones’ secretion, and balancing excitatory signals in cells [32].
Changes in the cell cycle in tumor cells have been related to loss of function or altered expression of K+ channels in several tumor types [26]. At least in the case of VGKC and KCa, the control of cancer cell proliferation can occur through the modulation of membrane potential (Vm), which, in turn, regulates transmembrane calcium (Ca+2) flow. Intracellular Ca+2 levels participate in cell cycle checkpoints’ control in normal and neoplastic proliferation [33].
1.2.2. Sodium Channels
There are two very different types of sodium (Na+) channels: (I) voltage-gated sodium channels (VGSC) and (II) epithelial sodium channels (ENaC). Present in absorptive epithelia (such as distal twisted tubules of the kidneys, colon, lungs, and ducts of the salivary gland), the ENaC are involved in Na+ absorption and a play key role in maintaining Na+ homeostasis, which is linked directly to the volume of extracellular fluid [34]. However, VGSC are involved in the initial phase of the action potential in most cells, being important for the generation and propagation of the action potential [26].
VGSC are formed mainly by a pore-forming multi-spanning integral membrane glycoprotein (α subunit) that can be associated with one or more regulatory β subunits. The β subunits are single-span integral membrane proteins that modulate the sodium current. They can also act as cell adhesion molecules in terms of interaction with extracellular matrix molecules, communication between adjacent cells, regulation of cell migration, cellular aggregation, and interaction with the cytoskeleton [35]. Aberrant expression/function of VGSCs is related to cell migration, invasion, and tumor metastasis [26].
1.2.3. Chloride Channels
Chloride (Cl−) is the most abundant anion in the extra- and intracellular spaces. Cl− transport through the plasma membrane is involved in numerous physiological processes, from homeostasis to volume control and regulation of excitable cells [36]. The chloride channels (ClC) are a family of anion channels that mediate the transport of Cl− ions across the cell. They can act through voltage dependency, triggered by calcium, or activated by several ligands and second messengers, and can be divided into two major classes: voltage-dependent Cl− channels of the ClC family, and the cystic fibrosis transmembrane conductance regulator (CFTR) [37].
Dysregulation of Cl− channels has been reported in multiple cancer types related to cell migration, invasion, and metastasis [26,27]. The Cl− intracellular ion channels (CLICs) are an emerging class involved in cancer development [38]. CFTR is expressed in the epithelial cells of various tissues and organs. Although defective CFTR leads to cystic fibrosis, dysregulation of CFTR can promote or suppress cancer progression [39].
1.2.4. Calcium Channels
Calcium is an important signaling molecule and serves as a second messenger for several fundamental cellular processes such as cell cycle control, migration, and apoptosis [40]. Regulation of intracellular Ca+2 levels involves the flow of Ca+2 through the plasmatic membrane and the release of intracellular Ca+2 stocks in the endoplasmic reticulum and mitochondria [26].
The Ca+2 channels are generally activated in response to membrane depolarization and mediate the influx of calcium in response to action potentials and depolarizing signals [41]. Calcium channels can be classified according to their activation mechanism: (I) voltage-gated calcium channels (VGCCs), (II) receptor-operated calcium channels (ROCCs), (III) store-operated calcium channels (SOCCs), (IV) transient receiver potential channels (TRPs), (V) acid-sensing ion channels (ASICs), and (VI) stretch-activated ion channels (SAICs) [42]. These channels play important roles in human physiology and it is not a surprise that calcium channel disorders are associated with tumor cell growth, survival, angiogenesis, and migration [26,42,43].
1.2.5. Porins
Voltage-dependent anion channel (VDAC), also known as a mitochondrial porin, regulates metabolites exchange between cytosol and mitochondria, cellular energy homeostasis, and is involved in mitochondria-mediated apoptosis. There are three VDAC isoforms (VDAC1, VDAC2, and VDAC3), and alterations in VDAC expression have been reported already in human pathologies, including cancer [44].
Aquaporins (AQP) are integral membrane proteins that serve as channels and enable the regulated transport of water essential to homeostasis in response to osmotic gradients created by the active transport of solutes. AQP isoforms identified in mammals present in multiple organs and tissues are involved in many biological functions. Altered AQP expression is related to carcinogenesis in diverse tissues, especially motility, invasiveness, and angiogenesis [45,46].
2. ICs’ Expression in Endometrial Cancer
Ion channels play an important regulatory role in receptivity and embryo implantation in the endometrium. Abnormalities in ion transport are related to endometrial diseases such as infertility and cancer [47]. The roles of ion channels in cancer-related cellular behaviors and the specific expression and functional profiles of various channels characteristic of certain human cancers have been studied as potential diagnostic and therapeutic targets [28]. Table 1 shows evidence collected to date about the role of ICT in endometrial cancer (Table 1).
2.1. Potassium Channels
The human EAG-related gene (hERG), which encodes the alpha subunit of the Kv11.1 channel, belongs to the EAG (ether-à-go-go) family, a subfamily of the Kv channels encoded by the KCNH gene family [48]. Aberrant hERG expression in various cancer cells has been correlated with cancer progression [49]. Cherubini et al. (2000) analyzed human samples and found high gene and protein hERG expression in endometrial cancers compared to normal and hyperplastic endometrium. The authors suggested the possible use of hERG K+ channels’ expression as a discriminatory molecular marker between cancerous and non-cancerous endometrium [50]. Suzuki et al. (2004) evaluated the multiple pore-forming and regulatory subunits of voltage-gated potassium (Kv) channel gene expression in uterine cancer cells. Although they did not use non-tumor endometrial cells, hERG-KCNE channel complexes may be selectively involved in the proliferation of endometrial cancer cells. However, the hERG channel blocker E-4031 did not reduce endometrial cancer cell proliferation [51].
hERG plays a role in depolarizing and hyperpolarizing the membrane potential. K+ channel-dependent hyperpolarization seems critical to cell cycle progression (G1 to S phases). Ca+2 influx evoked by hyperpolarization and the opening of more KCa is associated with mitogenic factors’ synthesis [52]. KCa are divided into three subfamilies: big conductance (BKCa; activated by depolarization and/or by increases in intracellular [Ca+2]), intermediate conductance (IKCa; activated by low intracellular [Ca+2]), and small conductance (SKCa; activated by low intracellular [Ca+2]) [53].

Table 1.
Expression of ICTs in endometrial cancer.
Table 1.
Expression of ICTs in endometrial cancer.
| Ion Channel or Transporter | Cellular Process(es) or Pathway(s) | Methods of Analysis | Type of Alteration | Reference |
|---|---|---|---|---|
| Potassium Channels | ||||
| Kv11.1 alpha subunit (hERG) | Differentiation and growth | Endometrial samples: RT-PCR and IHC | ▲frequency of hERG gene and protein expression in EC compared to NE | [50] |
| Kv11.1 alpha subunit (hERG) | Differentiation and growth | In vitro: RT-PCR and specific K+ channel blockers | (+) expression of hERG channel, and their potential auxiliary KCNE subunits are involved in cell proliferation ▼hERG did not reduce cell proliferation | [51] |
| IKCa1 | Tumor progression | Endometrial samples: RT-PCR and WB In vitro: Downregulation and activity inhibition of IKCa1 In vivo: Mouse model of EC | ▲gene and protein expression of IKCa1 in EC specimens compared to NE ▼IKCa1 suppressed cell proliferation and restrained cancer growth | [54] |
| KCa3.1 | Cell proliferation, migration, and invasion | In vitro: Downregulation and activity inhibition of KCa3.1 | ▼KCa3.1 channel inhibits cell proliferation, cell cycle progression, migration, and cellular invasion | [55] |
| BKCa | Cancer initiation and development | Endometrial samples: IHC In vitro: Downregulation of BKCa | ▲ BKCa expression in EC tissues compared to NE ▼ BKCa inhibited cell proliferation and migration | [56] |
| BKCa | Cell proliferation and migration | In vitro: Overexpression and downregulation of BKCa In vivo: Mouse xenograft model | ▲ BKCa stimulated proliferation and migration ▼ BKCa inhibited cell proliferation and migration and impaired tumor growth in vivo | [57] |
| K2P | Cell proliferation | Endometrial samples: RT-PCR and IHC In vitro: K2P activity inhibition | ▲ TREK-1 expression in proliferative phase of endometrium ▼ Cell proliferation by K2P channel blockers | [58] |
| Calcium channels | ||||
| Cav1.3 | Cell proliferation and migration | Endometrial samples: IHC In vitro: Downregulation of Cav1.2 channel and E2 treatment | ▲ expression of Cav1.3 in EC and AEH specimens compared to NE ▼ Cav1.3 inhibited cell proliferation and migration | [59] |
| Cav1.3 | Cell proliferation, apoptosis, and autophagy | In vitro: Cav1.3-antagonist | ▼ Cav1.3 suppressed cell proliferation and migration ▼ Cav1.3 increased apoptosis and autophagy | [60] |
| CACNA2D3 | Cell proliferation and migration | Endometrial samples: RT-PCR and IHC In vitro: Overexpression of CACNA2D3 and P4 treatment In vivo: Mouse xenograft model | ▼ expression of CACNA2D3 in EC tissues and cells ▲ CACNA2D3 inhibited cell proliferation and migration ▲ CACNA2D3 suppressed tumor growth in vivo | [61] |
| TRPM4 | Cell proliferation and migration | In silico: Bioinformatics analysis In vitro: Downregulation of TRPM4 channel and E2 treatment | ▼ TRPM4 expression levels correlated with poor clinical outcomes and EC cell proliferation ▼ TRPM4 promoted proliferation and migration | [62] |
| TRP | Mobility and invasiveness | Endometrial samples: RT-PCR In vitro: Primary endometrial stromal and epithelial cell culture | ▲TRPV2 and TRPC1 expression in EC is associated with high-risk cancer and high EMT status ▲TRPM4 mRNA expression was related to lower-risk EC and low EMT status | [63] |
| TRPV4 | Cell proliferation and metastasis | In silico: Proteomic and bioinformatics analysis In vitro: Downregulation and overexpression of TRPV4 In vivo: Mouse xenograft model | ▼ TRPV4 decreased Ca+2 influx and metastatic ability ▼ TRPV4 reduced peritoneal nodules in vivo ▲ TRPV4 showed the opposite effects in vitro and in vivo models | [64] |
| Chloride channels | ||||
| CFTR | Cell proliferation and migration | Endometrial samples: RT-PCR and IHC In vitro: Downregulation of CFTR | ▲ CFTR expression in EC compared to NE ▼ CFTR increases proliferation and migration | [65] |
| Sodium channels | ||||
| Nav1.7 | Tumor progression | Endometrial samples: RT-PCR In vitro: Primary EC cell culture and inhibition of Nav 1.7 | ▲ Nav1.7 expression in EC tissues ▲ Nav1.7 associated with poor prognosis ▼ Nav1.7 induced apoptosis and reduced the invasiveness ability | [66] |
| Porins | ||||
| AQP1 | Angiogenesis | Endometrial samples: IHC | (+) AQP1 expression in small vessels and microvessels ▲ AQP1 expression in EC compared to NE ▲ AQP1 correlated with tumor angiogenesis and poor prognosis | [67] |
| AQP2 | Cell migration, invasion, and adhesion | Endometrial samples: IHC and WB In vitro: Downregulation of AQP2 | ▲ AQP2 expression in EC compared to NE ▼ AQP2 attenuated migration, invasion, and adhesion, but not proliferation | [68] |
| AQP5 | Cell migration | In vitro: Downregulation of AQP5 | ▼AQP5 attenuated cell migration | [69] |
| AQP3 | Cancer cell differentiation | Endometrial samples: IHC | AQP3 expression is correlated with EC at an earlier stage and lower histological grade | [70] |
| VDAC | Tumor progression | Endometrial samples: RT-PCR and WB | ▲ VCAC1 and VDAC3 expression in EC compared to NE VCAC1 and VDAC3 expression correlates with tumor progression | [71] |
▲ increase; ▼ decrease; (+) positive. RT-PCR (reverse transcription-polymerase chain reaction); IHC (immunohistochemistry); WB (Western blot); NE (normal endometrium); E2 (estrogen); AEH (atypical endometrial hyperplasia).
Wang et al. (2007) demonstrated higher mRNA and protein expression of IKCa1 in endometrial cancer specimens than in normal endometrium and atypical hyperplasia specimens. The pharmacological inhibition of IKCa1 (clotrimazole and TRAM-34) and the downregulation by siRNA against IKCa1 suppressed the EC cell proliferation and arrested the cell cycle. Nude mice treated with clotrimazole and TRAM-34 showed restrained endometrial cancer growth, suggesting that IKCa1 channels may be a new target for the treatment of EC [54]. Similarly, Zhang et al. (2015) evaluated the role of the intermediate-conductance KCa3.1 channel in HEC-1-A and Ishikawa endometrial cancer cells. The gene silencing and pharmacological blockage of the KCa3.1 suppressed cell proliferation and cell cycle progression, and decreased the expression of cyclin D1 and MMP-2, proteins involved in tumor migration and invasion [55].
Wang et al. (2018) revealed higher expression of BKCa in endometrial adenocarcinoma tissues compared to normal endometrium and atypical endometrial hyperplasia. Furthermore, in vitro assays showed that RNAi-mediated knockdown of BKCa inhibited endometrial cancer cell (Ishikawa) growth, possibly via inactivation of the MEK/ERK pathway [56]. On the other hand, overexpression of BKCa promoted proliferation and migration of endometrial cancer HEC-1-B cells. BKCa knockdown decreased these pro-carcinogenic effects and suppressed the growth of the HEC-1-B xenografts in nude mice. The treatment with the selective BKCa channel inhibitor Iberiotoxin (IbTX) decreased HEC-1-B cell proliferation and migration [57].
K2P is a ‘‘leak channel’’ essential for maintaining a negative resting membrane potential [72]. TWIK-related K+ (TREK) channels, a subgroup of K2P channels, have been related to endometrial cancer. According to Patel et al. (2013), the proliferative endometrium expresses higher TREK-1 levels compared to the secretory endometrium, possibly linked to increased cell division in this phase of the menstrual cycle. The K2P channel blockers (methanandamide, lidocaine, zinc, and curcumin) showed antiproliferative effects in endometrial cancer in vitro [58]. K2P channels are expressed in a variety of human cell types. Aberrant expression and function are related to human diseases, such as cancer, and therapeutic regulation of K2P channel activity has been studied in different pathologies [73].
2.2. Calcium Channels
Different subunits of VGCCs demonstrated some degree of participation in cancer progression and development [74]. The L-type calcium channel α 1D subunit (Cav1.3) belongs to the family of VGCC channels. Immunohistochemical results showed high Cav1.3 expression in endometrial carcinoma and atypical endometrial hyperplasia tissues compared to benign endometrial tissues [59]. Sex steroid hormones, including estrogens, can modulate the expression of ion channels in cancer cells, especially in hormone-sensitive tissues [75]. According to Hao et al. (2015), shRNA-mediated Cav1.3 silencing suppressed endometrial cancer cell proliferation and migration. Although E2 treatment increased cell migration, its effect was partly inhibited by Cav1.3 deletion in EC cells. Bao et al. (2012) demonstrated the Cav1.3-antagonist nifedipine significantly suppressed endometrial carcinoma Hec-1A cells’ proliferation and migration in vitro. However, beyond apoptosis, autophagy was also induced in Hec-1A cells by nifedipine as a mechanism of cell survival. Autophagy inhibitor 3-MA enhanced nifedipine-induced cell death [60].
A recent work evaluated the role of CACNA2D3 (calcium voltage-gated channel auxiliary subunit α2δ3) in endometrial cancer. Kong et al. (2020) reported low expression of CACNA2D3 in endometrial cancer tissues and endometrial cell lines (Ishikawa and RL95-2) compared to adjacent healthy endometrial tissues. Unlike the other channels, overexpression of CACNA2D3 decreased cell proliferation and migration, and increased apoptosis and Ca+2 influx in EC cells. Overexpression of CACNA2D3 also decreases tumor growth in a mouse xenograft model. Progesterone (P4) signaling seemed to act in the upregulation of CACNA2D3 expression (in vivo and in vitro) since CACNA2D3 knockdown blocked the function of P4 [61].
Also studied in EC are TRP channels, which are Ca+2-permeable ion channels. Li et al. (2020) evaluated endometrial cancer calcium-activated TRPM4 channel gene expression data through The Cancer Genome Atlas (TCGA) datasets. Low TRPM4 expression levels were correlated with poor clinical outcomes and survival. The TRPM4 silencing in endometrial cancer AN3CA cells promoted proliferation and migration [62]. Recently, Eynde et al. (2022) investigated the TRP channel mRNA expression patterns in malignant endometrial tissues and tumor microenvironment epithelial and mesenchymal cells. The study cross-referenced TRP channel expression data with the epithelial to mesenchymal transition (EMT) status, a change that allows cells to acquire mobility and invasiveness. Calcium-permeable TRPV2 and canonical TRPC1 channels’ expression in both endometrial cancer biopsies and cancer cells were associated with high-risk biopsies and a high EMT status. In contrast, TRPM4 mRNA expression was higher in low-risk cancer tissues and cancer cells and with lower EMT status [63].
Li et al. (2020) also demonstrated that high expression of TRPV4 (transient receptor potential vanilloid 4) is associated with EC progression in vitro and in vivo. TRPV4 depletion (shTRPV4) decreased the calcium influx and metastatic ability in Ishikawa cells, and TRPV4-overexpression (OETRPV4) increased calcium levels and metastatic ability in HEC-1A cells. In vivo tumor xenograft models allowed for an evaluation of the number of metastatic peritoneal nodules. The xenograft model with Ishikawa cells (higher TRPV4 expression) showed a reduction in peritoneal nodules, while xenograft model HEC-1A cells (lower TRPV4 expression) increased the peritoneal nodules. Treatment with a TRPV2 agonist (GSK1016790A) and antagonist (HC067047) reverted the results. The authors also proposed that TRPV4 and Ca+2 could promote metastasis by regulating the cytoskeleton through the RhoA/ROCK1 pathway [64].
2.3. Chloride and Sodium Channels
Although less studied in endometrial cancer, Cl− and Na+ channels have been demonstrated to be involved in cancer progression. According to Xia et al. (2017), CFTR chloride channel expression is upregulated in endometrial carcinoma tissue compared to non-tumoral tissues. However, the specificity inhibitor CFTR(inh)-172 intensified the proliferative and migrative capability of endometrial Ishikawa cells in vitro [65]. Although not directly studied in endometrial cancer, overexpression of chloride channel-3 (CLC-3) was associated with migration and invasion in ectopic endometrial cells from patients with endometriosis [76] and progression of human cervical carcinoma [77].
Voltage-gated sodium channel Nav1.7 was highly expressed in endometrial carcinoma compared to adjacent non-tumoral tissue. Results from Liu et al. (2019) associated Nav1.7 levels with the tumor size, local lymph node metastasis, and patient survival. In vitro experiments with Nav1.7 blocker (PF-05089771) induced cancer cell apoptosis and reduced the invasion ability of isolated cells from EC biopsies [66].
2.4. Porins
Accumulating evidence has been suggesting that aquaporins are involved in the tumorigenesis process [45]. Aquaporin-1 (AQP1) was widely expressed in most secretory and absorptive epithelia and in the endothelial cells of microvessels. An imbalance in AQP1 could indicate a possible involvement in tumor angiogenesis and cell proliferation [78]. Pan et al. (2008) analyzed the AQP1 expression and intratumoral microvessel density (IMD) in endometrioid adenocarcinoma, endometrial hyperplasia, and a normal endometrium. AQP1 was found only in small vessels and microvessels. The AQP1/IMD ratio was significantly higher in endometrioid adenocarcinoma and positively correlated with the histologic grade, invasion, and metastasis [67]. Differently from the AQP1 distribution pattern in endometrial tissue, aquaporin-2 (AQP2) expression is found in the luminal and glandular epithelial cells [79]. Immunohistochemical and Western blot analyses demonstrated a significantly higher expression of AQP2 in EC tissues compared to control samples. In vitro, AQP2 knockdown attenuated migration, invasion, and adhesion but not proliferation in Ishikawa cells [68]. Downregulation of aquaporin-5 (AQP5) showed a reduction in endometrial cancer cells’ migration capacity [69]. Watanabe et al. (2020) associated clinicopathological parameters with AQP3 expression in endometrial cancer samples. Although non-tumoral tissues were not analyzed, the authors demonstrated a significant correlation between AQP3 expression and early tumor stages with lower histological grades [70].
VDAC, also known as a mitochondrial porin, acts as a gatekeeper of mitochondrial metabolites [44]. Jóźwiak et al. (2020) revealed that the isoforms VDAC1 and VDAC3 are upregulated in endometrial cancer tissue compared to a non-tumoral endometrium. Increased expression of VDAC1 was associated with infiltrative endometrial tumors. However, high VDAC3 levels were expressed in poorly differentiated endometrial cancers and low VDAC3 levels in metastatic or advanced tumor stages [71].
3. IC Regulation by Steroids Hormones and Growth Factors
Cancer development involves proliferative signaling, resistance to growth suppressors and death, replicative immortality, angiogenesis, and activation of invasion and metastasis pathways [7]. Ion transport mechanisms are implicated in these cell functions by the modulation of ion flux across cell membranes, cell volume, signal transduction pathways, cellular transport [80], and homeostatic maintenance in subcellular organelles [81]. Ion channels’ and transporters’ dysregulation has been related to pathophysiologic processes, especially in epithelial cells [80]. Interestingly, epithelial tissue is the most common site for the development of cancers. Specifically, those epithelia with secretory capacities, such as the uterus, seem to be frequent sites of cancer [82].
The uterus consists of two different layers: the endometrium and myometrium. The endometrium is mainly constituted of endometrial epithelial cells (luminal and glandular cells) underlying stromal cells [83]. In response to monthly reproductive hormone fluctuations and growth factors, endometrial cells possess remarkable plasticity and regenerative capacity to facilitate pregnancy [84]. However, abnormal human endometrium remodeling and regeneration lead to a range of uterine pathologies such as adenomyosis, endometriosis, and endometrial carcinoma [85]. Prolonged exposure to endogenous estrogen effects means an early age at menarche and advanced age at menopause are considered risk factors for EC [86,87]. Although EC mainly affects postmenopausal women, a rare subset of patients is diagnosed during pregnancy [88].
Therefore, various factors are associated with cancer development and progression, such as the modulation of ion channels’ expression through hormones and growth factors [75]. The potassium channels, followed by calcium, sodium, and chloride channels, are the most investigated in several pathologies [89]. The expression of these channels can be modulated by growth factors and hormones, such as the ovarian steroid hormones E2 and P4 [90,91]. There are two isoforms of estrogen receptors (ER): ERα, which predominantly stands out in normal endometrium and early-stage endometrial cancer, and ERβ, which is more evident in late-stage disease and metastasis [92]. P4 functions through two major progesterone receptor (PR) isoforms: PRA and PRB [93].
Since progesterone can suppress the growth of EC cells [94], the expression of PR is inversely related to the clinical grade and stage: lower levels of PR are related to more advanced disease [95]. Endometrial cells treated with P4 increased the expression of CACNA2D3 and the intracellular Ca+2 levels, preventing endometrial cancer cell proliferation and inducing apoptosis. In a mouse xenograft model, the treatment with P4 also upregulated the expression of CACNA2D3 and attenuated tumor growth [61].
Hao et al. (2015) identified that 17-β estradiol acts directly in the regulation of calcium Cav1.3 and Cav1.4 channels’ expression. Moreover, 17-β estradiol hormone has been reported to increase Cav1.3 expression in endometrial cancer cells. Furthermore, the decrease in Cav1.3 levels negatively interfered with estrogen-stimulated calcium influx, cell proliferation, and migration of endometrial cancer cells. Therefore, it is suggested that the Cav1.3 channel plays a role in 17-β estradiol-induced carcinogenesis in endometrial cells [59]. According to Bolanz et al. (2008), 17-β estradiol upregulates, in a time-dependent manner, TRPV6 expression in T-47D breast cancer, suggesting that TRPV6 channels facilitate the calcium influx and are part of the molecular mechanism of the 17-β estradiol-induced proliferation in breast cancer cells. [96]. In vitro experiments showed a decline in TRPM4 expression in response to estrogen stimuli in endometrial cancer, possibly involved in cancer cell proliferation and migration [62].
Wang et al. (2018) showed that 17-β estradiol regulated the expression of the KCa1.1 potassium channel in endometrial cancer. Decreased expression of KCa1.1 led to reduced levels of phosphorylated ERK and MEK (p-ERK and p-MEK) proteins. The reduction of KCa1.1 was also related to a decrease in proliferation, migration, and invasion of Ishikawa cells, suggesting that ion channels may be essential regulatory factors to mediate the effects of 17-β estradiol on endometrial cancer cells [56].
Liu et al. (2019) demonstrated that sodium channels provide increased motility, endocytosis, and cell invasion. These channels increase their expression in cancers that are hormone-dependent, such as endometrial cancer, for example [66]. Chlorine channels play a role in cell proliferation, migration, invasion, and metastasis [38]. Studies have suggested that the expression of CLC-3 chloride channels is regulated by 17-β estradiol in breast cancer cells [57,97]. Associations between Na+ and Cl- channels have already been described in breast cancer [75]. Zou et al. (2011) demonstrated that AQP2 expression in endometrial Ishikawa cancer cells increased dose-dependently with E2 stimuli; however, AQP2-specific siRNA attenuated E2-enhanced migration, invasion, and adhesion [68].
Ion channels can also be modulated by growth factors such as the vascular endothelial growth factor (VEGF). VEGFs are secreted by fibroblasts and inflammatory cells and bind to their receptors on endothelial cells to promote angiogenesis. However, VEGF receptors’ expression can also be found in tumor cells, resulting in autocrine tumor growth and angiogenesis induction [98]. Several angiogenic factors and their receptors have been studied in a wide variety of tumor types, including breast, pancreatic, lung, prostate, colorectal, brain, and ovarian cancer [99,100,101,102,103,104,105]. Pan et al. (2008) indicated possible signaling cooperation between AQP1 and VEGF to promote angiogenesis in endometrial cancer, facilitating tumor growth and spread [67].
Insulin-like growth factor 1 (IGF1) is associated with a phenotypic change from normal cells to neoplastic cells. There is already an association between IGF1 expression through the action of estrogen in endometrial cancer [106]. Hyperplasic endometrium and endometrial carcinoma tissues express high levels of IGF-I receptor (IGF-IR) [107]. Downregulation of IGF-1R expression inhibits the growth of endometrial carcinoma in vitro [108]. Borowiec et al. (2011) demonstrated that IGF-1 increases the activity and the expression of hEAG channels in breast cancer cells, possibly involved with mitogenic signaling [109]. Furthermore, hERGs can act in mechanisms of tumor metastasis and angiogenesis. K+ channels appear to regulate cellular factors involved in cell adhesion signaling, such as β1 integrin, and in increasing basal levels of hypoxia-inducible factor 1α (HIF-1α) and VEGF secretion in the hypoxic tumor microenvironment [49].
4. Ion Channels: Biomarkers or Potential Targets for EC?
Tumor-specific expression of certain channel types can form molecular markers of malignancy. By providing a classification for cancer, biomarkers can help to define the clinical prognosis and guide therapeutic strategies [110]. The emergence of new tools such as proteomics allows for identifying molecular fingerprints in EC and serves as a source for clinically relevant biomarkers’ discovery. In addition to assisting in clinical diagnosis and prognostics, proteomics analysis contributes to the evaluation of potential therapeutic targets and mechanisms of therapeutic resistance [111,112]. A prognostic factor has been defined as a patient or disease characteristic/variable that provides an estimation of the recovery or disease relapse chances [113]. EC prognostic factors include the tumoral staging and size, histological cell type determination, and the presence of myometrial and lymphovascular space invasion [114].
Ion channels have also been shown to be involved in endometrial oncogenesis. Tissue analysis revealed different expression patterns of K+ [50,54,56], Ca+2 [59,61,63], Cl− [65], Na+ channels [66], and AQP [67,68,70,71] between endometrial cancer and a nontumoral endometrium. Possibly related to imbalanced hormonal signaling, the increased ion channel expression appears to be linked to the channel-mediated pathway required for endometrial tumor progression.
The different expression patterns of ion channels between tumor and non-tumor tissues/cells also highlighted the ion channels that may make potential targets for anticancer therapies [115]. Based on preclinical in vitro and in vivo studies, channel inhibitors or channel downregulation may suppress endometrial cancer cell proliferation, differentiation, migration, and invasion, leading to tumor growth suppression [51,54,55,56,57,58,59,60,62,64,65,66,68,69]. CACNA2D3 calcium channel expression showed the opposite effect to the other channels in endometrial cancer. Its downregulation showed involvement in proliferation, migration, and tumor growth [61]. Considering that ion channels are widely expressed in the tissues and have physiological importance for the body’s homeostasis regulation, these data highlighted the complexity and importance of tracking the expression patterns of ion channels according to the type of tumor under analysis.
However, ion channels as a therapeutic target could bring side effects and risks since many of the ion channels identified in cancer cells are expressed in healthy normal cells [115]. Yet, theoretically, a plausible treatment for cancer regarding the functions of ion channels should target those mechanisms involved in tumor progression, such as proliferation, migration, and invasion. Moreover, they are easily accessible because they are membrane proteins that are often overexpressed or activated in cancer [116]. In this way, TRP channels, Cav1.3, KCa3.1, and AQP2 are all candidates to be targeted by therapies. Indeed, several in vivo studies evaluated the systemic effect of ion channels as a pharmacological target. In general, Ca+2, K+, and Na+ channels’ inhibition or activation demonstrated a lack of specificity and side effects mainly on the cardiovascular system [117]. For example, although the use of hERG channel blockers triggers cell cycle arrest and apoptosis in cancer cell lines [49], hERG1 channels are essential for regulating the cardiac action potential. Inherited mutations or pharmacological blocks that cause loss of channel function can lead to life-threatening arrhythmias. These proarrhythmic side effects require significant attention in new cancer drug development [118].
Although ion channel-targeting strategies may have off-target effects, some early-phase clinical trials are under study for cancer treatment [115]. TM-601 is a synthetic version of peptide chlorotoxin, found in scorpion venom, which acts as a Cl- channel activity blocker. Intracavitary administration of TM-601 radiolabeled with Iodine-131 in patients with recurrent glioma (phase 1 clinical trial) demonstrated good tolerability and potential antitumoral effects [119]. Another therapeutic approach is the use of monoclonal antibodies targeting ion channels [120]. P2X7 is a transmembrane receptor expressed in various cell types that can form a nonselective channel for cations when activated by extracellular ATP [121]. A non-functional isoform of P2X7 (nfP2X7) appears significantly expressed in tumor cells, such as those of bladder, kidney, colorectal, and lung cancer [122]. The use of a monoclonal antibody targeting an epitope on the cancer-specific variant of nfP2X7 (phase 1 clinical trial) was well-tolerated and brought promising results in basal cell carcinoma treatment [123]. The use of SOR-C13, a TRPV6 calcium channel inhibitor, in patients with advanced solid tumors (phase 1 clinical trial), including ovarian, colorectal, non-small cell lung, and pancreatic, demonstrated disease stabilization and suggested potential antitumor activity [124].
Approved drug repurposing is another field to be explored in the targeting of ion channels for cancer therapy. Drugs currently used in hypertension and psychiatric disorders’ treatment, for example, have inhibitory effects on ion channels, and their redirection may be promising for cancer treatment [125]. For example, the imbalance in intracellular calcium levels’ homeostasis related to estrogen signaling in carcinogenesis highlights the promising use of calcium channel blockers in endometrial cancer treatment [126]. It is noteworthy that drug repurposing should include new drug delivery and formulation methods since the expected effects for cancer treatment require higher doses than those used for other conditions [126].
5. Conclusions
More than regulators of the flow, ion channels appear to be the leading figure in a myriad of processes, including carcinogenesis. Figure 1 summarizes the ion channels with a described role in endometrial cancer. Today, the ion channel research grand challenge consists of determining and selectively blocking ion channel subtypes or ion channel mutants according to the tumor type, along with searching for safer pharmacotherapy [127]. Although there are not any clinical trials to date that validate the use of ion channels as molecular markers or therapeutic targets in endometrial cancer, the data presented highlight the role of ion channels in endometrial tumor progression, with a promising therapeutic approach to be investigated.
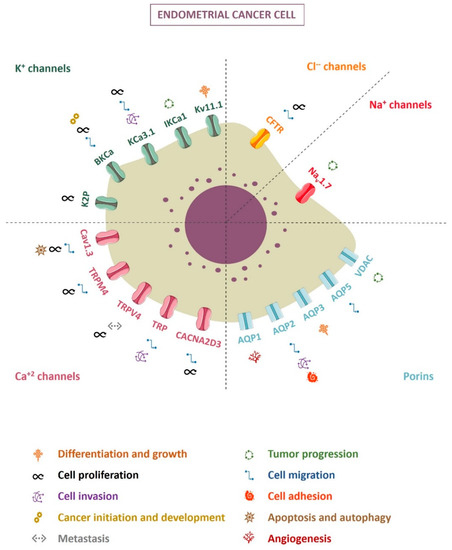
Figure 1.
Ion channels and their involvement in endometrial cancer cells.
Author Contributions
Conceptualization, G.B. and F.B.N.; investigation, B.P.C. and F.C.N.; writing—original draft preparation, B.P.C. and F.C.N.; writing—G.B. and F.B.N.; visualization, B.P.C.; supervision, G.B.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank the Graduate Program in Pathology’s staff for their support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- WHO. World Health Organization Fact Sheets: Cancer 2018. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 17 July 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial Cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO Staging for Carcinoma of the Vulva, Cervix, and Endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Urick, M.E.; Bell, D.W. Clinical Actionability of Molecular Targets in Endometrial Cancer. Nat. Rev. Cancer 2019, 19, 510–521. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Mutter, G.L. Endometrial Intraepithelial Neoplasia (EIN): Will It Bring Order to Chaos? Gynecol. Oncol. 2000, 76, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, P.A.; Critchley, H.O.D.; Williams, A.R.W.; Arends, M.J.; Saunders, P.T.K. New Concepts for an Old Problem: The Diagnosis of Endometrial Hyperplasia. Hum. Reprod. Update 2017, 23, 232–254. [Google Scholar] [CrossRef]
- Trimble, C.L.; Method, M.; Leitao, M.; Lu, K.; Ioffe, O.; Hampton, M.; Higgins, R.; Zaino, R.; Mutter, G.L. Management of Endometrial Precancers. Obstet. Gynecol. 2012, 120, 1160–1175. [Google Scholar] [CrossRef] [PubMed]
- Matias-Guiu, X.; Prat, J. Molecular Pathology of Endometrial Carcinoma. Histopathology 2013, 62, 111–123. [Google Scholar] [CrossRef]
- Gierisch, J.M.; Coeytaux, R.R.; Urrutia, R.P.; Havrilesky, L.J.; Moorman, P.G.; Lowery, W.J.; Dinan, M.; McBroom, A.J.; Hasselblad, V.; Sanders, G.D.; et al. Oral Contraceptive Use and Risk of Breast, Cervical, Colorectal, and Endometrial Cancers: A Systematic Review. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1931–1943. [Google Scholar] [CrossRef]
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, Endogenous Hormones, and Endometrial Cancer Risk: A Synthetic Review. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1531–1543. [Google Scholar]
- Wu, Q.-J.; Li, Y.-Y.; Tu, C.; Zhu, J.; Qian, K.-Q.; Feng, T.-B.; Li, C.; Wu, L.; Ma, X.-X. Parity and Endometrial Cancer Risk: A Meta-Analysis of Epidemiological Studies. Sci. Rep. 2015, 5, 14243. [Google Scholar] [CrossRef]
- Lacey, J.V.; Chia, V.M.; Rush, B.B.; Carreon, D.J.; Richesson, D.A.; Ioffe, O.B.; Ronnett, B.M.; Chatterjee, N.; Langholz, B.; Sherman, M.E.; et al. Incidence Rates of Endometrial Hyperplasia, Endometrial Cancer and Hysterectomy from 1980 to 2003 within a Large Prepaid Health Plan. Int. J. Cancer 2012, 131, 1921–1929. [Google Scholar] [CrossRef]
- de Haydu, C.; Black, J.D.; Schwab, C.L.; English, D.P.; Santin, A.D. An Update on the Current Pharmacotherapy for Endometrial Cancer. Expert Opin. Pharmacother. 2016, 17, 489–499. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.; et al. SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019. [Google Scholar]
- Fung-Kee-Fung, M.; Dodge, J.; Elit, L.; Lukka, H.; Chambers, A.; Oliver, T. Follow-up after Primary Therapy for Endometrial Cancer: A Systematic Review. Gynecol. Oncol. 2006, 101, 520–529. [Google Scholar] [CrossRef]
- Tangjitgamol, S.; See, H.T.; Kavanagh, J. Adjuvant Chemotherapy for Endometrial Cancer. Int. J. Gynecol. Cancer 2011, 21, 885–895. [Google Scholar] [CrossRef]
- Fleming, G.F. Systemic Chemotherapy for Uterine Carcinoma: Metastatic and Adjuvant. J. Clin. Oncol. 2007, 25, 2983–2990. [Google Scholar] [CrossRef]
- van Weelden, W.J.; Massuger, L.F.A.G.; Pijnenborg, J.M.A.; Romano, A. Anti-Estrogen Treatment in Endometrial Cancer: A Systematic Review. Front. Oncol. 2019, 9, 359. [Google Scholar] [CrossRef]
- Eritja, N.; Yeramian, A.; Chen, B.-J.; Llobet-Navas, D.; Ortega, E.; Colas, E.; Abal, M.; Dolcet, X.; Reventos, J.; Matias-Guiu, X. Endometrial Carcinoma: Specific Targeted Pathways. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; pp. 149–207. [Google Scholar]
- Djamgoz, M.B.A.; Coombes, R.C.; Schwab, A. Ion Transport and Cancer: From Initiation to Metastasis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130092. [Google Scholar] [CrossRef]
- Gadsby, D.C. Ion Channels versus Ion Pumps: The Principal Difference, in Principle. Nat. Rev. Mol. Cell Biol. 2009, 10, 344–352. [Google Scholar] [CrossRef]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.-S.; McNamara, J.O.; Williams, S.M. Neuroscience, 2nd ed.; Chapter 4—Channels and Transporters; Sinauer Associates Inc.: Sunderland, MA, USA, 2001; p. 681. ISBN 0878937420. [Google Scholar]
- Litan, A.; Langhans, S.A. Cancer as a Channelopathy: Ion Channels and Pumps in Tumor Development and Progression. Front. Cell. Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef]
- Arcangeli, A.; Becchetti, A. New Trends in Cancer Therapy: Targeting Ion Channels and Transporters. Pharmaceuticals 2010, 3, 1202–1224. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels and the Hallmarks of Cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef]
- Fraser, S.P.; Ozerlat-Gunduz, I.; Brackenbury, W.J.; Fitzgerald, E.M.; Campbell, T.M.; Coombes, R.C.; Djamgoz, M.B.A. Regulation of Voltage-Gated Sodium Channel Expression in Cancer: Hormones, Growth Factors and Auto-Regulation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130105. [Google Scholar] [CrossRef]
- Ramírez, A.; García-Quiroz, J.; Aguilar-Eslava, L.; Sánchez-Pérez, Y.; Camacho, J. Novel Therapeutic Approaches of Ion Channels and Transporters in Cancer. In Targets of Cancer Diagnosis and Treatment. Reviews of Physiology, Biochemistry and Pharmacology; Springer: Cham, Switzerland, 2020; Volume 183, pp. 45–101. [Google Scholar]
- Millar, I.D.; Bruce, J.I.; Brown, P.D. Ion Channel Diversity, Channel Expression and Function in the Choroid Plexuses. Cerebrospinal Fluid Res. 2007, 4, 8. [Google Scholar] [CrossRef]
- González, C.; Baez-Nieto, D.; Valencia, I.; Oyarzún, I.; Rojas, P.; Naranjo, D.; Latorre, R. K+ Channels: Function-Structural Overview. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 2087–2149. [Google Scholar]
- Yang, M.; Brackenbury, W.J. Membrane Potential and Cancer Progression. Front. Physiol. 2013, 4, 185. [Google Scholar] [CrossRef]
- Kellenberger, S.; Schild, L. Epithelial Sodium Channel/Degenerin Family of Ion Channels: A Variety of Functions for a Shared Structure. Physiol. Rev. 2002, 82, 735–767. [Google Scholar] [CrossRef]
- Savio-Galimberti, E.; Gollob, M.H.; Darbar, D. Voltage-Gated Sodium Channels: Biophysics, Pharmacology, and Related Channelopathies. Front. Pharmacol. 2012, 3, 124. [Google Scholar] [CrossRef]
- Stauber, T.; Jentsch, T.J. Chloride in Vesicular Trafficking and Function. Annu. Rev. Physiol. 2013, 75, 453–477. [Google Scholar] [CrossRef]
- Poroca, D.R.; Pelis, R.M.; Chappe, V.M. ClC Channels and Transporters: Structure, Physiological Functions, and Implications in Human Chloride Channelopathies. Front. Pharmacol. 2017, 8, 151. [Google Scholar] [CrossRef]
- Peretti, M.; Angelini, M.; Savalli, N.; Florio, T.; Yuspa, S.H.; Mazzanti, M. Chloride Channels in Cancer: Focus on Chloride Intracellular Channel 1 and 4 (CLIC1 AND CLIC4) Proteins in Tumor Development and as Novel Therapeutic Targets. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Guan, X.; Yang, Z.; Li, C. Emerging Role of Cystic Fibrosis Transmembrane Conductance Regulator—An Epithelial Chloride Channel in Gastrointestinal Cancers. World J. Gastrointest. Oncol. 2016, 8, 282. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The Versatility and Universality of Calcium Signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-Gated Calcium Channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef] [PubMed]
- Tajada, S.; Villalobos, C. Calcium Permeable Channels in Cancer Hallmarks. Front. Pharmacol. 2020, 11, 968. [Google Scholar] [CrossRef]
- Munaron, L. Intracellular Calcium, Endothelial Cells and Angiogenesis. Recent Pat. Anticancer. Drug Discov. 2006, 1, 105–119. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; De Pinto, V.; Zweckstetter, M.; Raviv, Z.; Keinan, N.; Arbel, N. VDAC, a Multi-Functional Mitochondrial Protein Regulating Cell Life and Death. Mol. Asp. Med. 2010, 31, 227–285. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.H.; Bowen, J.; Yool, A.J. Combined Systematic Review and Transcriptomic Analyses of Mammalian Aquaporin Classes 1 to 10 as Biomarkers and Prognostic Indicators in Diverse Cancers. Cancers 2020, 12, 1911. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ricciardelli, C.; Yool, A.J. Targeting Aquaporins in Novel Therapies for Male and Female Breast and Reproductive Cancers. Cells 2021, 10, 215. [Google Scholar] [CrossRef]
- Ruan, Y.C.; Chen, H.; Chan, H.C. Ion Channels in the Endometrium: Regulation of Endometrial Receptivity and Embryo Implantation. Hum. Reprod. Update 2014, 20, 517–529. [Google Scholar] [CrossRef]
- Vandenberg, J.I.; Perry, M.D.; Perrin, M.J.; Mann, S.A.; Ke, Y.; Hill, A.P. HERG K+ Channels: Structure, Function, and Clinical Significance. Physiol. Rev. 2012, 92, 1393–1478. [Google Scholar] [CrossRef]
- He, S.; Moutaoufik, M.T.; Islam, S.; Persad, A.; Wu, A.; Aly, K.A.; Fonge, H.; Babu, M.; Cayabyab, F.S. HERG Channel and Cancer: A Mechanistic Review of Carcinogenic Processes and Therapeutic Potential. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188355. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Taddei, G.L.; Crociani, O.; Paglierani, M.; Buccoliero, A.M.; Fontana, L.; Noci, I.; Borri, P.; Borrani, E.; Giachi, M.; et al. HERG Potassium Channels Are More Frequently Expressed in Human Endometrial Cancer as Compared to Non-Cancerous Endometrium. Br. J. Cancer 2000, 83, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takimoto, K. Selective Expression of HERG and Kv2 Channels Influences Proliferation of Uterine Cancer Cells. Int. J. Oncol. 2004, 25, 153–159. [Google Scholar] [CrossRef]
- Wonderlin, W.F.; Strobl, J.S. Potassium Channels, Proliferation and G1 Progression. J. Membr. Biol. 1996, 154, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Guéguinou, M.; Chantôme, A.; Fromont, G.; Bougnoux, P.; Vandier, C.; Potier-Cartereau, M. KCa and Ca2+ Channels: The Complex Thought. Biochim. Biophys. Acta-Mol. Cell Res. 2014, 1843, 2322–2333. [Google Scholar] [CrossRef]
- Wang, Z.H.; Shen, B.; Yao, H.L.; Jia, Y.C.; Ren, J.; Feng, Y.J.; Wang, Y.Z. Blockage of Intermediate-Conductance-Ca2+-Activated K+ Channels Inhibits Progression of Human Endometrial Cancer. Oncogene 2007, 26, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Chen, L.; Zhu, J. Effects of Intermediate-Conductance Ca2+-Activated K+ Channels on Human Endometrial Carcinoma Cells. Cell Biochem. Biophys. 2015, 72, 515–525. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Q.; Huang, G.; Guo, X.; Li, N.; Li, Y.; Li, B. BKCa Participates in E2 Inducing Endometrial Adenocarcinoma by Activating MEK/ERK Pathway. BMC Cancer 2018, 18, 1128. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, L.; Li, G.; Xia, M.; Du, C.; Zheng, Z. The Role of BKCa in Endometrial Cancer HEC-1-B Cell Proliferation and Migration. Gene 2018, 655, 42–47. [Google Scholar] [CrossRef]
- Patel, S.K.; Jackson, L.; Warren, A.Y.; Arya, P.; Shaw, R.W.; Khan, R.N. A Role for Two-Pore Potassium (K2P) Channels in Endometrial Epithelial Function. J. Cell. Mol. Med. 2013, 17, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Bao, X.; Jin, B.; Wang, X.; Mao, Z.; Li, X.; Wei, L.; Shen, D.; Wang, J.L. Ca2+ Channel Subunit a 1D Promotes Proliferation and Migration of Endometrial Cancer Cells Mediated by 17β-Estradiol via the G Protein-Coupled Estrogen Receptor. FASEB J. 2015, 29, 2883–2893. [Google Scholar] [CrossRef]
- Bao, X.X.; Xie, B.S.; Li, Q.; Li, X.P.; Wei, L.H.; Wang, J.L. Nifedipine Induced Autophagy through Beclin1 and MTOR Pathway in Endometrial Carcinoma Cells. Chin. Med. J. (Engl.) 2012, 125, 3120–3126. [Google Scholar] [CrossRef]
- Kong, X.; Li, M.; Shao, K.; Yang, Y.; Wang, Q.; Cai, M. Progesterone Induces Cell Apoptosis via the CACNA2D3/Ca2+/P38 MAPK Pathway in Endometrial Cancer. Oncol. Rep. 2020, 43, 121–132. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Yang, X.; Zhou, J.-Y.; Dong, Y.-Y.; Shen, B.-Q.; Wang, J.-Q.; Zhao, L.-J.; Wang, Z.-Q.; Li, X.-P.; et al. Decreased Expression of TRPM4 Is Associated with Unfavorable Prognosis and Aggressive Progression of Endometrial Carcinoma. Am. J. Transl. Res. 2020, 12, 3926–3939. [Google Scholar] [PubMed]
- Van den Eynde, C.; De Clercq, K.; Van Bree, R.; Luyten, K.; Annibali, D.; Amant, F.; Han, S.; Van Nieuwenhuysen, E.; Baert, T.; Peeraer, K.; et al. TRP Channel Expression Correlates with the Epithelial–Mesenchymal Transition and High-Risk Endometrial Carcinoma. Cell. Mol. Life Sci. 2022, 79, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, Y.; Wang, Z.; Zhou, J.; Jia, Y.; He, X.; Zhao, L.; Dong, Y.; Fan, Y.; Yang, X.; et al. Calcium and TRPV4 Promote Metastasis by Regulating Cytoskeleton through the RhoA/ROCK1 Pathway in Endometrial Cancer. Cell Death Dis. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Xia, X.; Wang, J.; Liu, Y.; Yue, M. Lower Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Promotes the Proliferation and Migration of Endometrial Carcinoma. Med. Sci. Monit. 2017, 23, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, H.; Yang, W.; Yao, S.; Hong, L. The Voltage-Gated Sodium Channel Na v 1.7 Associated with Endometrial Cancer. J. Cancer 2019, 10, 4954–4960. [Google Scholar] [CrossRef]
- Pan, H.; Sun, C.-C.; Zhou, C.-Y.; Huang, H.-F. Expression of Aquaporin-1 in Normal, Hyperplasic, and Carcinomatous Endometria. Int. J. Gynecol. Obstet. 2008, 101, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.B.; Zhang, R.J.; Tan, Y.J.; Ding, G.L.; Shi, S.; Zhang, D.; He, R.H.; Liu, A.X.; Wang, T.T.; Leung, P.C.K.; et al. Identification of Estrogen Response Element in the Aquaporin-2 Gene That Mediates Estrogen-Induced Cell Migration and Invasion in Human Endometrial Carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Xu, K.H.; Ma, J.Y.; Tian, Y.H.; Guo, X.Y.; Lin, J.; Wu, R.J. Reduced Migration of Ishikawa Cells Associated with Downregulation of Aquaporin-5. Oncol. Lett. 2012, 4, 257–261. [Google Scholar] [CrossRef]
- Watanabe, T.; Sato, K.; Kono, T.; Yamagishi, Y.; Kumazawa, F.; Miyamoto, M.; Takano, M.; Tsuda, H. Aquaporin 3 Expression in Endometrioid Carcinoma of the Uterine Body Correlated With Early Stage and Lower Grade. Pathol. Oncol. Res. 2020, 26, 2247–2253. [Google Scholar] [CrossRef]
- Jóźwiak, P.; Ciesielski, P.; Forma, E.; Kozal, K.; Wójcik-Krowiranda, K.; Cwonda, Ł.; Bieńkiewicz, A.; Bryś, M.; Krześlak, A. Expression of Voltage-Dependent Anion Channels in Endometrial Cancer and Its Potential Prognostic Significance. Tumor Biol. 2020, 42, 1010428320951057. [Google Scholar] [CrossRef]
- Huang, X.; Jan, L.Y. Targeting Potassium Channels in Cancer. J. Cell Biol. 2014, 206, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Mathie, A.; Veale, E.L.; Cunningham, K.P.; Holden, R.G.; Wright, P.D. Two-Pore Domain Potassium Channels as Drug Targets: Anesthesia and Beyond. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Lai, M.-D.; Phan, N.N.; Sun, Z.; Lin, Y.-C. Meta-Analysis of Public Microarray Datasets Reveals Voltage-Gated Calcium Gene Signatures in Clinical Cancer Patients. PLoS ONE 2015, 10, e0125766. [Google Scholar] [CrossRef]
- Restrepo-Angulo, I.; Bañuelos, C.; Camacho, J. Ion Channel Regulation by Sex Steroid Hormones and Vitamin D in Cancer: A Potential Opportunity for Cancer Diagnosis and Therapy. Front. Pharmacol. 2020, 11, 152. [Google Scholar] [CrossRef]
- Guan, Y.; Huang, Y.; Wu, J.; Deng, Z.; Wang, Y.; Lai, Z.; Wang, H.; Sun, X.; Zhu, Y.; Du, M.; et al. Overexpression of Chloride Channel-3 Is Associated with the Increased Migration and Invasion Ability of Ectopic Endometrial Cells from Patients with Endometriosis. Hum. Reprod. 2016, 31, 986–998. [Google Scholar] [CrossRef]
- Guan, Y.; Xie, Y.; Zhou, H.; Shi, H.; Zhu, Y.; Zhang, X.; Luan, Y.; Shen, X.; Chen, Y.; Xu, L.; et al. Overexpression of Chloride Channel-3 (ClC-3) Is Associated with Human Cervical Carcinoma Development and Prognosis. Cancer Cell Int. 2019, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Airley, R.; Hewitt, S.; Marples, D. Heterogeneous Expression of the Aquaporin 1 (AQP1) Water Channel in Tumors of the Prostate, Breast, Ovary, Colon and Lung: A Study Using High Density Multiple Human Tumor Tissue Microarrays. Int. J. Oncol. 2005, 26, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- He, R.-H.; Sheng, J.-Z.; Luo, Q.; Jin, F.; Wang, B.; Qian, Y.-L.; Zhou, C.-Y.; Sheng, X.; Huang, H.-F. Aquaporin-2 Expression in Human Endometrium Correlates with Serum Ovarian Steroid Hormones. Life Sci. 2006, 79, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Hoffmann, E.K.; Novak, I. Cell Volume Regulation in Epithelial Physiology and Cancer. Front. Physiol. 2013, 4, 233. [Google Scholar] [CrossRef]
- Leanza, L.; Biasutto, L.; Managò, A.; Gulbins, E.; Zoratti, M.; Szabò, I. Intracellular Ion Channels and Cancer. Front. Physiol. 2013, 4, 227. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Gray, C.A.; Bartol, F.F.; Tarleton, B.J.; Wiley, A.A.; Johnson, G.A.; Bazer, F.W.; Spencer, T.E. Developmental Biology of Uterine Glands. Biol. Reprod. 2001, 65, 1311–1323. [Google Scholar] [CrossRef]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The Role of Mesenchymal–Epithelial Transition in Endometrial Function. Hum. Reprod. Update 2019, 25, 114–133. [Google Scholar] [CrossRef]
- Gargett, C.E.; Nguyen, H.P.T.; Ye, L. Endometrial Regeneration and Endometrial Stem/Progenitor Cells. Rev. Endocr. Metab. Disord. 2012, 13, 235–251. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, W.; Liu, H.; Zhang, D. Age at Menopause and Risk of Developing Endometrial Cancer: A Meta-Analysis. Biomed Res. Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Gong, T.-T.; Wang, Y.-L.; Ma, X.-X. Age at Menarche and Endometrial Cancer Risk: A Dose-Response Meta-Analysis of Prospective Studies. Sci. Rep. 2015, 5, 14051. [Google Scholar] [CrossRef] [PubMed]
- Ilancheran, A.; Low, J.; Ng, J.S. Gynaecological Cancer in Pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Saczko, J.; Michel, O.; Chwiłkowska, A.; Sawicka, E.; Mączyńska, J.; Kulbacka, J. Estrogen Receptors in Cell Membranes: Regulation and Signaling. Adv. Anat. Embryol. Cell Biol. 2017, 227, 93–105. [Google Scholar] [CrossRef]
- Chabbert-Buffeta, N.; Skinner, D.C.; Caraty, A.; Bouchard, P. Neuroendocrine Effects of Progesterone. Steroids 2000, 65, 613–620. [Google Scholar] [CrossRef]
- Deng, Z.; Peng, S.; Zheng, Y.; Yang, X.; Zhang, H.; Tan, Q.; Liang, X.; Gao, H.; Li, Y.; Huang, Y.; et al. Estradiol Activates Chloride Channels via Estrogen Receptor-α in the Cell Membranes of Osteoblasts. Am. J. Physiol.-Cell Physiol. 2017, 313, C162–C172. [Google Scholar] [CrossRef]
- Gielen, S.C.J.P.; Hanekamp, E.E.; Hanifi-Moghaddam, P.; Sijbers, A.M.; van Gool, A.J.; Burger, C.W.; Blok, L.J.; Huikeshoven, F.J. Growth Regulation and Transcriptional Activities of Estrogen and Progesterone in Human Endometrial Cancer Cells. Int. J. Gynecol. Cancer 2006, 16, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Kastner, P.; Krust, A.; Turcotte, B.; Stropp, U.; Tora, L.; Gronemeyer, H.; Chambon, P. Two Distinct Estrogen-Regulated Promoters Generate Transcripts Encoding the Two Functionally Different Human Progesterone Receptor Forms A and B. EMBO J. 1990, 9, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.; Adami, H.O.; Bergkvist, L.; Lindgren, A.; Pettersson, B.; Hoover, R.; Schairer, C. Risk of Endometrial Cancer after Treatment with Oestrogens Alone or in Conjunction with Progestogens: Results of a Prospective Study. BMJ 1989, 298, 147–151. [Google Scholar] [CrossRef]
- Arnett-Mansfield, R.L.; DeFazio, A.; Wain, G.V.; Jaworski, R.C.; Byth, K.; Mote, P.A.; Clarke, C.L. Relative Expression of Progesterone Receptors A and B in Endometrioid Cancers of the Endometrium. Cancer Res. 2001, 61, 4576–4582. [Google Scholar]
- Bolanz, K.A.; Hediger, M.A.; Landowski, C.P. The Role of TRPV6 in Breast Carcinogenesis. Mol. Cancer Ther. 2008, 7, 271–279. [Google Scholar] [CrossRef]
- Yang, H.; Ma, L.; Wang, Y.; Zuo, W.; Li, B.; Yang, Y.; Chen, Y.; Chen, L.; Wang, L.; Zhu, L. Activation of ClC-3 Chloride Channel by 17β-estradiol Relies on the Estrogen Receptor α Expression in Breast Cancer. J. Cell. Physiol. 2018, 233, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Taylor, A.; Showeil, R.; Trivedi, P.; Horimoto, Y.; Bagwan, I.; Ewington, L.; Lam, E.W.F.; El-Bahrawy, M.A. Expression Profiling and Significance of VEGF-A, VEGFR2, VEGFR3 and Related Proteins in Endometrial Carcinoma. Cytokine 2014, 68, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Leon, A.; Perez-Quintela, B.V.; Iglesias-García, J.; Lariño-Noia, J.; Varo, E.; Forteza, J.; Domínguez-Muñoz, J.E. Ductal Adenocarcinoma of the Pancreas: Expression of Growth Factor Receptors, Oncogenes and Suppressor Genes, and Their Relationship to Pathological Features, Staging and Survival. Oncol. Lett. 2011, 2, 161–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rydén, L.; Jirstrom, K.; Haglund, M.; Stal, O.; Fernö, M. Epidermal Growth Factor Receptor and Vascular Endothelial Growth Factor Receptor 2 Are Specific Biomarkers in Triple-Negative Breast Cancer. Results from a Controlled Randomized Trial with Long-Term Follow-Up. Breast Cancer Res. Treat. 2010, 120, 491–498. [Google Scholar] [CrossRef]
- Kim, J.Y.; Bae, B.N.; Kwon, J.E.; Kim, H.J.; Park, K. Prognostic Significance of Epidermal Growth Factor Receptor and Vascular Endothelial Growth Factor Receptor in Colorectal Adenocarcinoma. Apmis 2011, 119, 449–459. [Google Scholar] [CrossRef]
- Ioannidou, E.; Moschetta, M.; Shah, S.; Parker, J.S.; Ozturk, M.A.; Pappas-Gogos, G.; Sheriff, M.; Rassy, E.; Boussios, S. Angiogenesis and Anti-Angiogenic Treatment in Prostate Cancer: Mechanisms of Action and Molecular Targets. Int. J. Mol. Sci. 2021, 22, 9926. [Google Scholar] [CrossRef]
- Krcek, R.; Matschke, V.; Theis, V.; Adamietz, I.A.; Bühler, H.; Theiss, C. Vascular Endothelial Growth Factor, Irradiation, and Axitinib Have Diverse Effects on Motility and Proliferation of Glioblastoma Multiforme Cells. Front. Oncol. 2017, 7, 182. [Google Scholar] [CrossRef]
- Masoumi Moghaddam, S.; Amini, A.; Morris, D.L.; Pourgholami, M.H. Significance of Vascular Endothelial Growth Factor in Growth and Peritoneal Dissemination of Ovarian Cancer. Cancer Metastasis Rev. 2012, 31, 143–162. [Google Scholar] [CrossRef]
- Frezzetti, D.; Gallo, M.; Maiello, M.R.; D’Alessio, A.; Esposito, C.; Chicchinelli, N.; Normanno, N.; De Luca, A. VEGF as a Potential Target in Lung Cancer. Expert Opin. Ther. Targets 2017, 21, 959–966. [Google Scholar] [CrossRef]
- Bruchim, I.; Sarfstein, R.; Werner, H. The IGF Hormonal Network in Endometrial Cancer: Functions, Regulation, and Targeting Approaches. Front. Endocrinol. 2014, 5, 76. [Google Scholar] [CrossRef]
- McCampbell, A.S.; Broaddus, R.R.; Loose, D.S.; Davies, P.J.A. Overexpression of the Insulin-like Growth Factor I Receptor and Activation of the AKT Pathway in Hyperplastic Endometrium. Clin. Cancer Res. 2006, 12, 6373–6378. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Yang, Y.; Li, X.; Li, T.; Zhang, Y.; Xu, C.; Liang, C.; Wang, X. Down-Regulation of IGF-1R Expression Inhibits Growth and Enhances Chemosensitivity of Endometrial Carcinoma in Vitro. Mol. Cell. Biochem. 2011, 353, 225–233. [Google Scholar] [CrossRef]
- Borowiec, A.-S.; Hague, F.; Harir, N.; Guénin, S.; Guerineau, F.; Gouilleux, F.; Roudbaraki, M.; Lassoued, K.; Ouadid-Ahidouch, H. IGF-1 Activates HEAG K+ Channels through an Akt-Dependent Signaling Pathway in Breast Cancer Cells: Role in Cell Proliferation. J. Cell. Physiol. 2007, 212, 690–701. [Google Scholar] [CrossRef]
- Conti, M. Targeting Ion Channels for New Strategies in Cancer Diagnosis and Therapy. Curr. Clin. Pharmacol. 2008, 2, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Njoku, K.; Chiasserini, D.; Whetton, A.D.; Crosbie, E.J. Proteomic Biomarkers for the Detection of Endometrial Cancer. Cancers 2019, 11, 1572. [Google Scholar] [CrossRef]
- Ghose, A.; Gullapalli, S.V.N.; Chohan, N.; Bolina, A.; Moschetta, M.; Rassy, E.; Boussios, S. Applications of Proteomics in Ovarian Cancer: Dawn of a New Era. Proteomes 2022, 10, 16. [Google Scholar] [CrossRef]
- Pavlou, M.P.; Diamandis, E.P.; Blasutig, I.M. The Long Journey of Cancer Biomarkers from the Bench to the Clinic. Clin. Chem. 2013, 59, 147–157. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martón, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Capatina, A.L.; Lagos, D.; Brackenbury, W.J. Targeting Ion Channels for Cancer Treatment: Current Progress and Future Challenges. In Targets of Cancer Diagnosis and Treatment. Reviews of Physiology, Biochemistry and Pharmacology; Springer: Cham, Switzerland, 2020; Volume 183, pp. 1–43. [Google Scholar]
- Schwab, A.; Stock, C. Ion Channels and Transporters in Tumour Cell Migration and Invasion. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130102. [Google Scholar] [CrossRef]
- Lynch, J.J.; Van Vleet, T.R.; Mittelstadt, S.W.; Blomme, E.A.G. Potential Functional and Pathological Side Effects Related to Off-Target Pharmacological Activity. J. Pharmacol. Toxicol. Methods 2017, 87, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Jehle, J.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Novel Roles for HERG K+ Channels in Cell Proliferation and Apoptosis. Cell Death Dis. 2011, 2, e193. [Google Scholar] [CrossRef]
- Mamelak, A.N.; Rosenfeld, S.; Bucholz, R.; Raubitschek, A.; Nabors, L.B.; Fiveash, J.B.; Shen, S.; Khazaeli, M.B.; Colcher, D.; Liu, A.; et al. Phase I Single-Dose Study of Intracavitary-Administered Iodine-131-TM-601 in Adults With Recurrent High-Grade Glioma. J. Clin. Oncol. 2006, 24, 3644–3650. [Google Scholar] [CrossRef]
- Haustrate, A.; Hantute-Ghesquier, A.; Prevarskaya, N.; Lehen’kyi, V. Monoclonal Antibodies Targeting Ion Channels and Their Therapeutic Potential. Front. Pharmacol. 2019, 10, 606. [Google Scholar] [CrossRef]
- Lara, R.; Adinolfi, E.; Harwood, C.A.; Philpott, M.; Barden, J.A.; Di Virgilio, F.; McNulty, S. P2X7 in Cancer: From Molecular Mechanisms to Therapeutics. Front. Pharmacol. 2020, 11, 793. [Google Scholar] [CrossRef]
- Gilbert, S.; Oliphant, C.; Hassan, S.; Peille, A.; Bronsert, P.; Falzoni, S.; Di Virgilio, F.; McNulty, S.; Lara, R. ATP in the Tumour Microenvironment Drives Expression of NfP2X7, a Key Mediator of Cancer Cell Survival. Oncogene 2019, 38, 194–208. [Google Scholar] [CrossRef]
- Gilbert, S.M.; Gidley Baird, A.; Glazer, S.; Barden, J.A.; Glazer, A.; Teh, L.C.; King, J. A Phase I Clinical Trial Demonstrates That NfP2X 7 -Targeted Antibodies Provide a Novel, Safe and Tolerable Topical Therapy for Basal Cell Carcinoma. Br. J. Dermatol. 2017, 177, 117–124. [Google Scholar] [CrossRef]
- Fu, S.; Hirte, H.; Welch, S.; Ilenchuk, T.T.; Lutes, T.; Rice, C.; Fields, N.; Nemet, A.; Dugourd, D.; Piha-Paul, S.; et al. First-in-Human Phase I Study of SOR-C13, a TRPV6 Calcium Channel Inhibitor, in Patients with Advanced Solid Tumors. Investig. New Drugs 2017, 35, 324–333. [Google Scholar] [CrossRef]
- Kale, V.P.; Amin, S.G.; Pandey, M.K. Targeting Ion Channels for Cancer Therapy by Repurposing the Approved Drugs. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 2747–2755. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, J.; Wang, J. Calcium and Calcium-Related Proteins in Endometrial Cancer: Opportunities for Pharmacological Intervention. Int. J. Biol. Sci. 2022, 18, 1065–1078. [Google Scholar] [CrossRef]
- Camerino, D.C.; Desaphy, J.-F. Grand Challenge for Ion Channels: An Underexploited Resource for Therapeutics. Front. Pharmacol. 2010, 1, 113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).