Simple Summary
Endometrial cancer is the most common gynecological tract malignancy in developed countries. Extrauterine disease, in particular lymph node metastasis, is an important prognostic factor. Nevertheless, pelvic lymphadenectomy is not considered to have a therapeutic benefit, as it did not improve survival in randomized studies. However, lymphadenectomy may have a therapeutic benefit if adjuvant therapy can be omitted without decreasing oncological outcomes, as the long-term quality of life is maintained by avoiding morbidities associated with adjuvant therapy. In intermediate- and high-risk endometrioid endometrial carcinomas, adjuvant therapy may be safely omitted without decreasing long-term survival by open surgery including systematic pelvic and para-aortic lymphadenectomy when patients are node-negative. Systematic lymphadenectomy may remove undetectable low-volume lymph node metastasis in both pelvic and para-aortic regions, and open surgery may reduce vaginal recurrence even without vaginal brachytherapy. However, lymphadenectomy may not improve survival in elderly patients and patients with p53-mutant tumors.
Abstract
Endometrial cancer is the most common gynecological tract malignancy in developed countries, and its incidence has been increasing globally with rising obesity rates and longer life expectancy. In endometrial cancer, extrauterine disease, in particular lymph node metastasis, is an important prognostic factor. Nevertheless, pelvic lymphadenectomy is not considered to have a therapeutic benefit, as it did not improve survival in randomized studies. However, lymphadenectomy may have a therapeutic benefit if adjuvant therapy can be omitted without decreasing oncological outcomes, as the long-term quality of life is maintained by avoiding morbidities associated with adjuvant therapy. In intermediate- and high-risk endometrioid endometrial carcinomas, adjuvant therapy may be safely omitted without decreasing long-term survival by open surgery including systematic pelvic and para-aortic lymphadenectomy when patients are node-negative. Systematic lymphadenectomy may remove undetectable low-volume lymph node metastasis in both pelvic and para-aortic regions, and open surgery may reduce vaginal recurrence even without vaginal brachytherapy. However, lymphadenectomy may not improve survival in elderly patients and patients with p53-mutant tumors. In this review, I discuss the characteristics of lymph node metastasis, the methods of lymph node assessment, and the therapeutic benefits of systematic lymphadenectomy in patients with intermediate- and high-risk endometrioid endometrial carcinoma.
1. Introduction
Endometrial cancer is the most common female genital tract malignancy in developed countries. Its incidence has been increasing globally because of rising obesity rates and longer life expectancy [1,2]. The majority of endometrial cancers are confined to the uterus, and patients with such diseases have a favorable prognosis. In contrast, patients with extrauterine disease, particularly lymph node metastasis, have a poorer survival. To assess lymph node status, lymphadenectomy or sentinel lymph node biopsy is performed. Lymphadenectomy has a diagnostic benefit by providing the knowledge of pathological lymph node status that is useful for tailoring the appropriate adjuvant therapy. However, pelvic lymphadenectomy has not been thought to have a therapeutic benefit, as it did not improve survival in two randomized controlled trials [3,4]. Thus, attempts have been made to reduce surgical morbidity of lymphadenectomy with the introduction of sentinel lymph node biopsy.
In contrast, attempts to reduce the use of adjuvant therapy in the management of endometrial cancer have been limited, although its effect on overall survival, similar to that of lymphadenectomy, has not been established [5,6,7,8,9]. In node-positive patient adjuvant therapy, radiation therapy and/or chemotherapy is given to eradicate residual diseases after surgery. However, even though lymph node metastasis is not detected by lymph node assessment, adjuvant therapy is recommended for patients with uterine-confined disease considered to be at risk of recurrence under current treatment guidelines [10,11,12].
Lymphadenectomy may have a therapeutic benefit in node-negative patients if adjuvant therapy can be omitted without decreasing oncological outcomes, because morbidities that decrease long-term quality of life of survivors associated with adjuvant therapy can be avoided [13,14,15,16,17,18,19]. In this review, I discuss the characteristics of lymph node metastasis in endometrial carcinoma, the methods of lymph node assessment, and the possibility of omitting adjuvant therapy in patients with node-negative uterine-confined endometrioid carcinoma who undergo systematic lymphadenectomy. In this review, I focus on endometrioid endometrial carcinoma, in particular intermediate- and high-risk diseases. A detailed discussion of the high-risk histological subtypes, such as serous carcinoma and carcinosarcoma, is beyond the scope of this review.
2. What Is the True Incidence of Lymph Node Metastasis?
Endometrial cancers can be classified into low-, intermediate-, and high-risk diseases based on the risk of recurrence (Table 1) [5,6,10,11,12]. The risk of recurrence varies according to uterine pathological factors, such as depth of myometrial invasion, tumor grade, cervical invasion, and lymphovascular space invasion [20]. Although the low-risk disease is almost identical, i.e., stage IA, Grade 1 or 2 endometrioid carcinoma, the definitions of intermediate- and high-risk disease are different by group. High-intermediate risk disease may be the same as high-risk disease without gross extracorporeal disease. The risk of recurrence, other than vaginal recurrence, is almost identical to that of lymph node metastasis [21].

Table 1.
Low-, intermediate-, high-intermediate, and high-risk endometrioid endometrial carcinoma without extrauterine disease.
Although the uterus is a pelvic organ, endometrial cancer metastasizes to both the pelvic and para-aortic lymph nodes. Pelvic lymph node metastasis is more often observed; however, para-aortic node metastasis is not rare [22,23,24,25,26]. In our previous study, lymph node metastasis was observed in 27 (9.2%) of the 292 patients with endometrioid endometrial carcinoma; pelvic and para-aortic node metastases were observed in 22 (7.5%) and 8 (2.7%) patients, respectively [26]. Most para-aortic node metastases develop in patients with pelvic node metastasis [22,24,26,27,28,29]. However, isolated para-aortic node metastasis is also observed [3,20,26,29,30,31,32,33,34].
Metastatic carcinomas in the lymph nodes are classified according to their size as macrometasasis (larger than 2 mm in diameter), micrometastasis (0.2 mm to 2 mm), and isolated tumor cells (smaller than 0.2 mm) [35,36]. As low-volume disease, i.e., micrometastasis and isolated tumor cells, may not be detected by standard pathological assessment, ultrastaging using serial sections of the nodes and immunohistochemical staining are necessary.
Thus, the true incidence of lymph node metastasis in endometrial carcinoma cannot be determined unless all pelvic and para-aortic lymph nodes are removed by systematic lymphadenectomy and pathologically assessed using the ultrastaging method. The incidences of lymph node metastasis detected by standard pathological evaluation have been underestimated.
3. Role of Adjuvant Therapy in Endometrial Cancer
There is no definitive evidence that adjuvant therapy improves the long-term survival of women with endometrial cancer apparently confined to the uterus. Randomized controlled trials have shown that external beam radiotherapy to the pelvis decreases local recurrence but does not improve overall survival [5,6,7]. Although external beam radiotherapy might improve survival in patients with high-intermediate risk disease [6], a meta-analysis indicated that in intermediate- and high-risk patients, adjuvant external beam radiotherapy did not improve overall survival [37]. In intermediate-risk disease, adjuvant vaginal brachytherapy is recommended to avoid gastrointestinal complications that are often associated with external beam radiotherapy [10,11,12,38]. In node-negative patients with deeply invasive grade 3 endometrioid tumors, no survival benefit was observed with pelvic radiation compared to vaginal brachytherapy alone [39]. However, the necessity of adjuvant vaginal brachytherapy is questioned, because most vaginal recurrences can be cured with salvage therapy [40,41,42].
Adjuvant chemotherapy in patients with high-risk stage I–II endometrioid carcinoma improved survival in one study [43], but did not in another study [44]. In high-intermediate and high-risk early-stage endometrial carcinoma, pelvic and para-aortic nodal recurrences were more common after chemotherapy and vaginal brachytherapy compared to pelvic radiation therapy [45]. Effects of chemotherapy may be limited in grade 3 tumors [46,47].
Late complications caused by adjuvant therapy may reduce the long-term quality of life of survivors. After external beam radiotherapy, gastrointestinal complications may lead to limitations in daily activities [38]. In particular, pelvic radiotherapy following surgery including lymphadenectomy significantly increased risk of serious complications compared to surgery alone [6]. Vaginal brachytherapy may cause vaginal atrophy and subsequent stenosis [17] and severe bowel complications such as rectal bleeding [16]. Chemotherapy including platinum and/or taxane often causes peripheral neuropathy that decreases quality of life [18,19].
Importantly, adjuvant therapy might not replace surgical removal as a treatment for positive nodes in some patients, as patients who underwent lymphadenectomy had an improved survival compared to those who did not undergo the procedure, even though adjuvant therapy was performed [48,49,50,51].
Theoretically, adjuvant therapy is not necessary for patients with node-negative uterine-confined disease, because residual diseases that otherwise need to be eradicated by adjuvant therapy are not left behind. Patients with clinically negative but histologically positive nodes can also become disease-free by both lymphadenectomy and/or sentinel lymph node biopsy followed by lymphadenectomy.
4. Sentinel Node-Negative Patients: Can Adjuvant Therapy Be Omitted?
Sentinel lymph node mapping has been increasingly performed instead of lymphadenectomy that may be associated with surgical morbidities and sequelae, such as vascular injury and lymphedema [52,53]. The sentinel lymph node is defined as the first node in the lymphatic basin that receives drainage from the primary tumor. If the sentinel lymph node is negative, a regional lymphadenectomy can be avoided [54]. In breast cancer and melanoma, both of which are superficial cancers with less complicated lymphatic drainage routes, the sentinel lymph node technique has been incorporated into standard practice [55,56]. In endometrial cancer, sentinel lymph node mapping has a high degree of diagnostic accuracy and detects low-volume metastasis in the sentinel nodes [57,58,59,60,61].
However, in intermediate- and high-risk endometrial cancers, the detection of the true sentinel nodes by the standard method, cervical injection of a tracer, may be difficult. Whereas low-risk diseases metastasize almost only to pelvic nodes, intermediate- and high-risk diseases metastasize to not only pelvic nodes, but also para-aortic nodes. The lymphatic network draining the uterus is complex and involves both pelvic and para-aortic nodes [62], as lymphatic channels draining from the uterine fundus course into the broad ligament and along the ovarian vessels [62,63]. The incidence of isolated para-aortic node metastasis (without pelvic node metastasis) in patients with intermediate- and high-risk diseases ranges from 2.6 to 3.1% [26,29,33], and the majority of para-aortic node metastases are observed above the inferior mesenteric artery [23]. Cervical injection is superior in detecting pelvic sentinel nodes [64] and achieves a higher sentinel node detection rate than fundal injection [65,66]. However, hysteroscopic or ultrasound-guided fundal sub-endometrial injection is necessary to detect para-aortic sentinel nodes [66,67,68,69,70,71].
Histopathological detection of metastatic lesions in the sentinel lymph nodes may be difficult during surgery. As a lymph node metastasis less than 2 mm was often missed at frozen section examination [72], ultrastaging is necessary to detect the majority of low-volume metastases in the sentinel nodes that account for 25–48% of sentinel node metastasis [57,73,74,75,76]. Many studies testing the sensitivity of sentinel lymph nodes have reported high sensitivity; however, in most studies, pathological evaluation of sentinel nodes was performed after back-up lymphadenectomy following sentinel lymph node biopsy [59,61,65,73,77,78,79,80].
Most importantly, negative sentinel nodes may lead to the omission of lymphadenectomy, but may not necessarily lead to the omission of adjuvant therapy. A false negative rate of 15% has been reported with a blue dye cervical injection [81]. Current studies on sentinel lymph node mapping were aimed at the accurate detection of metastatic lymph nodes by performing back-up lymphadenectomy; thus, lymphadenectomy was rarely omitted [59,61,65,73,77,78,79,80]. Adjuvant therapy was given even in patients with node-negative uterine-confined disease when they were considered to be at risk of recurrence based on uterine pathological factors [82,83]. In node-negative deeply invasive endometrioid carcinoma, patients who underwent sentinel lymph node mapping were more likely to receive adjuvant therapy than patients who underwent lymphadenectomy [84]. A previous study reported that of 54 sentinel node-negative patients treated with adjuvant therapy, eight (15%) developed recurrence [77]. If adjuvant therapy is given according to uterine pathological factors irrespective of nodal status, sentinel lymph node mapping, which significantly increases the costs compared to hysterectomy alone [53], might not be necessary. To evaluate the efficacy of sentinel lymph node biopsy, prospective studies evaluating the long-term oncologic outcome are necessary [85], particularly the long-term safety of omitting both systematic lymphadenectomy and adjuvant therapy in sentinel node-negative patients. Long-term follow-up is indispensable to detect lymph node recurrence, because it often develops five years or later [86].
5. Benefit of Systematic Lymphadenectomy in Node-Negative Patients: Omission of Adjuvant Therapy
For an accurate evaluation of the therapeutic effects of lymphadenectomy, appropriate patients need to undergo appropriate surgery, as selection of appropriate patients is necessary to detect survival difference between patients who undergo lymphadenectomy and those who do not. First, patients at low-risk for lymph node metastasis need to be excluded [87,88,89]. In these patients, the incidence of lymph node metastasis is at most 5.9%, when considering low-volume metastasis [74]; thus, survival difference cannot be detected without a sufficient power. It is plausible that effects of lymphadenectomy on survival cannot be detected in randomized trials, considering the rate of low-risk disease in endometrioid endometrial carcinoma [3,4,90,91]. Second, patients with high-risk histological subtypes, i.e., serous carcinoma and carcinosarcoma, also need to be excluded, as they often develop peritoneal and hematogenous spread independent of lymph node metastasis [92,93,94].
In intermediate- or high-risk endometrioid carcinomas, systematic lymphadenectomy, particularly pelvic with para-aortic lymphadenectomy, appears to improve survival [50,95,96,97,98,99,100]. Incidences of lymph node metastasis in intermediate- and high-risk endometrioid carcinomas were 17% and 25%, respectively, in our previous study [26]. These incidences may be underestimated, and when ultrastaging was performed, the incidence of lymph node metastasis in high-intermediate risk endometrioid endometrial carcinoma was 24–43% [101,102].
Additionally, for lymphadenectomy to have a therapeutic benefit, no positive lymph nodes are left behind at the completion of surgery. Namely, it is necessary to remove lymph nodes bearing not only macroscopic but also low-volume metastases. Moreover, lymph nodes in the para-aortic region as well as those in the pelvic region need to be removed in intermediate- and high-risk endometrial carcinoma when deep myometrial invasion is observed [103].
However, the procedures performed during lymphadenectomy range from a mere sampling of enlarged or suspicious lymph nodes for staging purposes to systematic removal of all accessible lymphatic tissue with a therapeutic intent [88]. Systematic complete lymphadenectomy is performed to remove enlarged nodes and to skeletonize the vessels of node-bearing tissue [30,88]. The number of nodes removed varies among studies: the median numbers of nodes removed in the pelvic and para-aortic region were in the ranges of 11–54 and 5–23, respectively [26,29,95,97,98,103,104]. The quality of surgical resection may be measured by a nodal count that is indicative of the extent of nodal dissection, although the number of nodes reported by the pathologist depends on surgical expertise, the comprehensiveness of pathological analysis, and anatomical variations in patients [88]. The removal of 10 or more regional lymph nodes was associated with improved survival in intermediate- and high-risk endometrioid carcinomas [48,49,105]. The number of nodal stations sampled may be a more accurate predictor of lymph node metastasis than lymph node count [106,107].
The number of nodes removed is associated with improved survival also in patients with other cancers, i.e., breast, lung, and cervical cancers, even when all regional lymph nodes are interpreted as pathologically negative [108,109,110,111]. In particular, with the removal of larger numbers of nodes, regional relapse was significantly decreased for breast cancer patients not receiving systemic therapy [109].
Node-negative patients consist of true node-negative and false node-negative patients. False node-negative patients have occult lymph node metastasis that cannot be detected by standard pathological evaluation or that cannot be removed by non-systematic lymphadenectomy.
Systematic lymphadenectomy can remove all lymph nodes bearing macroscopic and low-volume metastases, as patients with endometrioid carcinoma who undergo systematic lymphadenectomy rarely develop lymph node recurrence, even without adjuvant therapy [26,30,112,113,114]. Similarly, nodal recurrences were rare in patients who underwent systematic lymphadenectomy with subsequent vaginal brachytherapy alone [104,115,116,117].
More importantly, in intermediate- and high-risk endometrioid endometrial carcinomas, adjuvant therapy may be omitted without decreasing survival by open surgery with systematic pelvic and para-aortic lymphadenectomy when patients are node-negative (Table 2) [26,112,113,114]. In our prospective cohort study of 77 node-negative patients with intermediate- and high-risk endometrioid carcinoma, only two understaged high-risk patients died of disease [26]. Although hematogenous spread (pulmonary metastasis is most commonly observed) may develop in endometrioid carcinoma, its risk is low in patients without extrauterine diseases [118,119]. Thus node-negative patients undergoing systematic lymphadenectomy that can remove all lymph nodes including positive nodes containing undetectable low-volume metastasis may be considered to be true node-negative. In contrast, sentinel node-negative patients without back-up lymphadenectomy might have a higher possibility of having undetected residual lymph node metastasis at the completion of surgery. Of note, in patients with positive peritoneal cytology, adjuvant therapy may not be omitted, as positive peritoneal cytology in low-stage disease was associated with decreased survival [120].

Table 2.
Long-term outcomes of patients with intermediate- and high-risk endometrial carcinoma treated with open surgery alone, including pelvic and para-aortic lymphadenectomy.
Node-positive patients, in particular patients with low-volume metastasis, can be cured with surgery alone including systematic lymphadenectomy. A previous study has reported that five-year overall survival was 40% for node-positive patients treated with surgery alone [121]. We have experienced the long-term survival of a case with grade 3 endometrioid endometrial carcinoma with para-aortic node metastasis treated with surgery alone [122]. She had a pelvic node, a para-aortic node, and an adnexal metastasis, but all metastatic diseases were micrometastasis (<2 mm). Similarly, isolated tumor cells detected in removed sentinel nodes may not decrease survival, even without adjuvant therapy [123,124,125,126].
The patient age, which has been incorporated into the existing risk classification systems [5,6], may influence the effect of lymphadenectomy in endometrioid endometrial carcinoma (Table 3). In elderly women, lymphadenectomy may be less effective than in younger women, which might be indicated in German population-based studies where median age of the patients was 69 years or older [127,128]. This may be explained by the association of older age with adverse pathologic features [129,130], hematogenous dissemination [118], and immunosenescence [131]. Otherwise, the route of surgical approach, which was not described in some recent studies, might affect the results.

Table 3.
Effects of pelvic and para-aortic lymphadenectomy by age.
6. Patients Treated with Minimally Invasive Surgery: Can Adjuvant Therapy Be Omitted?
Minimally invasive surgery, laparoscopic or robot-assisted surgery, has been accepted as a standard surgical approach after two randomized controlled trials [132,133], as minimally invasive surgery has advantages including shorter hospital stays and lower surgical morbidity compared to open surgery. However, no firm evidence that the effect of minimally invasive surgery on long-term survival in intermediate- and high-risk patients is equivalent to that of open surgery has been established [132,133]. A randomized trial where lymphadenectomy was performed in all patients failed to show the non-inferiority of laparoscopy [132]. Another randomized trial showed that the use of open surgery and laparoscopic surgery resulted in equivalent survival outcomes [133]. However, in that study, only selected surgeons performed surgery on a highly selected group of endometrioid carcinoma patients [134]. A previous study showed that patients treated with minimally invasive surgery had a shorter recurrence-free survival than those treated with open surgery, though overall survival was similar between the two groups [135].
Postoperative recurrences, vaginal and intra-abdominal, may increase in patients treated with minimally invasive surgery, although patients treated with minimally invasive surgery are more likely to receive adjuvant pelvic radiotherapy [136]. Even low-risk patients develop vaginal recurrences after laparoscopic hysterectomy [137]. Cervical involvement was a higher risk of recurrence in patients with intermediate-risk diseases [138]. In patients with high-intermediate risk tumors, minimally invasive surgery was associated with a shorter time to and a higher risk of recurrence [139]. In high-grade endometrial cancers, extracting a large uterus is associated with an increased risk of intra-abdominal recurrence [140]. A recent study reported that robotic surgery was significantly associated with a higher recurrence rate in stage I intermediate-risk endometrioid carcinoma compared to laparotomy [141]. Of note, robotic surgery was significantly associated with poorer overall survival compared to laparoscopic surgery in stage I endometrial cancer [142].
Tumor spillage into the pelvic cavity and the vagina during surgery appears to cause an increased risk of recurrence associated with minimally invasive surgery in patients with endometrial cancer, as well as cervical cancer. Most gynecologic oncologists performing minimally invasive surgeries have experienced uterine perforation with an intra-uterine manipulator and tumor spill while making a colpotomy [143]. Minimally invasive surgery was associated with a higher incidence of positive peritoneal cytology [144]. In patients with polypoid, larger-size tumors or tumors involving the endocervix, vaginal smears collected during surgery were positive for tumor cells in 80% of the patients [145]. A healing wound provides a favorable environment for tumor cells to attach and grow [146], and the leakage of insufflation gas through the vagina and the impact of pneumoperitoneum on local immune reactions may also be associated with tumor development [147]. The poorer outcomes in patients treated with robot-assisted surgery compared to patients treated with laparoscopic surgery may be explained by prolonged operating times, Trendelenburg positioning, and the absence of tactile sensation on tissue manipulation [142]. In cervical cancer, minimally invasive surgery was significantly associated with decreased overall survival, both in a randomized trial and an epidemiological study [148,149]. Even among patients with prostate cancer and colorectal cancer where minimally invasive surgery is commonly used and rarely associated with peritoneal recurrence, patients with positive surgical margins and serous invasion developed peritoneal recurrence possibly due to tumor spillage [150,151]. Prevention of cancer cell dissemination and metastatic tumor formation is a major goal of cancer surgery.
Lower risk of recurrence associated with open surgery may partly be explained by the lower incidence of tumor spillage, because an intra-uterine manipulator that might be associated with an increased risk of recurrence [152,153] is not necessary. In addition, vaginal disinfection after colpotomy during open surgery may prevent tumor implantation. We perform vaginal disinfection using povidone-iodine, which has a cytotoxic effect on tumor cells even in low concentrations [154,155].
Although tumor recurrences after minimally invasive surgery may not decrease overall survival, comorbidities associated with salvage treatment may decrease quality of life in survivors.
However, in view of the ongoing obesity epidemic in some countries, in obese women, minimally invasive surgery appears a better surgical approach to reduce surgical complications [156,157]. In a randomized trial, minimally invasive surgery was associated with a better survival than open surgery in obese women, but the trend failed to reach statistical significance (p = 0.14). Conversely, non-obese women tended to have a worse long-term disease-free survival when treated with minimally invasive surgery (p = 0.06) [133]. Approximately 10% of patients with early-stage endometrial cancer are medically inoperable because of obesity-related comorbidities, such as cardiovascular disease and diabetes-related end-organ damage [158]. Morbidly obese women are more likely to die of their comorbidities and also of their endometrial cancers [159,160,161]. In women with high-risk disease, severe obesity was associated with a poorer recurrence-free survival, which may be a result of incomplete surgical staging and adapted adjuvant therapies [162].
7. Preoperative Prediction of Lymph Node Metastasis and Surgical Therapy
Preoperative detection of lymph node metastasis using imaging studies, i.e., computed tomography, magnetic resonance imaging (MRI), and positron emission tomography/computed tomography, is of limited value, because imaging studies have limited efficacy in detecting tumor cells in small lymph nodes. In contrast, a combination of MRI parameters of tumor, serum CA-125 levels, and tumor grade appears useful in identifying patients in whom lymphadenectomy can be safely omitted [163,164].
Preoperative risk classification using tumor grade may not be accurate, as the agreement between preoperative biopsy and final post-hysterectomy diagnosis for tumor grade was only modest in low-grade tumors (G1 or G2 endometrioid carcinoma) [165], whereas it was high in high-grade tumors (G3 endometrioid, serous, clear cell carcinoma, and carcinosarcoma) [166]. Clinically relevant upgrading from low grade to high grade was observed in 8%, specifically 4% of preoperative grade-1 samples and 14% of preoperative grade-2 samples [165]. In contrast, hormone receptor status determined by preoperative biopsy, i.e., loss of progesterone receptor (PR) and double loss of estrogen receptor and PR, were associated with lymph node metastasis [167,168].
Similarly to chemotherapy, surgical therapy should be individualized based on the molecular profiles of the tumor [169,170]. Patients with POLE exonuclease domain mutation (EDM) may not need to undergo lymphadenectomy, as none of the POLE EDM tumors had extrauterine disease [171]. Lymph node metastasis was observed in 45% of p53-mutant tumors [172], although p53 abnormality may not be accurately detected in preoperative biopsy specimens, as preoperative biopsy did not show abnormal p53 staining in 56% of the tumors that showed p53 overexpression in hysterectomy specimens [167]. Lymphadenectomy may not improve patients with p53-mutant tumors, as the poor prognosis of early-stage p53-mutant endometrial cancers [173,174,175,176] may not be due to undetected lymph node metastasis [175]. This may be associated with a high proportion of serous carcinoma in p53-mutant tumors, with 71–88% of serous carcinomas being p53 abnormal [175,177], and serous carcinomas often develop peritoneal dissemination [92]. In endometrioid carcinomas, p53 abnormality was also observed in 3%, 11%, and 26–37% of grade-1, grade-2, and grade-3 tumors, respectively [175,177]. A small group of endometrial cancers harbor more than one molecular classifying feature (multiple classifier), and in grade-3 endometrioid carcinoma with mismatch repair deficiency or POLE EDM, hence coexisting TP53 mutation may be a secondary event acquired during tumor progression and was not associated with poorer survival [178]. Thus, p53 abnormality detected with immunohistochemistry may not be associated with poorer survival in patients with node-negative uterine-confined endometrioid carcinoma who undergo systematic lymphadenectomy.
High-intermediate-risk tumors are associated with lymph node metastasis [100,179]. In patients with high-intermediate-risk disease, 15% had unfavorable features such as p53-mutant, 50% favorable features such as POLE-mutant, and 35% intermediate features such as microsatellite instability [180]. Molecular profiling revealed that women with POLE mutant tumors had a favorable prognosis, even though the tumor was grade-3 endometrioid [173,174,177,181].
Certain molecular subtypes are associated with more rapid recurrence when treated with minimally invasive surgery. Both microsatellite-stable endometrioid tumors and p53-mutant tumors were associated with shorter recurrence-free survival when treated with minimally invasive surgery [135,182].
8. Concluding Remarks and Future Direction
The most important goal of cancer therapy is cure. Maintenance of the long-term quality of life of survivors is another important goal. Lower extremity lymphedema was observed in 4.6–28.3% of endometrial cancer patients who underwent lymphadenectomy with or without adjuvant therapy [183,184,185,186] (Table 4). Of note, 3.4% of the patients who did not undergo lymphadenectomy developed lymphedema after endometrial cancer therapy [186]. As the use of postoperative radiation is associated with lower-extremity lymphedema, omission of radiation in node-negative patients decreases the incidence of lymphedema [187,188]. Removal of circumflex iliac nodes to the distal external iliac nodes is associated with the development of lymphedema, hence elimination of their dissection may be helpful [189]. In patients treated with surgery alone, lymph node staging procedures did not seem to affect quality of life or symptoms, suggesting that lymph node removal seems justified from the patient’s viewpoint [19]. Additionally, as external beam radiation may increase the risk of secondary cancer in the irradiated field, its use should be restricted, particularly in younger women [7,190]. In patients with intermediate- and high-risk endometrioid endometrial carcinomas, omitting unnecessary adjuvant therapy by performing systematic lymphadenectomy is a therapeutic benefit of lymphadenectomy (Figure 1).

Table 4.
Incidence of lower extremity lymphedema and lymphocele in patients with endometrial cancer.
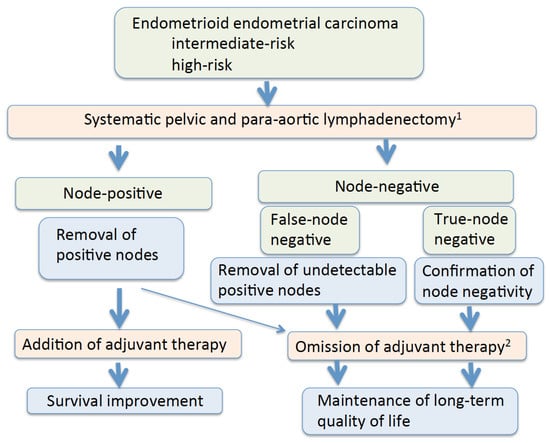
Figure 1.
Possible therapeutic benefits of systematic lymphadenectomy. 1 Lymphadenectomy may cause surgical morbidity and lymphedema. 2 Omission of adjuvant therapy may be possible in patients with node-negative uterine-confined disease and patients with isolated tumor cells.
Treatment guidelines for endometrial cancer based on the data from studies where a high percentage of patients had low-risk tumor cannot apply to patients with intermediate- and high-risk tumors. We believe open surgery with lymphadenectomy should be performed for patients with intermediate- and high-risk endometrioid endometrial carcinomas, and para-aortic lymphadenectomy is necessary for those with deep myometrial invasion [26]. To minimize the risk of lymph node recurrence, low-risk patients should also undergo lymph node assessment, excluding low-risk patients with tumors ≤ 2 cm who have a 0.6–0.8% risk of lymph node metastasis [191,192]. In addition, although postsurgical pathological examination may reveal lymphovascular space involvement that is an important risk factor of decreased survival [193,194], lymphovascular space involvement did not appear to reduce survival when lymph node metastasis was not detected [26,112,113,114,195]. These studies suggest that in endometrioid endometrial carcinoma, lymph node status determined by systematic lymphadenectomy separates patients into high-risk, i.e., node-positive, and low-risk, i.e., node-negative, groups [26,196].
The possibility of omitting adjuvant therapy in intermediate- and high-risk uterine-confined endometrioid endometrial carcinoma should be evaluated with real-world data and prospective randomized trials, comparing systematic lymphadenectomy with sentinel lymph node mapping, as well as comparing open surgery with minimally invasive surgery.
Funding
The APC was funded by Kameda Medical Center. This research received no external funding.
Acknowledgments
The author would like to thank his colleagues at the Department of Obstetrics and Gynecology, Kameda Medical Center for their help.
Conflicts of Interest
The author declares no conflict of interest.
References
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Basile, S.; Maneschi, F.; Lissoni, A.A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Kitchener, H.; Swart, A.M.C.; Qian, Q.; Amos, C.; Parmar, M.K.B. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [PubMed]
- Creutzberg, C.L.; van Putten, W.L.J.; Koper, P.C.M.; Lybeert, M.L.M.; Jobsen, J.J.; Warlam-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; van den Bergh, A.C.; van de Steen-Banasik, E.; et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre random trial. Lancet 2000, 355, 1404–1411. [Google Scholar] [CrossRef]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Onsrud, M.; Cvancarova, M.; Hellebust, T.P.; Trope, C.G.; Kristensen, G.B.; Lindemann, K. Long-term outcomes after pelvic radiation for early-stage endometrial cancer. J. Clin. Oncol. 2013, 31, 3951–3956. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.; Lissoni, A.; Spina, F.; Melpignano, M.; Zola, P.; Favalli, G.; Colombo, A.; Fossati, R. Adjuvant chemotherapy vs. radiotherapy in high-risk endometrial carcinoma: Results of a randomised trial. Br. J. Cancer 2006, 95, 266–271. [Google Scholar] [CrossRef]
- Susumu, N.; Sagae, S.; Udagawa, Y.; Niwa, K.; Kuramoto, H.; Satoh, S.; Kudo, R. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: A Japanese Gynecologic Oncology Group study. Gynecol. Oncol. 2008, 108, 226–233. [Google Scholar] [CrossRef]
- Ebina, Y.; Katabuchi, H.; Mikami, M.; Nagase, S.; Yaegashi, N.; Udagawa, Y.; Kato, H.; Kubushiro, K.; Takamatsu, K.; Ino, K.; et al. Japan Society of Gynecologic Oncology guidelines 2013 for the treatment of uterine body neoplasms. Int. J. Clin. Oncol. 2016, 21, 419–434. [Google Scholar] [CrossRef]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C. ESMO Guidelines Working Group (2013) Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. S6), vi33–vi38. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Corn, B.W.; Lanciano, R.M.; Greven, K.M.; Noumoff, J.; Schultz, D.; Hanks, G.E.; Fowble, B.L. Impact of improved irradiation technique, age, and lymph node sampling on the severe complication rate of surgically staged endometrial cancer patients: A multivariate analysis. J. Clin. Oncol. 1994, 12, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Nout, R.A.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; Van Der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; Nijman, H.W.; et al. Five-year quality of life of endometrial cancer patients treated in the randomized Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur. J. Cancer 2012, 48, 1638–1648. [Google Scholar] [CrossRef]
- van de Poll-Franse, L.V.; Pijnenborg, J.M.A.; Boll, D.; Vos, M.C.; van den Berg, H.; Lybeert, M.L.M.; de Winter, K.; Kruitwagen, R.F.P.M. Health related quality of life and symptoms after pelvic lymphadenectomy or radiotherapy vs. no adjuvant regional treatment in early-stage endometrial carcinoma: A large population-based study. Gynecol. Oncol. 2012, 127, 153–160. [Google Scholar] [CrossRef]
- Pellizzon, A.C.A.; Fogarolli, R.C.; Miziara, M.; Baraldi, H.; Soares, C.R. Morbidity of adjuvant high-dose-rate brachytherapy for low to intermediate risk endometrial adenocarcinoma completely resected. Int. J. Cancer 2001, 96, 105–108. [Google Scholar] [CrossRef]
- Harkenrider, M.M.; Block, A.M.; Siddiqui, Z.A.; Small, W., Jr. The role of vaginal cuff brachytherapy in endometrial cancer. Gynecol. Oncol. 2015, 136, 365–372. [Google Scholar] [CrossRef]
- Cella, D.; Huang, H.; Homesley, H.D.; Montag, A.; Salani, R.; De Geest, K.; Lee, R.; Spirtos, N.M. Patient-reported peripheral neuropathy of doxorubicin and cisplatin with and without paclitaxel in the treatment of advanced endometrial cancer: Results from GOG 184. Gynecol. Oncol. 2010, 119, 538–542. [Google Scholar] [CrossRef]
- Forsse, D.; Barbero, M.L.; Werner, H.M.J.; Woie, K.; Nordskar, N.; Nielsen, E.B.; Engh, M.E.; Vistad, I.; Rege, A.; Sævik-Lode, M.; et al. Longitudinal effects of adjuvant chemotherapy and lymph node staging on patient-reported outcomes in endometrial cancer survivors: A prospective cohort study. Am. J. Obstet. Gynecol. 2022, 226, 90.e1–90.e20. [Google Scholar] [CrossRef]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987, 60, 2035–2041. [Google Scholar] [CrossRef]
- AlHilli, M.M.; Podratz, K.C.; Dowdy, S.C.; Bakkum-Gamez, J.N.; Weaver, A.L.; McGree, M.E.; Keeney, G.L.; Cliby, W.A.; Mariani, A. Risk-scoring system for the individualized prediction of lymphatic dissemination in patients with endometrioid endometrial cancer. Gynecol. Oncol. 2013, 131, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Keeney, G.L.; Aletti, G.; Webb, M.J.; Haddock, M.G.; Podratz, K.C. Endometrial carcinoma: Paraaortic dissemination. Gynecol. Oncol. 2004, 92, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Dowdy, S.C.; Cliby, W.A.; Gostout, B.S.; Jones, M.B.; Wilson, T.O.; Podratz, K.C. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol. Oncol. 2008, 109, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Suzuki, Y.; Azuma, M.; Hatanaka, Y.; Konno, Y.; Watari, H.; Kato, H.; Matsuno, Y.; Yamashiro, K.; Sakuragi, N. Ultrastaging of para-aortic lymph nodes in stage IIIC1 endometrial cancer: A preliminary report. Gynecol. Oncol. 2012, 127, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, T.; Watari, H.; Kato, T.; Mitamura, T.; Hosaka, M.; Sudo, S.; Takeda, M.; Kobayashi, N.; Dong, P.; Todo, Y.; et al. Distribution of lymph node metastasis sites in endometrial cancer undergoing systematic pelvic and para-aortic lymphadenectomy: A proposal of optimal lymphadenectomy for future clinical trials. Ann. Surg. Oncol. 2014, 21, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, I.; Matsuura, T.; Mitani, T.; Otsuka, K.; Kanamoto, Y. Open surgery including lymphadenectomy without adjuvant therapy for uterine-confined intermediate- and high-risk endometrioid endometrial carcinoma. Curr. Oncol. 2022, 29, 298. [Google Scholar] [CrossRef]
- Fujimoto, T.; Nanjo, H.; Nakamura, A.; Yokoyama, Y.; Takano, T.; Shoji, T.; Nakahara, K.; Yamada, H.; Mizunuma, H.; Yaegashi, N.; et al. Para-aortic lymphadenectomy may improve disease-related survival in patients with multipositive pelvic lymph node stage IIIc endometrial cancer. Gynecol. Oncol. 2007, 107, 253–259. [Google Scholar] [CrossRef]
- Karube, Y.; Fujimoto, T.; Yakahashi, O.; Nanjyo, H.; Mizunuma, H.; Yaegashi, N.; Sugiyama, T.; Kurachi, H.; Sato, A.; Tanaka, T. Histopathologic prognostic factors predicting para-aortic lymph node metastasis in patients with endometrioid uterine cancer. Gynecol. Oncol. 2010, 118, 151–154. [Google Scholar] [CrossRef]
- Fotopoulou, C.; El-Balat, A.; du Bois, A.; Sehouli, J.; Harter, P.; Muallem, M.Z.; Krätschell, R.W.; Traut, A.; Heitz, F. Systematic pelvic and paraaortic lymphadenectomy in early high-risk or advanced endometrial cancer. Arch. Gynecol. Obstet. 2015, 292, 1321–1327. [Google Scholar] [CrossRef]
- Otsuka, I.; Kubota, T.; Aso, T. Lymphadenectomy and adjuvant therapy in endometrial carcinoma: Role of adjuvant chemotherapy. Br. J. Cancer 2002, 87, 377–380. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Gomez, J.D.; Alektiar, K.M.; Soslow, R.A.; Hensley, M.L.; Leitao, M.M., Jr.; Gardner, G.J.; Sonda, Y.; Chi, D.S.; Barakat, R.R. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol. Oncol. 2009, 115, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.J.; Yu, K.J.; Chao, K.C.; Teng, N.N.H. The incidence of isolated para-aortic nodal metastasis in completely staged endometrial cancer patients. Gynecol. Oncol. 2011, 121, 122–125. [Google Scholar] [CrossRef]
- Kumar, S.; Podratz, K.C.; Bakkum-Gamez, J.N.; Dowdy, S.C.; Weaver, A.L.; McGree, M.E.; Cliby, W.A.; Keeney, G.L.; Thomas, G.; Mariani, A. Prospective assessment of the prevalence of pelvic, paraaortic and high paraaortic lymph node metastasis in endometrial cancer. Gynecol. Oncol. 2014, 132, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Multinu, F.; Casarin, J.; Cappuccio, S.; Keeney, G.L.; Glaser, G.E.; Cliby, W.A.; Weaver, A.L.; McGree, M.E.; Angioni, S.; Faa, G.; et al. Ultrastaging of negative pelvic lymph nodes to decrease the true prevalence of isolated paraaortic dissemination in endometrial cancer. Gynecol. Oncol. 2019, 154, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, S.; Richer, L.; Souhami, L.; Arseneau, J.; Fu, L.; Gilbert, L.; Alfieri, J.; Jardon, K.; Zeng, X.Z. Clinical significance of isolated tumor cells and micrometastasis in low-grade, stage Iendometrial cancer. J. Surg. Oncol. 2018, 118, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Mariani, A.; Paolini, B.; Ditto, A.; Raspagliesi, F. Low-volume disease in endometrial cancer: The role of micrometastasis and isolated tumor cells. Gynecol. Oncol. 2019, 153, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.; Swart, A.M.; Orton, J.; Kitchener, H.; Whelan, T.; Lukka, H.; Eisenhauer, E.; Bacon, M.; Tu, D.; Parmar, M.K.B.; et al. The ASTEC/EN.5 writing committee on behalf of the ASTEC/EN.5 Study Group. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet 2009, 373, 137–146. [Google Scholar]
- Nout, R.A.; van de Poll-Francse, L.V.; Lybeert, M.L.M.; Warlam-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.M.; Lutgens, L.C.H.W.; Pras, B.; van Putten, W.L.J.; Creutzberg, C.L. Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the Post Operative Radiation Therapy in Endometrial Carcinoma 1 (PORTEC-1) trial. J. Clin. Oncol. 2011, 29, 1692–1700. [Google Scholar] [CrossRef]
- Onstad, M.; Ducie, J.; Fellman, B.M.; Abu-Rustum, N.R.; Leitao, M.; Mariani, A.; Multinu, F.; Lu, K.H.; Soliman, P. Adjuvant therapy for grade 3, deeply invasive endometrioid adenocarcinoma of the uterus. Int. J. Gynecol. Cancer 2020, 30, 485–490. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; van Putten, W.L.J.; Koper, P.C.; Lybeert, M.L.M.; Jobsen, J.J.; Wárlám-Rodenhius, C.C.; De Winter, K.A.L.; Lutgens, L.C.H.W.; van den Bergh, A.C.M.; van den Steen-Banasik, E.; et al. Survival after relapse in patients with endometrial cancer: Results from a randomized trial. Gynecol. Oncol. 2003, 89, 201–209. [Google Scholar] [CrossRef]
- Petignat, P.; Jolicoeur, M.; Alobaid, A.; Drouin, P.; Gauthier, P.; Provencher, D.; Donath, D.; Nguyen, T.V. Salvage treatment with high-dose-rate brachytherapy for isolated vaginal endometrial cancer recurrence. Gynecol. Oncol. 2006, 101, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, I.; Uno, M.; Wakabayashi, A.; Kameda, S.; Udagawa, H.; Kubota, T. Predictive factors for prolonged survival in recurrent endometrial carcinoma: Implications for follow-up protocol. Gynecol. Oncol. 2010, 119, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Watanabe, M.; Amikura, T.; Obata, H.; Sekine, M.; Yahata, T.; Fujita, K.; Tanaka, K. Adjuvant chemotherapy as treatment of high-risk stage I and II endometrial cancer. Gynecol. Oncol. 2004, 94, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Multinu, F.; Garzon, S.; Weaver, A.L.; McGree, M.E.; Sartori, E.; Landoni, F.; Zola, P.; Dinoi, G.; Aletti, G.; Block, M.S.; et al. Adjuvant Chemotherapy in Early Stage Endometrioid Endometrial Cancer with >50% Myometrial Invasion and Negative Lymph Nodes. Int. J. Gynecol. Cancer 2021, 31, 537–544. [Google Scholar] [CrossRef]
- Randall, M.E.; Filiaci, V.; McMeekin, D.S.; von Gruenigen, V.; Huang, H.; Yashar, C.M.; Mannel, R.S.; Kim, J.W.; Salani, R.; DiSilvestro, P.A.; et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J. Clin. Oncol. 2019, 37, 1810–1818. [Google Scholar] [CrossRef]
- Fujimoto, T.; Nanjyo, H.; Fukuda, J.; Nakamura, A.; Mizunuma, H.; Yaegashi, N.; Sugiyama, T.; Kurachi, H.; Sato, A.; Tanaka, T. Endometrioid uterine cancer: Histopathological risk factors of local and distant recurrence. Gynecol. Oncol. 2009, 112, 342–347. [Google Scholar] [CrossRef]
- Bakkum-Gamez, J.N.; Mariani, A.; Dowdy, S.C.; Weaver, A.L.; McGree, M.E.; Martin, J.R.; Keeney, G.L.; Jatoi, A.; Gostout, B.S.; Podratz, K.C. Efficacy of contemporary chemotherapy in stage IIIC endometrial cancer: A histologic dichotomy. Gynecol. Oncol. 2014, 132, 578–584. [Google Scholar] [CrossRef]
- Cragun, J.M.; Havrilesky, L.J.; Calingaert, B.; Synan, I.; Secord, A.A.; Soper, J.T.; Clarke-Pearson, D.L.; Berchuck, A. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J. Clin. Oncol. 2005, 23, 3668–3675. [Google Scholar] [CrossRef]
- Chan, J.K.; Cheung, M.K.; Huh, W.K.; Osann, K.; Husain, A.; Teng, N.N.; Kapp, D.S. Therapeutic role of lymph node resection in endometrioid corpus cancer. A study of 12,333 patients. Cancer 2006, 107, 1823–1830. [Google Scholar] [CrossRef]
- Jeong, N.H.; Lee, J.M.; Lee, J.K.; Kim, M.K.; Kim, Y.J.; Cho, C.H.; Kim, S.M.; Park, S.Y.; Park, C.Y.; Kim, K.T. Role of systematic lymphadenectomy and adjuvant radiation in early-stage endometrioid uterine cancer. Ann. Surg. Oncol. 2010, 17, 2951–2957. [Google Scholar] [CrossRef]
- Todo, Y.; Kato, H.; Minobe, S.; Okamoto, K.; Suzuki, Y.; Sudo, S.; Takeda, M.; Watari, H.; Kaneuchi, M.; Sakuragi, N. Initial failure site according to primary treatment with or without para-aortic lymphadenectomy in endometrial cancer. Gynecol. Oncol. 2011, 121, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M., Jr.; Zhou, Q.C.; Gomez-Hidalgo, N.R.; Iasonos, A.; Baser, R.; Mezzancello, M.; Chang, K.; Ward, J.; Chi, D.S.; Roche, K.L.; et al. Patient-reported outcomes after surgery for endometrial carcinoma: Prevalence of lower-extremity lymphedema after sentinel lymph node mapping versus lymphadenectomy. Gynecol. Oncol. 2020, 156, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Dioun, S.; Chen, L.; Melamed, A.; Gockley, A.; St Clair, C.M.; Hou, J.Y.; Khoury-Collado, F.; Hershman, D.L.; Wright, J.D. Uptake and outcomes of sentinel lymph node mapping in women undergoing minimally invasive surgery for endometrial cancer. BJOG 2022, 129, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Frimer, M.; Khoury-Collado, F.; Murray, M.P.; Barakat, R.R.; Abu-Rustum, N.R. Micrometastases of endometrial cancer to sentinel lymph nodes: Is it an artifact of uterine manipulation? Gynecol. Oncol. 2010, 119, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Nottegar, A.; Veronese, N.; Senthil, M.; Roumen, R.M.; Stubbs, B.; Choi, A.H.; Verheuvel, N.C.; Solmi, M.; Pea, A.; Capelli, P.; et al. Extra-nodal extension of sentinel lymph node metastasis is a marker of poor prognosis in breast cancer patients: A systematic review and an exploratory meta-analysis. Eur. J. Surg. Oncol. 2016, 42, 919–925. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Khoury-Collado, F.; Murray, M.P.; Hensley, M.L.; Sonoda, Y.; Alektiar, K.M.; Levine, D.A.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. Sentinel lymph node mapping for endometrial cancer improves the detection of metastatic disease to regional lymph nodes. Gynecol. Oncol. 2011, 122, 251–254. [Google Scholar] [CrossRef]
- Ballester, M.; Naoura, I.; Chéreau, E.; Seror, J.; Bats, A.S.; Bricou, A.; Daraï, E. Sentinel node biopsy upstages patients with presumed low- and intermediate-risk endometrial cancer: Results of a multicenter study. Ann. Surg. Oncol. 2013, 20, 407–412. [Google Scholar] [CrossRef]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Bodurtha Smith, A.J.; Fader, A.N.; Tanner, E.J. Sentinel lymph node assessment in endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 459–476. [Google Scholar] [CrossRef]
- Holloway, R.W.; Gupta, S.; Stavitzski, N.M.; Zhu, X.; Takimoto, E.L.; Gubbi, A.; Bigsby, G.E.; Brudie, L.A.; Kendrick, J.E.; Ahmad, S. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol. Oncol. 2016, 141, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.W.; Levenback, C.; Tornos, C.; Morris, M.; Wharton, J.T.; Gershenson, D.M. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: Results of a pilot study. Gynecol. Oncol. 1996, 62, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Coleman, R.C.; Soliman, P.T.; Ramirez, P.T.; Levenback, C.F. A case for caution in the pursuit of the sentinel node in women with endometrial carcinoma. Gynecol. Oncol. 2014, 132, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Ditto, A.; Casarin, J.; Pinelli, C.; Perrone, A.M.; Scollo, P.; Martinelli, F.; Bogani, G.; Maggiore, U.L.R.; Signorelli, M.; Chiappa, V.; et al. Hysteroscopic versus cervical injection for sentinel node detection in endometrial cancer: A multicenter prospective randomised controlled trial from the Multicenter Italian Trials in Ovarian cancer (MITO) study group. Eur. J. Cancer 2020, 140, 1–10. [Google Scholar] [CrossRef]
- Persson, J.; Salehi, S.; Bollino, M.; Lonnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)--the final step towards a paradigm shift in surgical staging. Eur. J. Cancer 2019, 116, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.M.; Casadio, P.; Formelli, G.; Levorato, M.; Ghi, T.; Costa, S.; Meriggiola, M.C.; Pelusi, G. Cervical and hysteroscopic injection for identification of sentinel lymph node in endometrial cancer. Gynecol. Oncol. 2008, 111, 62–67. [Google Scholar] [CrossRef]
- Kataoka, F.; Susumu, N.; Yamagami, W.; Kuwahata, M.; Takigawa, A.; Nomura, H.; Takeuchi, H.; Nakahara, T.; Kameyama, K.; Aoki, D. The importance of para-aortic lymph nodes in sentinel lymph node mapping for endometrial cancer by using hysteroscopic radio-isotope tracer injection combined with subserosal dye injection: Prospective study. Gynecol. Oncol. 2016, 140, 400–404. [Google Scholar] [CrossRef]
- Martinelli, F.; Ditto, A.; Signorelli, M.; Bogani, G.; Chiappa, V.; Lorusso, D.; Scaffa, C.; Recalcati, D.; Perotto, S.; Haeusler, E.; et al. Sentinel node mapping in endometrial cancer following Hysteroscopic injection of tracers: A single center evaluation over 200 cases. Gynecol. Oncol. 2017, 146, 525–530. [Google Scholar] [CrossRef]
- Angeles, M.A.; Migliorelli, F.; Vidal-Sicart, S.; Saco, A.; Ordi, J.; Ros, C.; Fuste, P.; Munmany, M.; Escura, S.; Carreras, N.; et al. Paraaortic sentinel lymph node detection in intermediate and high-risk endometrial cancer by transvaginal ultrasound-guided myometrial injection of radiotracer (TUMIR). J. Gynecol. Oncol. 2021, 32, e52. [Google Scholar] [CrossRef]
- Altın, D.; Taşkın, S.; Ortac, F.; Tokgozoğlu, N.; Vatansever, D.; Güler, A.H.; Güng, M.; Taşci, T.; Beşe, T.; Turan, H.; et al. Diagnostic accuracy of sentinel node biopsy in non-endometrioid, high-grade and/or deep myoinvasive endometrial cancer: A Turkish gynecologic oncology group study (TRSGO-SLN-006). Gynecol. Oncol. 2022, 164, 492–497. [Google Scholar] [CrossRef]
- Niikura, H.; Okamura, C.; Utsunomiya, H.; Yoshinaga, K.; Akahira, J.; Ito, K.; Yaegashi, N. Sentinel lymph node detection in patients with endometrial cancer. Gynecol. Oncol. 2004, 92, 669–674. [Google Scholar] [CrossRef]
- Pristauz, G.; Bader, A.A.; Regitnig, P.; Haas, J.; Winter, R.; Tamussino, K. How accurate is frozen section histology of pelvic lymph nodes in patients with endometrial cancer? Gynecol. Oncol. 2009, 115, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ballester, M.; Dubernard, G.; Lecuru, F.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; Rouzier, R.; et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: A prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011, 12, 469–476. [Google Scholar] [CrossRef]
- Kim, C.H.; Khoury-Collado, F.; Barber, E.L.; Soslow, R.A.; Makker, V.; Leitao, M.M., Jr.; Sonoda, Y.; Alektiar, K.M.; Barakat, R.R.; Abu-Rustum, N. Sentinel lymph node mapping with pathologic ultrastaging: A valuable tool for assessing nodal metastasis in low-grade endometrial cancer with superficial myoinvasion. Gynecol. Oncol. 2013, 131, 714–719. [Google Scholar] [CrossRef]
- Baiocchi, G.; Mantoan, H.; Goncalves, B.T.; Faloppa, C.C.; Kumagai, L.Y.; Badiglian-Filho, L.; da Costa, A.A.B.A.; De Brot, L. Size of Sentinel Node Metastasis Predicts Non-sentinel Node Involvement in Endometrial Cancer. Ann. Surg. Oncol. 2020, 27, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Casarin, J.; Maggiore, U.L.R.; Ditto, A.; Pinelli, C.; Dell’acqua, A.; Lopez, S.; Chiappa, V.; Brusadelli, C.; Guerrisi, R.; et al. Survival outcomes in endometrial cancer patients having lymphadenectomy, sentinel node mapping followed by lymphadectomy and sentinel node mapping alone: Long-term results of a propensity-matched analysis. Gynecol. Oncol. 2020, 158, 77–83. [Google Scholar] [CrossRef]
- Daraï, E.; Dubernard, G.; Bats, A.S.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Glfoier, F.; Leblanc, E.; Rouzier, R.; et al. Sentinel node biopsy for the management of early stage endometrial cancer: Long-term results of the SENTI-ENDO study. Gynecol. Oncol. 2015, 136, 54–59. [Google Scholar] [CrossRef]
- Cusimano, M.C.; Vicus, D.; Pulman, K.; Maganti, M.; Bernardini, M.Q.; Bouchard-Fortier, G.; Laframboise, S.; May, T.; Hogen, L.F.; Covens, A.L.; et al. Assessment of Sentinel Lymph Node Biopsy vs Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging. JAMA Surg. 2021, 156, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Di, S.D.; Lu, W.; He, Q.Z.; Li, Y.R.; Li, B.L.; Wang, Z.J.; Yan, Q.; Wan, X.P. A Prospective Study of Sentinel Lymph Node Mapping for Endometrial Cancer: Is It Effective in High-Risk Subtypes? Oncologist 2019, 24, e1381. [Google Scholar] [CrossRef]
- Torrent, A.; Amengual, J.; Sampol, C.M.; Ruiz, M.; Rioja, J.; Matheu, G.; Roca, P.; Cordoba, O. Sentinel Lymph Node Biopsy in Endometrial Cancer: Dual Injection, Dual Tracer—A Multidisciplinary Exhaustive Approach to Nodal Staging. Cancers 2022, 14, 929. [Google Scholar] [CrossRef]
- Barlin, J.N.; Khoury-Collado, F.; Kim, C.H.; Leitao, M.M., Jr.; Chi, D.S.; Sonoda, Y.; Alektiar, K.; DeLair, D.F.; Barakat, R.R.; Abu-Rustum, N.R. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol. Oncol. 2012, 125, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Buda, A.; Di Martino, G.; Restaino, S.; De Ponti, E.; Monterossi, G.; Giuliani, D.; Ercoli, A.; Dell’Orto, F.; Dinoi, G.; Grassi, T.; et al. The impact on survival of two different staging strategies in apparent early stage endometrial cancer comparing sentinel lymph nodes mapping algorithm and selective lymphadenectomy: An Italian retrospective analysis of two reference centers. Gynecol. Oncol. 2017, 147, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Buda, A.; Gasparri, M.L.; Puppo, A.; Mereu, L.; De Ponti, E.; Di Martino, G.; Novelli, A.; Tateo, S.; Muller, M.; Landoni, F.; et al. Lymph node evaluation in high-risk early stage endometrial cancer: A multi-institutional retrospective analysis comparing the sentinel lymph node (SLN) algorithm and SLN with selective lymphadenectomy. Lymph node evaluation in high-risk early stage endometrial cancer: A multi-institutional retrospective analysis comparing the sentinel lymph node (SLN) algorithm and SLN with selective lymphadenectomy. Gynecol. Oncol. 2018, 150, 261–266. [Google Scholar] [PubMed]
- Schlappe, B.A.; Weaver, A.L.; Ducie, J.A.; Eriksson, A.G.Z.; Dowdy, S.C.; Cliby, W.A.; Glaser, G.E.; Soslow, R.A.; Alektiar, K.M.; Makker, V.; et al. Multicenter study comparing oncologic outcomes between two nodal assessment methods in patients with deeply invasive endometrioid endometrial carcinoma: A sentinel lymph node algorithm versus a comprehensive pelvic and paraaortic lymphadenectomy. Gynecol. Oncol. 2018, 151, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Salman, L.; Cusimano, M.C.; Marchocki, Z.; Ferguson, S.E. Sentinel Lymph Node Mapping in High-Grade Endometrial Cancer. Curr. Oncol. 2022, 29, 96. [Google Scholar] [CrossRef]
- Takahashi, A.; Matsuura, M.; Matoda, M.; Nomura, H.; Okamoto, S.; Kanao, H.; Kondo, E.; Omatsu, K.; Kato, K.; Utsugi, K.; et al. Clinicopathological Features of Early and Late Recurrence of Endometrial Carcinoma After Surgical Resection. Int. J. Gynecol. Cancer 2017, 27, 967–972. [Google Scholar] [CrossRef]
- Podratz, K.C.; Mariani, A.; Webb, M.J. Staging and therapeutic value of lymphadenectomy in endometrial cancer. Gynecol. Oncol. 1998, 70, 163–164. [Google Scholar] [CrossRef]
- Chan, J.K.; Kapp, D.S. Role of complete lymphadenectomy in endometrioid uterine cancer. Lancet Oncol. 2007, 8, 831–841. [Google Scholar] [CrossRef]
- Dowdy, S.C.; Borah, B.J.; Bakkum-Gamez, J.N.; Weaver, A.L.; McGree, M.E.; Haas, L.R.; Keeney, G.L.; Mariani, A.; Podratz, K.C. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol. Oncol. 2012, 127, 5–10. [Google Scholar] [CrossRef]
- Creasman, W.T. ASTEC lymphadenectomy and radiation therapy studies: Are conclusions valid? Gynecol. Oncol. 2010, 116, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.W. The role of lymphadenectomy in endometrial cancer: Was the ASTEC trial doomed by design and are we destined to repeat that mistake? Gynecol. Oncol. 2012, 126, 5–11. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tang, Y.H.; Chiang, Y.C.; Wang, K.L.; Fu, H.C.; Ke, Y.M.; Lau, H.Y.; Hsu, K.F.; Wu, C.H.; Cheng, W.F. Impact of management on the prognosis of pure uterine papillary serous cancer—A Taiwanese Gynecologic Oncology Group (TGOG) study. Gynecol. Oncol. 2014, 133, 221–228. [Google Scholar] [CrossRef]
- Leath, C.A., III; Numnum, T.M.; Kendrick, J.E.; Frederick, P.J.; Rocconi, R.P.; Conner, M.G.; Straughn, J.M., Jr. Patterns of Failure for Conservatively Managed Surgical Stage I Uterine Carcinosarcoma. Implications for Adjuvant Therapy. Int. J. Gynecol. Cancer 2009, 19, 888–891. [Google Scholar] [CrossRef]
- Jeppesen, M.M.; Jensen, P.T.; Hansen, D.G.; Iachina, M.; Mogensen, O. The nature of early-stage endometrial cancer recurrence—national cohort study. Eur. J. Cancer 2016, 69, 51–60. [Google Scholar] [CrossRef]
- Mariani, A.; Webb, M.J.; Galli, L.; Podratz, K.C. Potential therapeutic role of para-aortic lymphadenectomy in node-positive endometrial cancer. Gynecol. Oncol. 2000, 76, 348–356. [Google Scholar] [CrossRef]
- Alektiar, K.M.; Venkatraman, E.; Chi, D.S.; Barakat, R.R. Intravaginal brachytherapy alone for intermediate-risk endometrial cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 111–117. [Google Scholar] [CrossRef]
- Todo, Y.; Kato, H.; Kaneuchi, M.; Watari, H.; Takeda, M.; Sakuragi, N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): A retrospective cohort analysis. Lancet 2010, 375, 1165–1172. [Google Scholar] [CrossRef]
- Huang, C.Y.; Ho, C.M.; Chen, Y.L.; You, S.L.; Chen, C.A.; Cheng, W.F. Impact of lymphadenectomy in uterine endometrioid carcinoma. Eur. J. Surg. Oncol. 2013, 39, 350–357. [Google Scholar] [CrossRef]
- Eggemann, H.; Ignatov, T.; Kaiser, K.; Burger, E.; Dan Costa, S.; Ignatov, A. Survival advantage of lymphadenectomy in endometrial cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1051–1060. [Google Scholar] [CrossRef]
- Nugent, E.K.; Bishop, E.A.; Mathews, C.A.; Moxley, K.M.; Tenney, M.; Mannel, R.S.; Walker, J.L.; Moore, K.N.; Landrum, L.M.; McMeekin, D.S. Do uterine risk factors or lymph node metastasis more significantly affect recurrence in patients with endometrioid adenocarcinoma? Gynecol. Oncol. 2012, 125, 94–98. [Google Scholar] [CrossRef]
- Ouldamer, L.; Bendifallah, S.; Body, G.; Canlorbe, G.; Touboul, C.; Graesslin, O.; Raimond, E.; Collinet, P.; Coutant, C.; Lavoué, V.; et al. Call for Surgical Nodal Staging in Women with ESMO/ESGO/ESTRO High-Intermediate Risk Endometrial Cancer: A Multicentre Cohort Analysis from the FRANCOGYN Study Group. Ann. Surg. Oncol. 2017, 24, 1660–1666. [Google Scholar] [CrossRef]
- Morrow, C.P.; Bundy, B.N.; Kurman, R.J.; Creasman, W.T.; Heller, P.; Homesley, H.D.; Graham, J.E. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1991, 40, 55–65. [Google Scholar] [CrossRef]
- Euscher, E.D.; Bassett, R.; Malpica, A. Lymph node counts in endometrial cancer: Expectations versus reality. Am. J. Surg. Pathol. 2011, 35, 913–918. [Google Scholar] [CrossRef]
- Fanning, J.; Nanavati, P.J.; Hilgers, R. Surgical staging and high dose rate brachytherapy for endometrial cancer: Limiting external radiotherapy to node-positive tumors. Obstet. Gynecol. 1996, 87, 1041–1044. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Iasonos, A.; Zhou, Q.; Oke, E.; Soslow, R.A.; Alektiar, K.M.; Chi, D.S.; Barakat, R.R. Is there a therapeutic impact to regional lymphadenectomy in the surgical treatment of endometrial carcinoma? Am. J. Obstet. Gynecol. 2008, 198, 457.e1–457.e6. [Google Scholar] [CrossRef]
- Huang, M.; Chadha, M.; Musa, F.; Friedmann, P.; Kolev, V.; Holcomb, K. Lymph nodes: Is total number or station number a better predictor of lymph node metastasis in endometrial cancer? Gynecol. Oncol. 2010, 119, 295–298. [Google Scholar] [CrossRef]
- Kilgore, L.C.; Partridge, E.E.; Alvarez, R.D.; Austin, J.M.; Shingleton, H.M.; Noojin, F.; Conner, W. Adenocarcinoma of the endometrium: Survival comparison of patients with and without pelvic node sampling. Gynecol. Oncol. 1995, 56, 29–33. [Google Scholar] [CrossRef]
- Sosa, J.A.; Diener-West, M.; Gusev, Y.; Choti, M.A.; Lange, J.R.; Dooley, W.C.; Zeiger, M.A. Association between extent of axillary lymph node dissection and survival in patients with stage I breast cancer. Ann. Surg. Oncol. 1998, 5, 140–149. [Google Scholar] [CrossRef]
- Weir, L.; Speers, C.; D’Yachkova, Y.; Olivotto, I.A. Prognostic significance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J. Clin. Oncol. 2002, 20, 1793–1799. [Google Scholar] [CrossRef]
- Gajra, A.; Newman, N.; Gamble, G.P.; Kohman, L.J.; Graziano, S.L. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J. Clin. Oncol. 2003, 21, 1029–1034. [Google Scholar] [CrossRef]
- Shah, M.; Lewin, S.N.; Deutsch, I.; Burke, W.M.; Sun, X.; Herzog, T.J.; Wright, J.D. Therapeutic role of lymphadenectomy for cervical cancer. Cancer 2011, 117, 310–317. [Google Scholar] [CrossRef]
- Chen, S.S. Operative treatment in stage I endometrial carcinoma with deep myometrial invasion and/or grade 3 tumor surgically limited to the corpus uteri. No recurrence with only primary surgery. Cancer 1989, 63, 1843–1845. [Google Scholar] [CrossRef]
- Ayhan, A.; Taskiran, C.; Celik, C.; Guney, I.; Yuce, K.; Ozyar, E.; Atahan, L.; Kucukali, T. Is there a survival benefit to adjuvant radiotherapy in high-risk surgical stage I endometrial cancer? Gynecol. Oncol. 2002, 86, 259–263. [Google Scholar] [CrossRef]
- Straughn, J.M.; Huh, W.K.; Orr, J.W.; Kelly, F.J.; Roland, P.Y.; Gold, M.A.; Powell, M.; Mutch, D.G.; Partridge, E.E.; Kilgore, L.C.; et al. Stage IC adenocarcinoma of the endometrium: Survival comparisons of surgically staged patients with and without adjuvant radiotherapy. Gynecol. Oncol. 2003, 89, 295–300. [Google Scholar] [CrossRef]
- Orr, J.W., Jr.; Holimon, J.L.; Orr, P.F. Stage I corpus cancer: Is teletherapy necessary? Am. J. Obstet. Gynecol. 1997, 176, 777–789. [Google Scholar] [CrossRef]
- Berclaz, G.; Hänggi, W.; Kratzer-Berger, A.; Altermatt, H.J.; Greiner, R.H.; Dreher, E. Lymphadenectomy in high risk endometrial carcinoma stage I and II: No more morbidity and no need for external pelvic radiation. Int. J. Gynecol. Cancer 1999, 9, 322–328. [Google Scholar] [CrossRef]
- Horowitz, N.S.; Peters, W.A., III; Smith, M.R.; Drescher, C.W.; Artwood, M.; Mate, T.P. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet. Gynecol. 2002, 99, 235–240. [Google Scholar]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Calori, G.; Podratz, K.C. Hematogenous dissemination in corpus cancer. Gynecol. Oncol. 2001, 80, 233–238. [Google Scholar] [CrossRef]
- Otsuka, I.; Ono, I.; Akamatsu, H.; Sunamori, K.; Aso, T. Pulmonary metastasis from endometrial carcinoma. Int. J. Gynecol. Cancer 2002, 12, 208–213. [Google Scholar] [CrossRef]
- Matsuo, K.; Yabuno, A.; Hom, M.S.; Shida, M.; Kakuda, M.; Adachi, S.; Mandelbaum, R.S.; Ueda, Y.; Hasegawa, K.; Enomoto, T.; et al. Significance of abnormal peritoneal cytology on survival of women with stage I–II endometrioid endometrial cancer. Gynecol. Oncol. 2018, 149, 301–309. [Google Scholar] [CrossRef]
- Brown, A.P.; Gaffney, D.K.; Dodson, M.K.; Soisson, A.P.; Belnap, T.W.; Alleman, K.; Sause, W.T. Survival analysis of endometrial cancer patients with positive lymph nodes. Int. J. Gynecol. Cancer 2013, 23, 861–868. [Google Scholar] [CrossRef]
- Otsuka, I.; Kadooka, M.; Matsuura, T. Long-term survival of a patient with stage IIIC2 grade 3 endometrioid endometrial carcinoma treated with surgery alone. Gynecol. Oncol. Rep. 2021, 38, 100869. [Google Scholar] [CrossRef]
- Goebel, E.A.; St Laurent, J.D.; Nucci, M.R.; Feltmate, C.M. Retrospective detection of isolated tumor cells by immunohistochemistry in sentinel lymph node biopsy performed for endometrial carcinoma: Is there clinical significance? Int. J. Gynecol. Cancer 2020, 30, 291–298. [Google Scholar] [CrossRef]
- Backes, F.J.; Felixb, A.S.; Plante, M.; Grégoire, J.; Sullivan, S.A.; Rossi, E.C.; Tanner, E.J., III; Stewart, K.I.; Soliman, P.T.; Holloway, R.W.; et al. Sentinel lymph node (SLN) isolated tumor cells (ITCs) in otherwise stage I/II endometrioid endometrial cancer: To treat or not to treat? Gynecol. Oncol. 2021, 161, 347–352. [Google Scholar] [CrossRef]
- Ghoniem, K.; Larisha, A.M.; Dinoi, G.; Zhou, X.C.; Alhilli, M.; Wallace, S.; Wohlmuth, C.; Baiocchi, G.; Tokgozoglu, N.; Raspagliesi, F.; et al. Oncologic outcomes of endometrial cancer in patients with low-volume metastasis in the sentinel lymph nodes: An international multi-institutional study. Gynecol. Oncol. 2021, 162, 590–598. [Google Scholar] [CrossRef]
- Plante, M.; Stanleigh, J.; Renaud, M.-C.; Sebastianelli, A.; Grondin, K.; Gregoire, J. Isolated tumor cells identified by sentinel lymph node mapping in endometrial cancer: Does adjuvant treatment matter? Gynecol. Oncol. 2017, 146, 240–246. [Google Scholar] [CrossRef]
- Ignatov, A.; Ivros, S.; Bozukova, T.; Papathemelis, T.; Ortmann, O.; Eggemann, H. Systematic lymphadenectomy in early stage endometrial cancer. Arch. Gynecol. Obstet. 2020, 302, 231–239. [Google Scholar] [CrossRef]
- Papathemelis, T.; Hassas, D.; Gerken, M.; Klinkhammer-Schalke, M.; Scharl, A.; Lux, M.P.; Beckmann, M.W.; Scharl, S. Is there a benefit of lymphadenectomy for overall and recurrence-free survival in type I FIGO IB G1-2 endometrial carcinoma? A retrospective population-based cohort analysis. J. Cancer Res. Clin. Oncol. 2018, 144, 2019–2027. [Google Scholar] [CrossRef]
- Gayar, O.H.; Robbins, J.R.; Parikh, K.; Lu, M.; Buekers, T.; Munkarah, A.; Elshaikh, M.A. Hysterectomy for uterine adenocarcinoma in the elderly: Tumor characteristics, and long-term outcome. Gynecol. Oncol. 2011, 123, 71–75. [Google Scholar] [CrossRef]
- Hachisuga, K.; Ohishi, Y.; Tomonobe, H.; Yahata, H.; Kato, K.; Oda, Y. Endometrial endometrioid carcinoma, G1, is more aggressive in the elderly than in the young. Histopathology 2021, 79, 708–719. [Google Scholar] [CrossRef]
- Pawelec, G. Does patient age influence anti-cancer immunity? Semin. Immunopathol. 2019, 41, 125–131. [Google Scholar] [CrossRef]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schalaerth, J.B.; Mannel, R.S.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J. Clin. Oncol. 2012, 30, 695–700. [Google Scholar] [CrossRef]
- Janda, M.V.; Gebski, L.C.; Davies, P.; Forder, A.; Brand, R.; Hogg, T.W.; Land, R.; Manolitsas, T.; Nascimento, M.; Neesham, D.; et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: A randomized clinical trial. JAMA 2017, 317, 1224–1233. [Google Scholar] [CrossRef]
- Wright, J.D. Laparoscopic Hysterectomy for Endometrial Cancer. A Procedure 25 Years in the Making. JAMA 2017, 317, 1215–1216. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Wang, J. Survival of microsatellite-stable endometrioid endometrial cancer patients after minimally invasive surgery: An analysis of the Cancer Genome Atlas data. Gynecol. Oncol. 2020, 158, 92–98. [Google Scholar] [CrossRef]
- Wright, J.D.; Burke, W.M.; Tergas, A.I.; Hou, J.Y.; Huang, Y.; Hu, J.C.; Hillyer, G.C.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Comparative Effectiveness of Minimally Invasive Hysterectomy for Endometrial Cancer. J. Clin. Oncol. 2016, 34, 1087–1096. [Google Scholar] [CrossRef]
- Chu, C.S.; Randall, T.C.; Bandera, C.A.; Rubin, S.C. Vaginal Cuff Recurrence of Endometrial Cancer Treated by Laparoscopic-Assisted Vaginal Hysterectomy. Gynecol. Oncol. 2003, 88, 62–65. [Google Scholar] [CrossRef]
- Kim, S.I.; Park, D.C.; Lee, S.J.; Song, M.J.; Kim, C.J.; Lee, H.N.; Yoon, J.H. Survival rates of patients who undergo minimally invasive surgery for endometrial cancer with cervical involvement. Int. J. Med. Sci. 2021, 18, 2204–2208. [Google Scholar] [CrossRef]
- Philp, I.; Tannenbaum, S.; Haber, H.; Saini, A.; St Laurent, J.; James, K.; Feltmate, C.; Russo, A.; Growdon, W. Effect of surgical approach on risk of recurrence after vaginal brachytherapy in early-stage high-intermediate risk endometrial cancer. Gynecol. Oncol. 2021, 160, 389–395. [Google Scholar] [CrossRef]
- Feigenberg, T.; Cormier, B.; Gotlieb, W.H.; Jegatheeswaran, K.; Helpman, L.; Kim, S.R.; Lau, S.; May, T.; Saab, D.; Plante, M.; et al. Factors associated with an increased risk of recurrence in patients diagnosed with high-grade endometrial cancer undergoing minimally invasive surgery: A study of the society of gynecologic oncology of Canada (GOC) community of practice (CoP). Gynecol. Oncol. 2021, 162, 606–612. [Google Scholar] [CrossRef]
- Song, J.; Le, T.; Hopkins, L.; Fung-Kee-Fung, M.; Lupe, K.; Gaudet, M.; Rajiv Samant, C.E. A comparison of disease recurrence between robotic versus laparotomy approach in patients with intermediate-risk endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 160–166. [Google Scholar] [CrossRef]
- Argenta, P.A.; Mattson, J.; Rivard, C.L.; Luther, E.; Schefter, A.; Vogel, R.I. Robot-assisted versus laparoscopic minimally invasive surgery for the treatment of stage I endometrial cancer. Gynecol. Oncol. 2022, 165, 347–352. [Google Scholar] [CrossRef]
- Chang, E.J.; Jooya, N.D.; Ciesielski, K.M.; Shahzad, M.M.; Roman, L.D.; Matsuo, K. Intraoperative tumor spill during minimally invasive hysterectomy for endometrial cancer: A survey study on experience and practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 256–261. [Google Scholar] [CrossRef]
- Nasioudis, D.; Ko, E.M.; Cory, L.; Latif, N. Impact of surgical approach on prevalence of positive peritoneal cytology and lymph-vascular invasion in patients with early-stage endometrial carcinoma: A National Cancer Database study. Int. J. Gynecol. Cancer 2021, 31, 1001–1006. [Google Scholar] [CrossRef]
- Stolnicu, S.; Terinte, C.; Ioanid, N.; Silva, E. Presence of tumor cells in the vagina during surgical treatment could be the source of vaginal recurrence in patients with endometrial carcinoma—A pilot prospective study. Ann. Diagn. Pathol. 2020, 46, 151503. [Google Scholar] [CrossRef]
- Otsuka, I. Cutaneous metastasis after surgery, injury, lymphadenopathy, and peritonitis: Possible mechanisms. Int. J. Mol. Sci. 2019, 20, 3286. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Wolf, J.K.; Levenback, C. Laparoscopic port-site metastases: Etiology and prevention. Gynecol. Oncol. 2003, 91, 179–189. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lope, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; del Carmen, M.G.; Yang, J.; Seagle, B.L.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef]
- Acar, O.; Esen, T.; Bavbek, S.; Pekerd, Ö.; Musaoglu, A. Port site and peritoneal metastases after robot-assisted radical prostatectomy. Int. J. Surg. Case Rep. 2014, 5, 131–134. [Google Scholar] [CrossRef]
- Jacquet, P.; Averbach, A.M.; Jacquet, N. Abdominal wall metastasis and peritoneal carcinomatosis after laparoscopic-assisted colectomy for colon cancer. Eur. J. Surg. Oncol. 1995, 21, 568–570. [Google Scholar] [CrossRef]
- Uccella, S.; Bonzini, M.; Malzoni, M.; Fanfani, F.; Palomba, S.; Aletti, G.; Corrado, G.; Ceccaroni, M.; Seracchioli, R.; Shakir, F.; et al. The effect of a uterine manipulator on the recurrence and mortality of endometrial cancer: A multi-centric study by the Italian Society of Gynecological Endoscopy. Am. J. Obstet. Gynecol. 2017, 216, 592.e1–592.e11. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Iserte, P.; Lago, V.; Tauste, C.; Diaz-Feijoo, B.; Gil-Moreno, A.; Oliver, R.; Coronado, P.; Salamanca, M.B.M.; Pantoja-Garrido, M.; Marcos-Sanmartin, J.; et al. Impact of uterine manipulator on oncological outcome in endometrial cancer surgery. Am. J. Obstet. Gynecol. 2021, 224, 65.e1–65.e11. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhao, J.M.; Luo, Z.C.; Zhang, P.; Chen, P.; Zhang, X.L.; Luo, S.; Yang, D.B.; Tan, J.; Zhou, Y.; et al. Diluted povidone-iodine inhibits tumor growth through apoptosis-induction and suppression of SOD activity. Oncol. Rep. 2012, 27, 383. [Google Scholar]
- Hah, J.H.; Roh, D.H.; Jung, Y.H.; Kim, K.H.; Sung, M.W. Selection of irrigation fluid to eradicate free cancer cells during head and neck cancer surgery. Head Neck 2012, 34, 546–550. [Google Scholar] [CrossRef]
- Bouwman, F.; Smits, A.; Lopes, A.; Das, N.; Pollard, A.; Massuger, L.; Bekkers, R.; Galaal, K. The impact of BMI on surgical complications and outcomes in endometrial cancer surgery—An institutional study and systematic review of the literature. Gynecol. Oncol. 2015, 139, 369–376. [Google Scholar] [CrossRef]
- Suidan, R.S.; He, W.; Sun, C.C.; Zhao, H.; Fleming, N.D.; Ramirez, P.T.; Soliman, P.T.; Westin, S.N.; Lu, K.H.; Giordano, S.H.; et al. Impact of body mass index and operative approach on surgical morbidity and costs in women with endometrial carcinoma and hyperplasia. Gynecol. Oncol. 2017, 145, 55–60. [Google Scholar] [CrossRef]
- Acharya, S.; Esthappan, J.; Badiyan, S.; DeWees, T.A.; Tanderup, K.; Schwarz, J.K.; Grigsby, P.W. Medically inoperable endometrial cancer in patients with a high body mass index (BMI): Patterns of failure after 3-D image-based high dose rate (HDR) brachytherapy. Radiother. Oncol. 2016, 118, 167–172. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Ko, E.M.; Walter, P.; Clark, L.; Jackson, A.; Franasiak, J.; Bolac, C.; Havrilesky, L.; Alvarez Secord, A.; Moore, D.T.; Gehrig, P.A.; et al. The complex triad of obesity, diabetes and race in Type I and II endometrial cancers: Prevalence and prognostic significance. Gynecol. Oncol. 2014, 133, 28–32. [Google Scholar] [CrossRef]
- Papatla, K.; Huang, M.; Slomovitz, B. The obese endometrial cancer patient: How do we effectively improve morbidity and mortality in this patient population? Ann. Oncol. 2016, 27, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Canlorbe, G.; Bendifallah, S.; Raimond, E.; Graesslin, O.; Hudry, D.; Coutant, C.; Touboul, C.; Bleu, G.; Collinet, P.; Darai, E.; et al. Severe Obesity Impacts Recurrence-Free Survival of Women with High-Risk Endometrial Cancer: Results of a French Multicenter Study. Ann. Surg. Oncol. 2015, 22, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, H.; Watari, H.; Todo, Y.; Kato, T.; Konno, Y.; Hosaka, M.; Sakuragi, N. Lymphadenectomy can be omitted for low-risk endometrial cancer based on preoperative assessments. J. Gynecol. Oncol. 2014, 25, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Kato, H.; Katayama, K.; Nakanishi, K.; Kawano, A.; Iura, A.; Konnai, K.; Onose, R.; Hirahara, F.; Miyagi, E. A preoperative risk-scoring system to predict lymph node metastasis in endometrial cancer and stratify patients for lymphadenectomy. Gynecol. Oncol. 2016, 142, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.C.M.; Reijnen, C.; Massuger, L.; Nagtegaal, I.D.; Bulten, J.; Pijnenborg, J.M.A. Accuracy of Endometrial Sampling in Endometrial Carcinoma: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2017, 130, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Gebb, J.S.; Einstein, M.H.; Shahabi, S.; Novetsky, A.P.; Goldberg, G.L. Accuracy of preoperative endometrial sampling for the detection of high-grade endometrial tumors. Am. J. Obstet. Gynecol. 2007, 196, 243.e1–243.e5. [Google Scholar] [CrossRef]
- Iwai, K.; Fukuda, K.; Hachisuga, T.; Mori, M.; Uchiyama, M.; Iwasaka, T.; Sugimori, H. Prognostic significance of progesterone receptor immunohistochemistry for lymph node metastases in endometrial carcinoma. Gynecol. Oncol. 1999, 72, 351–359. [Google Scholar] [CrossRef]
- Trovik, J.; Wik, E.; Werner, H.M.J.; Krakstad, C.; Helland, H.; Vandenput, I.; Njolstad, T.S.; Stefansson, I.M.; Marcickiewicz, J.; Tingulstad, S.; et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur. J. Cancer 2013, 49, 3431–3441. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar]
- Jamieson, A.; Huvila, J.; Thompson, E.F.; Leung, S.; Chiuc, D.; Lumc, A.; McConechy, M.; Grondin, K.; Aguirre-Hernandez, R.; Salvador, S.; et al. Variation in practice in endometrial cancer and potential for improved care and equity through molecular classification. Gynecol. Oncol. 2022, 165, 201–214. [Google Scholar] [CrossRef]
- Kolehmainen, A.M.; Pasanen, A.M.; Koivisto-Korander, R.L.; Bützow, R.C.; Loukovaara, M.J. Molecular characterization in the prediction of disease extent in endometrial carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Thompson, E.F.; Huvila, J.; Leung, S.; Lumb, A.; Morin, C.; Ennour-Idrissi, K.; Sebastianelli, A.; Renaud, M.C.; Gregoire, J.; et al. Endometrial carcinoma molecular subtype correlates with the presence of lymph node metastases. Gynecol. Oncol. 2022, 165, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, C.M.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Rush, C.M.; Lankes, H.A.; Creasman, W.T.; Miller, D.S.; Ramirez, N.C.; Geller, M.A.; et al. An NRG Oncology/GOG study of molecular classification for risk prediction in endometrioid endometrial cancer. Gynecol. Oncol. 2018, 148, 174–180. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; Horeweg, N.; Peters, E.E.M.; Rutten, T.; ter Haar, N.; Smit, V.T.H.B.M.; Kroon, C.D.; Boennelycke, M.; Hogdall, E.; Hogdall, C.; et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol. Oncol. 2022, 164, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; Nout, R.A.; McAlpine, J.N.; McConechy, M.K.; Britton, H.; Hussein, Y.R.; Gonzalez, C.; Ganesan, R.; Steele, J.C.; Harrison, B.T.; et al. Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am. J. Surg. Pathol. 2018, 42, 561–568. [Google Scholar] [CrossRef]
- Schultheis, A.M.; Martelotto, A.M.; De Filippo, M.R.; Piscuglio, S.; Ng, C.K.Y.; Hussein, Y.R.; Reis-Filho, J.S.; Soslow, R.A.; Weigelt, B. TP53 Mutational Spectrum in Endometrioid and Serous Endometrial Cancers. Int. J. Gynecol. Pathol. 2016, 35, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Leon-Castillo, A.; Gilvazquez, E.; Nout, R.; Smit, V.T.H.B.M.; McAlpine, J.N.; McConechy, M.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J. Pathol. 2020, 250, 312–322. [Google Scholar] [CrossRef]
- Guntupalli, S.R.; Zighelboim, I.; Kizer, N.T.; Zhang, Q.; Powell, M.A.; Thaker, P.H.; Goodfellow, P.J.; Mutch, D.G. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol. Oncol. 2012, 124, 31–35. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of PORTEC cohorts. Clin. Cancer. Res. 2006, 22, 4215–4224. [Google Scholar] [CrossRef]
- Billingsley, C.C.; Cohn, D.E.; Mutch, D.G.; Hade, E.M.; Goodfellow, P.J. Prognostic Significance of POLE Exonuclease Domain Mutations in High-Grade Endometrioid Endometrial Cancer on Survival and Recurrence. A Subanalysis. Int. J. Gynecol. Cancer 2016, 26, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, J.; Zhao, L.; Wang, Z.; Wang, J. Tumor Molecular Features Predict Endometrial Cancer Patients’ Survival after Open or Minimally Invasive Surgeries. Front. Oncol. 2021, 11, 634857. [Google Scholar] [CrossRef] [PubMed]
- Konno, Y.; Todo, Y.; Minobe, S.; Kato, H.; Okamoto, K.; Sudo, S.; Takeda, M.; Watar, I.H.; Kaneuchi, M.; Sakuragi, N. A Retrospective Analysis of Postoperative Complications with or Without Para-aortic Lymphadenectomy in Endometrial Cancer. Int. J. Gynecol. Cancer 2011, 21, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, F.; Uccella, S.; Cromi, A.; Bogani, G.; Robba, C.; Serati, M.; Bolis, P. Lymphoceles, lymphorrhea, and lymphedema after laparoscopic and open endometrial cancer staging. Ann. Surg. Oncol. 2012, 19, 259–267. [Google Scholar] [CrossRef]
- Menderes, G.; Azodi, M.; Schwartz, P.; Silasi, D.A. Comparison of Lymphedema Incidence Between 2 Lymphadenectomy Techniques in Patients With Uterine Cancer Undergoing Robotic Staging. Int. J. Gynecol. Cancer 2015, 25, 160–165. [Google Scholar] [CrossRef]
- Wedin, M.; Stalberg, K.; Marcickiewicz, J.; Ahlner, E.; Akesson, A.; Lindahl, G.; Kjolhede, P.; on behalf of the LASEC study group. Incidence of lymphedema in the lower limbs and lymphocyst formation within one year of surgery for endometrial cancer: A prospective longitudinal multicenter study. Gynecol. Oncol. 2020, 159, 201–208. [Google Scholar] [CrossRef]
- Tada, H.; Teramukai, S.; Fukushima, M.; Sasaki, H. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC Cancer 2009, 9, 47. [Google Scholar] [CrossRef]
- Mitra, D.; Catalano, P.J.; Cimbak, N.; Damato, A.L.; Muto, M.G.; Viswanathan, A.N. The risk of lymphedema after postoperative radiation therapy in endometrial cancer. J. Gynecol. Oncol. 2016, 27, e4. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, J.P.; Bryant, C.S.; Awonuga, A.O.; Imudia, A.N.; Ruterbusch, J.J.; Cote, M.L.; Ali-fehmi, R.; Morris, R.T.; Malone, J.M. Second neoplasms in survivors of endometrial cancer: Impact of radiation therapy. Gynecol. Oncol. 2009, 113, 233–239. [Google Scholar] [CrossRef]
- Todo, Y.; Yamazaki, H.; Takeshita, S.; Ohba, Y.; Sudo, S.; Minobe, S.; Okamoto, K.; Kato, H. Close relationship between removal of circumflex iliac nodes to distal external iliac nodes and postoperative lower-extremity lymphedema in uterine corpus malignant tumors. Gynecol. Oncol. 2015, 139, 160–164. [Google Scholar] [CrossRef]
- Milam, M.R.; Java, J.; Walker, J.L.; Metzinger, D.S.; Parker, L.P.; Coleman, R.L. Nodal metastasis risk in endometrioid endometrial cancer. Obstet. Gynecol. 2012, 119 2 Pt 1, 286–292. [Google Scholar] [CrossRef]
- AlHilli, M.M.; Podratz, K.C.; Dowdy, S.C.; Bakkum-Gamez, J.N.; Weaver, A.L.; McGree, M.E.; Kumar, S.; Keeney, G.L.; Cliby, W.A.; Mariani, A. Preoperative biopsy and intraoperative tumor diameter predict lymph node dissemination in endometrial cancer. Gynecol. Oncol. 2013, 128, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; Hou, J.Y.; Tergas, A.I.; Burke, W.M.; Huang, Y.; Hu, J.C.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Magnitude of risk for nodal metastasis associated with lymphvascular space invasion for endometrial cancer. Gynecol. Oncol. 2016, 140, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Stalberg, K.; Bjurberg, M.; Borgfeldt, C.; Carlson, J.; Dahm-Kähler, P.; Flöter-Rådestad, A.; Hellman, K.; Hjerpe, E.; Holmberg, E.; Kjølhede, P.; et al. Lymphovascular space invasion as a predictive factor for lymph node metastases and survival in endometrioid endometrial cancer—A Swedish Gynecologic Cancer Group (SweGCG) study. Acta Oncol. 2019, 58, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Matanes, E.; Eisenberg, N.; Lau, S.; Salvador, S.; Ferenczy, A.; Pelmus, M.; Gotlieb, W.H.; Kogan, L. Absence of prognostic value of lymphovascular space invasion in patients with endometrial cancer and negative sentinel lymph nodes. Gynecol. Oncol. 2021, 162, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.L. Adjuvant radiotherapy following properly staged endometrial cancer: What role? Gynecol. Oncol. 2004, 92, 737–739. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).