The Impact of Race–Ethnicity and Diagnosis of Alzheimer’s Disease and Related Dementias on Mammography Use
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
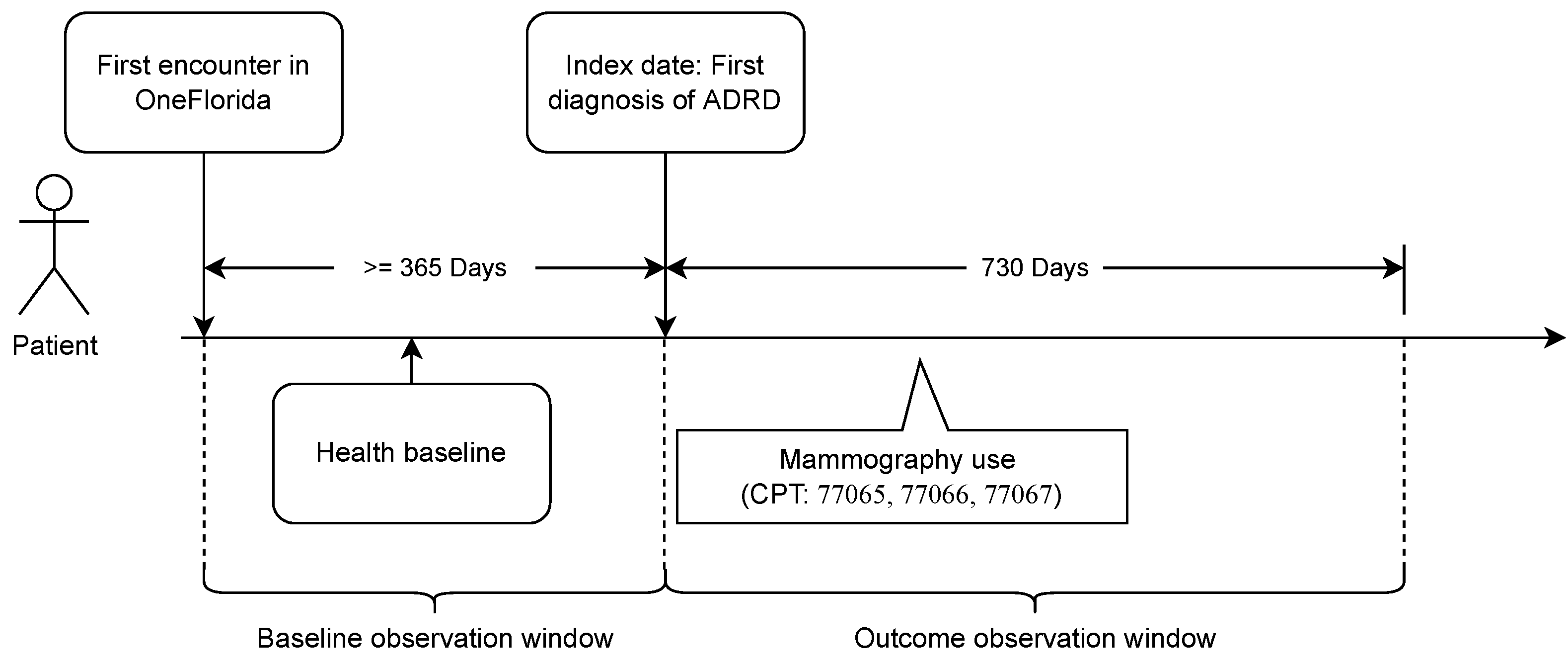
2.1. Data Source and Study Population
2.2. Study Outcome and Exposures
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Multivariable Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Disease | Code Type | Code | Concept | Used For |
|---|---|---|---|---|
| Alzheimer’s disease | ICD-9 | 331.0 | Alzheimer’s disease | Inclusion, exclusion |
| Alzheimer’s disease | ICD-10 | G30.0 | Alzheimer’s disease with early onset | Inclusion, exclusion |
| Alzheimer’s disease | ICD-10 | G30.1 | Alzheimer’s disease with late onset | Inclusion, exclusion |
| Alzheimer’s disease | ICD-10 | G30.8 | Other Alzheimer’s disease | Inclusion, exclusion |
| Alzheimer’s disease | ICD-10 | G30.9 | Alzheimer’s disease, unspecified | Inclusion, exclusion |
| Vascular dementia | ICD-9 | 290.40 | Vascular dementia, uncomplicated | Inclusion, exclusion |
| Vascular dementia | ICD-9 | 290.41 | Vascular dementia, with delirium | Inclusion, exclusion |
| Vascular dementia | ICD-9 | 290.42 | Vascular dementia, with delusions | Inclusion, exclusion |
| Vascular dementia | ICD-9 | 290.43 | Vascular dementia, with depressed mood | Inclusion, exclusion |
| Vascular dementia | ICD-10 | F01.50 | Vascular dementia without behavioral disturbance | Inclusion, exclusion |
| Vascular dementia | ICD-10 | F01.51 | Vascular dementia with behavioral disturbance | Inclusion, exclusion |
| Frontotemporal dementia | ICD-9 | 331.1 | Frontotemporal dementia | Inclusion, exclusion |
| Frontotemporal dementia | ICD-9 | 331.11 | Pick’s disease | Inclusion, exclusion |
| Frontotemporal dementia | ICD-9 | 331.19 | Other frontotemporal dementia | Inclusion, exclusion |
| Frontotemporal dementia | ICD-10 | G31.0 | Frontotemporal dementia | Inclusion, exclusion |
| Frontotemporal dementia | ICD-10 | G31.01 | Pick’s disease | Inclusion, exclusion |
| Frontotemporal dementia | ICD-10 | G31.09 | Other frontotemporal dementia | Inclusion, exclusion |
| Lewy body dementia | ICD-9 | 331.82 | Dementia with Lewy bodies | Inclusion, exclusion |
| Lewy body dementia | ICD-10 | G31.83 | Dementia with Lewy bodies | Inclusion, exclusion |
| Dementia | ICD-9 | 290.0 | Senile dementia, uncomplicated | Exclusion |
| Dementia | ICD-9 | 290.10 | Presenile dementia, uncomplicated | Exclusion |
| Dementia | ICD-9 | 290.11 | Presenile dementia with delirium | Exclusion |
| Dementia | ICD-9 | 290.12 | Presenile dementia with delusional features | Exclusion |
| Dementia | ICD-9 | 290.13 | Presenile dementia with depressive features | Exclusion |
| Dementia | ICD-9 | 290.20 | Senile dementia with delusional features | Exclusion |
| Dementia | ICD-9 | 290.21 | Senile dementia with depressive features | Exclusion |
| Dementia | ICD-9 | 290.3 | Senile dementia with delirium | Exclusion |
| Dementia | ICD-9 | 290.8 | Other specified senile psychotic conditions | Exclusion |
| Dementia | ICD-9 | 290.9 | Unspecified senile psychotic condition | Exclusion |
| Dementia | ICD-9 | 291.2 | Alcohol-induced persisting dementia | Exclusion |
| Dementia | ICD-9 | 292.82 | Drug-induced persisting dementia | Exclusion |
| Dementia | ICD-9 | 294.0 | Amnestic disorder in conditions classified elsewhere | Exclusion |
| Dementia | ICD-9 | 294.10 | Dementia in conditions classified elsewhere without behavioral disturbance | Exclusion |
| Dementia | ICD-9 | 294.11 | Dementia in conditions classified elsewhere with behavioral disturbance | Exclusion |
| Dementia | ICD-9 | 294.20 | Dementia, unspecified, without behavioral disturbance | Exclusion |
| Dementia | ICD-9 | 294.21 | Dementia, unspecified, with behavioral disturbance | Exclusion |
| Dementia | ICD-9 | 294.8 | Other persistent mental disorders due to conditions classified elsewhere | Exclusion |
| Dementia | ICD-9 | 294.9 | Unspecified persistent mental disorders due to conditions classified elsewhere | Inclusion, exclusion |
| Dementia | ICD-9 | 331.2 | Senile degeneration of brain | Exclusion |
| Dementia | ICD-9 | 331.3 | Communicating hydrocephalus | Exclusion |
| Dementia | ICD-9 | 331.4 | Obstructive hydrocephalus | Exclusion |
| Dementia | ICD-9 | 331.5 | Idiopathic normal pressure hydrocephalus (INPH) | Exclusion |
| Dementia | ICD-9 | 331.6 | Corticobasal degeneration | Exclusion |
| Dementia | ICD-9 | 331.7 | Cerebral degeneration in diseases classified elsewhere | Exclusion |
| Dementia | ICD-9 | 331.81 | Reye’s syndrome | Exclusion |
| Dementia | ICD-9 | 331.83 | Mild cognitive impairment, so stated | Inclusion, exclusion |
| Dementia | ICD-9 | 331.89 | Other cerebral degeneration | Exclusion |
| Dementia | ICD-9 | 437.0 | Cerebral atherosclerosis | Exclusion |
| Dementia | ICD-9 | 437.1 | Other generalized ischemic cerebrovascular disease | Exclusion |
| Dementia | ICD-9 | 437.2 | Hypertensive encephalopathy | Exclusion |
| Dementia | ICD-9 | 437.3 | Cerebral aneurysm, non-ruptured | Exclusion |
| Dementia | ICD-9 | 437.4 | Cerebral arteritis | Exclusion |
| Dementia | ICD-9 | 437.5 | Moyamoya disease | Exclusion |
| Dementia | ICD-9 | 437.6 | Nonpyogenic thrombosis of intracranial venous sinus | Exclusion |
| Dementia | ICD-9 | 437.7 | Transient global amnesia | Exclusion |
| Dementia | ICD-9 | 437.8 | Other ill-defined cerebrovascular disease | Exclusion |
| Dementia | ICD-9 | 437.9 | Unspecified cerebrovascular disease | Exclusion |
| Dementia | ICD-9 | 797 | Senility without mention of psychosis | Exclusion |
| Dementia | ICD-10 | F02.80 | Dementia in other diseases classified elsewhere without behavioral disturbance | Exclusion |
| Dementia | ICD-10 | F02.81 | Dementia in other diseases classified elsewhere with behavioral disturbance | Exclusion |
| Dementia | ICD-10 | F03.90 | Unspecified dementia without behavioral disturbance | Exclusion |
| Dementia | ICD-10 | F03.91 | Unspecified dementia with behavioral disturbance | Exclusion |
| Dementia | ICD-10 | F04 | Amnestic disorder due to known physiological condition | Exclusion |
| Dementia | ICD-10 | F05 | Delirium due to known physiological condition | Exclusion |
| Dementia | ICD-10 | F06.0 | Psychotic disorder with hallucinations due to known physiological condition | Exclusion |
| Dementia | ICD-10 | F06.1 | Catatonic disorder due to known physiological condition | Exclusion |
| Dementia | ICD-10 | F06.8 | Other specified mental disorders due to known physiological condition | Exclusion |
| Dementia | ICD-10 | F09 | Unspecified mental disorder due to known physiological condition | Inclusion, exclusion |
| Dementia | ICD-10 | F10.27 | Alcohol dependence with alcohol-induced persisting dementia | Exclusion |
| Dementia | ICD-10 | F10.97 | Alcohol use, unspecified with alcohol-induced persisting dementia | Exclusion |
| Dementia | ICD-10 | F13.27 | Sedative, hypnotic, or anxiolytic dependence with sedative, hypnotic or anxiolytic-induced persisting dementia | Exclusion |
| Dementia | ICD-10 | F13.97 | Sedative, hypnotic, or anxiolytic use, unspecified with sedative, hypnotic or anxiolytic-induced persisting dementia | Exclusion |
| Dementia | ICD-10 | F18.17 | Inhalant abuse with inhalant-induced dementia | Exclusion |
| Dementia | ICD-10 | F18.27 | Inhalant dependence with inhalant-induced dementia | Exclusion |
| Dementia | ICD-10 | F18.97 | Inhalant use, unspecified with inhalant-induced persisting dementia | Exclusion |
| Dementia | ICD-10 | F19.17 | Other psychoactive substance abuse with psychoactive substance-induced persisting dementia | Exclusion |
| Dementia | ICD-10 | F19.27 | Other psychoactive substance dependence with psychoactive substance-induced persisting dementia | Exclusion |
| Dementia | ICD-10 | F19.97 | Other psychoactive substance use, unspecified with psychoactive substance-induced persisting dementia | Exclusion |
| Dementia | ICD-10 | G13.8 | Systemic atrophy primarily affecting central nervous system in other diseases classified elsewhere | Exclusion |
| Dementia | ICD-10 | G31.1 | Senile degeneration of brain, not elsewhere classified | Exclusion |
| Dementia | ICD-10 | G31.2 | Degeneration of nervous system due to alcohol | Exclusion |
| Dementia | ICD-10 | G31.84 | Mild cognitive impairment, so stated | Inclusion, exclusion |
| Dementia | ICD-10 | G31.85 | Corticobasal degeneration | Exclusion |
| Dementia | ICD-10 | G31.89 | Other specified degenerative diseases of nervous system | Exclusion |
| Dementia | ICD-10 | G31.9 | Degenerative disease of nervous system, unspecified | Exclusion |
| Dementia | ICD-10 | G45.4 | Transient global amnesia | Exclusion |
| Dementia | ICD-10 | G91.0 | Communicating hydrocephalus | Exclusion |
| Dementia | ICD-10 | G91.1 | Obstructive hydrocephalus | Exclusion |
| Dementia | ICD-10 | G91.2 | (Idiopathic) normal pressure hydrocephalus | Exclusion |
| Dementia | ICD-10 | G93.7 | Reye’s syndrome | Exclusion |
| Dementia | ICD-10 | G94 | Other disorders of brain in diseases classified elsewhere | Exclusion |
| Dementia | ICD-10 | I67.1 | Cerebral aneurysm, non-ruptured | Exclusion |
| Dementia | ICD-10 | I67.2 | Cerebral atherosclerosis | Exclusion |
| Dementia | ICD-10 | I67.4 | Hypertensive encephalopathy | Exclusion |
| Dementia | ICD-10 | I67.5 | Moyamoya disease | Exclusion |
| Dementia | ICD-10 | I67.6 | Nonpyogenic thrombosis of intracranial venous system | Exclusion |
| Dementia | ICD-10 | I67.7 | Cerebral arteritis, not elsewhere classified | Exclusion |
| Dementia | ICD-10 | I67.81 | Acute cerebrovascular insufficiency | Exclusion |
| Dementia | ICD-10 | I67.82 | Cerebral ischemia | Exclusion |
| Dementia | ICD-10 | I67.89 | Other cerebrovascular disease | Exclusion |
| Dementia | ICD-10 | I67.9 | Cerebrovascular disease, unspecified | Exclusion |
| Dementia | ICD-10 | R41.81 | Age-related cognitive decline | Exclusion |
| Dementia | ICD-10 | R54 | Age-related physical debility | Exclusion |
| Conditions that cause dementia | ICD-9 | 332 | Parkinson’s disease | Exclusion |
| Conditions that cause dementia | ICD-9 | 332.0 | Paralysis agitans | Exclusion |
| Conditions that cause dementia | ICD-9 | 332.1 | Secondary Parkinsonism | Exclusion |
| Conditions that cause dementia | ICD-10 | G20 | Parkinson’s disease | Exclusion |
| Conditions that cause dementia | ICD-10 | G21 | Secondary Parkinsonism | Exclusion |
| Conditions that cause dementia | ICD-10 | G21.1 | Other drug-induced secondary parkinsonism | Exclusion |
| Conditions that cause dementia | ICD-10 | G21.11 | Neuroleptic induced parkinsonism | Exclusion |
| Conditions that cause dementia | ICD-10 | G21.19 | Other drug induced secondary parkinsonism | Exclusion |
| Conditions that cause dementia | ICD-10 | G21.2 | Secondary parkinsonism due to other external agents | Exclusion |
| Conditions that cause dementia | ICD-10 | G21.3 | Postencephalitic parkinsonism | Exclusion |
| Conditions that cause dementia | ICD-10 | G21.4 | Vascular parkinsonism | Exclusion |
| Conditions that cause dementia | ICD-10 | G21.8 | Other secondary parkinsonism | Exclusion |
| Conditions that cause dementia | ICD-10 | G21.9 | Secondary parkinsonism, unspecified | Exclusion |
| Conditions that cause dementia | ICD-9 | 348.81 | Temporal sclerosis | Exclusion |
| Conditions that cause dementia | ICD-10 | G93.81 | Temporal sclerosis | Exclusion |
| Disease | Code Type | Code |
|---|---|---|
| Acquired immunodeficiency syndrome | ICD-9 | 42, 79571 |
| Acquired immunodeficiency syndrome | ICD-10 | V08, B20 |
| Any malignancy, includes leukemia and lympyoma | ICD-9 | 140, 140.0, 140.1, 140.3, 140.4, 140.5, 140.6, 140.8, 140.9, 141.0, 141.1, 141.2, 141.3, 141.4, 141.5, 141.6, 141.8, 141.9, 142, 142.0, 142.1, 142.2, 142.8, 142.9, 143, 143, 143.1, 143.8, 143.9, 144, 144, 144.1, 144.8, 144.9, 145, 145, 145.1, 145.2, 145.3, 145.4, 145.5, 145.6, 145.8, 145.9, 146, 146, 146.1, 146.2, 146.3, 146.4, 146.5, 146.6, 146.7, 146.8, 146.9, 147, 147.0, 147.1, 147.2, 147.3, 147.8, 147.9, 148, 148, 148.1, 148.2, 148.3, 148.8, 148.9, 149, 149, 149.1, 149.8, 149.9, 150, 150.0, 150.1, 150.2, 150.3, 150.4, 150.5, 150.8, 150.9, 151, 151, 151.1, 151.2, 151.3, 151.4, 151.5, 151.6, 151.8, 151.9, 152, 152.0, 152.1, 152.2, 152.3, 152.8, 152.9, 153, 153, 153.1, 153.2, 153.3, 153.4, 153.5, 153.6, 153.7, 153.8, 153.9, 154, 154.0, 154.1, 154.2, 154.3, 154.8, 155, 155, 155.1, 155.2, 156, 156, 156.1, 156.2, 156.8, 156.9, 157, 157.0, 157.1, 157.2, 157.3, 157.4, 157.8, 157.9, 158, 158, 158.8, 158.9, 159, 159, 159.1, 159.8, 159.9, 160, 160.0, 160.1, 160.2, 160.3, 160.4, 160.5, 160.8, 160.9, 161, 161.0, 161.1, 161.2, 161.3, 161.8, 161.9, 162, 162, 162.2, 162.3, 162.4, 162.5, 162.8, 162.9, 163, 163, 163.1, 163.8, 163.9, 164, 164, 164.1, 164.2, 164.3, 164.8, 164.9, 165, 165, 165.8, 165.9, 170, 170, 170.1, 170.2, 170.3, 170.4, 170.5, 170.6, 170.7, 170.8, 170.9, 171, 171, 171.2, 171.3, 171.4, 171.5, 171.6, 171.7, 171.8, 171.9, 172, 172, 172.1, 172.2, 172.3, 172.4, 172.5, 172.6, 172.7, 172.8, 172.9, 174, 174, 174.1, 174.2, 174.3, 174.4, 174.5, 174.6, 174.8, 174.9, 175, 175, 175.9, 176, 176, 176.1, 176.2, 176.3, 176.4, 176.5, 176.8, 176.9, 179, 180, 180, 180.1, 180.8, 180.9, 181, 182, 182, 182.1, 182.8, 183, 183, 183.2, 183.3, 183.4, 183.5, 183.8, 183.9, 184, 184, 184.1, 184.2, 184.3, 184.4, 184.8, 184.9, 185, 186, 186, 186.9, 187, 187.1, 187.2, 187.3, 187.4, 187.5, 187.6, 187.7, 187.8, 187.9, 188, 188, 188.1, 188.2, 188.3, 188.4, 188.5, 188.6, 188.7, 188.8, 188.9, 189, 189, 189.1, 189.2, 189.3, 189.4, 189.8, 189.9, 190, 190, 190.1, 190.2, 190.3, 190.4, 190.5, 190.6, 190.7, 190.8, 190.9, 191, 191, 191.1, 191.2, 191.3, 191.4, 191.5, 191.6, 191.7, 191.8, 191.9, 192, 192, 192.1, 192.2, 192.3, 192.8, 192.9, 193, 194, 194, 194.1, 194.3, 194.4, 194.5, 194.6, 194.8, 194.9, 195, 195, 195.1, 195.2, 195.3, 195.4, 195.5, 195.8, 200, 200, 200, 200.01, 200.02, 200.03, 200.04, 200.05, 200.06, 200.07, 200.08, 200.1, 200.1, 200.11, 200.12, 200.13, 200.14, 200.15, 200.16, 200.17, 200.18, 200.2, 200.2, 200.21, 200.22, 200.23, 200.24, 200.25, 200.26, 200.27, 200.28, 200.3, 200.3, 200.31, 200.32, 200.33, 200.34, 200.35, 200.36, 200.37, 200.38, 200.4, 200.4, 200.41, 200.42, 200.43, 200.44, 200.45, 200.46, 200.47, 200.48, 200.5, 200.5, 200.51, 200.52, 200.53, 200.54, 200.55, 200.56, 200.57, 200.58, 200.6, 200.6, 200.61, 200.62, 200.63, 200.64, 200.65, 200.66, 200.67, 200.68, 200.7, 200.7, 200.71, 200.72, 200.73, 200.74, 200.75, 200.76, 200.77, 200.78, 200.8, 200.8, 200.81, 200.82, 200.83, 200.84, 200.85, 200.86, 200.87, 200.88, 201, 201, 201, 201.01, 201.02, 201.03, 201.04, 201.05, 201.06, 201.07, 201.08, 201.1, 201.1, 201.11, 201.12, 201.13, 201.14, 201.15, 201.16, 201.17, 201.18, 201.2, 201.2, 201.21, 201.22, 201.23, 201.24, 201.25, 201.26, 201.27, 201.28, 201.4, 201.4, 201.41, 201.42, 201.43, 201.44, 201.45, 201.46, 201.47, 201.48, 201.5, 201.5, 201.51, 201.52, 201.53, 201.54, 201.55, 201.56, 201.57, 201.58, 201.6, 201.6, 201.61, 201.62, 201.63, 201.64, 201.65, 201.66, 201.67, 201.68, 201.7, 201.7, 201.71, 201.72, 201.73, 201.74, 201.75, 201.76, 201.77, 201.78, 201.9, 201.9, 201.91, 201.92, 201.93, 201.94, 201.95, 201.96, 201.97, 201.98, 202, 202, 202, 202.01, 202.02, 202.03, 202.04, 202.05, 202.06, 202.07, 202.08, 202.1, 202.1, 202.11, 202.12, 202.13, 202.14, 202.15, 202.16, 202.17, 202.18, 202.2, 202.2, 202.21, 202.22, 202.23, 202.24, 202.25, 202.26, 202.27, 202.28, 202.3, 202.3, 202.31, 202.32, 202.33, 202.34, 202.35, 202.36, 202.37, 202.38, 202.4, 202.4, 202.41, 202.42, 202.43, 202.44, 202.45, 202.46, 202.47, 202.48, 202.5, 202.5, 202.51, 202.52, 202.53, 202.54, 202.55, 202.56, 202.57, 202.58, 202.6, 202.6, 202.61, 202.62, 202.63, 202.64, 202.65, 202.66, 202.67, 202.68, 202.7, 202.7, 202.71, 202.72, 202.73, 202.74, 202.75, 202.76, 202.77, 202.78, 202.8, 202.8, 202.81, 202.82, 202.83, 202.84, 202.85, 202.86, 202.87, 202.88, 202.9, 202.9, 202.91, 202.92, 202.93, 202.94, 202.95, 202.96, 202.97, 202.98, 203, 203, 203, 203.01, 203.02, 203.1, 203.1, 203.11, 203.12, 203.8, 203.8, 203.81, 203.82, 204, 204, 204, 204.01, 204.02, 204.1, 204.1, 204.11, 204.12, 204.2, 204.2, 204.21, 204.22, 204.8, 204.8, 204.81, 204.82, 204.9, 204.9, 204.91, 204.92, 205, 205, 205, 205.01, 205.02, 205.1, 205.1, 205.11, 205.12, 205.2, 205.2, 205.21, 205.22, 205.3, 205.3, 205.31, 205.32, 205.8, 205.8, 205.81, 205.82, 205.9, 205.9, 205.91, 205.92, 206, 206, 206, 206.01, 206.02, 206.1, 206.1, 206.11, 206.12, 206.2, 206.2, 206.21, 206.22, 206.8, 206.8, 206.81, 206.82, 206.9, 206.9, 206.91, 206.92, 207, 207, 207, 207.01, 207.02, 207.1, 207.1, 207.11, 207.12, 207.2, 207.2, 207.21, 207.22, 207.8, 207.8, 207.81, 207.82, 208, 208, 208, 208.01, 208.02, 208.1, 208.1, 208.11, 208.12, 208.2, 208.2, 208.21, 208.22, 208.8, 208.8, 208.81, 208.82, 208.9, 208.9, 208.91, 208.92 |
| Any malignancy, includes leukemia and lympyoma | ICD-10 | C00, C00.0, C00.1, C00.2, C00.3, C00.4, C00.5, C00.6, C00.8, C00.9, C01, C02, C02.0, C02.1, C02.2, C02.3, C02.4, C02.8, C02.9, C03, C03.0, C03.1, C03.9, C04, C04.0, C04.1, C04.8, C04.9, C05, C05.0, C05.1, C05.2, C05.8, C05.9, C06, C06.0, C06.1, C06.2, C06.8, C06.80, C06.89, C06.9, C07, C08, C08.0, C08.1, C08.9, C09, C09.0, C09.1, C09.8, C09.9, C10, C10.0, C10.1, C10.2, C10.3, C10.4, C10.8, C10.9, C11, C11.0, C11.1, C11.2, C11.3, C11.8, C11.9, C12, C13, C13.0, C13.1, C13.2, C13.8, C13.9, C14, C14.0, C14.2, C14.8, C15, C15.3, C15.4, C15.5, C15.8, C15.9, C16, C16.0, C16.1, C16.2, C16.3, C16.4, C16.5, C16.6, C16.8, C16.9, C17, C17.0, C17.1, C17.2, C17.3, C17.8, C17.9, C18, C18.0, C18.1, C18.2, C18.3, C18.4, C18.5, C18.6, C18.7, C18.8, C18.9, C19, C20, C21, C21.0, C21.1, C21.2, C21.8, C22, C22.0, C22.1, C22.2, C22.3, C22.4, C22.7, C22.8, C22.9, C23, C24, C24.0, C24.1, C24.8, C24.9, C25, C25.0, C25.1, C25.2, C25.3, C25.4, C25.7, C25.8, C25.9, C26, C26.0, C26.1, C26.9, C30, C30.0, C30.1, C31, C31.0, C31.1, C31.2, C31.3, C31.8, C31.9, C32, C32.0, C32.1, C32.2, C32.3, C32.8, C32.9, C33, C34, C34.0, C34.00, C34.01, C34.02, C34.1, C34.10, C34.11, C34.12, C34.2, C34.3, C34.30, C34.31, C34.32, C34.8, C34.80, C34.81, C34.82, C34.9, C34.90, C34.91, C34.92, C37, C38, C38.0, C38.1, C38.2, C38.3, C38.4, C38.8, C39, C39.0, C39.9, C40, C40.0, C40.00, C40.01, C40.02, C40.1, C40.10, C40.11, C40.12, C40.2, C40.20, C40.21, C40.22, C40.3, C40.30, C40.31, C40.32, C40.8, C40.80, C40.81, C40.82, C40.9, C40.90, C40.91, C40.92, C43, C43.0, C43.1, C43.10, C43.11, C43.111, C43.112, C43.12, C43.121, C43.122, C43.2, C43.20, C43.21, C43.22, C43.3, C43.30, C43.31, C43.39, C43.4, C43.5, C43.51, C43.52, C43.59, C43.6, C43.60, C43.61, C43.62, C43.7, C43.70, C43.71, C43.72, C43.8, C43.9, C45, C45.0, C45.1, C45.2, C45.7, C45.9, C46, C46.0, C46.1, C46.2, C46.3, C46.4, C46.5, C46.50, C46.51, C46.52, C46.7, C46.9, C47, C47.0, C47.1, C47.10, C47.11, C47.12, C47.2, C47.20, C47.21, C47.22, C47.3, C47.4, C47.5, C47.6, C47.8, C47.9, C48, C48.0, C48.1, C48.2, C48.8, C49, C49.0, C49.1, C49.10, C49.11, C49.12, C49.2, C49.20, C49.21, C49.22, C49.3, C49.4, C49.5, C49.6, C49.8, C49.9, C49.A, C49.A0, C49.A1, C49.A2, C49.A3, C49.A4, C49.A5, C49.A9, C50, C50.0, C50.01, C50.011, C50.012, C50.019, C50.02, C50.021, C50.022, C50.029, C50.1, C50.11, C50.111, C50.112, C50.119, C50.12, C50.121, C50.122, C50.129, C50.2, C50.21, C50.211, C50.212, C50.219, C50.22, C50.221, C50.222, C50.229, C50.3, C50.31, C50.311, C50.312, C50.319, C50.32, C50.321, C50.322, C50.329, C50.4, C50.41, C50.411, C50.412, C50.419, C50.42, C50.421, C50.422, C50.429, C50.5, C50.51, C50.511, C50.512, C50.519, C50.52, C50.521, C50.522, C50.529, C50.6, C50.61, C50.611, C50.612, C50.619, C50.62, C50.621, C50.622, C50.629, C50.8, C50.81, C50.811, C50.812, C50.819, C50.82, C50.821, C50.822, C50.829, C50.9, C50.91, C50.911, C50.912, C50.919, C50.92, C50.921, C50.922, C50.929, C51, C51.0, C51.1, C51.2, C51.8, C51.9, C52, C53, C53.0, C53.1, C53.8, C53.9, C54, C54.0, C54.1, C54.2, C54.3, C54.8, C54.9, C55, C56, C56.1, C56.2, C56.9, C57, C57.0, C57.00, C57.01, C57.02, C57.1, C57.10, C57.11, C57.12, C57.2, C57.20, C57.21, C57.22, C57.3, C57.4, C57.7, C57.8, C57.9, C58, C60, C60.0, C60.1, C60.2, C60.8, C60.9, C61, C62, C62.0, C62.00, C62.01, C62.02, C62.1, C62.10, C62.11, C62.12, C62.9, C62.90, C62.91, C62.92, C63, C63.0, C63.00, C63.01, C63.02, C63.1, C63.10, C63.11, C63.12, C63.2, C63.7, C63.8, C63.9, C64, C64.1, C64.2, C64.9, C65, C65.1, C65.2, C65.9, C66, C66.1, C66.2, C66.9, C67, C67.0, C67.1, C67.2, C67.3, C67.4, C67.5, C67.6, C67.7, C67.8, C67.9, C68, C68.0, C68.1, C68.8, C68.9, C69, C69.0, C69.00, C69.01, C69.02, C69.1, C69.10, C69.11, C69.12, C69.2, C69.20, C69.21, C69.22, C69.3, C69.30, C69.31, C69.32, C69.4, C69.40, C69.41, C69.42, C69.5, C69.50, C69.51, C69.52, C69.6, C69.60, C69.61, C69.62, C69.8, C69.80, C69.81, C69.82, C69.9, C69.90, C69.91, C69.92, C70, C70.0, C70.1, C70.9, C71, C71.0, C71.1, C71.2, C71.3, C71.4, C71.5, C71.6, C71.7, C71.8, C71.9, C72, C72.0, C72.1, C72.2, C72.20, C72.21, C72.22, C72.3, C72.30, C72.31, C72.32, C72.4, C72.40, C72.41, C72.42, C72.5, C72.50, C72.59, C72.9, C73, C74, C74.0, C74.00, C74.01, C74.02, C74.1, C74.10, C74.11, C74.12, C74.9, C74.90, C74.91, C74.92, C75, C75.0, C75.1, C75.2, C75.3, C75.4, C75.5, C75.8, C75.9, C81, C81.0, C81.00, C81.01, C81.02, C81.03, C81.04, C81.05, C81.06, C81.07, C81.08, C81.09, C81.1, C81.10, C81.11, C81.12, C81.13, C81.14, C81.15, C81.16, C81.17, C81.18, C81.19, C81.2, C81.20, C81.21, C81.22, C81.23, C81.24, C81.25, C81.26, C81.27, C81.28, C81.29, C81.3, C81.30, C81.31, C81.32, C81.33, C81.34, C81.35, C81.36, C81.37, C81.38, C81.39, C81.4, C81.40, C81.41, C81.42, C81.43, C81.44, C81.45, C81.46, C81.47, C81.48, C81.49, C81.7, C81.70, C81.71, C81.72, C81.73, C81.74, C81.75, C81.76, C81.77, C81.78, C81.79, C81.9, C81.90, C81.91, C81.92, C81.93, C81.94, C81.95, C81.96, C81.97, C81.98, C81.99, C82, C82.0, C82.00, C82.01, C82.02, C82.03, C82.04, C82.05, C82.06, C82.07, C82.08, C82.09, C82.1, C82.10, C82.11, C82.12, C82.13, C82.14, C82.15, C82.16, C82.17, C82.18, C82.19, C82.2, C82.20, C82.21, C82.22, C82.23, C82.24, C82.25, C82.26, C82.27, C82.28, C82.29, C82.3, C82.30, C82.31, C82.32, C82.33, C82.34, C82.35, C82.36, C82.37, C82.38, C82.39, C82.4, C82.40, C82.41, C82.42, C82.43, C82.44, C82.45, C82.46, C82.47, C82.48, C82.49, C82.5, C82.50, C82.51, C82.52, C82.53, C82.54, C82.55, C82.56, C82.57, C82.58, C82.59, C82.6, C82.60, C82.61, C82.62, C82.63, C82.64, C82.65, C82.66, C82.67, C82.68, C82.69, C82.8, C82.80, C82.81, C82.82, C82.83, C82.84, C82.85, C82.86, C82.87, C82.88, C82.89, C82.9, C82.90, C82.91, C82.92, C82.93, C82.94, C82.95, C82.96, C82.97, C82.98, C82.99, C83, C83.0, C83.00, C83.01, C83.02, C83.03, C83.04, C83.05, C83.06, C83.07, C83.08, C83.09, C83.1, C83.10, C83.11, C83.12, C83.13, C83.14, C83.15, C83.16, C83.17, C83.18, C83.19, C83.3, C83.30, C83.31, C83.32, C83.33, C83.34, C83.35, C83.36, C83.37, C83.38, C83.39, C83.5, C83.50, C83.51, C83.52, C83.53, C83.54, C83.55, C83.56, C83.57, C83.58, C83.59, C83.7, C83.70, C83.71, C83.72, C83.73, C83.74, C83.75, C83.76, C83.77, C83.78, C83.79, C83.8, C83.80, C83.81, C83.82, C83.83, C83.84, C83.85, C83.86, C83.87, C83.88, C83.89, C83.9, C83.90, C83.91, C83.92, C83.93, C83.94, C83.95, C83.96, C83.97, C83.98, C83.99, C84, C84.0, C84.00, C84.01, C84.02, C84.03, C84.04, C84.05, C84.06, C84.07, C84.08, C84.09, C84.1, C84.10, C84.11, C84.12, C84.13, C84.14, C84.15, C84.16, C84.17, C84.18, C84.19, C84.4, C84.40, C84.41, C84.42, C84.43, C84.44, C84.45, C84.46, C84.47, C84.48, C84.49, C84.6, C84.60, C84.61, C84.62, C84.63, C84.64, C84.65, C84.66, C84.67, C84.68, C84.69, C84.7, C84.70, C84.71, C84.72, C84.73, C84.74, C84.75, C84.76, C84.77, C84.78, C84.79, C84.A, C84.A0, C84.A1, C84.A2, C84.A3, C84.A4, C84.A5, C84.A6, C84.A7, C84.A8, C84.A9, C84.Z, C84.Z0, C84.Z1, C84.Z2, C84.Z3, C84.Z4, C84.Z5, C84.Z6, C84.Z7, C84.Z8, C84.Z9, C84.9, C84.90, C84.91, C84.92, C84.93, C84.94, C84.95, C84.96, C84.97, C84.98, C84.99, C85, C85.1, C85.10, C85.11, C85.12, C85.13, C85.14, C85.15, C85.16, C85.17, C85.18, C85.19, C85.2, C85.20, C85.21, C85.22, C85.23, C85.24, C85.25, C85.26, C85.27, C85.28, C85.29, C85.8, C85.80, C85.81, C85.82, C85.83, C85.84, C85.85, C85.86, C85.87, C85.88, C85.89, C85.9, C85.90, C85.91, C85.92, C85.93, C85.94, C85.95, C85.96, C85.97, C85.98, C85.99, C88, C88.0, C88.2, C88.3, C88.4, C88.8, C88.9, C90, C90.0, C90.00, C90.01, C90.02, C90.1, C90.10, C90.11, C90.12, C90.2, C90.20, C90.21, C90.22, C90.3, C90.30, C90.31, C90.32, C91, C91.0, C91.00, C91.01, C91.02, C91.1, C91.10, C91.11, C91.12, C91.3, C91.30, C91.31, C91.32, C91.4, C91.40, C91.41, C91.42, C91.5, C91.50, C91.51, C91.52, C91.6, C91.60, C91.61, C91.62, C91.A, C91.A0, C91.A1, C91.A2, C91.Z, C91.Z0, C91.Z1, C91.Z2, C91.9, C91.90, C91.91, C91.92, C92, C92.0, C92.00, C92.01, C92.02, C92.1, C92.10, C92.11, C92.12, C92.2, C92.20, C92.21, C92.22, C92.3, C92.30, C92.31, C92.32, C92.4, C92.40, C92.41, C92.42, C92.5, C92.50, C92.51, C92.52, C92.6, C92.60, C92.61, C92.62, C92.A, C92.A0, C92.A1, C92.A2, C92.Z, C92.Z0, C92.Z1, C92.Z2, C92.9, C92.90, C92.91, C92.92, C93, C93.0, C93.00, C93.01, C93.02, C93.1, C93.10, C93.11, C93.12, C93.3, C93.30, C93.31, C93.32, C93.Z, C93.Z0, C93.Z1, C93.Z2, C93.9, C93.90, C93.91, C93.92, C94, C94.0, C94.00, C94.01, C94.02, C94.2, C94.20, C94.21, C94.22, C94.3, C94.30, C94.31, C94.32, C94.4, C94.40, C94.41, C94.42, C94.6, C94.8, C94.80, C94.81, C94.82, C95, C95.0, C95.00, C95.01, C95.02, C95.1, C95.10, C95.11, C95.12, C95.9, C95.90, C95.91, C95.92, C96, C96.0, C96.2, C96.20, C96.21, C96.22, C96.29, C96.4, C96.5, C96.6, C96.A, C96.Z, C96.9 |
| Cerebrovascular Disease | ICD-9 | 430, 431, 432, 432.0, 432.1, 432.9, 433, 433.0, 433.00, 433.01, 433.1, 433.1, 433.11, 433.2, 433.2, 433.21, 433.3, 433.3, 433.31, 433.8, 433.8, 433.81, 433.9, 433.9, 433.91, 434, 434, 434, 434.01, 434.1, 434.1, 434.11, 434.9, 434.9, 434.91, 435, 435, 435.1, 435.2, 435.3, 435.8, 435.9, 436, 437, 437, 437.1, 437.2, 437.3, 437.4, 437.5, 437.6, 437.7, 437.8, 437.9, 438, 438, 438.1, 438.1, 438.11, 438.12, 438.13, 438.14, 438.19, 438.2, 438.2, 438.21, 438.22, 438.3, 438.3, 438.31, 438.32, 438.4, 438.4, 438.41, 438.42, 438.5, 438.5, 438.51, 438.52, 438.53, 438.6, 438.7, 438.8, 438.81, 438.82, 438.83, 438.84, 438.85, 438.89, 438.9 |
| Cerebrovascular Disease | ICD-10 | I60, I60.0, I60.00, I60.01, I60.02, I60.1, I60.10, I60.11, I60.12, I60.2, I60.3, I60.30, I61, I61.0, I61.1, I61.2, I61.3, I61.4, I61.5, I61.6, I61.8, I61.9, I62, I62.0, I62.00, I62.01, I62.02, I62.03, I62.1, I62.9, I63, I63.0, I63.00, I63.01, I63.011, I63.012, I63.013, I63.019, I63.02, I63.03, I63.031, I63.032, I63.033, I63.039, I63.09, I63.1, I63.10, I63.11, I63.111, I63.112, I63.113, I63.119, I63.12, I63.13, I63.131, I63.132, I63.133, I63.139, I63.19, I63.2, I63.20, I63.21, I63.211, I63.212, I63.213, I63.219, I63.22, I63.23, I63.231, I63.232, I63.233, I63.239, I63.29, I63.3, I63.30, I63.31, I63.311, I63.312, I63.313, I63.319, I63.32, I63.321, I63.322, I63.323, I63.329, I63.33, I63.331, I63.332, I63.333, I63.339, I63.34, I63.341, I63.342, I63.343, I63.349, I63.39, I63.4, I63.40, I63.41, I63.411, I63.412, I63.413, I63.419, I63.42, I63.421, I63.422, I63.423, I63.429, I63.43, I63.431, I63.432, I63.433, I63.439, I63.44, I63.441, I63.442, I63.443, I63.449, I63.49, I63.5, I63.50, I63.51, I63.511, I63.512, I63.513, I63.519, I63.52, I63.521, I63.522, I63.523, I63.529, I63.53, I63.531, I63.532, I63.533, I63.539, I63.54, I63.541, I63.542, I63.543, I63.549, I63.59, I63.6, I63.8, I63.81, I63.89, I63.9, I65, I65.0, I65.01, I65.02, I65.03, I65.09, I65.1, I65.2, I65.21, I65.22, I65.23, I65.29, I65.8, I65.9, I66, I66.0, I66.01, I66.02, I66.03, I66.09, I66.1, I66.11, I66.12, I66.13, I66.19, I66.2, I66.21, I66.22, I66.23, I66.29, I66.3, I66.8, I66.9, I67, I67.0, I67.1, I67.2, I67.3, I67.4, I67.5, I67.6, I67.7, I67.8, I67.81, I67.82, I67.83, I67.84, I67.841, I67.848, I67.85, I67.850, I67.858, I67.89, I67.9, I68, I68.0, I68.2, I68.8 |
| Chronic Pulmonary Disease | ICD-9 | 416.8, 416.9, 506.4, 519.1, 519.11, 519.19, 490, 491, 491, 491.1, 491.2, 491.2, 491.21, 491.22, 491.8, 491.9, 492, 4920, 492.8, 493, 493, 493, 493.01, 493.02, 493.1, 493.1, 493.11, 493.12, 493.2, 493.2, 493.21, 493.22, 493.8, 493.81, 493.82, 493.9, 493.9, 493.91, 493.92, 494, 494, 494.1, 495, 495, 495.1, 495.2, 495.3, 495.4, 495.5, 495.6, 495.7, 495.8, 495.9, 496, 500, 501, 502, 503, 504, 505 |
| Chronic Pulmonary Disease | ICD-10 | I27.8, I27.9, J40, J41, J41.0, J41.1, J41.8, J42, J43, J43.0, J43.1, J43.2, J43.8, J43.9, J44, J44.0, J44.1, J44.9, J45, J45.2, J45.20, J45.21, J45.22, J45.3, J45.30, J45.31, J45.32, J45.4, J45.40, J45.41, J45.42, J45.5, J45.50, J45.51, J45.52, J45.9, J45.90, J45.901, J45.902, J45.909, J45.99, J45.990, J45.991, J45.998, J47, J47.0, J47.1, J47.9, J60, J61, J62, J62.0, J62.8, J63, J63.0, J63.1, J63.2, J63.3, J63.4, J63.5, J63.6, J64, J65, J66, J66.0, J66.1, J66.2, J66.8, J67, J67.0, J67.1, J67.2, J67.3, J67.4, J67.5, J67.6, J67.7, J67.8, J67.9, J68.4 |
| Congestive Heart Failure | ICD-9 | 398.91, 425.4, 425.5, 425.7, 425.8, 425.9, 428, 428, 428.1, 428.2, 428.2, 428.21, 428.22, 428.23, 428.3, 428.3, 428.31, 428.32, 428.33, 428.4, 428.4, 428.41, 428.42, 428.43, 428.9 |
| Congestive Heart Failure | ICD-10 | I09.81, I42.5, I42.6, I42.7, I42.8, I42.9, I43, I50, I50.1, I50.2, I50.20, I50.21, I50.22, I50.23, I50.3, I50.30, I50.31, I50.32, I50.33, I50.4, I50.40, I50.41, I50.42, I50.43, I50.8, I50.81, I50.810, I50.811, I50.812, I50.813, I50.814, I50.82, I50.83, I50.84, I50.89, I50.9 |
| Dementia | ICD-9 | 290, 290.0, 290.1, 290.1, 290.11, 290.12, 290.13, 290.2, 290.2, 290.21, 290.3, 290.4, 290.4, 290.41, 290.42, 290.43, 290.8, 290.9, 291, 291.1, 291.2, 292.82, 294.1, 294.1, 294.11, 331, 331.1, 331.11, 331.19, 331.2, 331.82 |
| Dementia | ICD-10 | F01, F01.5, F01.50, F01.51, F02, F02.8, F02.80, F02.81, F03, F03.9, F03.90, F03.91, F05.1, G30, G30.0, G30.1, G30.8, G30.9, G31.1 |
| Diabetes | ICD-9 | 250, 250.0, 250.01, 250.02, 250.03, 250.1, 250.1, 250.11, 250.12, 250.13, 250.2, 250.2, 250.21, 250.22, 250.23, 250.3, 250.3, 250.31, 250.32, 250.33 |
| Diabetes | ICD-10 | E10.1, E10.10, E10.11, E10.6, E10.61, E10.610, E10.618, E10.62, E10.620, E10.621, E10.622, E10.628, E10.63, E10.630, E10.638, E10.64, E10.641, E10.649, E10.65, E10.69, E10.8, E10.9, E11.0, E11.00, E11.01, E11.1, E11.10, E11.11, E11.6, E11.61, E11.610, E11.618, E11.62, E11.620, E11.621, E11.622, E11.628, E11.63, E11.630, E11.638, E11.64, E11.641, E11.649, E11.65, E11.69, E11.8, E11.9, E13.0, E13.0, E13.00, E13.01, E13.1, E13.10, E13.11, E13.6, E13.61, E13.610, E13.618, E13.62, E13.620, E13.621, E13.622, E13.628, E13.63, E13.630, E13.638, E13.64, E13.641, E13.649, E13.65, E13.69, E13.8, E13.9 |
| Diabetes with Chronic Complications | ICD-9 | 250.8, 250.80, 250.81, 250.82, 250.83, 250.9, 250.9, 250.91, 250.92, 250.93, 250.4, 250.4, 250.41, 250.42, 250.43, 250.5, 250.5, 250.51, 250.52, 250.53, 250.6, 250.6, 250.61, 250.62, 250.63, 250.7, 250.7, 250.71, 250.72, 250.73, 362, 362.01, 362.02, 362.03, 362.04, 362.05, 362.06, 362.07 |
| Diabetes with Chronic Complications | ICD-10 | E10.2, E10.21, E10.22, E10.29, E10.3, E10.31, E10.311, E10.319, E10.32, E10.321, E10.3211, E10.3212, E10.3213, E10.3219, E10.329, E10.3291, E10.3292, E10.3293, E10.3299, E10.33, E10.331, E10.3311, E10.3312, E10.3313, E10.3319, E10.339, E10.3391, E10.3392, E10.3393, E10.3399, E10.34, E10.341, E10.3411, E10.3412, E10.3413, E10.3419, E10.349, E10.3491, E10.3492, E10.3493, E10.3499, E10.35, E10.351, E10.3511, E10.3512, E10.3513, E10.3519, E10.352, E10.3521, E10.3522, E10.3523, E10.3529, E10.353, E10.3531, E10.3532, E10.3533, E10.3539, E10.354, E10.3541, E10.3542, E10.3543, E10.3549, E10.355, E10.3551, E10.3552, E10.3553, E10.3559, E10.359, E10.3591, E10.3592, E10.3593, E10.3599, E10.36, E10.37, E10.37X1, E10.37X2, E10.37X3, E10.37X9, E10.39, E10.4, E10.40, E10.41, E10.42, E10.43, E10.44, E10.49, E10.5, E10.51, E10.52, E10.59, E11.2, E11.21, E11.22, E11.29, E11.3, E11.31, E11.311, E11.319, E11.32, E11.321, E11.3211, E11.3212, E11.3213, E11.3219, E11.329, E11.3291, E11.3292, E11.3293, E11.3299, E11.33, E11.331, E11.3311, E11.3312, E11.3313, E11.3319, E11.339, E11.3391, E11.3392, E11.3393, E11.3399, E11.34, E11.341, E11.3411, E11.3412, E11.3413, E11.3419, E11.349, E11.3491, E11.3492, E11.3493, E11.3499, E11.35, E11.351, E11.3511, E11.3512, E11.3513, E11.3519, E11.352, E11.3521, E11.3522, E11.3523, E11.3529, E11.353, E11.3531, E11.3532, E11.3533, E11.3539, E11.354, E11.3541, E11.3542, E11.3543, E11.3549, E11.355, E11.3551, E11.3552, E11.3553, E11.3559, E11.359, E11.3591, E11.3592, E11.3593, E11.3599, E11.36, E11.37, E11.37X1, E11.37X2, E11.37X3, E11.37X9, E11.39, E11.4, E11.40, E11.41, E11.42, E11.43, E11.44, E11.49, E11.5, E11.51, E11.52, E11.59, E13.2, E13.21, E13.22, E13.29, E13.3, E13.31, E13.311, E13.319, E13.32, E13.321, E13.3211, E13.3212, E13.3213, E13.3219, E13.329, E13.3291, E13.3292, E13.3293, E13.3299, E13.33, E13.331, E13.3311, E13.3312, E13.3313, E13.3319, E13.339, E13.3391, E13.3392, E13.3393, E13.3399, E13.34, E13.341, E13.3411, E13.3412, E13.3413, E13.3419, E13.349, E13.3491, E13.3492, E13.3493, E13.3499, E13.35, E13.351, E13.3511, E13.3512, E13.3513, E13.3519, E13.352, E13.3521, E13.3522, E13.3523, E13.3529, E13.353, E13.3531, E13.3532, E13.3533, E13.3539, E13.354, E13.3541, E13.3542, E13.3543, E13.3549, E13.355, E13.3551, E13.3552, E13.3553, E13.3559, E13.359, E13.3591, E13.3592, E13.3593, E13.3599, E13.36, E13.37, E13.37X1, E13.37X2, E13.37X3, E13.37X9, E13.39, E13.4, E13.40, E13.41, E13.42, E13.43, E13.44, E13.49, E13.5, E13.51, E13.52, E13.59 |
| Hemiplegia or Paraplegia | ICD-9 | 342, 342, 342, 342.01, 342.02, 342.1, 342.1, 342.11, 342.12, 342.8, 342.8, 342.81, 342.82, 342.9, 342.9, 342.91, 342.92, 344, 344, 344.01, 344.02, 344.03, 344.04, 344.09, 344.1, 344.2, 344.3, 344.3, 344.31, 344.32, 344.4, 344.4, 344.41, 344.42, 344.5, 344.6, 344.6, 344.61, 344.9 |
| Hemiplegia or Paraplegia | ICD-10 | G04.1, G80.1, G80.2, G81, G81.0, G81.00, G81.01, G81.02, G81.03, G81.04, G81.1, G81.10, G81.11, G81.12, G81.13, G81.14, G81.9, G81.90, G81.91, G81.92, G81.93, G81.94, G82, G82.2, G82.20, G82.21, G82.22, G82.5, G82.50, G82.51, G82.52, G82.53, G82.54, G83.0, G83.1, G83.10, G83.11, G83.12, G83.13, G83.14, G83.2, G83.20, G83.21, G83.22, G83.23, G83.24, G83.3, G83.30, G83.31, G83.32, G83.33, G83.34, G83.4, G83.9 |
| Metastaic solid tumor | ICD-9 | 196, 196.1, 196.2, 196.3, 196.5, 196.6, 196.8, 196.9, 197, 197.1, 197.2, 197.3, 197.4, 197.5, 197.6, 197.7, 197.8, 198, 198.1, 198.2, 198.3, 198.4, 198.5, 198.6, 198.7, 198.8, 198.81, 198.82, 198.89, 199, 199.1 |
| Metastaic solid tumor | ICD-10 | C77, C77.0, C77.1, C77.2, C77.3, C77.4, C77.5, C77.8, C77.9, C78, C78.0, C78.00, C78.01, C78.02, C78.1, C78.2, C78.3, C78.30, C78.39, C78.4, C78.5, C78.6, C78.7, C78.8, C78.80, C78.89, C79, C79.0, C79.00, C79.01, C79.02, C79.1, C79.10, C79.11, C79.19, C79.2, C79.3, C79.31, C79.32, C79.4, C79.40, C79.49, C79.5, C79.51, C79.52, C79.6, C79.60, C79.61, C79.62, C79.7, C79.70, C79.71, C79.72, C79.8, C79.81, C79.82, C79.89, C79.9, C80, C80.0, C80.1, C80.2 |
| Mild Liver Disease | ICD-9 | 70.32, 70.33, 70.54, 571.2, 571.4, 571.4, 571.41, 571.42, 571.49, 571.5, 571.6 |
| Mild Liver Disease | ICD-10 | B18, B18.0, B18.1, K70.2, K70.3, K70.30, K70.31, K73, K73.0, K73.1, K73.2, K73.8, K73.9, K74.0, K74.3, K74.4, K74.5, K74.6, K74.60, K74.69 |
| Moderate or Severe Liver Disease | ICD-9 | 456, 456.1, 456.2, 456.2, 456.21, 572.2, 572.3, 572.4, 572.8, V42.7, 70.22, 70.23, 70.44 |
| Moderate or Severe Liver Disease | ICD-10 | I85.0, I85.00, I85.01, I85.9, I86.4, K70.4, K70.40, K70.41, K71.1, K71.10, K71.11, K72.1, K72.10, K72.11, K72.9, K72.90, K72.91, K76.5, K76.6, K76.7, Z94.4 |
| Myocardial Infarction | ICD-9 | 410, 410, 410, 410.01, 410.02, 410.1, 410.1, 410.11, 410.12, 410.2, 410.2, 410.21, 410.22, 410.3, 410.3, 410.31, 410.32, 410.4, 410.4, 410.41, 410.42, 410.5, 410.5, 410.51, 410.52, 410.6, 410.6, 410.61, 410.62, 410.7, 410.7, 410.71, 410.72, 410.8, 410.8, 410.81, 410.82, 410.9, 410.9, 410.91, 410.92, 412 |
| Myocardial Infarction | ICD-10 | I21, I21.0, I21.01, I21.02, I21.09, I21.1, I21.11, I21.19, I21.2, I21.21, I21.29, I21.3, I21.4, I21.9, I21.A, I21.A1, I21.A9, I22, I22.0, I22.1, I22.2, I22.8, I22.9, I25.2 |
| Peptic Ulcer Disease | ICD-9 | 531, 531, 531, 531.01, 531.1, 531.1, 531.11, 531.2, 531.2, 531.21, 531.3, 531.3, 531.31, 531.4, 531.4, 531.41, 531.5, 531.5, 531.51, 531.6, 531.6, 531.61, 531.7, 531.7, 531.71, 531.9, 531.9, 531.91, 532, 532, 532, 532.01, 532.1, 532.1, 532.11, 532.2, 532.2, 532.21, 532.3, 532.3, 532.31, 532.4, 532.4, 532.41, 532.5, 532.5, 532.51, 532.6, 532.6, 532.61, 532.7, 532.7, 532.71, 532.9, 532.9, 532.91, 533, 533, 533, 533.01, 533.1, 533.1, 533.11, 533.2, 533.2, 533.21, 533.3, 533.3, 533.31, 533.4, 533.4, 533.41, 533.5, 533.5, 533.51, 533.6, 533.6, 533.61, 533.7, 533.7, 533.71, 533.9, 533.9, 533.91, 534, 534, 534, 534.01, 534.1, 534.1, 534.11, 534.2, 534.2, 534.21, 534.3, 534.3, 534.31, 534.4, 534.4, 534.41, 534.5, 534.5, 534.51, 534.6, 534.6, 534.61, 534.7, 534.7, 534.71, 534.9, 534.9, 534.91 |
| Peptic Ulcer Disease | ICD-10 | K25, K25.0, K25.1, K25.2, K25.3, K25.4, K25.5, K25.6, K25.7, K25.9, K26, K26.0, K26.1, K26.2, K26.3, K26.4, K26.5, K26.6, K26.7, K26.9, K27, K27.0, K27.1, K27.2, K27.3, K27.4, K27.5, K27.6, K27.7, K27.9, K28, K28.0, K28.1, K28.2, K28.3, K28.4, K28.5, K28.6, K28.7, K28.9 |
| Peripheral Vascular Disease | ICD-9 | 93, 440, 440, 440.1, 440.2, 440.2, 440.21, 440.22, 440.23, 440.24, 440.29, 440.3, 440.3, 440.31, 440.32, 440.4, 440.8, 440.9, 441, 441, 441, 441.01, 441.02, 441.03, 441.1, 441.2, 441.3, 441.4, 441.5, 441.6, 441.7, 441.9, 442, 442.1, 442.2, 442.3, 442.8, 442.81, 442.82, 442.83, 442.84, 442.89, 443.1, 443.2, 443.8, 443.9, 443.21, 443.22, 443.23, 443.24, 443.29, 443.81, 443.82, 443.89, 447.7, 447.71, 447.72, 447.73, 785.4, V43.4 |
| Peripheral Vascular Disease | ICD-10 | I70, I70.0, I70.1, I70.2, I70.20, I70.201, I70.202, I70.203, I70.208, I70.209, I70.21, I70.211, I70.212, I70.213, I70.218, I70.219, I70.22, I70.221, I70.222, I70.223, I70.228, I70.229, I70.23, I70.231, I70.232, I70.233, I70.234, I70.235, I70.238, I70.239, I70.24, I70.241, I70.242, I70.243, I70.244, I70.245, I70.248, I70.249, I70.25, I70.260, I70.261, I70.262, I70.263, I70.268, I70.269, I70.29, I70.291, I70.292, I70.293, I70.298, I70.299, I70.3, I70.30, I70.301, I70.302, I70.303, I70.308, I70.309, I70.31, I70.311, I70.312, I70.313, I70.318, I70.319, I70.32, I70.321, I70.322, I70.323, I70.328, I70.329, I70.33, I70.331, I70.332, I70.333, I70.334, I70.338, I70.339, I70.34, I70.341, I70.342, I70.343, I70.344, I70.345, I70.348, I70.349, I70.35, I70.36, I70.361, I70.362, I70.363, I70.368, I70.369, I70.39, I70.391, I70.392, I70.393, I70.398, I70.399, I70.4, I70.40, I70.401, I70.402, I70.403, I70.408, I70.409, I70.41, I70.411, I70.412, I70.413, I70.418, I70.419, I70.42, I70.421, I70.422, I70.423, I70.428, I70.429, I70.43, I70.431, I70.432, I70.433, I70.434, I70.435, I70.438, I70.439, I70.44, I70.441, I70.442, I70.443, I70.444, I70.445, I70.448, I70.449, I70.45, I70.46, I70.461, I70.462, I70.463, I70.468, I70.469, I70.49, I70.491, I70.492, I70.493, I70.498, I70.499, I70.5, I70.50, I70.501, I70.502, I70.503, I70.508, I70.509, I70.51, I70.511, I70.512, I70.513, I70.518, I70.519, I70.52, I70.521, I70.522, I70.523, I70.528, I70.529, I70.53, I70.531, I70.532, I70.533, I70.534, I70.535, I70.538, I70.539, I70.54, I70.541, I70.542, I70.543, I70.544, I70.545, I70.548, I70.549, I70.55, I70.56, I70.561, I70.562, I70.563, I70.568, I70.569, I70.59, I70.591, I70.592, I70.593, I70.598, I70.599, I70.6, I70.60, I70.601, I70.602, I70.603, I70.608, I70.609, I70.61, I70.611, I70.612, I70.613, I70.618, I70.619, I70.62, I70.621, I70.622, I70.623, I70.628, I70.629, I70.63, I70.631, I70.632, I70.633, I70.634, I70.635, I70.638, I70.639, I70.64, I70.641, I70.642, I70.643, I70.644, I70.645, I70.648, I70.649, I70.65, I70.66, I70.661, I70.662, I70.663, I70.668, I70.669, I70.69, I70.691, I70.692, I70.693, I70.698, I70.699, I70.7, I70.70, I70.701, I70.702, I70.703, I70.708, I70.709, I70.71, I70.711, I70.712, I70.713, I70.718, I70.719, I70.72, I70.721, I70.722, I70.723, I70.728, I70.729, I70.73, I70.731, I70.732, I70.733, I70.734, I70.735, I70.738, I70.739, I70.74, I70.741, I70.742, I70.743, I70.744, I70.745, I70.748, I70.749, I70.75, I70.76, I70.761, I70.762, I70.763, I70.768, I70.769, I70.79, I70.791, I70.792, I70.793, I70.798, I70.799, I70.8, I70.9, I70.90, I70.91, I70.92, I71, I71.0, I71.00, I71.01, I71.02, I71.03, I71.1, I71.2, I71.3, I71.4, I71.5, I71.6, I71.8, I71.9, I73.1, I73.8, I73.81, I73.89, I73.9, I77.1, I79.0, I79.1, I79.8 |
| Renal Disease | ICD-9 | 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, 582, 582, 582.1, 582.2, 582.3, 582.4, 582.8, 582.81, 582.89, 582.9, 583, 583, 583.1, 583.2, 583.4, 583.6, 583.7, 585, 585.1, 585.2, 585.3, 585.4, 585.5, 585.6, 585.9, 586, 588, V42.0, V45.1, V45.11, V45.12, V56, V56.0, V56.1, V56.2, V56.3, V56.31, V56.32, V56.8 |
| Renal Disease | ICD-10 | I12.0, I13.11, N18.5, N18.6, N19, Z49.0, Z49.01, Z49.02, Z99.2, N25.0 |
| Rheumatologic Disease | ICD-9 | 710, 710.1, 710.4, 714.81, 725, 714, 714.1, 714.2 |
| Rheumatologic Disease | ICD-10 | M05, M05.0, M05.00, M05.01, M05.011, M05.012, M05.019, M05.02, M05.021, M05.022, M05.029, M05.03, M05.031, M05.032, M05.039, M05.04, M05.041, M05.042, M05.049, M05.05, M05.051, M05.052, M05.059, M05.06, M05.061, M05.062, M05.069, M05.07, M05.071, M05.072, M05.079, M05.09, M05.1, M05.10, M05.11, M05.111, M05.112, M05.119, M05.12, M05.121, M05.122, M05.129, M05.13, M05.131, M05.132, M05.139, M05.14, M05.141, M05.142, M05.149, M05.15, M05.151, M05.152, M05.159, M05.16, M05.161, M05.162, M05.169, M05.17, M05.171, M05.172, M05.179, M05.19, M05.2, M05.20, M05.21, M05.211, M05.212, M05.219, M05.22, M05.221, M05.222, M05.229, M05.23, M05.231, M05.232, M05.239, M05.24, M05.241, M05.242, M05.249, M05.25, M05.251, M05.252, M05.259, M05.26, M05.261, M05.262, M05.269, M05.27, M05.271, M05.272, M05.279, M05.29, M05.3, M05.30, M05.31, M05.311, M05.312, M05.319, M05.32, M05.321, M05.322, M05.329, M05.33, M05.331, M05.332, M05.339, M05.34, M05.341, M05.342, M05.349, M05.35, M05.351, M05.352, M05.359, M05.36, M05.361, M05.362, M05.369, M05.37, M05.371, M05.372, M05.379, M05.39, M05.4, M05.40, M05.41, M05.411, M05.412, M05.419, M05.42, M05.421, M05.422, M05.429, M05.43, M05.431, M05.432, M05.439, M05.44, M05.441, M05.442, M05.449, M05.45, M05.451, M05.452, M05.459, M05.46, M05.461, M05.462, M05.469, M05.47, M05.471, M05.472, M05.479, M05.49, M05.5, M05.50, M05.51, M05.511, M05.512, M05.519, M05.52, M05.521, M05.522, M05.529, M05.53, M05.531, M05.532, M05.539, M05.54, M05.541, M05.542, M05.549, M05.55, M05.551, M05.552, M05.559, M05.56, M05.561, M05.562, M05.569, M05.57, M05.571, M05.572, M05.579, M05.59, M05.6, M05.60, M05.61, M05.611, M05.612, M05.619, M05.62, M05.621, M05.622, M05.629, M05.63, M05.631, M05.632, M05.639, M05.64, M05.641, M05.642, M05.649, M05.65, M05.651, M05.652, M05.659, M05.66, M05.661, M05.662, M05.669, M05.67, M05.671, M05.672, M05.679, M05.69, M05.7, M05.70, M05.71, M05.711, M05.712, M05.719, M05.72, M05.721, M05.722, M05.729, M05.73, M05.731, M05.732, M05.739, M05.74, M05.741, M05.742, M05.749, M05.75, M05.751, M05.752, M05.759, M05.76, M05.761, M05.762, M05.769, M05.77, M05.771, M05.772, M05.779, M05.79, M05.8, M05.80, M05.81, M05.811, M05.812, M05.819, M05.82, M05.821, M05.822, M05.829, M05.83, M05.831, M05.832, M05.839, M05.84, M05.841, M05.842, M05.849, M05.85, M05.851, M05.852, M05.859, M05.860], M05.861, M05.862, M05.869, M05.87, M05.871, M05.872, M05.879, M05.89, M05.9, M06, M06.0, M06.00, M06.01, M06.011, M06.012, M06.019, M06.02, M06.021, M06.022, M06.029, M06.03, M06.031, M06.032, M06.039, M06.04, M06.041, M06.042, M06.049, M06.05, M06.051, M06.052, M06.059, M06.06, M06.061, M06.062, M06.069, M06.07, M06.071, M06.072, M06.079, M06.08, M06.09, M06.1, M06.2, M06.20, M06.21, M06.211, M06.212, M06.219, M06.22, M06.221, M06.222, M06.229, M06.23, M06.231, M06.232, M06.239, M06.24, M06.241, M06.242, M06.249, M06.25, M06.251, M06.252, M06.259, M06.26, M06.261, M06.262, M06.269, M06.27, M06.271, M06.272, M06.279, M06.28, M06.29, M06.3, M06.30, M06.31, M06.311, M06.312, M06.319, M06.32, M06.321, M06.322, M06.329, M06.33, M06.331, M06.332, M06.339, M06.34, M06.341, M06.342, M06.349, M06.35, M06.351, M06.352, M06.359, M06.36, M06.361, M06.362, M06.369, M06.37, M06.371, M06.372, M06.379, M06.38, M06.39, M06.4, M06.8, M06.80, M06.81, M06.811, M06.812, M06.819, M06.82, M06.821, M06.822, M06.829, M06.83, M06.831, M06.832, M06.839, M06.84, M06.841, M06.842, M06.849, M06.85, M06.851, M06.852, M06.859, M06.86, M06.861, M06.862, M06.869, M06.87, M06.871, M06.872, M06.879, M06.88, M06.89, M06.9, M31.5, M32, M32.0, M32.1, M32.10, M32.11, M32.12, M32.13, M32.14, M32.15, M32.19, M32.8, M32.9, M34, M34.0, M34.1, M34.2, M34.8, M34.81, M34.82, M34.83, M34.89, M34.9, M35.3, M36.0 |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Seely, J.M.; Alhassan, T. Screening for Breast Cancer in 2018-What Should We Be Doing Today? Curr. Oncol. 2018, 25, S115–S124. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.W.; Vulkan, D.; Cuckle, H.; Parmar, D.; Sheikh, S.; Smith, R.A.; Evans, A.; Blyuss, O.; Johns, L.; Ellis, I.O.; et al. Effect of Mammographic Screening from Age 40 Years on Breast Cancer Mortality (UK Age Trial): Final Results of a Randomised, Controlled Trial. Lancet Oncol. 2020, 21, 1165–1172. [Google Scholar] [CrossRef]
- Eddy, D.M. Screening for Breast Cancer. Ann. Intern. Med. 1989, 111, 389–399. [Google Scholar] [CrossRef]
- Siu, A.L. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 279–296. [Google Scholar] [CrossRef]
- Nyström, L.; Andersson, I.; Bjurstam, N.; Frisell, J.; Nordenskjöld, B.; Rutqvist, L.E. Long-Term Effects of Mammography Screening: Updated Overview of the Swedish Randomised Trials. Lancet 2002, 359, 909–919. [Google Scholar] [CrossRef]
- Nelson, H.D.; Cantor, A.; Humphrey, L.; Fu, R.; Pappas, M.; Daeges, M.; Griffin, J. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation; U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2016.
- Nelson, H.D.; Fu, R.; Cantor, A.; Pappas, M.; Daeges, M.; Humphrey, L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-Analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016, 164, 244–255. [Google Scholar] [CrossRef]
- IARC. Breast Cancer Screening; IARC: Lion, France, 2016; ISBN 978-92-832-3015-1. [Google Scholar]
- Oeffinger, K.C.; Fontham, E.T.H.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.-C.T.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update from the American Cancer Society. JAMA 2015, 314, 1599–1614. [Google Scholar] [CrossRef]
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S.; et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J. Clin. 2007, 57, 75–89. [Google Scholar] [CrossRef]
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Niell, B.; Monsees, B.; Sickles, E.A. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J. Am. Coll. Radiol. 2018, 15, 408–414. [Google Scholar] [CrossRef]
- Monticciolo, D.L.; Newell, M.S.; Hendrick, R.E.; Helvie, M.A.; Moy, L.; Monsees, B.; Kopans, D.B.; Eby, P.R.; Sickles, E.A. Breast Cancer Screening for Average-Risk Women: Recommendations from the ACR Commission on Breast Imaging. J. Am. Coll. Radiol. 2017, 14, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Brayne, C.; Matthews, F.E. Survival Times in People with Dementia: Analysis from Population Based Cohort Study with 14 Year Follow-Up. BMJ 2008, 336, 258. [Google Scholar] [CrossRef] [PubMed]
- Brodaty, H.; Seeher, K.; Gibson, L. Dementia Time to Death: A Systematic Literature Review on Survival Time and Years of Life Lost in People with Dementia. Int. Psychogeriatr. 2012, 24, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.B.; Shadlen, M.-F.; Wang, L.; McCormick, W.C.; Bowen, J.D.; Teri, L.; Kukull, W.A. Survival after Initial Diagnosis of Alzheimer Disease. Ann. Intern. Med. 2004, 140, 501–509. [Google Scholar] [CrossRef]
- Sachs, G.A.; Carter, R.; Holtz, L.R.; Smith, F.; Stump, T.E.; Tu, W.; Callahan, C.M. Cognitive Impairment: An Independent Predictor of Excess Mortality. Ann. Intern. Med. 2011, 155, 300–308. [Google Scholar] [CrossRef]
- Todd, S.; Barr, S.; Roberts, M.; Passmore, A.P. Survival in Dementia and Predictors of Mortality: A Review. Int. J. Geriatr. Psychiatry 2013, 28, 1109–1124. [Google Scholar] [CrossRef]
- Tom, S.E.; Hubbard, R.A.; Crane, P.K.; Haneuse, S.J.; Bowen, J.; McCormick, W.C.; McCurry, S.; Larson, E.B. Characterization of Dementia and Alzheimer’s Disease in an Older Population: Updated Incidence and Life Expectancy with and without Dementia. Am. J. Public Health 2015, 105, 408–413. [Google Scholar] [CrossRef]
- Zhang, Y.; Bian, J.; Huo, J.; Yang, S.; Guo, Y.; Shao, H. Comparing the Downstream Costs and Healthcare Utilization Associated with the Use of Low-Dose Computed Tomography (LDCT) in Lung Cancer Screening in Patients with and without Alzheimer’s Disease and Related Dementias (ADRD). Curr. Med. Res. Opin. 2021, 37, 1731–1737. [Google Scholar] [CrossRef]
- Advani, S.; Braithwaite, D. Optimizing Selection of Candidates for Lung Cancer Screening: Role of Comorbidity, Frailty and Life Expectancy. Transl. Lung Cancer Res. 2019, 8, S454. [Google Scholar] [CrossRef]
- Weng, X.; Shen, C.; Vasekar, M.; Gupta, S.; Wang, L. Compare Breast Cancer Screening, Diagnosis, and Treatment Between Medicare Patients with and without ADRD. Innov. Aging 2020, 4, 146. [Google Scholar] [CrossRef]
- Hogan, W.R.; Shenkman, E.A.; Robinson, T.; Carasquillo, O.; Robinson, P.S.; Essner, R.Z.; Bian, J.; Lipori, G.; Harle, C.; Magoc, T.; et al. The OneFlorida Data Trust: A Centralized, Translational Research Data Infrastructure of Statewide Scope. J. Am. Med. Inform. Assoc. 2022, 29, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug. Benefits 2019, 12, 188–197. [Google Scholar] [PubMed]
- Douthit, N.; Kiv, S.; Dwolatzky, T.; Biswas, S. Exposing Some Important Barriers to Health Care Access in the Rural USA. Public Health 2015, 129, 611–620. [Google Scholar] [CrossRef]
- Levit, L.A.; Byatt, L.; Lyss, A.P.; Paskett, E.D.; Levit, K.; Kirkwood, K.; Schenkel, C.; Schilsky, R.L. Closing the Rural Cancer Care Gap: Three Institutional Approaches. JCO Oncol. Pract. 2020, 16, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Rural-Urban Commuting Area (RUCA) Codes, U.S. Department of Agriculture. 2010. Available online: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/ (accessed on 29 June 2022).
- Yang, S.; Bian, J.; George, T.J.; Daily, K.; Zhang, D.; Braithwaite, D.; Guo, Y. The Association between Cognitive Impairment and Breast and Colorectal Cancer Screening Utilization. BMC Cancer 2021, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Soysal, P.; Rongve, A.; Isik, A.T.; Thompson, T.; Maggi, S.; Smith, L.; Basso, C.; Stewart, R.; Ballard, C.; et al. Survival Time and Differences between Dementia with Lewy Bodies and Alzheimer’s Disease Following Diagnosis: A Meta-Analysis of Longitudinal Studies. Ageing Res. Rev. 2019, 50, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Survival Following a Diagnosis of Alzheimer Disease|Dementia and Cognitive Impairment|JAMA Neurology|JAMA Network. Available online: https://jamanetwork.com/journals/jamaneurology/fullarticle/783112 (accessed on 31 August 2022).
| BCS-Eligible Women with ADRD n = 21,715 | BCS-Eligible Women without ADRD n = 65,145 | p-Value | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 64.8 (6.4) | 64.8 (6.4) | =1.000 |
| Race–ethnicity | =0.114 | ||
| NHW | 9261 (42.7%) | 30,700(47.1%) | |
| NHB | 3545 (16.3%) | 9919 (15.2%) | |
| NHO | 758 (3.5%) | 3198 (4.9%) | |
| Hispanic | 4365 (20.1%) | 8809 (20.7%) | |
| Unknown | 3786 (17.4%) | 8156 (12.5%) | |
| Rurality | <0.001 | ||
| Urban | 19,829 (91.3%) | 57,266 (87.9%) | |
| Rural | 1886 (8.7%) | 7879 (12.1%) | |
| Charlson Comorbidity Index | <0.001 | ||
| 0 | 15,544 (71.6%) | 54,914(82.3%) | |
| 1 | 3987 (18.4%) | 6759 (10.4%) | |
| >1 | 2184 (10.1%) | 3472 (5.3%) | |
| Social Vulnerability Index | |||
| SVI-SS (SD) | 0.57 (0.20) | 0.55 (0.21) | <0.001 |
| SVI-HCD (SD) | 0.52 (0.19) | 0.49 (0.19) | <0.001 |
| SVI-MSL (SD) | 0.60 (0.26) | 0.59 (0.26) | <0.001 |
| SVI-HTT (SD) | 0.57 (0.17) | 0.50 (0.17) | <0.001 |
| Mammography use | <0.001 | ||
| Yes | 2074 (10.0%) | 4999 (8.0%) | |
| No | 19,641 (90.0%) | 60,146 (92.0%) |
| BCS-Eligible Women with ADRD n = 21,715 | BCS-Eligible Women without ADRD n = 65,145 | |
|---|---|---|
| NHW | ||
| SVI-SS (SD) | 0.52 (0.19) | 0.47 (0.20) |
| SVI-HCD (SD) | 0.54 (0.19) | 0.49 (0.20 |
| SVI-MSL (SD) | 0.46 (0.23) | 0.48 (0.23) |
| SVI-HTT (SD) | 0.54 (0.16) | 0.49 (0.16) |
| NHB | ||
| SVI-SS (SD) | 0.61 (0.20) | 0.63 (0.20) |
| SVI-HCD (SD) | 0.55 (0.19) | 0.56 (0.18) |
| SVI-MSL (SD) | 0.60 (0.23) | 0.61 (0.23) |
| SVI-HTT (SD) | 0.60 (0.15) | 0.59 (0.16) |
| NHO | ||
| SVI-SS (SD) | 0.51 (0.20) | 0.47 (0.20) |
| SVI-HCD (SD) | 0.50 (0.17) | 0.46 (0.19) |
| SVI-MSL (SD) | 0.55 (0.24) | 0.57 (0.23) |
| SVI-HTT (SD) | 0.52 (0.17) | 0.48 (0.16) |
| Hispanic | ||
| SVI-SS (SD) | 0.59 (0.20) | 0.57 (0.20) |
| SVI-HCD (SD) | 0.47 (0.18) | 0.46 (0.18) |
| SVI-MSL (SD) | 0.77 (0.22) | 0.75 (0.21) |
| SVI-HTT (SD) | 0.58 (0.18) | 0.55 (0.18) |
| Unknown | ||
| SVI-SS (SD) | 0.60 (0.20) | 0.59 (0.20) |
| SVI-HCD (SD) | 0.48 (0.17) | 0.50 (0.18) |
| SVI-MSL (SD) | 0.75 (0.24) | 0.69 (0.27) |
| SVI-HTT (SD) | 0.59 (0.17) | 0.58 (0.17) |
| Variables | Base Model | Interaction Model | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Primary Exposures | ||||
| ADRD diagnosis | ||||
| Yes vs. No | 1.19 (1.13, 1.26) | <0.001 | - | - |
| Race–ethnicity | ||||
| NHB vs. NHW | 1.43 (1.33, 1.55) | <0.001 | - | - |
| Hispanic vs. NHW | 1.32 (1.22, 1.42) | <0.001 | - | - |
| NHO vs. NHW | 1.24 (1.08, 1.41) | =0.016 | - | - |
| Unknown vs. NHW | 3.06 (2.85, 3.29) | <0.001 | - | - |
| ADRD diagnosis by Race–ethnicity | ||||
| Yes vs. No in NHW | - | - | 1.04 (0.94, 1.15) | =0.479 |
| Yes vs. No in NHB | - | - | 0.72 (0.62, 0.83) | <0.001 |
| Yes vs. No in NHO | - | - | 0.65 (0.45, 0.93) | =0.019 |
| Yes vs. No in Hispanics | - | - | 1.56 (1.39, 1.75) | <0.001 |
| Yes vs. No in Unknown | - | - | 1.55 (1.40, 1.71) | <0.001 |
| Covariates | ||||
| Age | 0.95 (0.95, 0.96) | <0.001 | 0.95 (0.95, 0.96) | <0.001 |
| Charlson Comorbidity Index | ||||
| >1 vs. 0 | 0.81 (0.72, 0.90) | =0.001 | 0.83 (0.74, 0.93) | =0.009 |
| 1 vs. 0 | 0.85 (0.79, 0.92) | =0.001 | 0.86 (0.79, 0.93) | =0.002 |
| Rurality | ||||
| Urban vs. Rural | 0.96 (0.88, 1.06) | =0.449 | 0.98 (0.89, 1.08) | =0.651 |
| Social Vulnerability Index | ||||
| SVI-SS | 0.77 (0.60, 0.99) | =0.038 | 0.77 (0.60, 0.99) | =0.040 |
| SVI-HCD | 0.98 (0.80, 1.20) | =0.832 | 0.99 (0.80, 1.21) | =0.900 |
| SVI-MSL | 1.96 (1.68, 2.27) | <0.001 | 1.89 (1.62, 2.19) | <0.001 |
| SVI-HTT | 1.59 (1.30, 1.94) | <0.001 | 1.62 (1.32, 1.99) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Li, Y.; Woodard, J.N.; Islam, J.Y.; Yang, S.; George, T.J.; Shenkman, E.A.; Bian, J.; Guo, Y. The Impact of Race–Ethnicity and Diagnosis of Alzheimer’s Disease and Related Dementias on Mammography Use. Cancers 2022, 14, 4726. https://doi.org/10.3390/cancers14194726
Chen A, Li Y, Woodard JN, Islam JY, Yang S, George TJ, Shenkman EA, Bian J, Guo Y. The Impact of Race–Ethnicity and Diagnosis of Alzheimer’s Disease and Related Dementias on Mammography Use. Cancers. 2022; 14(19):4726. https://doi.org/10.3390/cancers14194726
Chicago/Turabian StyleChen, Aokun, Yongqiu Li, Jennifer N. Woodard, Jessica Y. Islam, Shuang Yang, Thomas J. George, Elizabeth A. Shenkman, Jiang Bian, and Yi Guo. 2022. "The Impact of Race–Ethnicity and Diagnosis of Alzheimer’s Disease and Related Dementias on Mammography Use" Cancers 14, no. 19: 4726. https://doi.org/10.3390/cancers14194726
APA StyleChen, A., Li, Y., Woodard, J. N., Islam, J. Y., Yang, S., George, T. J., Shenkman, E. A., Bian, J., & Guo, Y. (2022). The Impact of Race–Ethnicity and Diagnosis of Alzheimer’s Disease and Related Dementias on Mammography Use. Cancers, 14(19), 4726. https://doi.org/10.3390/cancers14194726








