Clinical Application of the New Prostate Imaging for Recurrence Reporting (PI-RR) Score Proposed to Evaluate the Local Recurrence of Prostate Cancer after Radical Prostatectomy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- PSA level (available in 109 patients)
- Results of PET-CT (46 patients were submitted to choline PET-CT, and 22 to Ga-PSMA PET-CT within one month from mpMRI) that were dichotomized as positive or negative depending on the clinical report
- Results of transrectal ultrasonography (TRUS)-guided biopsy within 3 months from mpMRI (19 patients)
- Histopathological data of the primary tumor were collected, when available (39 patients).
2.2. mpMRI Protocol Study
- Morphological study: Fast Relaxation Fast Spin Echo T2-weighted (T2w) sequences in the sagittal, axial and coronal planes, covering the prostate lodge.
- Diffusion-weighted imaging (DWI): a single-shot echo-planar sequence with a high b-value (2000 s/mm2) and another single-shot echo-planar sequence with two different b-values (50 and 1000 s/mm2), this latter for the calculation of the apparent diffusion coefficient (ADC) map.
- DCE acquisition: three-dimensional (3D) T1-weighted Time-of-Flight Spoiled Gradient-Recalled sequence on the axial plane during the intravenous injection of a gadolinium-based contrast agent at a flow rate of 3 mL/sec followed by 15 mL of saline solution. The 3D data sets were acquired with a 10 s temporal resolution; the acquisitions before the contrast agent administration were analyzed to detect foci of hemorrhage.
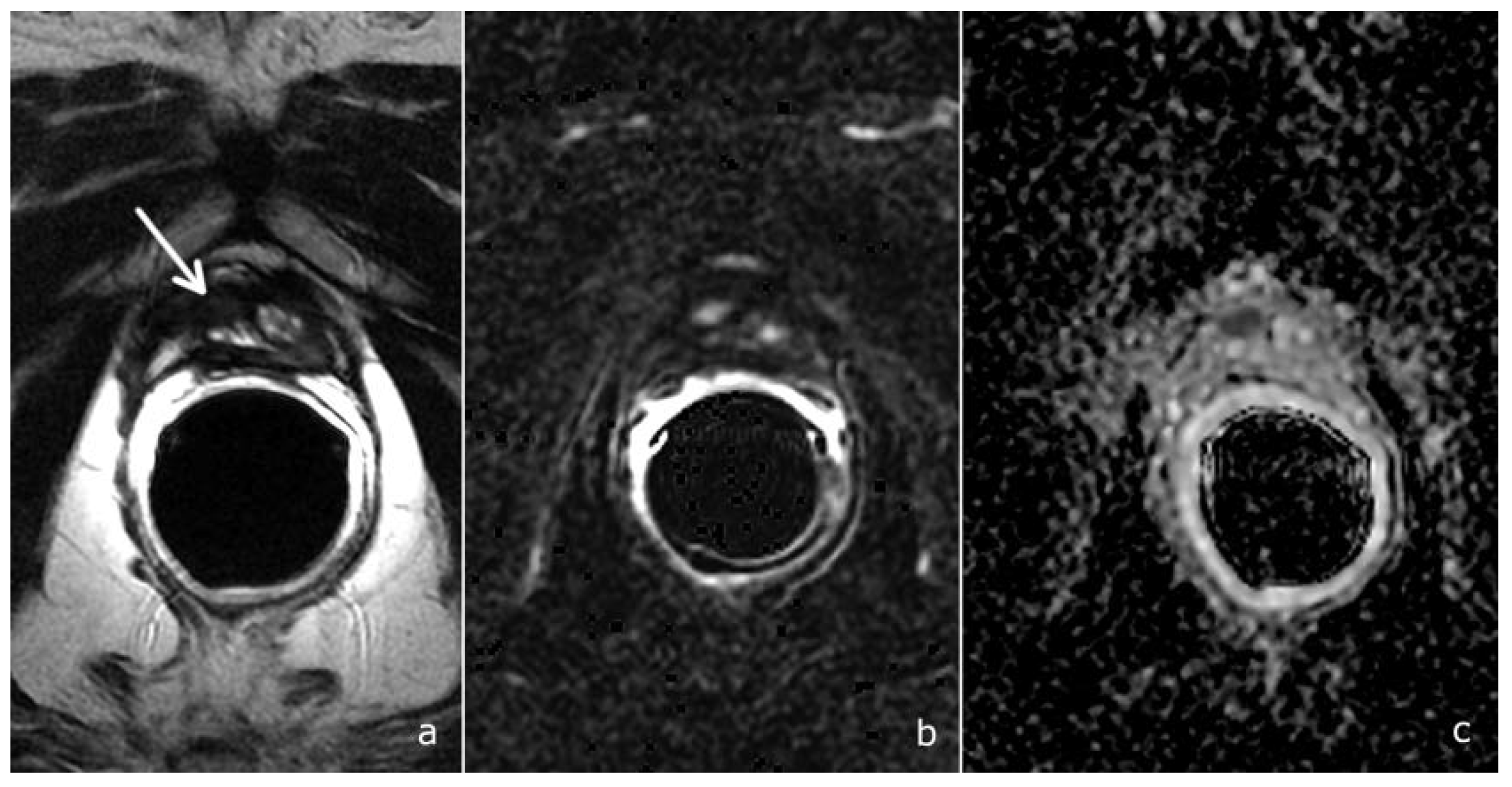
2.3. Image Analysis
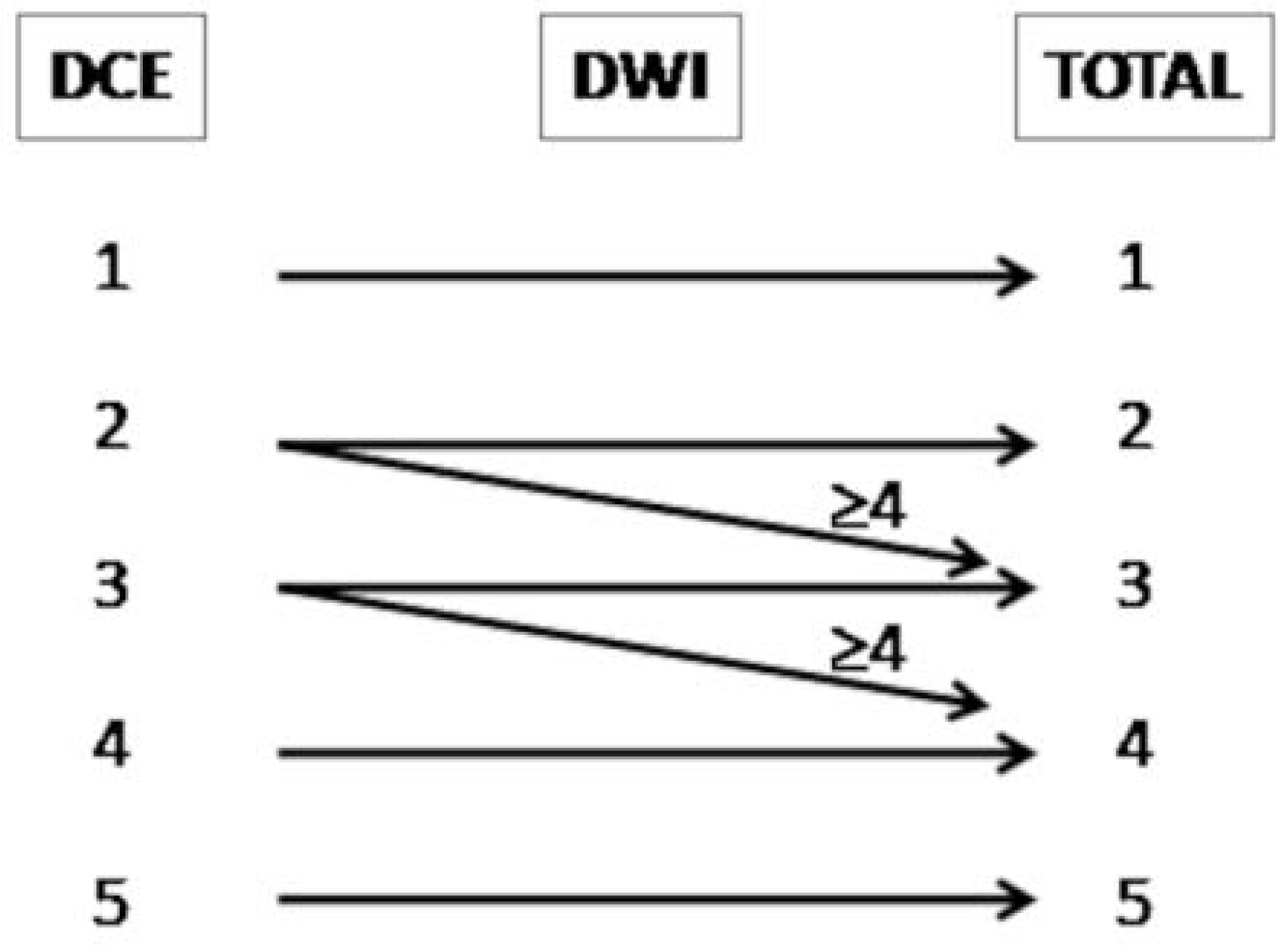
- Scores of 1 and 2 were assigned to lesions with a very low and low likelihood of recurrence
- Score 3 was assigned if the presence of recurrence was uncertain
- Scores 4 and 5 were assigned when the likelihood of recurrence was high and very high
2.4. Statistical Analysis
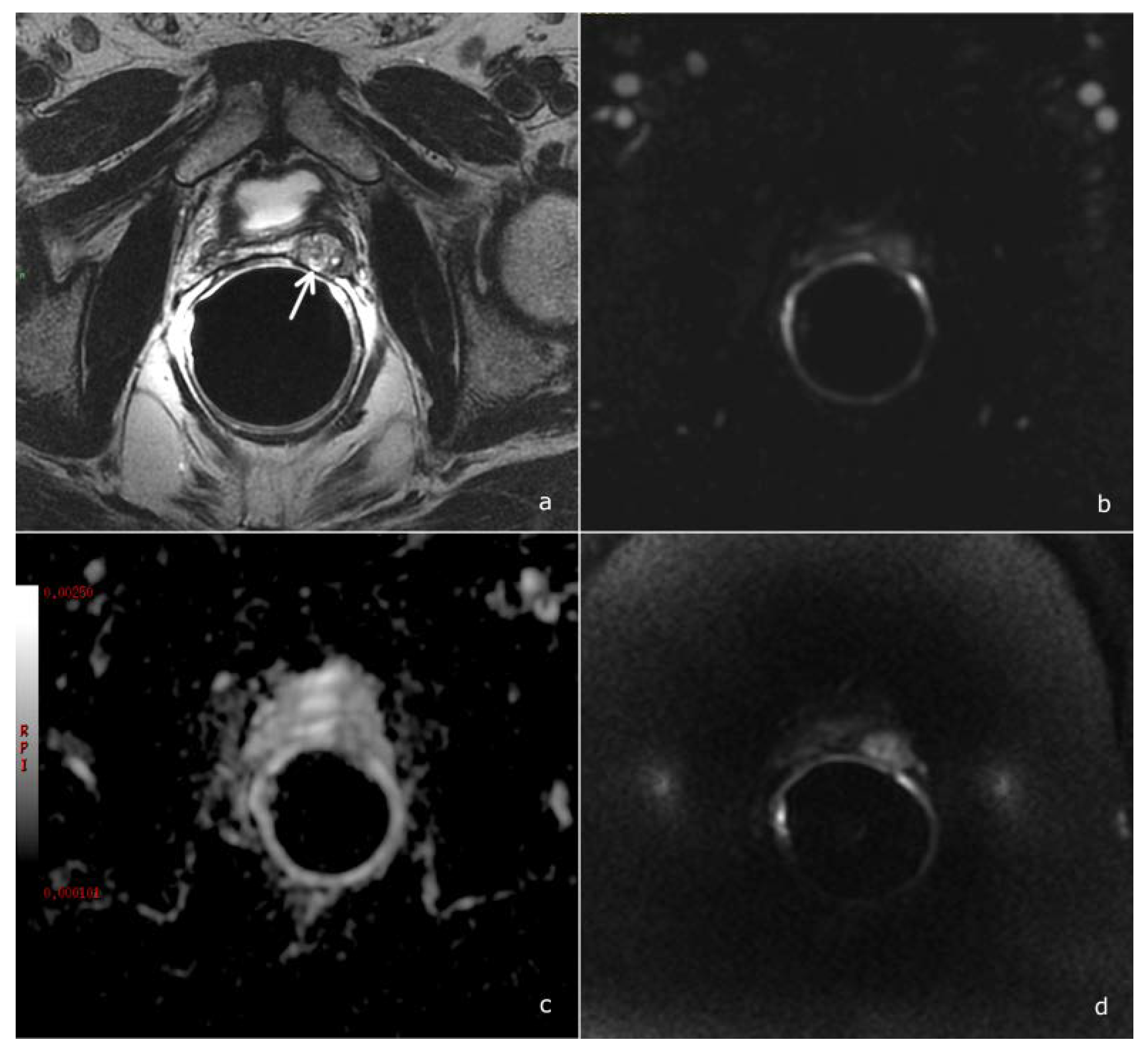
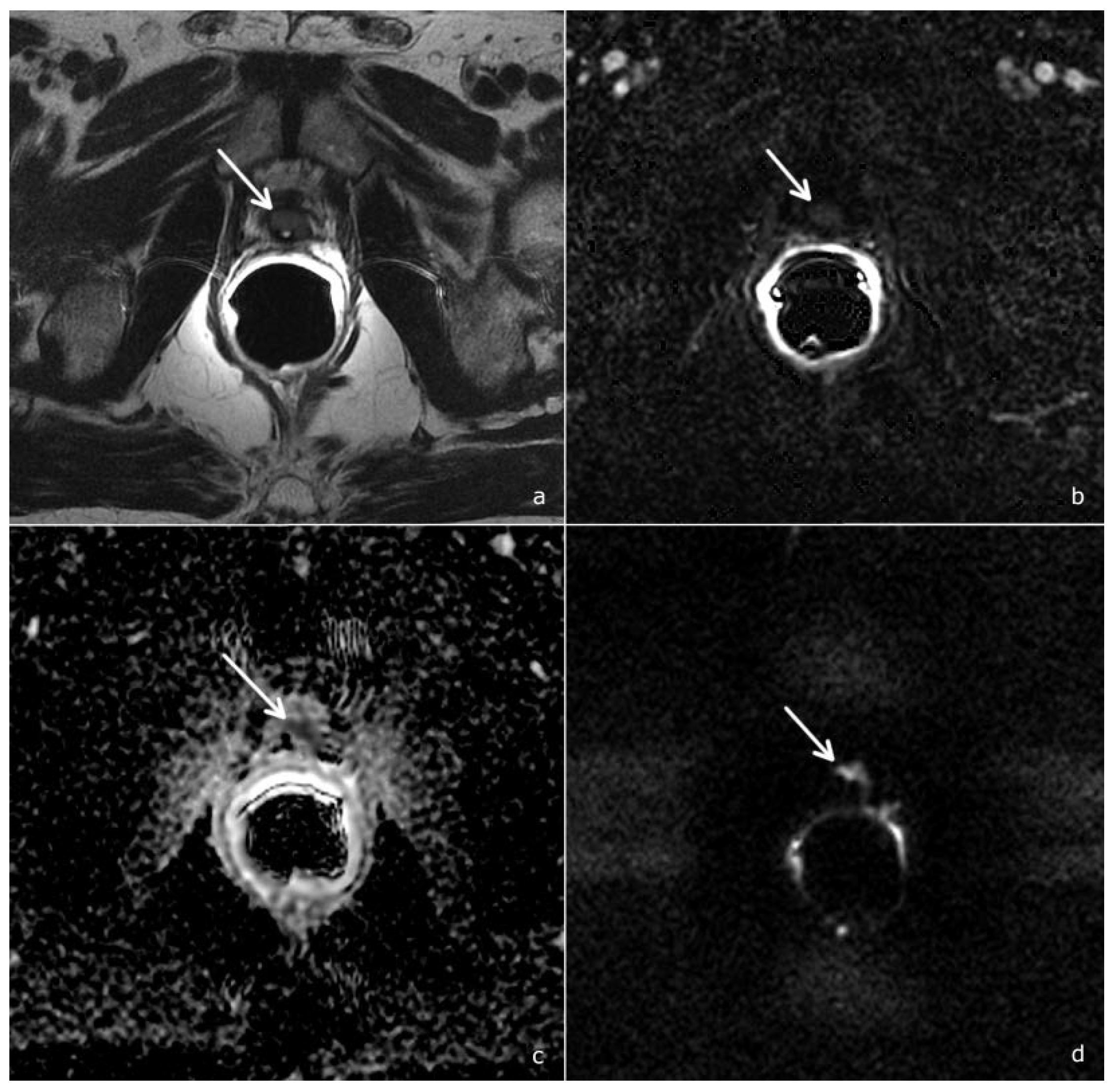
3. Results
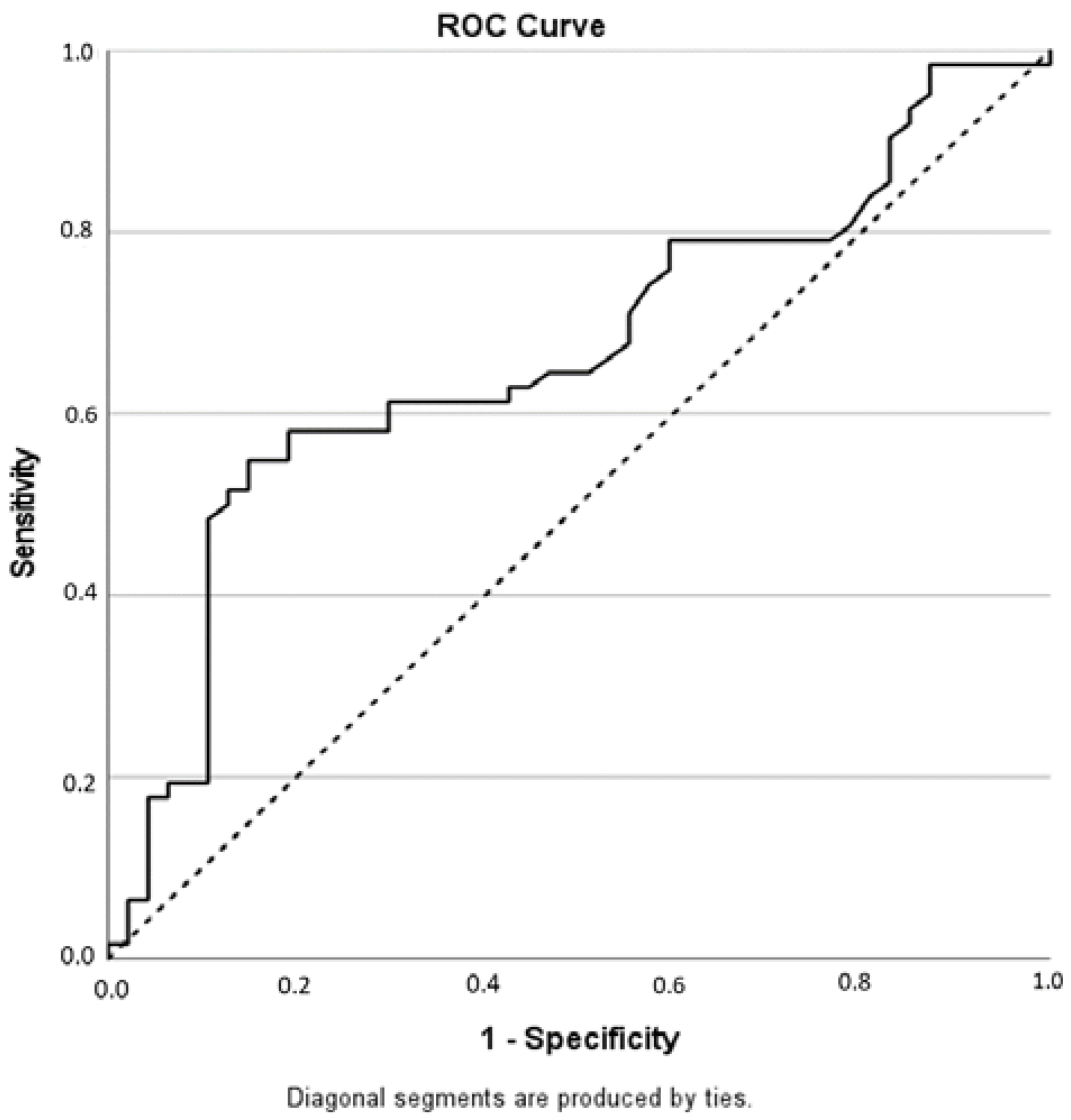
3.1. Diagnostic Accuracy of the PI-RR Score and Inter-Observer Agreement
3.2. Agreement between mpMRI and PET-CT
3.2.1. Comparison between mpMRI and Choline PET-CT
3.2.2. Comparison between mpMRI and Ga-PSMA PET-CT
3.3. Correlation between PI-RR and PSA Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EAU Guidelines. In Proceedings of the EAU Annual Congress, Amsterdam, The Netherlands, 1–4 July 2022; ISBN 978-94-92671-16-5.
- Bianchi, L.; Chessa, F.; Angiolini, A.; Cercenelli, L.; Lodi, S.; Bortolani, B.; Molinaroli, E.; Casablanca, C.; Droghetti, M.; Gaudiano, C.; et al. The Use of Augmented Reality to Guide the Intraoperative Frozen Section During Robot-assisted Radical Prostatectomy. Eur. Urol. 2021, 80, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Schiavina, R.; Borghesi, M.; Casablanca, C.; Chessa, F.; Bianchi, F.M.; Pultrone, C.; Vagnoni, V.; Ercolino, A.; Dababneh, H.; et al. Patterns of positive surgical margins after open radical prostatectomy and their association with clinical recurrence. Minerva Urol. Nefrol. 2020, 72, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.J.; Kattan, M.W.; Eastham, J.A.; Dotan, Z.A.; Bianco, F.J.B., Jr.; Lilja, H.; Scardino, P.T. Defining Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy: A Proposal for a Standardized Definition. J. Clin. Oncol. 2006, 24, 3973–3978. [Google Scholar] [CrossRef] [PubMed]
- Froemming, A.T.; Verma, S.; Eberhardt, S.C.; Oto, A.; Alexander, L.F.; Allen, B.C.; Coakley, F.V.; Davis, B.J.; Fulgham, P.F.; Hosseinzadeh, K.; et al. ACR Appropriateness Criteria ® Post-treatment Follow-up Prostate Cancer. J. Am. Coll. Radiol. 2018, 15, S132–S149. [Google Scholar] [CrossRef]
- Panebianco, V.; Sciarra, A.; Lisi, D.; Galati, F.; Buonocore, V.; Catalano, C.; Gentile, V.; Laghi, A.; Passariello, R. Prostate cancer: 1HMRS-DCEMR at 3T versus [(18)F]choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatectomy (RRP). Eur. J. Radiol. 2012, 81, 700–708. [Google Scholar] [CrossRef]
- Crocerossa, F.; Marchioni, M.; Novara, G.; Carbonara, U.; Ferro, M.; Russo, G.I.; Porpiglia, F.; Di Nicola, M.; Damiano, R.; Autorino, R.; et al. Detection Rate of Prostate Specific Membrane Antigen Tracers for Positron Emission Tomography/Computerized Tomography in Prostate Cancer Biochemical Recurrence: A Systematic Review and Network Meta-Analysis. J. Urol. 2021, 205, 356–369. [Google Scholar] [CrossRef]
- Panebianco, V.; Villeirs, G.; Weinreb, J.C.; Turkbey, B.I.; Margolis, D.J.; Richenberg, J.; Schoots, I.G.; Moore, C.M.; Futterer, J.; Macura, K.J.; et al. Prostate Magnetic Resonance Imaging for Local Recurrence Reporting (PI-RR): International Consensus -based Guidelines on Multiparametric Magnetic Resonance Imaging for Prostate Cancer Recurrence after Radiation Therapy and Radical Prostatectomy. Eur. Urol. Oncol. 2021, 4, 868–876. [Google Scholar] [CrossRef]
- Pecoraro, M.; Turkbey, B.I.; Purysko, A.S.; Girometti, R.; Giannarini, G.; Villeirs, G.; Roberto, M.; Catalano, C.; Padhani, A.R.; Barentsz, J.O.; et al. Diagnostic Accuracy and Observer Agreement of the MRI Prostate Imaging for Recurrence Reporting Assessment Score. Radiology 2022, 304, 212252. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Patel, P.; Mathew, M.S.; Trilisky, I.; Oto, A. Multiparametric MR Imaging of the Prostate after Treatment of Prostate Cancer. RadioGraphics 2018, 38, 437–449. [Google Scholar] [CrossRef]
- Potretzke, T.A.; Froemming, A.T.; Gupta, R.T. Post-treatment prostate MRI. Abdom. Radiol. 2020, 45, 2184–2197. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Takahashi, N.; Karnes, R.J.; Froemming, A.T. Prostatic Remnant After Prostatectomy: MR Findings and Prevalence in Clinical Practice. Am. J. Roentgenol. 2020, 214, W37–W43. [Google Scholar] [CrossRef]
- Sella, T.; Schwartz, L.H.; Swindle, P.W.; Onyebuchi, C.N.; Scardino, P.T.; Scher, H.I.; Hricak, H. Suspected Local Recurrence after Radical Prostatectomy: Endorectal Coil MR Imaging. Radiology 2004, 231, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Casciani, E.; Polettini, E.; Carmenini, E.; Floriani, I.; Masselli, G.; Bertini, L.; Gualdi, G.F. Endorectal and Dynamic Contrast-Enhanced MRI for Detection of Local Recurrence After Radical Prostatectomy. Am. J. Roentgenol. 2008, 190, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, S.; Petracchini, M.; Scotti, L.; Gallo, T.; Macera, A.; Bona, M.C.; Ortega, C.; Gabriele, P.; Regge, D. Endorectal magnetic resonance imaging at 1.5 Tesla to assess local recurrence following radical prostatectomy using T2-weighted and contrast-enhanced imaging. Eur. Radiol. 2009, 19, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; Barchetti, F.; Sciarra, A.; Musio, D.; Forte, V.; Gentile, V.; Tombolini, V.; Catalano, C. Prostate cancer recurrence after radical prostatectomy: The role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. Eur. Radiol. 2013, 23, 1745–1752. [Google Scholar] [CrossRef]
- Kitajima, K.; Hartman, R.P.; Froemming, A.T.; Hagen, C.E.; Takahashi, N.; Kawashima, A. Detection of Local Recurrence of Prostate Cancer After Radical Prostatectomy Using Endorectal Coil MRI at 3 T: Addition of DWI and Dynamic Contrast Enhancement to T2-Weighted MRI. Am. J. Roentgenol. 2015, 205, 807–816. [Google Scholar] [CrossRef]
- Gaudiano, C.; Ciccarese, F.; Bianchi, L.; Corcioni, B.; De Cinque, A.; Giunchi, F.; Schiavina, R.; Fiorentino, M.; Brunocilla, E.; Golfieri, R. The role of MRI in the detection of local recurrence: Added value of multiparametric approach and Signal Inten-sity/Time Curve analysis. Arch. Ital. Urol. Androl. 2022, 94, 25–31. [Google Scholar] [CrossRef]
- De Visschere, P.J.; Standaert, C.; Fütterer, J.J.; Villeirs, G.M.; Panebianco, V.; Walz, J.; Maurer, T.; Hadaschik, B.A.; Lecouvet, F.E.; Giannarini, G.; et al. A Systematic Review on the Role of Imaging in Early Recurrent Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 47–76. [Google Scholar] [CrossRef]
- Tendulkar, R.D.; Agrawal, S.; Gao, T.; Efstathiou, J.A.; Pisansky, T.M.; Michalski, J.M.; Koontz, B.F.; Hamstra, D.A.; Feng, F.Y.; Liauw, S.L.; et al. Contemporary Update of a Multi-Institutional Predictive Nomogram for Salvage Radiotherapy After Radical Prostatectomy. J. Clin. Oncol. 2016, 34, 3648–3654. [Google Scholar] [CrossRef]
- Radzina, M.; Tirane, M.; Roznere, L.; Zemniece, L.; Dronka, L.; Kalnina, M.; Mamis, E.; Biederer, J.; Lietuvietis, V.; Freimanis, A.; et al. Accuracy of 68Ga-PSMA-11 PET/CT and multiparametric MRI for the detection of local tumor and lymph node metastases in early biochemical recurrence of prostate cancer. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 106–118. [Google Scholar] [PubMed]
- Couñago, F.; Del Cerro, E.; Recio, M.; Díaz, A.A.; Marcos, F.J.; Cerezo, L.; Maldonado, A.; Rodríguez-Luna, J.M.; Thuissard, I.; Martín, J.L.R. Role of 3T multiparametric magnetic resonance imaging without endorectal coil in the detection of local recurrent prostate cancer after radical prostatectomy: The radiation oncology point of view. Scand. J. Urol. 2015, 49, 360–365. [Google Scholar] [CrossRef]
- Liauw, S.L.; Pitroda, S.P.; Eggener, S.E.; Stadler, W.M.; Pelizzari, C.A.; Vannier, M.W.; Oto, A. Evaluation of the Prostate Bed for Local Recurrence After Radical Prostatectomy Using Endorectal Magnetic Resonance Imaging. Int. J. Radiat. Oncol. 2013, 85, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.J.; Kawashima, A.; Woodrum, D.A.; Tollefson, M.K.; Karnes, J.; Davis, B.J.; Rangel, L.J.; King, B.F.; Mynderse, L.A. Early localization of recurrent prostate cancer after prostatectomy by endorectal coil magnetic resonance imaging. Can. J. Urol. 2014, 21, 7283–7289. [Google Scholar]
- Ceci, F.; Bianchi, L.; Borghesi, M.; Polverari, G.; Farolfi, A.; Briganti, A.; Schiavina, R.; Brunocilla, E.; Castellucci, P.; Fanti, S. Prediction nomogram for 68Ga-PSMA-11 PET/CT in different clinical settings of PSA failure after radical treatment for prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 136–146. [Google Scholar] [CrossRef]
- Bianchi, L.; Borghesi, M.; Schiavina, R.; Castellucci, P.; Ercolino, A.; Bianchi, F.M.; Barbaresi, U.; Polverari, G.; Brunocilla, E.; Fanti, S.; et al. Predictive accuracy and clinical benefit of a nomogram aimed to predict 68Ga-PSMA PET/CT positivity in patients with prostate cancer recurrence and PSA < 1 ng/ml external validation on a single institution database. Eur. J. Pediatr. 2020, 47, 2100–2105. [Google Scholar] [CrossRef]
| Sequence | Score | Pattern |
|---|---|---|
| DWI | 1 | No signal abnormality |
| 2 | Diffuse moderate hyperintensity on high b-value and diffuse moderate hypointensity on the ADC map | |
| 3 | Focal marked hyperintensity on high b-value or focal marked hypointensity on the ADC map | |
| 4 | Focal marked hyperintensity on high b-value and focal marked hypointensity on the ADC map, not on the same site as that of the primary tumour, or tumour site not known | |
| 5 | Focal marked hyperintensity on high b-value and focal marked hypointensity on the ADC map, on the same site as that of the primary tumour | |
| DCE | 1 | No enhancement |
| 2 | Diffuse enhancement | |
| 3 | Focal late enhancement | |
| 4 | Focal early enhancement, not on the same site as that of the primary tumour, or tumour site not known | |
| 5 | Focal early enhancement, on the same site as that of the primary tumour |
| PI-RR | Biopsy Result | Total | |
|---|---|---|---|
| (N = 19) | |||
| Negative | Positive | ||
| 1 | 0 | 1 | 1 |
| 2 | 2 | 1 | 3 |
| 3 | 1 | 3 | 4 |
| 4 | 2 | 7 | 9 |
| 5 | 1 | 1 | 2 |
| PI-RR | Reader 2 | Total | ||||
|---|---|---|---|---|---|---|
| (N = 134) | ||||||
| Reader 1 | 1 | 2 | 3 | 4 | 5 | |
| 1 | 42 | 0 | 0 | 0 | 0 | 42 |
| −100.00% | 0.00% | 0.00% | 0.00% | 0.00% | ||
| 2 | 0 | 17 | 3 | 0 | 0 | 20 |
| 0.00% | −85.00% | −15.00% | 0.00% | 0.00% | ||
| 3 | 0 | 0 | 15 | 2 | 0 | 17 |
| 0.00% | 0.00% | −88.20% | −11.80% | 0.00% | ||
| 4 | 0 | 0 | 6 | 31 | 0 | 37 |
| 0.00% | 0.00% | −16.20% | 83.8% | 0.00% | ||
| 5 | 0 | 0 | 1 | 0 | 17 | 18 |
| 0.00% | 0.00% | −5.60% | 0.00% | −94.40% | ||
| Total | 42 | 17 | 25 | 33 | 17 | 134 |
| CHOLINE PET-CT | mpMRI | Total | |
|---|---|---|---|
| (N = 46) | |||
| Negative | Positive | ||
| N (%) | N (%) | ||
| Negative | 14 | 23 | 37 |
| −37.80% | −62.20% | ||
| Positive | 0 | 9 | 9 |
| 0.00% | −100% | ||
| Ga-PSMA PET-CT | mpMRI | Total | |
|---|---|---|---|
| (N = 22) | |||
| Negative | Positive | ||
| N (%) | N (%) | ||
| Negative | 8 | 9 | 17 |
| −47.10% | −52.90% | ||
| Positive | 1 | 4 | 5 |
| −20.00% | −80.00% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccarese, F.; Corcioni, B.; Bianchi, L.; De Cinque, A.; Paccapelo, A.; Galletta, G.L.; Schiavina, R.; Brunocilla, E.; Golfieri, R.; Gaudiano, C. Clinical Application of the New Prostate Imaging for Recurrence Reporting (PI-RR) Score Proposed to Evaluate the Local Recurrence of Prostate Cancer after Radical Prostatectomy. Cancers 2022, 14, 4725. https://doi.org/10.3390/cancers14194725
Ciccarese F, Corcioni B, Bianchi L, De Cinque A, Paccapelo A, Galletta GL, Schiavina R, Brunocilla E, Golfieri R, Gaudiano C. Clinical Application of the New Prostate Imaging for Recurrence Reporting (PI-RR) Score Proposed to Evaluate the Local Recurrence of Prostate Cancer after Radical Prostatectomy. Cancers. 2022; 14(19):4725. https://doi.org/10.3390/cancers14194725
Chicago/Turabian StyleCiccarese, Federica, Beniamino Corcioni, Lorenzo Bianchi, Antonio De Cinque, Alexandro Paccapelo, Giovanni Luca Galletta, Riccardo Schiavina, Eugenio Brunocilla, Rita Golfieri, and Caterina Gaudiano. 2022. "Clinical Application of the New Prostate Imaging for Recurrence Reporting (PI-RR) Score Proposed to Evaluate the Local Recurrence of Prostate Cancer after Radical Prostatectomy" Cancers 14, no. 19: 4725. https://doi.org/10.3390/cancers14194725
APA StyleCiccarese, F., Corcioni, B., Bianchi, L., De Cinque, A., Paccapelo, A., Galletta, G. L., Schiavina, R., Brunocilla, E., Golfieri, R., & Gaudiano, C. (2022). Clinical Application of the New Prostate Imaging for Recurrence Reporting (PI-RR) Score Proposed to Evaluate the Local Recurrence of Prostate Cancer after Radical Prostatectomy. Cancers, 14(19), 4725. https://doi.org/10.3390/cancers14194725






