Identification of Biomarkers Associated with Liver Metastasis Progression from Colorectal Cancer Using Exosomal RNA Profiling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
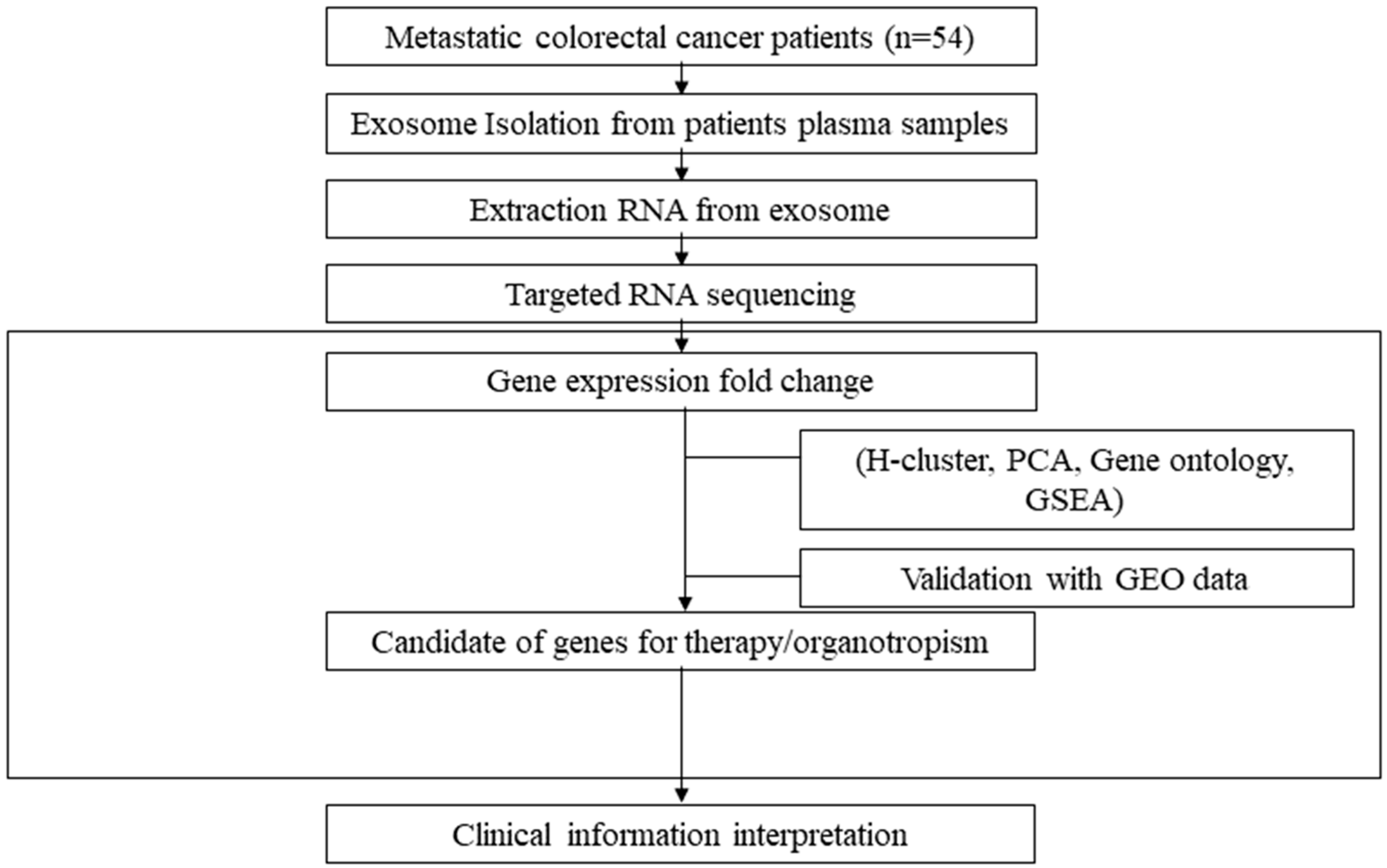
2.1. Study Design and Participants
2.2. Sample Preparation and the Isolation and Identification of Exosomes
2.3. Exosomal RNA Extraction and Sequencing
2.4. Data Processing and Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
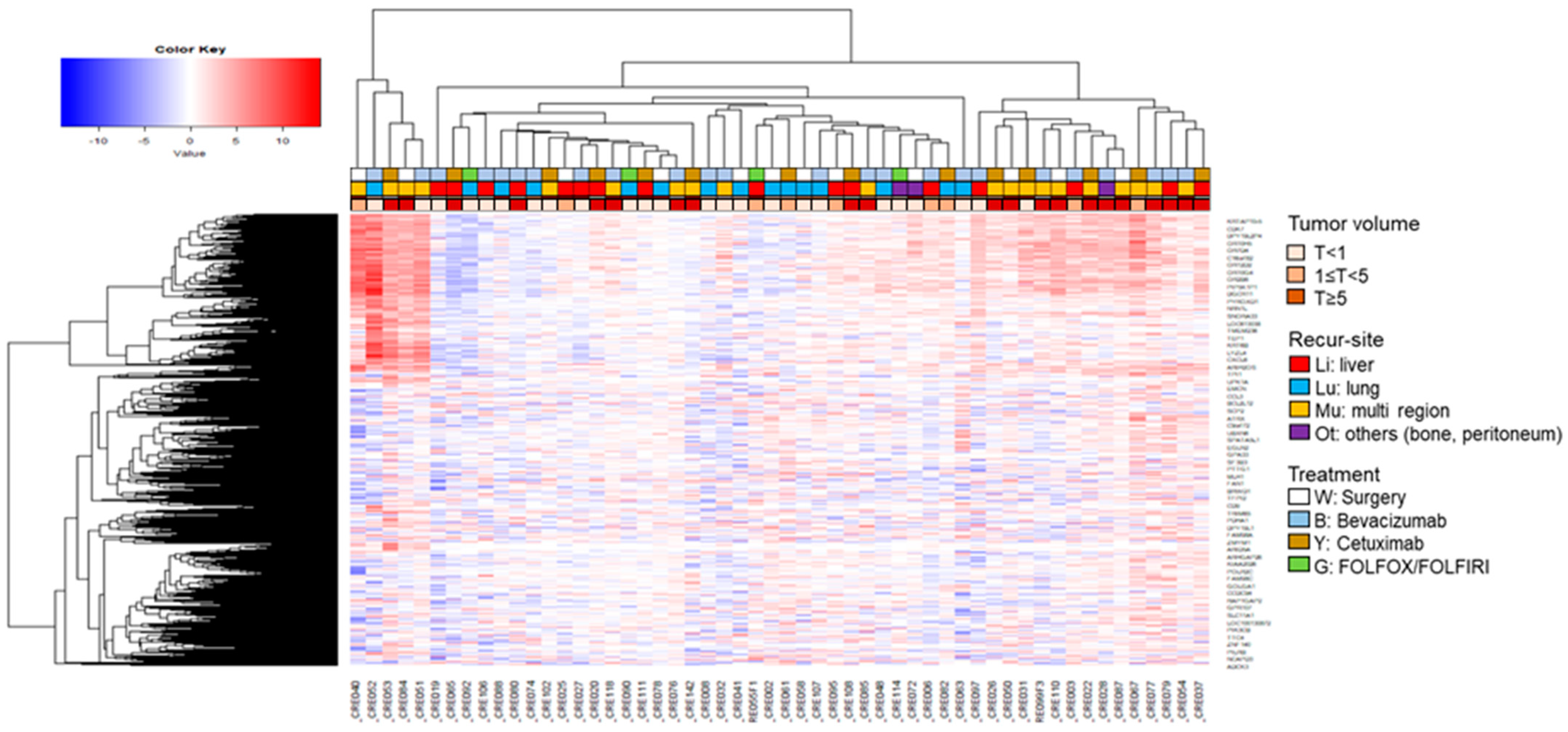
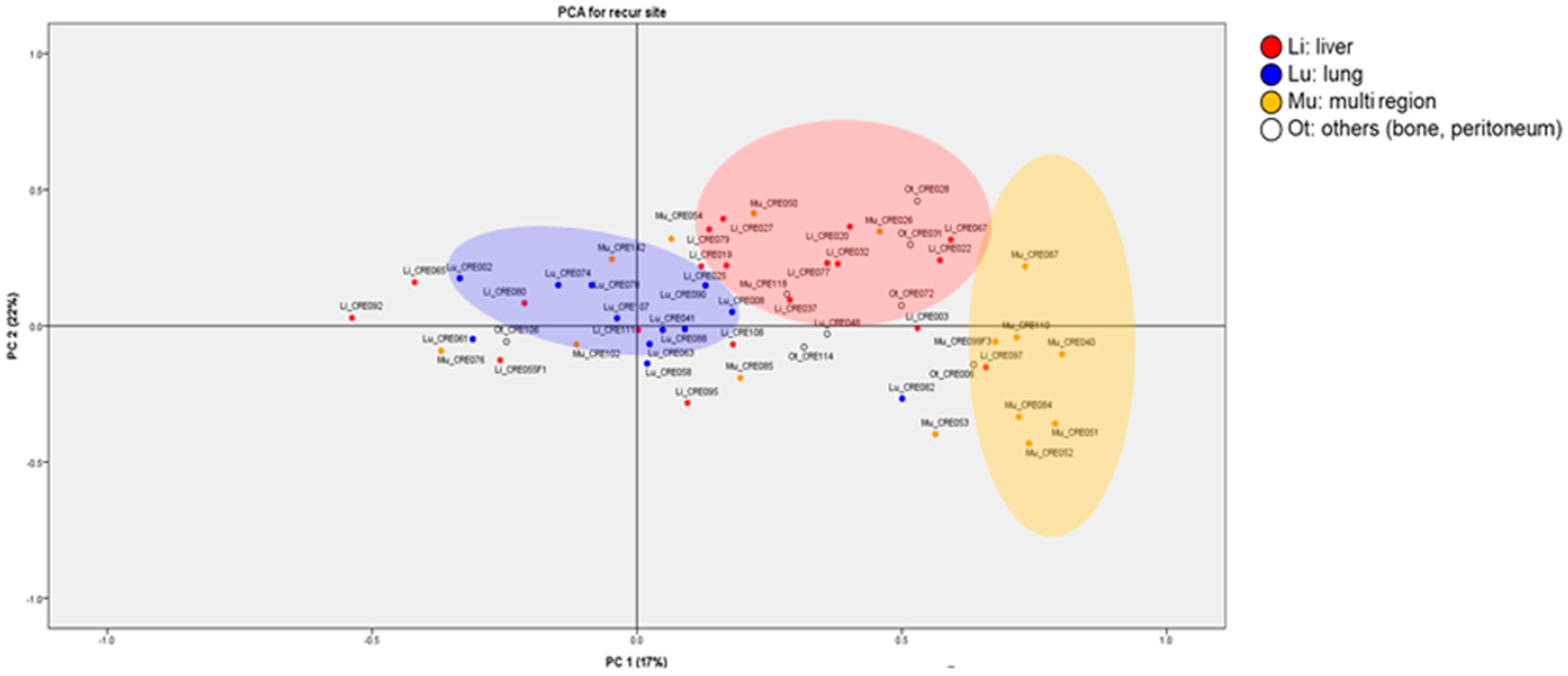
3.2. Exosomal RNA Expression Patterns Analysis Using H-Clustering and PCA
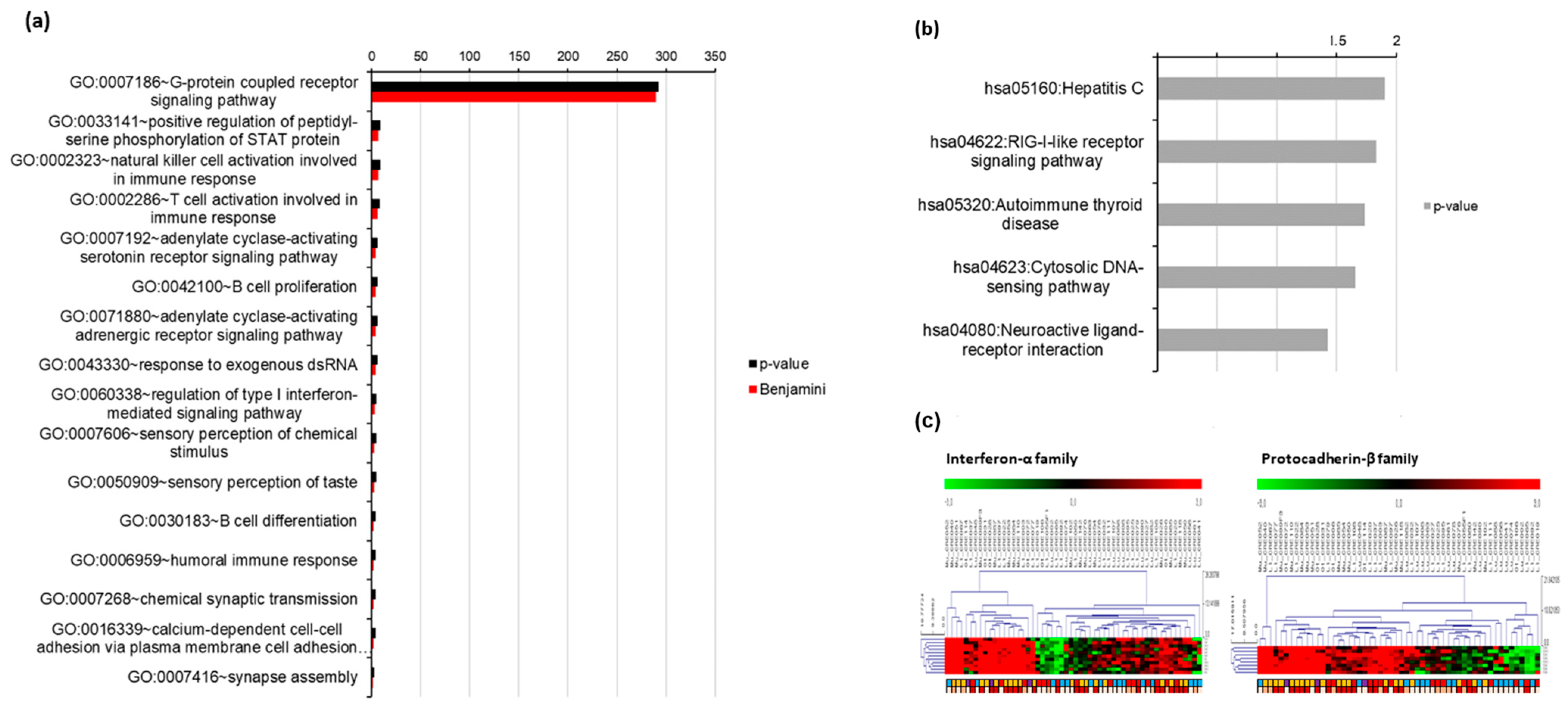
3.3. Gene Oncology (GO) Analysis of Exosomal RNA Expression
3.4. Validation of Tumor RNA Expression Using GSE 41258
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, S.; Won, Y.J.; Park, Y.R.; Jung, K.W.; Kong, H.J.; Lee, E.S.; The Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2017. Cancer Res. Treat. 2020, 52, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Kahi, C.J.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Lieberman, D.; Levin, T.R.; Robertson, D.J.; Rex, D.K. Colonoscopy Surveillance after Colorectal Cancer Resection: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2016, 111, 337–346. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- Sinha, D.; Roy, S.; Saha, P.; Chatterjee, N.; Bishayee, A. Trends in Research on Exosomes in Cancer Progression and Anticancer Therapy. Cancers 2021, 13, 326. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Sinicropi, D.; Qu, K.; Collin, F.; Crager, M.; Liu, M.L.; Pelham, R.J.; Pho, M.; Dei Rossi, A.; Jeong, J.; Scott, A.; et al. Whole transcriptome RNA-Seq analysis of breast cancer recurrence risk using formalin-fixed paraffin-embedded tumor tissue. PLoS ONE 2012, 7, e40092. [Google Scholar] [CrossRef] [PubMed]
- San Lucas, F.A.; Allenson, K.; Bernard, V.; Castillo, J.; Kim, D.U.; Ellis, K.; Ehli, E.A.; Davies, G.E.; Petersen, J.L.; Li, D.; et al. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann. Oncol. 2016, 27, 635–641. [Google Scholar] [CrossRef]
- Treasure, T.; Farewell, V.; Macbeth, F.; Batchelor, T.; Milosevic, M.; King, J.; Zheng, Y.; Leonard, P.; Williams, N.R.; Brew-Graves, C.; et al. The Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) burden of care study: Analysis of local treatments for lung metastases and systemic chemotherapy in 220 patients in the PulMiCC cohort. Color. Dis. 2021, 23, 2911–2922. [Google Scholar] [CrossRef]
- Oweira, H.; Mehrabi, A.; Reissfelder, C.; Abdel-Rahman, O. A Real-World, Population-Based Analysis of the Outcomes of Colorectal Cancer Patients with Isolated Synchronous Liver or Lung Metastases Treated with Metastasectomy. World J. Surg. 2020, 44, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.; Treasure, T. Surgical management and outcomes of colorectal cancer liver metastases. Cancer Epidemiol. 2018, 52, 160–161. [Google Scholar] [CrossRef]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.P.; Goh, B.C. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Chen, M.W.; Vacherot, F.; De La Taille, A.; Gil-Diez-De-Medina, S.; Shen, R.; Friedman, R.A.; Burchardt, M.; Chopin, D.K.; Buttyan, R. The emergence of protocadherin-PC expression during the acquisition of apoptosis-resistance by prostate cancer cells. Oncogene 2002, 21, 7861–7871. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, M.W.; Terry, S.; Vacherot, F.; Chopin, D.K.; Bemis, D.L.; Kitajewski, J.; Benson, M.C.; Guo, Y.; Buttyan, R. A human- and male-specific protocadherin that acts through the wnt signaling pathway to induce neuroendocrine transdifferentiation of prostate cancer cells. Cancer Res. 2005, 65, 5263–5271. [Google Scholar] [CrossRef]
- Zhou, W.; Guan, W.; Zhou, Y.; Rao, Y.; Ji, X.; Li, J. Weighted genes associated with the progression of retinoblastoma: Evidence from bioinformatic analysis. Exp. Eye Res. 2021, 211, 108730. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, S.; Stiles, J.K. The emerging role of CXCL10 in cancer (Review). Oncol. Lett. 2011, 2, 583–589. [Google Scholar] [CrossRef]
- Vazirinejad, R.; Ahmadi, Z.; Kazemi Arababadi, M.; Hassanshahi, G.; Kennedy, D. The biological functions, structure and sources of CXCL10 and its outstanding part in the pathophysiology of multiple sclerosis. Neuroimmunomodulation 2014, 21, 322–330. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Wang, Z.; Ao, X.; Shen, Z.; Ao, L.; Wu, X.; Pu, C.; Guo, W.; Xing, W.; He, M.; Yuan, H.; et al. TNF-alpha augments CXCL10/CXCR3 axis activity to induce Epithelial-Mesenchymal Transition in colon cancer cell. Int. J. Biol. Sci. 2021, 17, 2683–2702. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, W.; Chen, R.; Xiang, B.; Zhou, S.; Lan, L. CXCL10 is a Tumor Microenvironment and Immune Infiltration Related Prognostic Biomarker in Pancreatic Adenocarcinoma. Front. Mol. Biosci. 2021, 8, 611508. [Google Scholar] [CrossRef]
- Delitto, D.; Perez, C.; Han, S.; Gonzalo, D.H.; Pham, K.; Knowlton, A.E.; Graves, C.L.; Behrns, K.E.; Moldawer, L.L.; Thomas, R.M.; et al. Downstream mediators of the intratumoral interferon response suppress antitumor immunity, induce gemcitabine resistance and associate with poor survival in human pancreatic cancer. Cancer Immunol. Immunother. 2015, 64, 1553–1563. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.H.; Li, Y.; Guan, X.Y. Identification of chemokine CXCL10 in tumor microenvironment by antibody array as a prognostic marker in hepatocellular carcinoma. Neoplasma 2017, 64, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yin, H.; Han, C.; Mao, Q.; Tang, J.; Ji, Z.; Yan, X.; Wang, L.; Liu, S.; Ai, C. The role of CXCL10 in prognosis of patients with colon cancer and tumor microenvironment remodeling. Medicine 2021, 100, e27224. [Google Scholar] [CrossRef]
- Guldner, I.H.; Wang, Q.; Yang, L.; Golomb, S.M.; Zhao, Z.; Lopez, J.A.; Brunory, A.; Howe, E.N.; Zhang, Y.; Palakurthi, B.; et al. CNS-Native Myeloid Cells Drive Immune Suppression in the Brain Metastatic Niche through Cxcl10. Cell 2020, 183, 1234–1248.e25. [Google Scholar] [CrossRef]
- Ren, J.; Ding, L.; Zhang, D.; Shi, G.; Xu, Q.; Shen, S.; Wang, Y.; Wang, T.; Hou, Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 2018, 8, 3932–3948. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, X.; Si, M.; Yang, J.; Sun, S.; Wu, H.; Cui, S.; Qu, X.; Yu, X. Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett. 2020, 474, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020, 13, 156. [Google Scholar] [CrossRef]

| Clinical Variables | Number of Patients (N, %) | ||
|---|---|---|---|
| Age | <65 years | 39 (72.2) | |
| ≥65 years | 15 (27.8) | ||
| Gender | Male | 31 (57.4) | |
| Female | 23 (42.6) | ||
| Perioperative therapy | Surgery only | 12 (22.2) | |
| Cytotoxic chemotherapy | 4 (7.4) | ||
| Cetuximab-based therapy | 14 (25.9) | ||
| Bevacizumab-based therapy | 24 (44.4) | ||
| Metastatic sites | Liver | 16 (29.6) | |
| Lung | 15 (27.9) | ||
| Multiple (only liver and lung) | 20 (37.0) | ||
| Others (bone, peritoneum) | 3 (5.5) | ||
| Metastasis tumor size | <1 cm3 | 25 (46.3) | |
| Liver | 5 (20.0) | ||
| Lung | 13 (52.0) | ||
| Multiple | 5 (20.0) | ||
| Others | 2 (8.0) | ||
| ≥1 cm3 | 29 (53.7) | ||
| Liver | 11 (38.0) | ||
| Lung | 2 (2.9) | ||
| Multiple | 15 (51.7) | ||
| Others | 1 (3.5) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Park, Y.S.; Kim, J.H.; Lim, A.R.; Hyun, M.H.; Kim, B.; Lee, J.W.; Lee, S.B.; Kim, Y.H. Identification of Biomarkers Associated with Liver Metastasis Progression from Colorectal Cancer Using Exosomal RNA Profiling. Cancers 2022, 14, 4723. https://doi.org/10.3390/cancers14194723
Lee S, Park YS, Kim JH, Lim AR, Hyun MH, Kim B, Lee JW, Lee SB, Kim YH. Identification of Biomarkers Associated with Liver Metastasis Progression from Colorectal Cancer Using Exosomal RNA Profiling. Cancers. 2022; 14(19):4723. https://doi.org/10.3390/cancers14194723
Chicago/Turabian StyleLee, Soohyeon, Young Soo Park, Jwa Hoon Kim, Ah Reum Lim, Myung Han Hyun, Boyeon Kim, Jong Won Lee, Saet Byeol Lee, and Yeul Hong Kim. 2022. "Identification of Biomarkers Associated with Liver Metastasis Progression from Colorectal Cancer Using Exosomal RNA Profiling" Cancers 14, no. 19: 4723. https://doi.org/10.3390/cancers14194723
APA StyleLee, S., Park, Y. S., Kim, J. H., Lim, A. R., Hyun, M. H., Kim, B., Lee, J. W., Lee, S. B., & Kim, Y. H. (2022). Identification of Biomarkers Associated with Liver Metastasis Progression from Colorectal Cancer Using Exosomal RNA Profiling. Cancers, 14(19), 4723. https://doi.org/10.3390/cancers14194723








