Prognostic Relevance of NPM1 and FLT3 Mutations in Acute Myeloid Leukaemia, Longterm Follow-Up—A Single Center Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Endpoints
2.2. Diagnostic Workup and Molecular and Cytogenetic Analysis
2.3. Treatment Plan, Risk Assessment, and Criteria of Response
2.4. Statistical Analysis
3. Results
3.1. Prevalence of NPM1 and FLT3 Mutations
3.2. Study Cohort: Characteristics of Patients
3.3. Response and Toxicities
3.4. Relapse: Relapse Rate (RR) and Relapse-Free Survival (RFS)
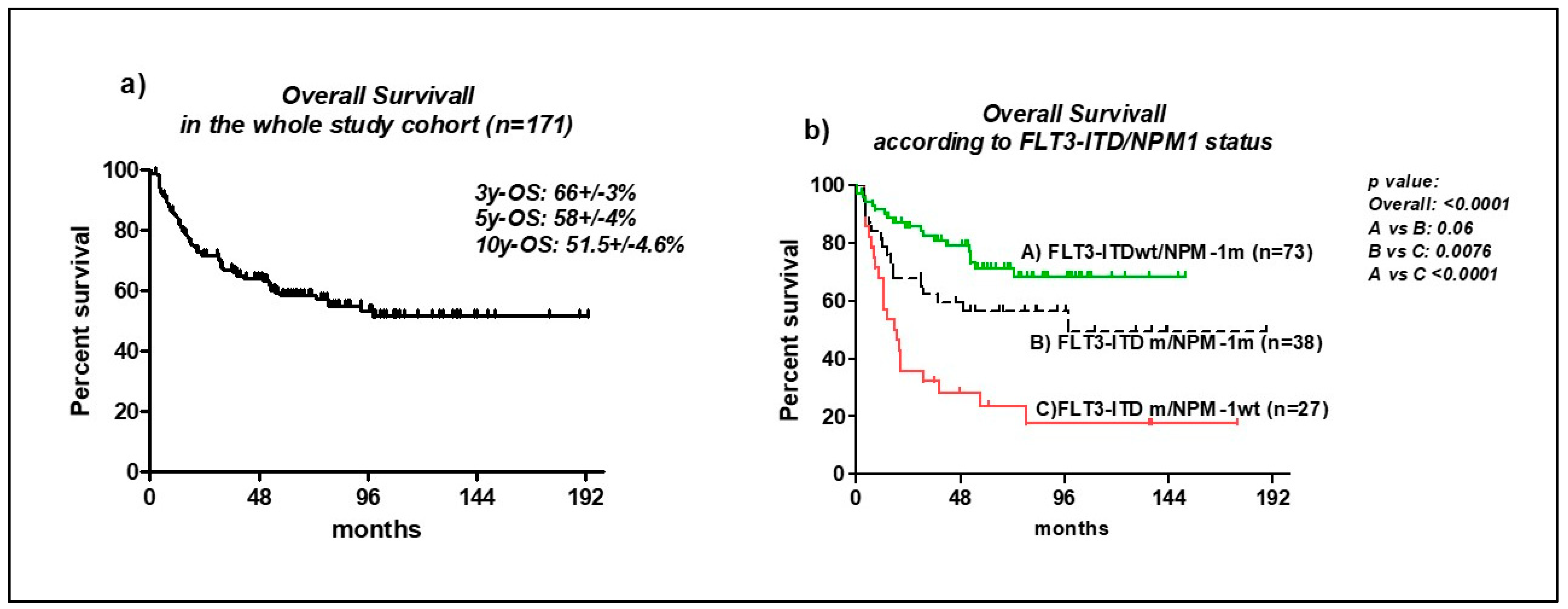
3.5. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Döhner, K.; Krauter, J.; Fröhling, S.; Corbacioglu, A.; Bullinger, L.; Habdank, M.; Späth, D.; Morgan, M.; Benner, A.; et al. German-Austrian Acute Myeloid Leukemia Study Group. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N. Engl. J. Med. 2008, 358, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Kayser, S.; Schlenk, R.F.; Londono, M.C.; Breitenbuecher, F.; Wittke, K.; Du, J.; Groner, S.; Späth, D.; Krauter, J.; Ganser, A.; et al. German-Austrian AML Study Group (AMLSG). Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood 2009, 114, 2386–2392. [Google Scholar] [CrossRef]
- Patel, J.P.; Gonen, M.; Figueroa, M.E.; Fernandez, H.; Sun, Z.; Racevskis, J.; Van Vlierberghe, P.; Dolgalev, I.; Thomas, S.; Aminova, O.; et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 2012, 366, 1079–1089. [Google Scholar] [CrossRef]
- Scholl, S.; Theuer, C.; Scheble, V.; Kunert, C.; Heller, A.; Mügge, L.O.; Fricke, H.J.; Höffken, K.; Wedding, U. Clinical impact of nucleophosmin mutations and Flt3 internal tandem duplication in patents older than 60yr with acute myeloid leukemia. Eur. J. Haematol. 2008, 80, 208–215. [Google Scholar] [CrossRef]
- Thiede, C.; Koch, S.; Creutzig, E.; Steudel, C.; Illmer, T.; Schaich, M.; Ehninger, G. Prevalence and prognostic impact of NPM1 mutations in 1485 adult with acute myeloid leukemia (AML). Blood 2006, 107, 4011–4020. [Google Scholar] [CrossRef]
- Schnittger, S.; Schoch, C.; Kern, W.; Mecucci, C.; Tschulik, C.; Martelli, M.F.; Haferlach, T.; Hiddemann, W.; Falini, B. Nucleophosfomin gene mutations are predictor of favourable prognosis in acute myelogenous leukemia with normal karyotype. Blood 2005, 106, 3733–3739. [Google Scholar] [CrossRef]
- Oran, B.; Cortes, J.; Beitinjaneh, A.; Chen, H.C.; de Lima, M.; Patel, K.; Ravandi, F.; Wang, X.; Brandt, M.; Andersson, B.S.; et al. Allogeneic transplantation in first remission improves outcomes irrespective of FLT3-ITD allelic ratio in FLT3-ITD positive acute myelogenous leukemia. Biol. Blood Marrow. Transplant. 2016, 22, 1218–1226. [Google Scholar] [CrossRef]
- Gale, R.E.; Hills, R.; Kottaridis, P.D.; Srirangan, S.; Wheatley, K.; Burnett, A.K.; Linch, D.C. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): An analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood 2005, 106, 3658–3665. [Google Scholar] [CrossRef]
- Bornhauser, M.; Illmer, T.; Schaich, M.; Soucek, S.; Ehninger, G.; Thiede, C. AML SHG 96 study group. Improved outcome after stem-cell transplantation in FLT3/ITDpositive AML. Blood 2007, 109, 2264–2265. [Google Scholar] [CrossRef] [PubMed]
- Brunet, S.; Labopin, M.; Esteve, J.; Cornelissen, J.; Socié, G.; Iori, A.P.; Verdonck, L.F.; Volin, L.; Gratwohl, A.-; Sierra, J.; et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: A retrospective analysis. J. Clin. Oncol. 2012, 30, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Gale, R.E.; Green, C.; Allen, C.; Mead, A.J.; Burnett, A.K.; Hills, R.K.; Linch, D.C. Medical Research Council Adult Leukaemia Working Party. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008, 111, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, S.; Bacher, U.; Kern, W.; Alpermann, T.; Haferlach, C.; Haferlach, T. Prognostic impact of FLT3-ITD load in NPM1 mutated acute myeloid leukemia. Leukemia 2011, 25, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Pratcorona, M.; Brunet, S.; Nomdedéu, J.; Ribera, J.M.; Tormo, M.; Duarte, R.; Escoda, L.; Guàrdia, R.; de Llano, M.P.Q.; Salamero, O.; et al. Grupo Cooperativo Para el Estudio y Tratamiento de las Leucemias Agudas Mieloblásticas. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: Relevance to post-remission therapy. Blood 2013, 121, 2734–2738. [Google Scholar]
- Bacher, U.; Haferlach, C.; Kern, W.; Haferlach, T.; Schnittger, S. Prognostic relevance of FLT3–TKD mutations in AML: The combination matters-an analysis of 3082 patients. Blood 2008, 111, 2527–2537. [Google Scholar] [CrossRef]
- Thiede, C.; Steudel, C.; Mohr, B.; Schaich, M.; Schäkel, U.; Platzbecker, U.; Wermke, M.; Bornhäuser, M.; Ritter, M.; Neubauer, A.; et al. Analysis of FLT3- activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002, 99, 4326–4335. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Voso, M.T.; Larson, R.A.; Jones, D.; Marcucci, G.; Prior, T.; Krauter, J.; Heuser, M.; Lavorgna, S.; Nomdede, J.; Geyer, S.M.; et al. Midostaurin in patients with acute myelod leukemia and FLT3-TKD mutations: A sub analysis from the Ratify trial. Blood Adv. 2020, 13, 4945–4954. [Google Scholar] [CrossRef]
- Doöhner, K.; Thiede, C.; Jahn, N.; Panina, E.; Gambietz, A.; Larson, R.A.; Prior, T.W.; Marcucci, G.-; Jones, D.; Krauter, J.; et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood 2020, 135, 371–380. [Google Scholar] [CrossRef]
- International System for Cytogenetic Nomenclature. Guidelines for Cancer Cytogenetics. Supplement to: An International System for Human Cytogenetic Nomenclature; Felix Mitelmann: Memphis, TN, USA, 1995. [Google Scholar]
- Grimwade, D.; Hills, R.K.; Moorman, A.V.; Walker, H.; Chatters, S.; Goldstone, A.H.; Wheatley, K.; Harrison, C.J.; Burnett, A.K. National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010, 116, 354–365. [Google Scholar] [PubMed]
- Gorello, P.; Cazzaniga, G.; Alberti, F.; Dell’Oro, M.G.; Gottardi, E.; Specchia, G.; Roti, G.; Rosati, R.; Martelli, M.F.; Diverio, D.; et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying Nucleophosmin (NPM1) gene mutations. Leukemia 2006, 20, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Yokota, S.; Iwai, T.; Kaneko, H.; Horiike, S.; Kashima, K.; Sonoda, Y.; Fujimoto, T.; Misawa, S. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996, 10, 1911–1918. [Google Scholar] [PubMed]
- Schuurhuis, J.G.; Heuser, M.; Freeman, S.; Béné, M.C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef]
- Bassan, R.; Intermesoli, T.; Masciulli, A.; Pavoni, C.; Boschini, C.; Gianfaldoni, G.; Marmont, F.; Cavattoni, I.; Mattei, D.; Terruzzi, E.; et al. Randomized trial comparing standard vs sequential high-dose chemotherapy for inducing early CR in adult AML. Blood Adv. 2019, 3, 1103–1117. [Google Scholar] [CrossRef]
- Mandelli, F.; Vignetti, M.; Suciu, S.; Stasi, R.; Petti, M.C.; Meloni, G.; Muus, P.; Marmont, F.; Marie, J.P.; Labar, B.; et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: The EORTC and GIMEMA Groups Study AML-10. J. Clin. Oncol. 2009, 27, 5397–5403. [Google Scholar] [CrossRef]
- The AML Collaborative Group. A systematic collaborative overview of randomized trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction therapy for acute myeloid leukaemia. Br. J. Haematol. 1998, 103, 100–109. [Google Scholar] [CrossRef]
- Aurelius, J.; Möllgård, L.; Kiffin, R.; Sander, E.F.; Nilsson, S.; Thorén, F.B.; Hellstrand, K.; Martner, A. Anthracycline-based consolidation may determine outcome of post-consolidation immunotherapy in AML. Leuk. Lymphoma 2019, 60, 2771–2778. [Google Scholar] [CrossRef]
- Minetto, P.; Candoni, A.; Guolo, F.; Clavio, M.; Zannier, M.E.; Miglino, M.; Dubbini, M.V.; Carminati, E.; Sicuranza, A.; Ciofini, S.; et al. Fludarabine, High-Dose Cytarabine and Idarubicin-Based Induction May Overcome the Negative Prognostic Impact of FLT3-ITD in NPM1 Mutated AML, Irrespectively of FLT3-ITD Allelic Burden. Cancers 2020, 24, 34. [Google Scholar] [CrossRef]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.-N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Acute Leukemia French Association. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Grech, G.A.; Patel, G.Y.; Bhudia, N.; Farah, H.; Mason, J.; Wall, K.; Akiki, S.; et al. UK National Cancer Research Institute AML Working Group. Assessment of minimal residual disease in standard-risk AML. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Balsat, M.; Renneville, A.; Thomas, X.; de Botton, S.; Caillot, D.; Marceau, A.; Lemasle, E.; Marolleau, J.P.; Nibourel, O.; Berthon, C.; et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: A study by the Acute Leukemia French Association Group. J. Clin. Oncol. 2017, 35, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.; Grigg, A.; Singh, J.; Droogleever, M.P.; Zhang, L.; Lim, A.; Fong, C.Y.; Ting, S.B.; Schwarer, A.; Tiong, I.S.; et al. Treatment practice and outcome in FLT3-mutant acute myeloid leukemia in the re-midostaurin era: A real world experience from Australian tertiary hospitals. Leuk. Lymphoma 2020, 61, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 ELN Recommendations from an International Expert Panel. Blood 2022, online ahead of print. [CrossRef]
- Erba, H.; Montesinos, P.; Vrhovac, R.; Patkowska, E.; Kim, H.J.; Zak, P.; Wang, P.N.; Mitov, T.; Hanyok, J.; Liu, L.; et al. Quizartinib prolunged survival vs placebo plus intensive induction and consolidation therapy followed by single agent continuation in patients aged 18–75 years with newly diagnosed FLT3-ITD+ AML. In Proceedings of the 27th Congress of European Hematology Association (EHA 2022), Wien, Austria, 9–12 June 2022. [Google Scholar]
- Borlenghi, E.; Cattaneo, C.; Cerqui, E.; Archetti, S.; Bertoli, D.; Bellotti, D.; Gramegna, D.; Soverini, G.; Oberti, M.; Schieppati, F.; et al. Postremission therapy with repeated courses of high-dose cytarabine, idarubicin, and limited autologous stem cell support achieves a very good long-term outcome in European leukemia net favorable and intermediate-risk acute myeloid leukemia. Hematol. Oncol. 2020, 38, 754–762. [Google Scholar] [CrossRef]


| ITDm/NPM1wt n 27 | ITDm/NPM1m n 38 | TKDm/NPM1wt n 12 | TKDm/NPM1m n 21 | FLT3wt/NPM1m n 73 | Total n 171 | |
|---|---|---|---|---|---|---|
| Median age (years) | 55 | 55 | 54 | 54.5 | 55 | 54.5 |
| Male n (%) | 15 (55) | 16 (42.1) | 7 (58) | 13 (61.9) | 35 (47.9) | 86 (50.3) |
| Extramedullary disease n (%) | 0 | 1 (2.6) | 2 (16) | 2 (9.5) | 6 (8.2) | 11 (6.4) |
| Favorable K n (%) | 1 (3.7) | - | 2 (16.7) | - | - | 3 (1.8) |
| Intermediate K n (%) | 23 (85.2) | 38 (100) | 8 (66.6) | 21 (100) | 71 (97.3) | 161 (94.2) |
| Adverse K n (%) | 3 (11.1) | - | 2 (16.7) | - | 2 (2.7) | 7 (4) |
| ITDm/NPM1wt n (%) 27 (15.8) | ITDm/NPM1m n (%) 38 (22.2) | TKDm/NPM1wt n (%) 12 (7) | TKDm/NPM1m n (%) 21 (12.3) | FLT3wt/NPM1m n (%) 73 (42.7) | Total n 171 | |
|---|---|---|---|---|---|---|
| CR rate after ICE | 18 (66.7) | 37 (97.3) | 9 (75) | 21 (100) | 66 (90) | 151 (88.3) |
| CR rate after 2 courses | 24 (88.9) | 38 (100) | 11 (92) | - | 68 (93.2) | 162 (94.7) |
| Relapse rate | 17 (70.8) | 18 (47) | 6 (54.5) | 11 (52.4) | 25 (36.7) | 77 (52.1) |
| Duration CR <6 months | 6 (25) | 8 (21) | 1 (9) | 2 (9.5) | 2 (2.9) | 19/162 (11.7) |
| Median duration CR (months) | 11 | 35 | 15.9 | 19.7 | 48 | 32.5 |
| Median RFS (months) | 10 | 35 | 32 | 33 | undef | 42 |
| Median OS (months) | 18.46 | 98 | undef | 93 | undef | undef |
| 5y-OS (% +/− SE) | 23 +/− 8 | 56 +/− 8 | 54 +/− 15 | 70.8 +/− 11 | 71 +/− 6 | 58 +/− 4 |
| Allo-SCT in CR1 during disease course | 15 12 (80) 3 | 11 6 (67) 5 | 7 4 (57) 3 | 6 - 6 | 22 4(18) 18 | 61 26 (42.6) 35 (57) |
| Median RFS (months) censored at allo-SCT | 10 | undef | 11.5 | 48 | undef | undef |
| Median OS (months) censored at allo-SCT | 10 | undef | 31 | 93 | undef | undef |
| 5y-OS (% +/− SE) censored at allo-SCT | 16.7 +/− 11 | 57 +/− 9 | 40 +/− 21 | 68 +/− 13 | 75 +/− 7 | 60 +/− 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borlenghi, E.; Cattaneo, C.; Bertoli, D.; Cerqui, E.; Archetti, S.; Passi, A.; Oberti, M.; Zollner, T.; Giupponi, C.; Pagani, C.; et al. Prognostic Relevance of NPM1 and FLT3 Mutations in Acute Myeloid Leukaemia, Longterm Follow-Up—A Single Center Experience. Cancers 2022, 14, 4716. https://doi.org/10.3390/cancers14194716
Borlenghi E, Cattaneo C, Bertoli D, Cerqui E, Archetti S, Passi A, Oberti M, Zollner T, Giupponi C, Pagani C, et al. Prognostic Relevance of NPM1 and FLT3 Mutations in Acute Myeloid Leukaemia, Longterm Follow-Up—A Single Center Experience. Cancers. 2022; 14(19):4716. https://doi.org/10.3390/cancers14194716
Chicago/Turabian StyleBorlenghi, Erika, Chiara Cattaneo, Diego Bertoli, Elisa Cerqui, Silvana Archetti, Angela Passi, Margherita Oberti, Tatiana Zollner, Carlotta Giupponi, Chiara Pagani, and et al. 2022. "Prognostic Relevance of NPM1 and FLT3 Mutations in Acute Myeloid Leukaemia, Longterm Follow-Up—A Single Center Experience" Cancers 14, no. 19: 4716. https://doi.org/10.3390/cancers14194716
APA StyleBorlenghi, E., Cattaneo, C., Bertoli, D., Cerqui, E., Archetti, S., Passi, A., Oberti, M., Zollner, T., Giupponi, C., Pagani, C., Bianchetti, N., Bottelli, C., Bagnasco, S., Sciumè, M., Tucci, A., & Rossi, G. (2022). Prognostic Relevance of NPM1 and FLT3 Mutations in Acute Myeloid Leukaemia, Longterm Follow-Up—A Single Center Experience. Cancers, 14(19), 4716. https://doi.org/10.3390/cancers14194716






