Simple Summary
Patients with active malignancies have an increased risk for severe SARS-CoV-2 infection and high mortality from COVID-19. Additionally, due to the underlying immune deficiency, prolonged replication and a higher rate of escape mutations are seen. Thus, it is crucial to introduce direct antiviral agents, whereas there is only limited knowledge of their efficacy and optimal regimens mainly from small series in oncologic patients. In this real-world experience study, 252 patients with active malignancy were found among 4890 hospitalized patients for COVID-19. We have shown that patients with malignancy benefit from early remdesivir therapy, resulting in a decrease in 28-day in-hospital mortality by 80%. Factors independently associated with a worse prognosis include low glomerular filtration rate and low peripheral oxygen saturation at baseline. The results not only confirm the lifesaving effect of remdesivir in oncologic patients, but also underline the need to optimize therapy, including kidney protection and early oxygen therapy.
Abstract
Data on the use of remdesivir, the first antiviral agent against SARS-CoV-2, are limited in oncologic patients. We aimed to analyze contributing factors for mortality in patients with malignancies in the real-world CSOVID-19 study. In total, 222 patients with active oncological disorders were selected from a nationwide COVID-19 study of 4890 subjects. The main endpoint of the current study was the 28-day in-hospital mortality. Approximately half of the patients were male, and the majority had multimorbidity (69.8%), with a median age of 70 years. Baseline SpO2 < 85% was observed in 25%. Overall, 59 (26.6%) patients died before day 28 of hospitalization: 29% due to hematological, and 20% due to other forms of cancers. The only factor increasing the odds of death in the multivariable model was eGFR < 60 mL/min/m2 (4.621, p = 0.02), whereas SpO2 decreased the odds of death at baseline (0.479 per 5%, p = 0.002) and the use of remdesivir (0.425, p = 0.03). This study shows that patients with COVID-19 and malignancy benefit from early remdesivir therapy, resulting in a decrease in early mortality by 80%. The prognosis was worsened by low glomerular filtration rate and low peripheral oxygen saturation at baseline underlying the role of kidney protection and early hospitalization.
1. Introduction
Since the beginning of the COVID-19 pandemic declared by the WHO on 11 March 2020, more than 450 million cases of SARS-CoV-2 infection and more than 6 million deaths due to it have been registered worldwide. Certain groups of patients with COVID-19, with an increased risk of disease progression and mortality, have been determined. In the opinion of most researchers, patients with cancer are highly vulnerable to SARS-CoV-2 infection due to frequent contact with the healthcare system, an immunocompromised state from cancer and its therapy, older age, and comorbidities, partially caused by anti-tumor treatment [1,2]. According to various studies, patients with serious malignancies showing COVID-19 symptoms have a high probability of early mortality [3].
Several pharmacological approaches have been suggested for the treatment of COVID-19. Some therapeutic approaches for COVID-19 concentrated on the repurposing of the already approved drugs, which have been known to have activity against other viruses. They included remdesivir (RDV), which had been earlier studied for the treatment of the Ebola virus, as well as SARS-CoV-1 and Middle East respiratory syndrome (MERS) coronaviruses [4,5]. Based on findings from phase III clinical trials and real-world experience studies, RDV received both American and European authorization [6,7,8].
RDV appears to be less prone to significant drug interactions than the other drugs proposed for COVID-19 treatment [9]. It also has a good profile of tolerance and shows few drug interactions, all of which make RDV a suitable drug for use in humans. Nevertheless, when the efficacy of RDV was evaluated in RCTs, the real advantage of RDV was questioned. A meta-analysis, including five RCTs studying over 7000 COVID-19 patients failed to demonstrate that RDV administration was associated with an improvement in survival. Other meta-analyses pointed out that RDV improved clinical outcomes but did not reduce mortality, in comparison to untreated patients [10,11,12]. These conflicting results are attributable to many factors (i.e., steroid use, age, immunosuppression, and timing of administration), which can influence the effects of antiviral treatments, mostly because of the difficulties in the selection of optimal patient populations and finding the appropriate stage of the disease for remdesivir administration.
IDSA recommends remdesivir for treatment of severe COVID-19 in hospitalized patients with SpO2 (oxygen peripheral blood saturation) < 94% on room air. However, the guideline panel’s recommendation is against the routine initiation of remdesivir among patients on invasive ventilation and/or ECMO (extracorporeal membrane oxygenation). Immunocompromised patients who are unable to control viral replication may still benefit from remdesivir despite SpO2 that exceeds 94% on room air or a requirement for mechanical ventilation [13]. The literature describes cases of successful use of remdesivir also in combination with high-titer convalescent plasma or with monoclonal antibodies in immunocompromised patients [14,15]. Patients with malignancies in the severely immunocompromised group might benefit from RDV therapy outside of the commonly accepted framework. Therefore, we aimed to analyze contributing factors for mortality in a large real-world COVID-19 study with a special focus on the effect of antiviral therapy.
2. Materials and Methods
Patients who were analyzed in the current study originated from the SARSTer national database, which included 4890 COVID-19 subjects treated between 1 March 2020 and 30 June 2021 in 30 Polish centers. SARSTer is an ongoing national real-world experience study assessing treatment outcomes in patients with COVID-19. This project is supported by the Polish Association of Epidemiologists and Infectiologists (PTEiLChZ) and the Medical Research Agency.
All the patients were diagnosed with SARS-CoV-2 based on positive results of the real-time reverse transcriptase–polymerase chain reaction (RT-PCR) from the nasopharyngeal swab specimen and required hospitalization due to COVID-19. The decision concerning the treatment regimen was taken entirely by the treating physician based on current knowledge and national recommendations [16,17]. If selected remdesivir was administered intravenously once daily for 5 days, with a loading dose of 200 mg on day 1, followed by a maintenance dose of 100 mg; tocilizumab also iv in a single dose of 800 mg if PBW > 90 kg; 600 mg if PBW > 65 kg and ≤90 kg; 400 mg if PBW > 40 kg and ≤65 kg; and dexamethasone usually orally or intravenously with doses 4–8 mg per day.
The current study included 255 adults (≥18 yrs) patients with active oncological disorders, who constituted 5.2% of subjects included in the SARSTer database (N = 4890) at the time of analyses. Patients with oncological disease indicated in their medical history or who were in remission (n = 24), and without information on COVID-19 outcome (n = 9), were excluded from the study. In total, 222 patients underwent further analyses. Data were entered retrospectively and submitted online by a web-based platform operated by Tiba sp. z o.o. Baseline data included age, gender, body mass index (BMI), comorbidities, and other medications.
COVID-19, SpO2, and laboratory measures of inflammation, including C-reactive protein (CRP), procalcitonin, white blood cells (WBC), platelets, interleukin 6 (IL-6), and d-dimers. In the current study as an endpoint of treatment, efficacy served early hospital mortality before day 28 of hospitalization. Multimorbidity was defined as having at least one of the following diagnoses in addition to ongoing malignancy: diabetes mellitus, cardiovascular disease, and pulmonary disease.
The results were expressed as n (%) or median (interquartile range, IQR). In statistical analyses groups were compared with non-parametric tests, Fisher’s exact test for categorical and the Kruskal–Wallis test for continuous variables. All tests of significance were two-sided. Logistic regression models were used to identify factors associated with the odds of early hospital death. Factors significant in univariate models (p < 0.1) were included in the final multivariate model. A confidence interval (CI) of 95% was accepted. For continuous variables, odds ratios (OR) in uni- and multivariate analyses were shown per incremental unit of the parameter from the median. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Medical University of Białystok (29 October 2020, number APK.002.303.2020). Additionally, the local bioethics committees approved the experimental use of tocilizumab in patients with COVID-19, and written informed consent was obtained from each patient before the treatment initiation.
3. Results
Of 222 patients included in the analyses, 60 (27.0%) had hematological and 162 (73.0%) other types of malignancy. Approximately half of the patients were male (51.8%), the majority had multimorbidities (69.8%), the median age was 70 (IQR: 63.0–78.0) years, and the median BMI was 26.5 (23.5–29.8) kg/m2. Baseline laboratory values are presented in Table 1. In terms of disease severity 25% of patients had SpO2 below 85% (median 92% IQR: 85–95%). In terms of disease progression, 59 (26.6%) of patients died before or on day 28 of hospitalization, 47 (29%) in the group with hematological cancer, and 12 (20%) in the group with other cancers (p = 0.231).

Table 1.
Baseline characteristics of studied cohort stratified by early hospital death.
Since gender is a well-known factor affecting mortality in patients with malignancy we also compared baseline characteristics for both genders (Supplementary Table S1). Interestingly enough, woman who died of cancer were older than those who survived (73 (68–81) vs. 69 (62–78) years old, p = 0.09), whereas such a trend was not observed in men (69 (63–81) vs. 70 (65–76) years old, p = 0.54). This might further suggest an additional influence of gender, irrespective of age, in COVID-19 patients.
Fifty-eight (26.1%) patients received remdesivir, in the majority of cases for five days with a median time from symptoms onset of 6 days and a median time since diagnosis of 5 days. One hundred and ten (49.5%) patients received dexamethasone, with a median time of therapy duration of 9 days and a median time of treatment initiation since diagnosis of 2 days. Additionally, thirty-six (16.2%) patients were treated with tocilizumab, and forty-three (19.4%) were treated with convalescent plasma (Table 1).
Patients with hematological cancers were comparable to patients with other types of cancer in respect to gender, age, BMI, and SpO2 at hospital admission, as well as in most other characteristics. However, patients with hematological malignancies were significantly more likely to have low neutrophils (2700 vs. 4180/μL, p = 0.0002) and platelet (1042 vs. 192.000/μL, p < 0.0001) counts and less likely to have multimorbidity (56.7% vs. 74.7%, p = 0.0132). In terms of COVID-19-specific treatment, patients with hematological cancers were more likely to receive convalescent plasma (33.3% vs. 14.2%, p = 0.0021), as well as to receive dexamethasone and tocilizumab later after diagnosis of COVID-19 (3 vs. 2 (IQR: 1–7 vs. 1–5) days and 4 vs. 4 (9.5–15 vs. 2–7) days, p = 0.0423 and p = 0.0425, respectively), Table 2.

Table 2.
Baseline characteristics of studied cohort stratified by the type of cancer.
Interestingly enough, the baseline characteristics of patients who died were comparable to those who survived in terms of age, BMI, type of cancer, multimorbidity, and use of other medication (Table 1). However, patients who died were significantly more likely to be male (66.1% vs. 46.6%, p = 0.0146), to have lower SpO2 at hospital admission (84% vs. 92%, p < 0.0001), lower lymphocyte count (670 vs. 990/μL, p = 0.0014), more often eGFR < 60 mL/min/m2 (31% vs. 45%, p = 0.0004) and higher CRP (96.5 vs. 57.3 mg/dL, p = 0.0008), procalcitonin (0.27 vs. 0.11 ng/mL, p = 0.0017), IL-6 (139.7 vs. 43.9 pg/mL, p < 0.0001), neutrophils (5190 vs. 3260/μL, p = 0.0091).
In terms of COVID-19-specific treatment patients who died were less likely to receive remdesivir (15.5% vs. 30.1%, p = 0.0370); however, there was no difference in the time of starting treatment from the onset of symptoms or diagnosis. Those who died were also more likely to receive dexamethasone (62.7% vs. 44.8%, p = 0.0226) with a shorter time of treatment duration (8 vs. 9 days, p = 0.0233), but with no difference in the time since diagnosis. There were no statistical differences in terms of using tocilizumab and convalescent plasma between those who died and those who survived, Table 1.
In univariate logistic regression model factors associated with increased odds of early hospital death were male sex (OR 2.174 [95%CI:1.200–3.939], p = 0.0104), older age (1.309 [1.022–1.667] per 10 years increase, p = 0.0330) and eGFR < 60 mL/min/m2 (3.055 [1.671–5.584], p = 0.0003). In terms of COVID-19-specific treatment, the only factors associated with the likelihood of death were remdesivir, which decreased (0.425 [0.201–0.895], p = 0.0243) the odds, and dexamethasone, which increased (2.121 [1.175–3.827], p = 0.0125) the odds. In addition, several laboratory values were associated with the odds of death including CRP (1.272 [1.082–1.496] per 50 mg/dL increase, p = 0.0036). procalcitonin (1.210 (1.019–1.435] per 2 ng/mL increase, p = 0.0292), neutrophil count (1.120 [1.050–1.195], per 1000/μL decrease, p = 0.8045), IL-6 (1.239 [1.059–1.450] per 200 pg/mL increase, p = 0.0074) and d-dimers (1.159 [1.025–1.309] per 2000 ug/mL increase, p = 0.0185) (Table 3).

Table 3.
Univariate and multivariate logistic regression models for the odds of death.
The final multivariate model included 126 patients and 22 deaths. After including all significant factors from the univariable model, the only factor increasing the odds of death in the multivariable model was eGFR < 60 mL/min/m2 (4.621 [1.244–17.17], p = 0.0223). The only independent factor significantly decreasing the odds of death among patients with malignancy and COVID-19 was SpO2 (0.479 [0.303–0758] per 5% increase, p = 0.0017) and the use of remdesivir (0.425 [0.201–0.895], p = 0.0314), Figure 1.
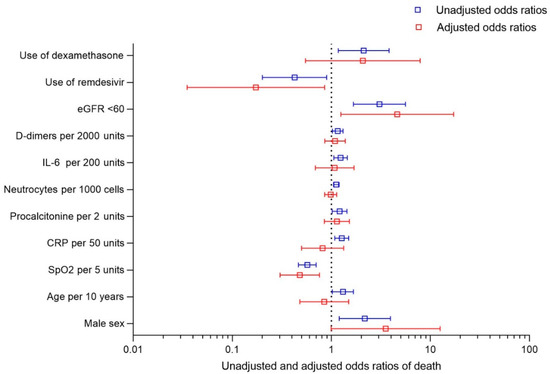
Figure 1.
Unadjusted and adjusted odds ratio of 28-day mortality in COVID-19 patients with active malignancy.
4. Discussion
In this cohort of patients with various malignancies, including almost one-third with hematologic cancer in the univariate analysis, we found male sex and age to be risk factors for early hospital death. Similar observations were made in a number of analyses. In a large cohort analysis published by Lee et al. and Williams et al., the risk of death was significantly associated with age and male sex [18,19]. On the other hand, when we performed a multivariate logistic regression model, both sex and age lost their significance. Similar results were observed in cohorts of patients with COVID-19 and thoracic cancers. The TERAVOLT registry included patients from eight countries, and in univariate analyses identified age > 65, current or former smokers, presence of any comorbidities, and chemotherapy as significant risk-of-death factors; however, multivariate analysis only revealed smoking history as associated with an increased risk of death [20]. One may conclude that the type of statistical analysis, especially the need for multifactorial analyses, may have a great impact on final conclusions. In this case, the lack of influence of age may result from the fact that most of the patients in our cohort were already at high risk, with all having baseline active malignancy, most being over 60 years old.
SpO2 at hospital admission and decreased remained crucial factors associated with odds of death. These data do not require an explanation as it reflects the severity of the disease course prior to hospitalization and/or preexisting comorbidities, particularly kidney insufficiency and respiratory failure. Both are well known and described in multiple studies. For example, Mamlouk O et al. found the overall mortality rate of cancer patients with CKD and COVID-19 was almost 1.7 times higher than the rate of patients with no CKD at the tertiary cancer center in Houston [21]. Additionally, in one of the studies from the SARSTER project, we were able to find that lower eGFR was a leading driving factor of mortality in the analysis of 2322 COVID-19 patients where a subject with eGFR < 30 mL/min had six-times higher mortality compared with those >60 mL/min [22].
We did not find multimorbidity as an independent predictor of early hospital death, which may reflect that most of the patients in our cohort were already at high risk with all having as baseline disease active cancer. In addition, BMI was not identified as a risk factor; however, we believe that in cancer patients, who might be underweight, the increase in BMI reflects an improvement in underlying disease rather than deterioration.
In most analyses, the type of cancer might be a critical prognostic factor for both course severity and final outcome. Retrospective studies suggest that higher rates of case fatality are observed in patients with hematologic malignancies than in those with solid tumors [23]. Our study group consisted of patients with different types of cancer. Importantly, we could not find a difference in 28-day mortality between the subjects with hematologic vs. other types of cancers, most likely due to the relatively low number of patients. Importantly, however, groups of patients with hematological cancer and other types of cancer were comparable with respect to gender, age, BMI, and, most importantly, SpO2 at hospital admission. Furthermore, we were not able to analyze separately prognostic factors of death among patients with specific types of cancers.
In our analyses, the use of remdesivir was the only independent factor significantly decreasing the odds of early hospital death by over 80% among patients with malignancy. For the study group, irrespective of the type of cancer, the median number of days from symptom onset for introducing antiviral remdesivir treatment was 6 days. After comparison of those who died with patients who survived, the initiation of remdesivir treatment was slightly delayed, although there was no significant difference. This may indicate that prompt and timely initiation of antiviral therapy may have a beneficial effect in some subgroups of patients, such as those with immunosuppression. Importunately, for the number of hematologic disorders, prolonged viral shedding (detection of SARS-CoV-2 by molecular testing) may occur, although it how it impacts hospital deaths remains unknown [24].
According to general and focused cancer patient guidelines, remdesivir is recommended for inpatients hospitalized for COVID-19 pneumonia, with hypoxia, but who are not yet requiring mechanical ventilation or ECMO [25]. In those hospitalized with COVID-19 pneumonia, patients receiving remdesivir had a shorter time to recovery, clinical improvement, and lower mortality [6]. A study of patients without hypoxia (with oxygen saturation >94% on room air) found inconsistent results, with a 5-day course of therapy with remdesivir resulting in improved clinical status but no impact with longer courses. Patients with COVID-19 without reduced oxygen saturation included in the SIMPLE-moderate phase III trial treated with remdesivir for 5 days improved clinically when compared with those receiving standard of care alone. Similar results were obtained for patients with the severe form of COVID-19 defined as oxygen saturation SpO2 < 95% or receiving oxygen support, treated with remdesivir in the SIMPLE-severe phase III trial [26,27].
The ACTT-1 and SIMPLE trials also showed that the safety profile of remdesivir was similar to that of placebo. Although some open-label trials involving hospitalized patients showed conflicting results of clinical efficacy, refs. [26,28,29] data from the ACTT-1 and SIMPLE trials and multiple real-world studies, including previous SARSTer analysis [8], together with the results of the current trial, proved the clinical benefit of treatment with remdesivir in different populations of COVID-19 patients.
In none of the previously published studies were oncology patients analyzed. There is a number of case studies with single or a series of cases discussing rationales for the use and results of remdesivir treatment in cancer patients. Hence, our study is one of the first oriented at oncologic patients.
Surprising results were shown with respect to the use of dexamethasone. In univariate analysis, this treatment was associated with worse prognosis and higher early hospitalization death in cancer patients (p = 0.0243), whereas in multivariate this significance was lost (p = 0.2824). It is contrary to most of the recommendations and a number of studies. A meta-analysis of seven clinical trials representing 1703 critically ill patients found decreased mortality when different corticosteroids were used for COVID-19 treatment; nevertheless, the effect only reached statistical significance with dexamethasone [30]. Some important observations were reported in the RECOVERY trial. Corticosteroids were found to be harmful in patients who did not require supplemental oxygen support at baseline [31]. A clinical trial of 299 hospitalized patients with moderate-to-severe COVID-19 reported improved outcomes (less need for mechanical ventilation and better Sequential Organ Failure Assessment [SOFA] scores), but no significant impact on mortality [32]. The beneficial effect of dexamethasone was reported for severe and/or late COVID-19 patients, whereas in our study, dexamethasone was introduced relatively early, during the first 5 days from diagnosis (median 2 days). We may conclude that early dexamethasone treatment showed no benefit to the final disease outcome.
As mentioned, our study is one of the first to analyze treatment effectiveness in cancer patients. We are aware of the limitations of our study. Among them, the impact of multiple factors related to cancer and COVID-19 treatment should be pointed out. One of the major confounding factors was the type of cancer and oncologic treatment. One should mention also that comedications of COVID-19 and their impact on the patient’s condition may influence the course of the disease and potential complications. To eliminate this imbalance as a confounding factor, we performed an analysis using multivariate logistic regression. On the other hand, the major strength of the study lies in the collection of data from a real-world, heterogeneous population, thus being representative of routine practice. We believe that our observations may help to improve our understanding of the complexity of COVID-19 cancer patient management. As SARSTer is an ongoing nationwide study, we will re-evaluate these observations with increasing numbers of patients in the cohort.
5. Conclusions
In conclusion, this real-world experience study shows that patients with malignancies could be among one of the populations to especially benefit from early remdesivir therapy, with a decrease in 28-day in-hospital mortality of 80%. On the other hand, leading factors independently associated with worse prognosis in patients with cancers include low glomerular filtration rate and low peripheral oxygen saturation, which underline the role of kidney protection and early hospitalization in this subgroup of COVID-19 patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14194720/s1, Table S1: Baseline characteristics of studied cohort stratified by gender and early hospital death.
Author Contributions
Conceptualization, J.J., J.K., M.P., R.F. and K.T.; Data curation, M.R. (Magdalena Rogalska), D.Z.-M., M.R. (Marta Rorat), B.L., P.C., K.S., A.P., A.D., I.Z., W.M., D.K., K.K., R.P., G.A., B.O.-G., M.F., B.S., B.B., P.F.-C. and K.T.; Formal analysis, J.J., J.K. and K.T.; Funding acquisition, R.F.; Investigation, J.J. and J.K.; Methodology, J.J., J.K., M.P., R.F. and K.T.; Supervision, J.J. and R.F.; Writing—original draft, J.J., J.K., M.P. and K.T.; Writing—review and editing, J.J., J.K., M.P., M.R. (Magdalena Rogalska), D.Z.-M., M.R. (Marta Rorat), B.L., P.C., K.S., A.P., A.D., I.Z., W.M., D.K., K.K., R.P., G.A., B.O.-G., M.F., B.S., B.B., P.F.-C., R.F. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
SARSTer project was supported by Polish Association of Epidemiologists and Infectiologists (PTEiLChZ).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Medical University of Białystok (29 October 2020, number APK.002.303.2020). Additionally, the local bioethics committees approved the experimental use of tocilizumab in patients with COVID-19 and written informed consent was obtained from each patient before the treatment initiation.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data can be shared up on request.
Conflicts of Interest
Jerzy Jaroszewicz—received lectures honoraria and travel grants from Abbvie, Bausch Health, Gilead, MSD and Roche Diagnostics and served in advisory boards of Abbvie, Gilead and MSD; Małgorzata Pawłowska—lectures honoraria from Abbvie, Bausch Health, Gilead, MSD and Roche, member of advisory boards of AbbVie, Gilead, MSD, Roche; DZM—received lectures honoraria from Abbvie, Gilead; Katarzyna Sikorska received lectures honoraria from Gilead; Anna Piekarska research grants and honoraria for ABBVIE, Gilead, ROCHE, MSD; WM—received lectures honoraria and travel grants from Abbvie, Gilead, MSD, Janssen and Roche and served in advisory boards of Abbvie, Gilead and MSD; Dorota Kozielewicz received lectures honoraria from Gilead, Bausch Health; Regina Podlasin received lectures honoraria from Gilead; Bartosz Szetela received lectures honoraria and travel grants from Gilead, MSD, Glaxo and served in advisory boards of Gilead and Glaxo; Robert Flisiak received research grants and honoraria for lectures from AbbVie, Gilead, MSD, Roche; Krzysztof Tomasiewicz received personal fees from Gilead, Abbvie, Merck, Promed, and Roche. All remaining authors have declared no conflict of interest.
References
- Tian, Y.; Qiu, X.; Wang, C.; Zhao, J.; Jiang, X.; Niu, W.; Huang, J.; Zhang, F. Cancer associates with risk and severe events of COVID-19: A systematic review and meta-analysis. Int. J. Cancer 2021, 148, 363–374. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, J.; de la Cruz, J.; Cedillo, Á.; Llamas, P.; Duarte, R.; Jiménez-Yuste, V.; Hernández-Rivas, J.Á.; Gil-Manso, R.; Kwon, M.; Sánchez-Godoy, P.; et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: Lessons from a large population-based registry study. J. Hematol. Oncol. 2020, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.S.; Tagliamento, M.; Lambertini, M.; McNally, R.; Romano, M.; Leone, M.; Curigliano, G.; de Azambuja, E. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur. J. Cancer 2020, 139, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mulangu, S.; Dodd, L.E.; Davey, R.T., Jr.; Tshiani Mbaya, O.; Proschan, M.; Mukadi, D.; Lusakibanza Manzo, M.; Nzolo, D.; Tshomba Oloma, A.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9, eaal3653. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dood, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Preliminary Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 83, 1827–1837. [Google Scholar] [CrossRef]
- Flisiak, R.; Zarębska-Michaluk, D.; Berkan-Kawińska, A.; Tudrujek-Zdunek, M.; Rogalska, M.; Piekarska, A.; Kozielewicz, D.; Kłos, K.; Rorat, M.; Bolewska, B.; et al. Remdesivir-based therapy improved recovery of patients with COVID-19 in the SARSTer multicentre, real-world study. Pol. Arch. Intern. Med. 2021, 131, 103–110. [Google Scholar] [CrossRef]
- Pagliano, P.; Sellitto, C.; Scarpati, G.; Ascione, T.; Conti, V.; Franci, G.; Piazza, O.; Filippelli, A. An overview of the preclinical discovery and development of remdesivir for the treatment of coronavirus disease 2019 (COVID-19). Expert Opin. Drug Discov. 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Okoli, G.N.; Rabbani, R.; Copstein, L.; Al-Juboori, A.; Askin, N.; Abou-Setta, A.M. Remdesivir for coronavirus disease 2019 (COVID-19): A systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Infect. Dis. 2021, 53, 691–699. [Google Scholar] [CrossRef]
- Gholamhoseini, M.T.; Yazdi-Feyzabadi, V.; Goudarzi, R.; Mehrolhassani, M.H. Safety and Efficacy of Remdesivir for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. J. Pharm. Pharm. Sci. 2021, 24, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khera, D.; Chugh, A.; Khera, P.S.; Chugh, V.K. Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: A systematic review and meta-analysis. BMJ Open 2021, 11, e048416. [Google Scholar] [CrossRef] [PubMed]
- IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed on 7 March 2022).
- Helleberg, M.; Niemann, C.U.; Moestrup, K.S.; Kirk, O.; Lebech, A.M.; Lane, C.; Lundgren, J. Persistent COVID-19 in an Immunocompromised Patient Temporarily Responsive to Two Courses of Remdesivir Therapy. J. Infect. Dis. 2020, 222, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Palomba, E.; Carrabba, M.; Zuglian, G.; Alagna, L.; Saltini, P.; Fortina, V.; Hu, C.; Bandera, A.; Fabio, G.; Gori, A.; et al. Treatment of SARS-CoV-2 relapse with remdesivir and neutralizing antibodies cocktail in a patient with X-linked agammaglobulinaemia. Int. J. Infect. Dis. 2021, 110, 338–340. [Google Scholar] [CrossRef]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Parczewski, M.; Piekarska, A.; Simon, K.; Tomasiewicz, K.; Zarębska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of 31 March 2020. Pol. Arch. Intern. Med. 2020, 130, 352–357. [Google Scholar]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Management of SARS-CoV-infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of 26 April 2021. Pol. Arch. Intern. Med. 2021, 131, 487–496. [Google Scholar] [CrossRef]
- Lee, L.Y.; Cazier, J.B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef]
- Williams, M.; Mi, E.; Le Calvez, K.; Chen, J.; Pakzad-Shahabi, L.; Dadhania, S.; Wang, J.; Ho, A.; Rabinowicz, S. Estimating the Risk of Death from COVID-19 in Adult Cancer Patients. Clin. Oncol. 2021, 33, e172–e179. [Google Scholar] [CrossRef]
- Garassino, M.C.; Whisenant, J.G.; Huang, L.C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C.; et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol. 2020, 21, 914–922. [Google Scholar] [CrossRef]
- Mamlouk, O.; Turin, A.; D’Achiardi, D.; Simbaqueba, C.; Dickson, K.; Franco-Vega, M.; Halm, J.; Mandayam, S. Clinical characteristics and outcomes of cancer patients with chronic kidney disease and coronavirus disease 2019. J. Clin. Oncol. 2021, 39, e18815. [Google Scholar] [CrossRef]
- Zarębska-Michaluk, D.; Jaroszewicz, J.; Rogalska, M.; Lorenc, B.; Rorat, M.; Szymanek-Pasternak, A.; Piekarska, A.; Berkan-Kawińska, A.; Sikorska, K.; Tudrujek-Zdunek, M.; et al. Impact of Kidney Failure on the Severity of COVID-19. J. Clin. Med. 2021, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.W.; Cazier, J.B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. UK Coronavirus Cancer Monitoring Project Team. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef]
- Shah, V.; Ko Ko, T.; Zuckerman, M.; Vidler, J.; Sharif, S.; Mehra, V.; Gandhi, S.; Kuhnl, A.; Yallop, D.; Avenoso, D.; et al. Poor outcome and prolonged persistence of SARS-CoV-2 RNA in COVID-19 patients with haematological malignancies; King’s College Hospital experience. Br. J. Haematol. 2020, 190, e279–e282. [Google Scholar] [CrossRef]
- NCCN Best Practices Guidance: Management of COVID-19 Infection in Patients with Cancer. Available online: https://www.nccn.org/docs/default-source/covid-19/2021-covid-infectious-disease-management.pdf?sfvrsn=63f70c30_7 (accessed on 15 January 2022).
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Poissy, J.; Belhadi, D.; Diallo, A.; Lê, M.P.; Peytavin, G.; Staub, T.; et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2021, 22, 209–221. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R.; Henao-Restrepo, A.M.; Preziosi, M.P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernández García, C.; Kieny, M.P.; et al. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [PubMed]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
