Cranial MRI in Childhood Acute Leukemia during Treatment and Follow-Up Including the Impact of Intrathecal MTX—A Single-Center Study and Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
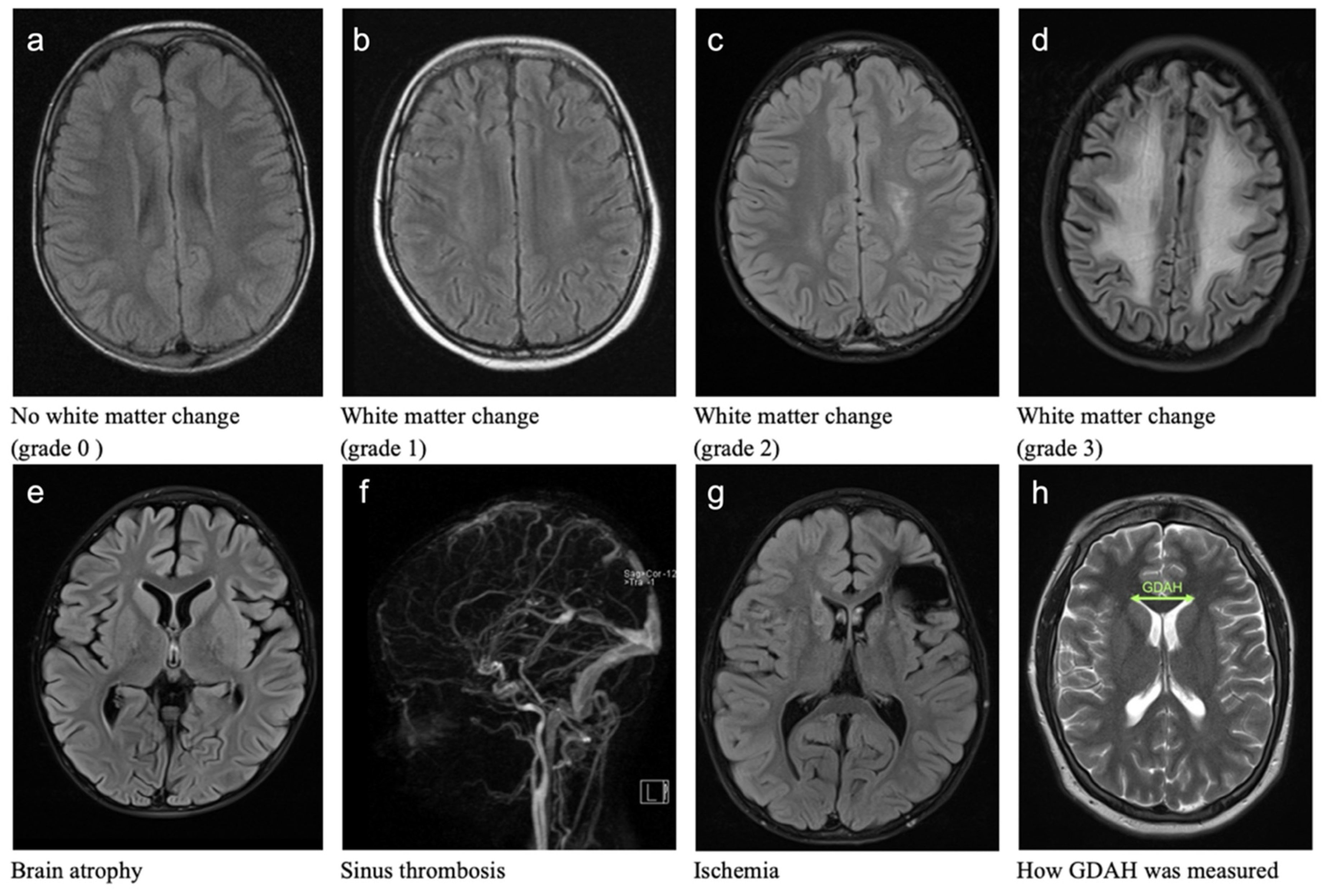
3.1. Cerebral Pathomorphologies
3.1.1. General Cerebral Pathomorphologies (CP)
3.1.2. White Matter Changes (WMC)
3.1.3. Sinus Vein Thrombosis (ST)
3.1.4. Brain Atrophy (BA)
3.1.5. Cerebral Ischemia
3.1.6. Ventricular Width (GDAH)
3.2. Intrathecal Methotrexate (ith. MTX) in Patients with Acute Leukemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossig, C.; Juergens, H.; Schrappe, M.; Moericke, A.; Henze, G.; von Stackelberg, A.; Reinhardt, D.; Burkhardt, B.; Woessmann, W.; Zimmermann, M.; et al. Effective Childhood Cancer Treatment: The Impact of Large Scale Clinical Trials in Germany and Austria. Pediatr. Blood Cancer 2013, 60, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Anastasopoulou, S.; Eriksson, M.A.; Heyman, M.; Wang, C.; Niinimäki, R.; Mikkel, S.; Vaitkevičienė, G.E.; Johannsdottir, I.M.; Myrberg, I.H.; Jonsson, O.G.; et al. Posterior Reversible Encephalopathy Syndrome in Children with Acute Lymphoblastic Leukemia: Clinical Characteristics, Risk Factors, Course, and Outcome of Disease. Pediatr. Blood Cancer 2019, 66, e27594. [Google Scholar] [CrossRef] [PubMed]
- Zając-Spychała, O.; Pawlak, M.; Karmelita-Katulska, K.; Pilarczyk, J.; Jończyk-Potoczna, K.; Przepióra, A.; Derwich, K.; Wachowiak, J. Anti-Leukemic Treatment-Induced Neurotoxicity in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia: Impact of Reduced Central Nervous System Radiotherapy and Intermediate- to High-Dose Methotrexate. Leuk. Lymphoma 2018, 59, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Zając-Spychała, O.; Pawlak, M.A.; Karmelita-Katulska, K.; Pilarczyk, J.; Derwich, K.; Wachowiak, J. Long-Term Brain Structural Magnetic Resonance Imaging and Cognitive Functioning in Children Treated for Acute Lymphoblastic Leukemia with High-Dose Methotrexate Chemotherapy Alone or Combined with CNS Radiotherapy at Reduced Total Dose to 12 Gy. Neuroradiology 2017, 59, 147–156. [Google Scholar] [CrossRef]
- Duffner, P.K.; Armstrong, F.D.; Chen, L.; Helton, K.J.; Brecher, M.L.; Bell, B.; Chauvenet, A.R. Neurocognitive and Neuroradiologic Central Nervous System Late Effects in Children Treated on Pediatric Oncology Group (POG) P9605 (Standard Risk) and P9201 (Lesser Risk) Acute Lymphoblastic Leukemia Protocols (ACCL0131). J. Pediatr. Hematol. Oncol. 2014, 36, 8–15. [Google Scholar] [CrossRef]
- Steinberg, S.; Hartmann, R.; Wisniewski, S.; Berger, K.; Beck, J.; Henze, G. Untersuchung von Spätfolgen Nach ZNS-Rezidiv Einer Akuten Lymphoblastischen Leukämie Im Kindesalter. Klin. Pädiatrie 1998, 210, 200–206. [Google Scholar] [CrossRef]
- Hertzberg, H.; Huk, W.J.; Ueberall, M.A.; Langer, T.; Meier, W.; Dopfer, R.; Skalej, M.; Lackner, H.; Bode, U.; Janssen, G.; et al. CNS Late Effects after ALL Therapy in Childhood. Part I: Neuroradiological Findings in Long-Term Survivors of Childhood ALL—An Evaluation of the Interferences between Morphology and Neuropsychological Performance. Med. Pediatr. Oncol. 1997, 28, 387–400. [Google Scholar] [CrossRef]
- Gandy, K.; Scoggins, M.A.; Jacola, L.M.; Litten, M.; Reddick, W.E.; Krull, K.R. Structural and Functional Brain Imaging in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia Treated with Chemotherapy: A Systematic Review. JNCI Cancer Spectr. 2021, 5, pkab069. [Google Scholar] [CrossRef]
- Lin, W.; Xie, J.; Zhang, J.; Cheng, H.; Cui, H.; Zhang, Y.; Wu, Y.; Yu, J.; Qi, P.; Fan, J.; et al. Posterior Reversible Encephalopathy Syndrome in Children with Acute Lymphoblastic Leukemia during Remission Induction Chemotherapy: A Single-Center Retrospective Study. Minerva Pediatr. 2019. [Google Scholar] [CrossRef]
- Bondarenko, V.P.; Tereschenko, G.V.; Andrianov, M.M.; Rumyantseva, Y.V.; Karachunsky, A.I.; Kasatkin, V.N.; Karelin, A.F.; Anisimov, V.N.; Zhukovskaya, E.V.; Rumyantsev, A.G. Magnetic Resonance Imaging of Changes in the Brain of Children Cured of Acute Lymphoblastic Leukemia. Hematol. Rep. 2019, 11, 70–74. [Google Scholar] [CrossRef]
- Follin, C.; Svärd, D.; van Westen, D.; Björkman-Burtscher, I.M.; Sundgren, P.C.; Fjalldal, S.; Lätt, J.; Nilsson, M.; Johanson, A.; Erfurth, E.M. Microstructural White Matter Alterations Associated to Neurocognitive Deficits in Childhood Leukemia Survivors Treated with Cranial Radiotherapy–a Diffusional Kurtosis Study. Acta Oncol. 2019, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Sabin, N.D.; Reddick, W.E.; Bhojwani, D.; Liu, W.; Brinkman, T.M.; Glass, J.O.; Hwang, S.N.; Srivastava, D.; Pui, C.H.; et al. Leukoencephalopathy and Long-Term Neurobehavioral, Neurocognitive andBrain Imaging Outcomes in Survivors of Childhood Acute Lymphoblastic Leukemia with Chemotherapy: A Longitudinal Analysis. Lancet Haematol. 2016, 3, e456–e466. [Google Scholar] [CrossRef]
- Svärd, D.; Erfurth, E.M.; Hellerstedt, R.; Mannfolk, P.; Mårtensson, J.; Sundgren, P.; Follin, C. Cognitive Interference Processing in Adult Survivors of Childhood Acute Lymphoblastic Leukemia Using Functional Magnetic Resonance Imaging. Acta Oncol. 2022, 61, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Guenette, J.P.; Tirumani, S.H.; Keraliya, A.R.; Shinagare, A.B.; Ramaiya, N.H.; Jagannathan, J.P. MRI Findings in Patients With Leukemia and Positive CSF Cytology: A Single-Institution 5-Year Experience. Am. J. Roentgenol. 2016, 207, 1278–1282. [Google Scholar] [CrossRef]
- Morioka, S.; Morimoto, M.; Yamada, K.; Hasegawa, T.; Morita, T.; Moroto, M.; Isoda, K.; Chiyonobu, T.; Imamura, T.; Nishimura, A.; et al. Effects of Chemotherapy on the Brain in Childhood: Diffusion Tensor Imaging of Subtle White Matter Damage. Neuroradiology 2013, 55, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Parasole, R.; Petruzziello, F.; Menna, G.; Mangione, A.; Cianciulli, E.; Buffardi, S.; Marchese, L.; Nastro, A.; Misuraca, A.; Poggi, V. Central Nervous System Complications during Treatment of Acute Lymphoblastic Leukemia in a Single Pediatric Institution. Leuk. Lymphoma 2010, 51, 1063–1071. [Google Scholar] [CrossRef]
- Ficek, K.; Blamek, S.; Syguła, D.; Miszczyk, L.; Sońta-Jakimczyk, D.; Tarnawski, R. Evaluation of the Late Effects of CNS Prophylactic Treatment in Childhood Acute Lymphoblastic Leukemia (ALL) Using Magnetic Resonance Spectroscopy. In Acta neurochirurgica. Supplement; Springer: Vienna, Austria, 2010; Volume 106, pp. 195–197. [Google Scholar] [CrossRef]
- Porto, L.; Preibisch, C.; Hattingen, E.; Bartels, M.; Lehrnbecher, T.; Dewitz, R.; Zanella, F.; Good, C.; Lanfermann, H.; DuMesnil, R.; et al. Voxel-Based Morphometry and Diffusion-Tensor MR Imaging of the Brain in Long-Term Survivors of Childhood Leukemia. Eur. Radiol. 2008, 18, 2691–2700. [Google Scholar] [CrossRef]
- Porto, L.; Kieslich, M.; Schwabe, D.; Zanella, F.E.; Lanfermann, H. Central Nervous System Imaging in Childhood Leukaemia. Eur. J. Cancer 2004, 40, 2082–2090. [Google Scholar] [CrossRef]
- Dellani, P.R.; Eder, S.; Gawehn, J.; Vucurevic, G.; Fellgiebel, A.; Müller, M.J.; Schmidberger, H.; Stoeter, P.; Gutjahr, P. Late Structural Alterations of Cerebral White Matter in Long-Term Survivors of Childhood Leukemia. J. Magn. Reson. Imaging 2008, 27, 1250–1255. [Google Scholar] [CrossRef]
- Lo Nigro, L.; Di Cataldo, A.; Schiliro, G. Acute Neurotoxicity in Children with B-Lineage Acute Lymphoblastic Leukemia (B-ALL) Treated with Intermediate Risk Protocols. Med. Pediatr. Oncol. 2000, 35, 449–455. [Google Scholar] [CrossRef]
- Mahoney, D.H.; Shuster, J.J.; Nitschke, R.; Lauer, S.J.; Steuber, C.P.; Winick, N.; Camitta, B. Acute Neurotoxicity in Children with B-Precursor Acute Lymphoid Leukemia: An Association with Intermediate-Dose Intravenous Methotrexate and Intrathecal Triple Therapy—A Pediatric Oncology Group Study. J. Clin. Oncol. 1998, 16, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Zimmerman, R.A.; Faro, S.; Bilaniuk, L.T.; Chou, T.Y.; Molloy, P.T. Childhood Leukemia: Central Nervous System Abnormalities during and after Treatment. AJNR Am. J. Neuroradiol. 1996, 17, 295–310. [Google Scholar] [PubMed]
- Pääkköö, E.; Talvensaari, K.; Pyhtinen, J.; Lanning, M. Late Cranial MRI after Cranial Irradiation in Survivors of Childhood Cancer. Neuroradiology 1994, 36, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Pääkköö, E.; Vainionpää, L.; Pyhtinen, J.; Lanning, M. Minor Changes on Cranial MRI during Treatment in Children with Acute Lymphoblastic Leukaemia. Neuroradiology 1996, 38, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.; Hamborg-Pedersen, B. Computed Tomography of the Brain Following Prophylactic Treatment with Irradiation Therapy and Intraspinal Methotrexate in Children with Acute Lymphoblastic Leukemia. Neuroradiology 1984, 26, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, K.; Gutjahr, P.; Kutzner, J. CT Studies before and after CNS Treatment for Acute Lymphoblastic Leukemia and Malignant Non-Hodgkin’s Lymphoma in Childhood. Neuroradiology 1980, 20, 173–180. [Google Scholar] [CrossRef]
- Phillips, N.S.; Cheung, Y.T.; Glass, J.O.; Scoggins, M.A.; Liu, W.; Ogg, R.J.; Mulrooney, D.A.; Pui, C.; Robison, L.L.; Reddick, W.E.; et al. Neuroanatomical Abnormalities Related to Dexamethasone Exposure in Survivors of Childhood Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2020, 67, 1–7. [Google Scholar] [CrossRef]
- Greiner, J.; Schrappe, M.; Claviez, A.; Zimmermann, M.; Niemeyer, C.; Kolb, R.; Eberl, W.; Berthold, F.; Bergsträsser, E.; Gnekow, A.; et al. THROMBOTECT—A Randomized Study Comparing Low Molecular Weight Heparin, Antithrombin and Unfractionated Heparin for Thromboprophylaxis during Induction Therapy of Acute Lymphoblastic Leukemia in Children and Adolescents. Haematologica 2019, 104, 756–765. [Google Scholar] [CrossRef]
- Attarbaschi, A.; Panzer-Grümayer, R.; Mann, G.; Möricke, A.; König, M.; Mecklenbräuker, A.; Teigler-Schlegel, A.; Bradtke, J.; Harbott, J.; Göhring, G.; et al. Minimal Residual Disease–Based Treatment Is Adequate for Relapse-Prone Childhood Acute Lymphoblastic Leukemia with an Intrachromosomal Amplification of Chromosome 21: The Experience of the ALL-BFM 2000 Trial. Klin. Pädiatrie 2014, 226, 338–343. [Google Scholar] [CrossRef]
- Ratei, R.; Basso, G.; Dworzak, M.; Gaipa, G.; Veltroni, M.; Rhein, P.; Biondi, A.; Schrappe, M.; Ludwig, W.-D.; Karawajew, L. Monitoring Treatment Response of Childhood Precursor B-Cell Acute Lymphoblastic Leukemia in the AIEOP-BFM-ALL 2000 Protocol with Multiparameter Flow Cytometry: Predictive Impact of Early Blast Reduction on the Remission Status after Induction. Leukemia 2009, 23, 528–534. [Google Scholar] [CrossRef]
- Creutzig, U.; Dworzak, M.; Zimmermann, M.; Bourquin, J.-P.; Gruhn, B.; Fleischhack, G.; Graf, N.; Klingebiel, T.; Kremens, B.; Lehrnbecher, T.; et al. Randomised Introduction of 2-CDA as Intensification during Consolidation for Children with High-Risk AML—Results from Study AML-BFM 2004. Klin. Pädiatrie 2015, 227, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Bermel, R.A.; Bakshi, R. The Measurement and Clinical Relevance of Brain Atrophy in Multiple Sclerosis. Lancet Neurol. 2006, 5, 158–170. [Google Scholar] [CrossRef]
- Iuvone, L.; Mariotti, P.; Colosimo, C.; Guzzetta, F.; Ruggiero, A.; Riccardi, R. Long-Term Cognitive Outcome, Brain Computed Tomography Scan, and Magnetic Resonance Imaging in Children Cured for Acute Lymphoblastic Leukemia. Cancer 2002, 95, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A. MR Signal Abnormalities at 1.5 T in Alzheimer’s Dementia and Normal Aging. Am. J. Neuroradiol. 1987, 8, 421–426. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Turner Ramli, L.D.; Blumhardt, T.; Jaspan, B.N.; Turner, B.; Blumhardt, L.; Ramli, N.; Jaspan, T. Ventricular Enlargement in Multiple Sclerosis: A Comparison of Three-Dimensional and Linear MRI Estimates. Neuroradiology 2001, 43, 608–614. [Google Scholar] [CrossRef]
- Sleurs, C.; Lemiere, J.; Vercruysse, T.; Nolf, N.; Van Calster, B.; Deprez, S.; Renard, M.; Vandecruys, E.; Benoit, Y.; Uyttebroeck, A. Intellectual Development of Childhood ALL Patients: A Multicenter Longitudinal Study. Psychooncology 2017, 26, 508–514. [Google Scholar] [CrossRef]
- Kanellopoulos, A.; Andersson, S.; Zeller, B.; Tamnes, C.K.; Fjell, A.M.; Walhovd, K.B.; Westlye, L.T.; Fosså, S.D.; Ruud, E. Neurocognitive Outcome in Very Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia After Treatment with Chemotherapy Only. Pediatr. Blood Cancer 2016, 63, 133–138. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Krull, K.R. Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia Treated on Contemporary Treatment Protocols: A Systematic Review. Neurosci. Biobehav. Rev. 2015, 53, 108–120. [Google Scholar] [CrossRef]
- Badr, M.A.; Hassan, T.H.; El-Gerby, K.M.; Lamey, M.E. Magnetic Resonance Imaging of the Brain in Survivors of Childhood Acute Lymphoblastic Leukemia. Oncol. Lett. 2013, 5, 621–626. [Google Scholar] [CrossRef]
- Rijmenams, I.; Moechars, D.; Uyttebroeck, A.; Radwan, A.; Blommaert, J.; Deprez, S.; Sunaert, S.; Segers, H.; Gillebert, C.R.; Lemiere, J.; et al. Age-and Intravenous Methotrexate-Associated Leukoencephalopathy and Its Neurological Impact in Pediatric Patients with Lymphoblastic Leukemia. Cancers 2021, 13, 1939. [Google Scholar] [CrossRef]
- van der Plas, E.; Spencer Noakes, T.L.; Butcher, D.T.; Weksberg, R.; Galin-Corini, L.; Wanstall, E.A.; Te, P.; Hopf, L.; Guger, S.; Spiegler, B.J.; et al. Quantitative MRI Outcomes in Child and Adolescent Leukemia Survivors: Evidence for Global Alterations in Gray and White Matter. Neuroimage 2020, 28, 102428. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, E.; Schachar, R.J.; Hitzler, J.; Crosbie, J.; Guger, S.L.; Spiegler, B.J.; Ito, S.; Nieman, B.J. Brain Structure, Working Memory and Response Inhibition in Childhood Leukemia Survivors. Brain Behav. 2017, 7, e00621. [Google Scholar] [CrossRef] [PubMed]
- Çetingül, N.; Aydinok, Y.; Kantar, M.; Öniz, H.; Kavakli, K.; Yalman, O.; Erermiş, S.; Cȩlebisoy, N.; Akyürekli, Ö.; Öztop, S.; et al. Neuropsychologic sequelae in the long-term survivors of childhood acute lymphoblastic leukemia. Pediatric Hematol. Oncol. 2009, 16, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kingma, A.; Van Dommelen, R.I.; Mooyaart, E.L.; Wilmink, J.T.; Deelman, B.G.; Kamps, W.A. Slight Cognitive Impairment and Magnetic Resonance Imaging Abnormalities but Normal School Levels in Children Treated for Acute Lymphoblastic Leukemia with Chemotherapy Only. J. Pediatr. 2001, 139, 413–420. [Google Scholar] [CrossRef]
- Yim, Y.S.; Mahoney, D.H.; Oshman, D.G. Hemiparesis and Ischemic Changes of the White Matter after Intrathecal Therapy for Children with Acute Lymphocytic Leukemia. Cancer 1991, 67, 2058–2061. [Google Scholar] [CrossRef]
- Salkade, P.; Lim, T. Methotrexate-Induced Acute Toxic Leukoencephalopathy. J. Cancer Res. Ther. 2012, 8, 292. [Google Scholar] [CrossRef]


| n MTX/Patient | ALL | AML | All | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Sum MTX | Patients | Sum MTX | Patients | Sum MTX | ||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| ≤12 | 7 | 12 | 70.6 | 84 | 43.7 | 12 | 12.8 | 84 | 6.0 | ||||
| 12 | 53 | 68.8 | 636 | 53.0 | 1 | 5.9 | 12 | 6.3 | 54 | 57.4 | 648 | 46.5 | |
| >12 | 14 | 1 | 5.9 | 14 | 7.3 | 1 | 1.0 | 14 | 1.0 | ||||
| 15 | 10 | 13.0 | 150 | 12.5 | 10 | 10.6 | 150 | 10.8 | |||||
| 16 | 1 | 1.3 | 16 | 1.3 | 1 | 1.1 | 16 | 1.1 | |||||
| 20 | 1 | 5.9 | 20 | 10.4 | 1 | 1.1 | 20 | 1.4 | |||||
| 28 | 4 | 5.2 | 112 | 9.3 | 4 | 4.3 | 112 | 8.1 | |||||
| 31 | 6 | 7.8 | 186 | 15.5 | 2 | 11.8 | 62 | 32.3 | 8 | 8.5 | 248 | 17.8 | |
| 32 | 2 | 2.6 | 64 | 5.3 | 2 | 2.1 | 64 | 4.6 | |||||
| 37 | 1 | 1.3 | 37 | 3.1 | 1 | 1.1 | 37 | 2.7 | |||||
| sum | 77 | 100 | 1201 | 100 | 17 | 100 | 192 | 100 | 94 | 100 | 1393 | 100 | |
| Mean MTX/patient | 15.6 | p = 0.000 | 11.3 | 14.8 | |||||||||
| Subgroup | Number of ith. MTX Applications | Chi-Quadrat Test | |
|---|---|---|---|
| ≤12 [n] | >12 [n] | ||
| Gender | |||
| Male/Female | 33/20 | 9/15 | 0.038 |
| Risk Group | |||
| IR/HR | 52/1 | 7/17 | 0.000 |
| Relapse | |||
| No/Yes | 53/0 | 11/13 | 0.000 |
| SCT | |||
| No/Yes | 53/0 | 9/15 | 0.000 |
| CNS positive | |||
| No/Yes | 52/1 | 19/5 | 0.010 |
| CNS irradiation | |||
| No/Yes | 51/2 | 20/4 | 0.072 |
| Brain atrophy | |||
| No/Yes | 29/24 | 15/9 | n.s |
| White matter changes | |||
| No/Yes | 44/9 | 18/6 | n.s |
| Sinus vein thrombosis | |||
| No/Yes | 52/1 | 22/2 | n.s |
| Ischemia | |||
| No/Yes | 51/2 | 21/3 | n.s |
| Ventricular Width (GDAH) | Time Period ≤ 30 Days | Time Period > 30 Days | ||
|---|---|---|---|---|
| Number of ith. MTX Applications | Number of ith. MTX Applications | |||
| ≤12 [n] | >12 [n] | ≤12 [n] | >12 [n] | |
| Mean at timepoint 1 (mm) | 32.9 | 33.4 | 33.6 | 34.6 |
| Mean at timepoint 2 (mm) | 33.7 | 34.3 | 34.4 | 36.3 |
| Mean at timepoint 3 (mm) | 32.0 | 35.9 | 33.3 | 35.5 |
| Median at timepoint 1 (mm) | 32.6 | 32.6 | 33.1 | 34.8 |
| Median at timepoint 2 (mm) | 35.3 | 33.9 | 35.7 | 36.3 |
| Median at timepoint 3 (mm) | 32.8 | 35.1 | 32.9 | 35.6 |
| Mean difference (t3-t1) (mm) | 1.8 | 1.0 | −0.3 | 0.9 |
| Mean difference (t2-t1) (mm) | −0.8 | −1.4 | 1.7 | 0.8 |
| Median difference (t3-t1) (mm) | 1.1 | 0.4 | 0.0 | 0.8 |
| Median difference (t2-t1) (mm) | 0.0 | 0.0 | 1.4 | 1.1 |
| Summary of Pathomorphologies in ALL | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Age | Follow-Up Time | Specific Imaging Findings | Neurological Findings and Symptoms | Percentage of Pathologic MRIs | Explanation | Recommendations | References | |
| WMC | 4483 | 0 y–19 y | 0.1 y–65 y | leukoencephalopathy (frontal and temporal), hyperdense regions, calcifications, meningeal enhancement, grey matter changes, smaller hippocampus and impaired microstructural white matter integrity in frontal brain regions, impaired white matter integrity, altered functional connectivity, microstructural damage in white matter, fornix, uncinate fasciculus, and ventral cingulum MRS, DTI, DKI are more sensitive than cranial MRI | headache, seizures, change in mental status neurocognitive deficits (vocabulary, memory, learning capacity, spatial ability, executive functions, and attention), lower IQ-performance, speech disorders, disorder in fine motor skills, coordination, widespread reductions in brain activation during cognitive tasks, poorer memory and fine-motor functioning outcome, long-term neurobehavioral problems, no significant relationships between MRI outcome and test scores, school placement, or education level, no correlation to neurocognitive impairment | 4–100% In 78.8% persistence | leukemia, relapse, low age, treatment, cranial irradiation, chemotherapy, i.v. MTX, ith. MTX, dexamethasone, infections |
| [5,6,8,10,11,12,15,17,18,19,22,23,34,41,42,45,46] |
| ST | 112 | 3 y–16 y | 9 d–6 y | superior sagittal sinus, sigmoid sinus | headache, seizures, hemiparesis, change in mental status | 2–14% | leukemia, relapse, treatment, infection |
| [19,23] |
| BA | 495 | 0.3 y–21.7 y | 0.1 y–28 y | decreased hippocampal, thalamus, temporal, occipital lobe, nucleus caudatus and cerebelli volume, grey and white matter atrophy | cognitive impairment, lower IQ, poorer verbal abilities, disorder in fine motor skills and coordination, more poorly in working memory and response inhibition, no significant correlation to imaging (2 studies) correlations between working memory and volume of amygdale, thalamus, striatum, and corpus callosum (1 study) | 4–100% | leukemia, ith. MTX, (high dose) chemotherapy, dexamethasone, cranial irradiation, females |
| [3,4,6,18,20,28,41,43,44] |
| Ischemia | 25 | 6.9 y ± 3.0 y | 6 y | old infarct and hemorrhage | 4% | cranial irradiation, HR | prospective studies | [41] | |
| Other | 2787 | 1 m–17.9 y | 10 d–37 y | PRES, stroke, hemorrhage meningioma, osteoma CNS lymphoma, inflammation, infections, no altered fMRI activity | seizures, visual disturbances, conscious disturbances, dizziness, headache, fever, ataxia, flaccid paralysis, altered mental status, neurocognitive impairment, longer response times and reduced accuracy performance during cognitive interference processing, no effect on IQ and cognitive development, no correlation to imaging | 1.5–28.6% | older age, T-cell ALL, relapse, CNS involvement, HR, induction chemotherapy, TiT, cranial irradiation, infection |
| [2,9,13,16,19,21,23,41] |
| Summary of pathomorphologies in AML | |||||||||
| Patients | Age | Follow-up time | Specific imaging findings | Neurological findings and symptoms | Percentage of pathologic MRIs | Explanation | Recommendations | References | |
| WMC | No reports | ||||||||
| ST | 5 | 3 y–16 y | under treatment | sigmoid sinus | Seizure, limb weakness | 1/5 | leukemia, treatment | early diagnosis | [23] |
| BA | No reports | ||||||||
| Ischemia | 5 | 0.6 y–13 y | under treatment | disseminated tiny lesions in thalamus and cerebral white matter | encephalopathy | 1/5 | leukemia, treatment, infection | early diagnosis | [23] |
| Other | 55 | 0.6 y–16 y | 0 d–5 y | disseminated microinfarcts, vasculopathy, hemorrhage, infections, aspergillosis, late: chordoma and other tumors | headache, ataxia, dizziness, altered mental status, hemiplegia, seizures, fever, sepsis | 24–28.6% | treatment, leukemia, CNS involvement, relapse, infection | early diagnosis studies to genetic polymorphism for risk factors | [19,23] |
| Patients (n) | Age at Diagnosis (Years) | ith. MTX Applications (n) | Increase in Ventricular Width (No. or % of Patients) | Other Findings | Follow-Up Time | Explanation | Recommendation | Reference |
|---|---|---|---|---|---|---|---|---|
| 118 | Mean: 5.8 0.3–16.1 | 6–8 | 37% with irradiation 24% without irradiation | Any MRI abnormality: 61/118 patients (51.7%) | 7 years | Cranial irradiation, ith. MTX | Prospective studies | [7] |
| 27 | Mean: 3.6 0.3–14 | unknown | 10/11 (irradiated patients) | In irradiated patients: -4 patients.: WMC -2 patients: meningioma -8 patients: low/heterogeneous intensity foci | 2–20 years | Cranial irradiation | No specific recommendation | [24] |
| 18 | Mean: 5.5 2.4–14.4 | Yes, but unknown n of applications | 13 (transient) | 2 patients (CNS negative) with WMC | 1–29 months | Steroid treatment ith. MTX | No follow-up with MRI is indicated | [25] |
| 28 | 3.9–14.4 | 6, but also 24 Gy cranial irradiation | 2 patients with severe cerebral atrophy had enlarged ventricles | 12 patients: slight atrophic changes 9 patients: severe cerebral atrophy | 1–10 years | Disease severity and treatment | Restrictive cranial irradiation | [26] |
| 60 | 1–14 | unknown | 10 increased at diagnosis 8/21 1 year after CNS treatment | No cranial pathomorphologies | up to 8 years | Cranial irradiation, ith. MTX | Prospective studies | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mergen, M.; Scheid, S.; Schubmehl, H.; Backens, M.; Reith, W.; Graf, N. Cranial MRI in Childhood Acute Leukemia during Treatment and Follow-Up Including the Impact of Intrathecal MTX—A Single-Center Study and Review of the Literature. Cancers 2022, 14, 4688. https://doi.org/10.3390/cancers14194688
Mergen M, Scheid S, Schubmehl H, Backens M, Reith W, Graf N. Cranial MRI in Childhood Acute Leukemia during Treatment and Follow-Up Including the Impact of Intrathecal MTX—A Single-Center Study and Review of the Literature. Cancers. 2022; 14(19):4688. https://doi.org/10.3390/cancers14194688
Chicago/Turabian StyleMergen, Marvin, Sascha Scheid, Hannah Schubmehl, Martin Backens, Wolfgang Reith, and Norbert Graf. 2022. "Cranial MRI in Childhood Acute Leukemia during Treatment and Follow-Up Including the Impact of Intrathecal MTX—A Single-Center Study and Review of the Literature" Cancers 14, no. 19: 4688. https://doi.org/10.3390/cancers14194688
APA StyleMergen, M., Scheid, S., Schubmehl, H., Backens, M., Reith, W., & Graf, N. (2022). Cranial MRI in Childhood Acute Leukemia during Treatment and Follow-Up Including the Impact of Intrathecal MTX—A Single-Center Study and Review of the Literature. Cancers, 14(19), 4688. https://doi.org/10.3390/cancers14194688






