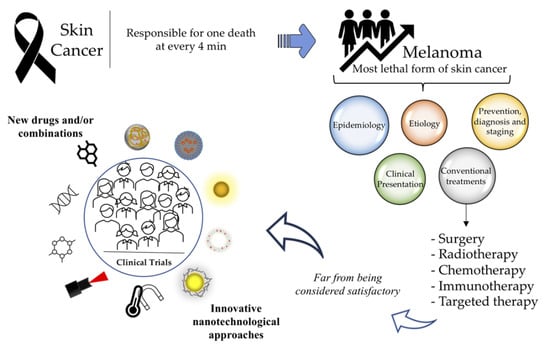
Melanoma Management: From Epidemiology to Treatment and Latest Advances
Abstract
Simple Summary
Abstract
1. Introduction
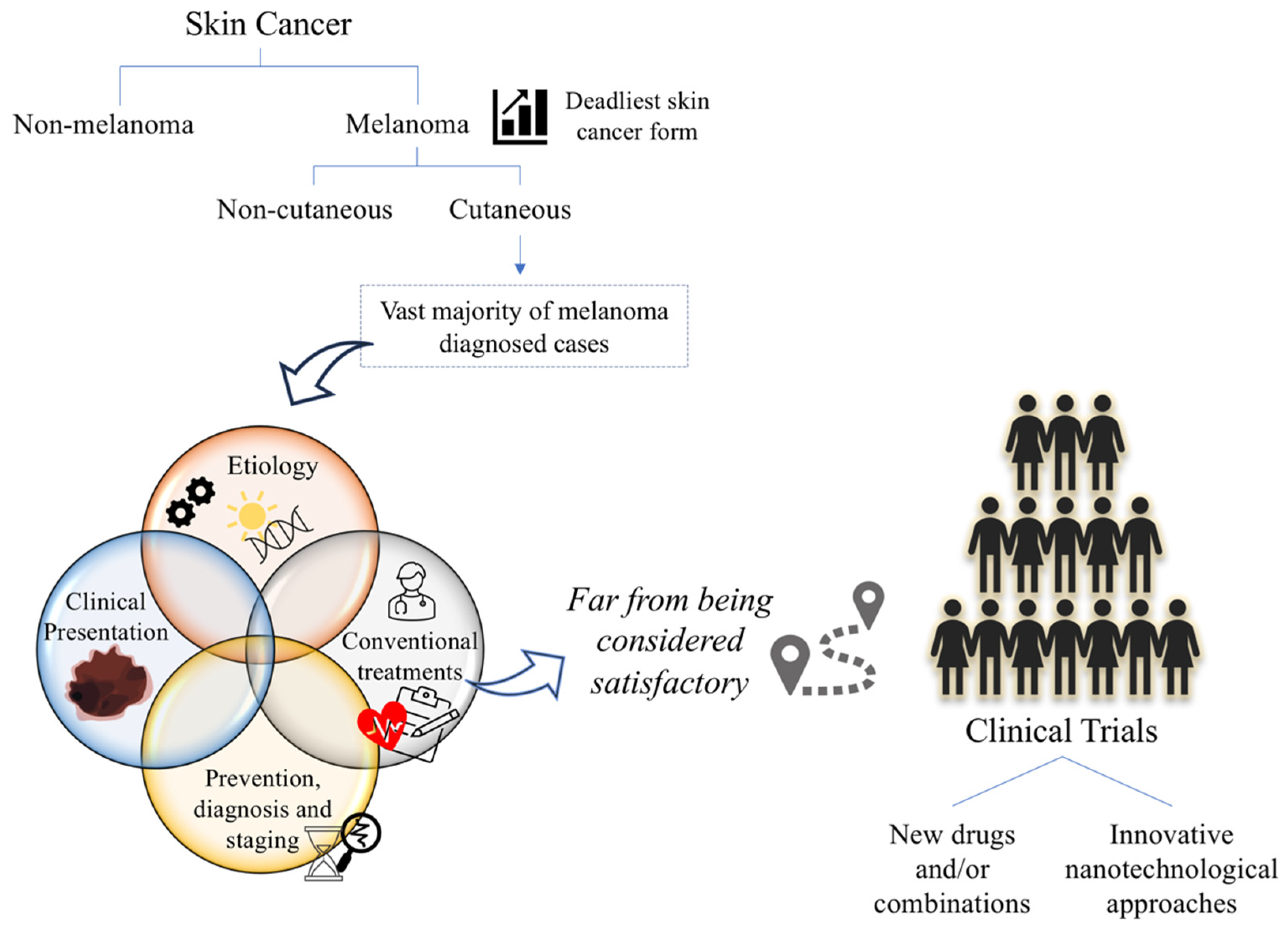
2. Cutaneous Melanoma
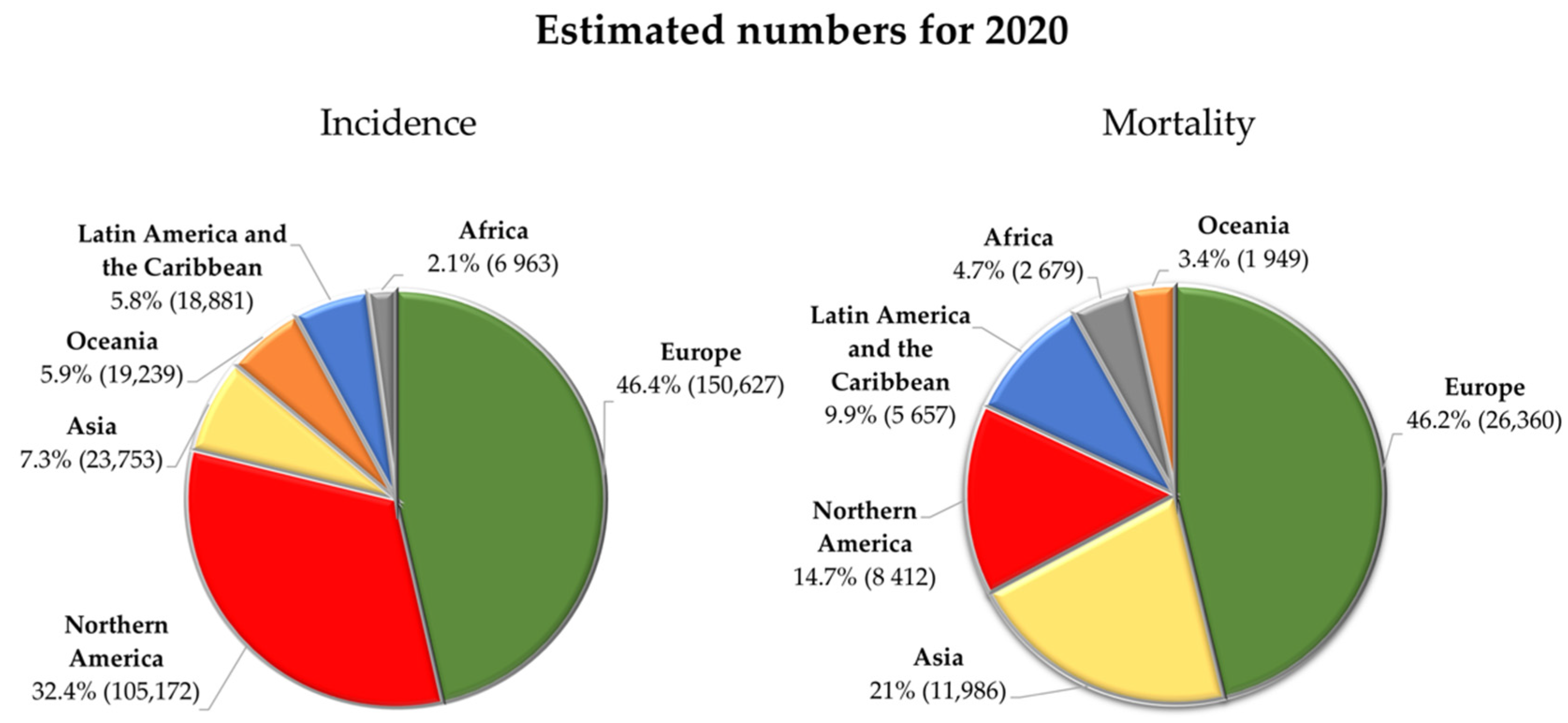
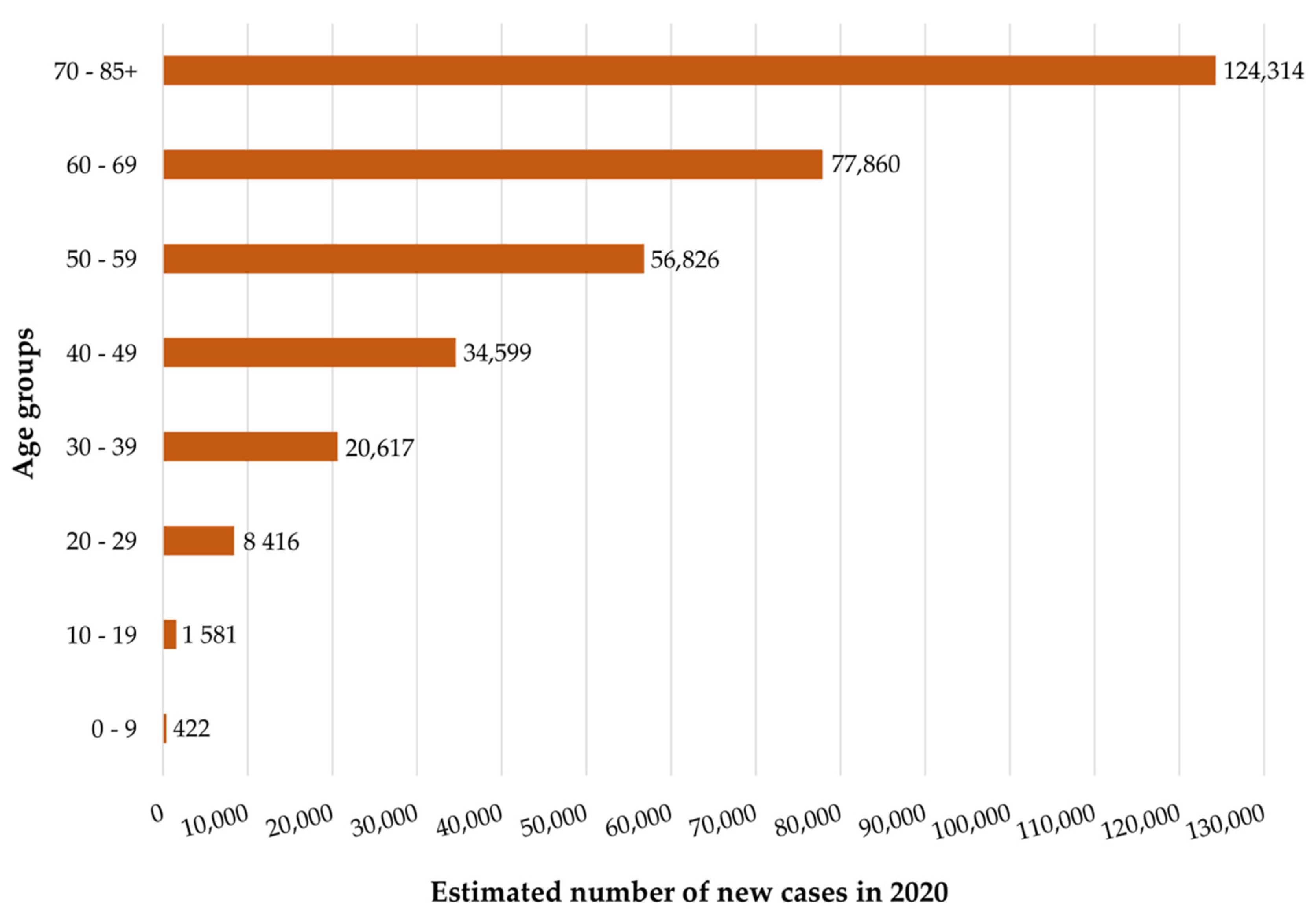
2.1. Epidemiology and Etiology
| Modifiable risk factors | Exposure to UV radiation (e.g., sunlight or use of tanning devices) |
| History of blistering sunburns at a young age | |
| Medications (e.g., psoralen or immunosuppressive drugs) | |
| Environmental exposure to chemicals (e.g., heavy metals or pesticides) | |
| Non-modifiable risk factors | Age |
| Sex | |
| Ethnicity | |
| Individual phenotypic characteristics (e.g., skin and light eyes, red or blond hair and high density of freckles) | |
| Clinical characteristics of the patient (e.g., increased number of common nevi or presence of atypical nevi) | |
| Personal and family history of skin cancers | |
| Personal history of diseases that compromise the immune system (e.g., hematologic malignancies or infection by HIV) | |
| Genetic alterations | |
| Specific genetic conditions (e.g., albinism or xeroderma pigmentosum) |
2.2. Clinical Presentation
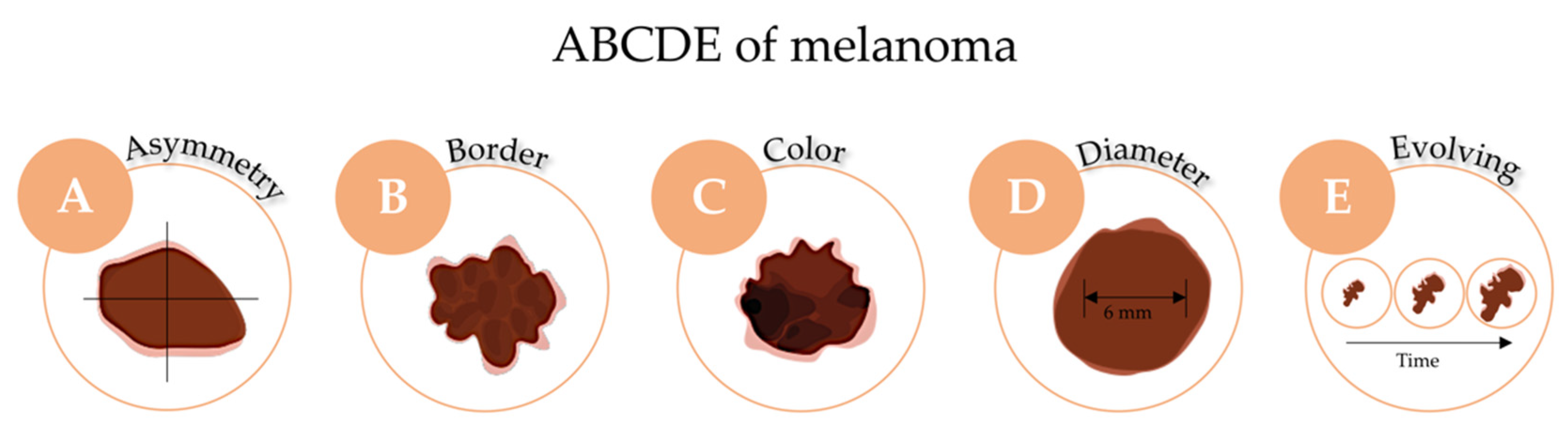
2.3. Prevention, Diagnosis and Staging
3. Challenges and Opportunities for Cutaneous Melanoma Treatment
3.1. Current Available Strategies
3.1.1. Surgery Resection
3.1.2. Radiotherapy
3.1.3. Chemotherapy
3.1.4. Immunotherapy
3.1.5. Targeted Therapy
3.1.6. Combination of Therapeutic Approaches
3.2. Ongoing Clinical Trials
3.3. Completed and Undergoing Innovative Nanotechnological Approaches on Clinical Trials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and Function of the Skin. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–14. ISBN 978-0-12-802926-8. [Google Scholar]
- Park, S. Biochemical, structural and physical changes in aging human skin, and their relationship. Biogerontology 2022, 23, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Batheja, P.; Kaushik, D.; Michniak, B. Structural and Biochemical Changes in Aging Skin and Their Impact on Skin Permeability Barrier. In Skin Aging Handbook; Dayan, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 55–90. ISBN 978-0-8155-1584-5. [Google Scholar]
- Mota, A.H.; Rijo, P.; Molpeceres, J.; Reis, C.P. Broad overview of engineering of functional nanosystems for skin delivery. Int. J. Pharm. 2017, 532, 710–728. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Collins, S.A.B.; Resneck, J.S.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e2. [Google Scholar] [CrossRef]
- Basra, M.K.; Shahrukh, M. Burden of skin diseases. Expert Rev. Pharmacoecon. Outcomes Res. 2014, 9, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.A.; Paul, C.; Nijsten, T.; Gisondi, P.; Salavastru, C.; Taieb, C.; Trakatelli, M.; Puig, L.; Stratigos, A. Prevalence of most common skin diseases in Europe: A population-based study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer Cancer Tomorrow. Estimated Number of New Cases from 2020 to 2040 of Melanoma of Skin and Non-Melanoma Skin Cancer. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16_17&single_unit=50000&group_cancers=1&multiple_cancers=1 (accessed on 9 August 2022).
- International Agency for Research on Cancer Cancer Tomorrow. Estimated Number of Deaths from 2020 to 2040 of Melanoma of Skin and Non-Melanoma Skin Cancer. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16_17&single_unit=5000&group_cancers=1&multiple_cancers=1&types=1 (accessed on 9 August 2022).
- Euro Melanoma and Global Coaliation for Melanoma Patient Advocacy. 2020 Melanoma Skin Cancer Report: Stemming the Global Epidemic; Global Coalition for Melanoma Patient Advocacy: Oldham, UK, 2020. [Google Scholar]
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin Cancer. Prim. Care 2015, 42, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Lai, V.; Cranwell, W.; Sinclair, R. Epidemiology of skin cancer in the mature patient. Clin. Dermatol. 2018, 36, 167–176. [Google Scholar] [CrossRef]
- Rebecca, V.W.; Sondak, V.K.; Smalley, K.S.M. A brief history of melanoma: From mummies to mutations. Melanoma Res. 2012, 22, 114–122. [Google Scholar] [CrossRef]
- Matthews, N.H.; Li, W.-Q.; Qureshi, A.A.; Weinstock, M.A.; Cho, E. Epidemiology of Melanoma. In Cutaneous Melanoma, Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017; pp. 139–149. ISBN 978-0-9944381-4-0. [Google Scholar]
- Dimitriou, F.; Krattinger, R.; Ramelyte, E.; Barysch, M.J.; Micaletto, S.; Dummer, R.; Goldinger, S.M. The World of Melanoma: Epidemiologic, Genetic, and Anatomic Differences of Melanoma Across the Globe. Curr. Oncol. Rep. 2018, 20, 87. [Google Scholar] [CrossRef]
- Sommer, L. Generation of melanocytes from neural crest cells. Pigment. Cell Melanoma Res. 2011, 24, 411–421. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Caksa, S.; Baqai, U.; Aplin, A.E. The future of targeted kinase inhibitors in melanoma. Pharmacol. Ther. 2022, 239, 108200. [Google Scholar] [CrossRef] [PubMed]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A. Heterogeneity of neural crest-derived melanocytes. Cent. Eur. J. Biol. 2013, 8, 315–330. [Google Scholar] [CrossRef]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. N. Am. 2020, 100, 1–12. [Google Scholar] [CrossRef]
- Davey, M.G.; Miller, N.; McInerney, N.M. A Review of Epidemiology and Cancer Biology of Malignant Melanoma. Cureus 2021, 13, e15087. [Google Scholar] [CrossRef]
- Pérez-Guijarro, E.; Day, C.P.; Merlino, G.; Zaidi, M.R. Genetically engineered mouse models of melanoma. Cancer 2017, 123, 2089–2103. [Google Scholar] [CrossRef]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Bastholt, L.; Grob, J.-J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.J.; et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline—Update 2016. Eur. J. Cancer 2016, 63, 201–217. [Google Scholar] [CrossRef]
- Millet, A.; Martin, A.R.; Ronco, C.; Rocchi, S.; Benhida, R. Metastatic Melanoma: Insights Into the Evolution of the Treatments and Future Challenges. Med. Res. Rev. 2017, 37, 98–148. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics—Update 2019. Eur. J. Cancer 2020, 126, 141–158. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of Skin Cancer. In Sunlight, VitaminD and Skin Cancer; Reichrath, J., Ed.; Springer: New York, NY, USA, 2014; pp. 120–140. ISBN 978-1-4939-0437-2. [Google Scholar]
- Gupta, A.K.; Bharadwaj, M.; Mehrotra, R. Skin Cancer Concerns in People of Color: Risk Factors and Prevention. Asian Pac. J. Cancer Prev. 2016, 17, 5257. [Google Scholar]
- Zambrano-Román, M.; Padilla-Gutiérrez, J.R.; Valle, Y.; Muñoz-Valle, J.F.; Valdés-Alvarado, E. Non-Melanoma Skin Cancer: A Genetic Update and Future Perspectives. Cancers 2022, 14, 2371. [Google Scholar] [CrossRef] [PubMed]
- Mozzillo, N.; Ascierto, P.A. Melanoma: The role of surgery in the era of new therapies. J. Transl. Med. 2014, 12, 195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kozar, I.; Margue, C.; Rothengatter, S.; Haan, C.; Kreis, S. Many ways to resistance: How melanoma cells evade targeted therapies. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.M.; Fisher, D.E. Biology of Melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 29–56. [Google Scholar] [CrossRef]
- Matias, M.; Pinho, J.O.; Penetra, M.J.; Campos, G.; Reis, C.P.; Gaspar, M.M. The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval. Cells 2021, 10, 3088. [Google Scholar] [CrossRef]
- Pinho, J.O.; Lopes, J.; Albino, M.; Reis, C.; Matias, M.; Gaspar, M.M. Advances in Nanotechnology-Related Strategies Against Melanoma. In Mitochondrial Dysfunction and Nanotherapeutics; de Oliveira, M.R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 385–424. ISBN 978-0-323-85666-9. [Google Scholar]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. How to Treat Melanoma? The Current Status of Innovative Nanotechnological Strategies and the Role of Minimally Invasive Approaches like PTT and PDT. Pharmaceutics 2022, 14, 1817. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.; Caruana, P.; la Fuente, N.D.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- de Lázaro, I.; Mooney, D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021, 11, 1469–1479. [Google Scholar] [CrossRef]
- Beiu, C.; Giurcaneanu, C.; Grumezescu, A.M.; Holban, A.M.; Popa, L.G.; Mihai, M.M. Nanosystems for Improved Targeted Therapies in Melanoma. J. Clin. Med. 2020, 9, 318. [Google Scholar] [CrossRef]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta. Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Cancer Today. Estimated Number of New Cases Worlwide in 2020, Melanoma of Skin, Both Sexes, All Ages (Global Cancer Observatory). Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=16&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&g (accessed on 30 August 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Cancer Today. Estimated Number of Deaths Worlwide in 2020, Melanoma of Skin, Both Sexes, All Ages (Global Cancer Observatory). Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=16&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&g (accessed on 30 August 2022).
- Silva, G.S.; Rosenbach, M. Climate change and dermatology: An introduction to a special topic, for this special issue. Int. J. Women’s Dermatol. 2021, 7, 3–7. [Google Scholar] [CrossRef]
- Parker, E.R. The influence of climate change on skin cancer incidence—A review of the evidence. Int. J. Women’s Dermatol. 2021, 7, 17–27. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Cancer Tomorrow. Estimated Number of New Cases from 2020 to 2040 of Melanoma of Skin. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16&single_unit=50000&group_cancers=1&multiple_cancers=1 (accessed on 9 August 2022).
- International Agency for Research on Cancer Cancer Tomorrow. —Estimated Number of Deaths from 2020 to 2040 of Melanoma of Skin. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16&single_unit=5000&group_cancers=1&multiple_cancers=1&types=1 (accessed on 9 August 2022).
- Wróbel, S.; Przybyło, M.; Stępień, E. The Clinical Trial Landscape for Melanoma Therapies. J. Clin. Med. 2019, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Dzwierzynski, W.W. Melanoma Risk Factors and Prevention. Clin. Plast. Surg. 2021, 48, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Le Clair, M.Z.; Cockburn, M.G. Tanning bed use and melanoma: Establishing risk and improving prevention interventions. Prev. Med. Rep. 2016, 3, 139–144. [Google Scholar] [CrossRef] [PubMed]
- El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens-part D: Radiation. Lancet Oncol. 2009, 10, 751–752. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Cancer Today. Estimated Number of New Cases Worldwide in 2020, Melanoma of Skin, Both Sexes, Per Countries (Global Cancer Observatory). Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=population&mode_population=countries&population=900&populations=900&key=asr&sex=0&cancer=16&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer= (accessed on 2 September 2022).
- Emri, G.; Paragh, G.; Tósaki, Á.; Janka, E.; Kollár, S.; Hegedűs, C.; Gellén, E.; Horkay, I.; Koncz, G.; Remenyik, É. Ultraviolet radiation-mediated development of cutaneous melanoma: An update. J. Photochem. Photobiol. B. Biol. 2018, 185, 169–175. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Solar and Ultraviolet Radiation. In Radiation—A Review of Human Carcinogens, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Galichet, L., Ed.; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100D, pp. 35–90. ISBN 978-92-832-0136-6. [Google Scholar]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Paluncic, J.; Kovacevic, Z.; Jansson, P.J.; Kalinowski, D.; Merlot, A.M.; Huang, M.L.H.; Lok, H.C.; Sahni, S.; Lane, D.J.R.; Richardson, D.R. Roads to melanoma: Key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim. Biophys. Acta—Mol. Cell Res. 2016, 1863, 770–784. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Cancer Today. Estimated Number of New Skin Melanoma Cases Worldwide in 2020 for Both Sexes (Global Cancer Observatory). Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=16&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer (accessed on 1 September 2022).
- Morgese, F.; Sampaolesi, C.; Torniai, M.; Conti, A.; Ranallo, N.; Giacchetti, A.; Serresi, S.; Onofri, A.; Burattini, M.; Ricotti, G.; et al. Gender Differences and Outcomes in Melanoma Patients. Oncol. Ther. 2020, 8, 103–114. [Google Scholar] [CrossRef]
- Bellenghi, M.; Puglisi, R.; Pontecorvi, G.; De Feo, A.; Carè, A.; Mattia, G. Sex and Gender Disparities in Melanoma. Cancers 2020, 12, 1819. [Google Scholar] [CrossRef]
- Orthaber, K.; Pristovnik, M.; Skok, K.; Perić, B.; Maver, U. Skin Cancer and Its Treatment: Novel Treatment Approaches with Emphasis on Nanotechnology. J. Nanomater. 2018, 94, 409–420. [Google Scholar] [CrossRef]
- Naik, P.P.; Farrukh, S.N. Influence of Ethnicities and Skin Color Variations in Different Populations: A Review. Skin Pharmacol. Physiol. 2021, 35, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Xu, S. Adaptation of human skin color in various populations. Hereditas 2017, 155, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hessler, M.; Jalilian, E.; Xu, Q.; Reddy, S.; Horton, L.; Elkin, K.; Manwar, R.; Tsoukas, M.; Mehregan, D.; Avanaki, K. Melanoma Biomarkers and Their Potential Application for in Vivo Diagnostic Imaging Modalities. Int. J. Mol. Sci. 2020, 21, 9583. [Google Scholar] [CrossRef]
- Nasti, T.H.; Timares, L. MC1R, Eumelanin and Pheomelanin: Their Role in Determining the Susceptibility to Skin Cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A.; Sanguedolce, F.; Murray-Stewart, T. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Podlipnik, S.; Potrony, M.; Puig, S. Genetic markers for characterization and prediction of prognosis of melanoma subtypes: A 2021 update. Ital. J. Dermatol. Venereol. 2021, 156, 322–330. [Google Scholar] [CrossRef]
- Zocchi, L.; Lontano, A.; Merli, M.; Dika, E.; Nagore, E.; Quaglino, P.; Puig, S.; Ribero, S. Familial Melanoma and Susceptibility Genes: A Review of the Most Common Clinical and Dermoscopic Phenotypic Aspect, Associated Malignancies and Practical Tips for Management. J. Clin. Med. 2021, 10, 3760. [Google Scholar] [CrossRef]
- Potrony, M.; Badenas, C.; Aguilera, P.; Puig-Butille, J.A.; Carrera, C.; Malvehy, J.; Puig, S. Update in genetic susceptibility in melanoma. Ann. Transl. Med. 2015, 3, 210. [Google Scholar]
- Ming, Z.; Lim, S.Y.; Kefford, R.F.; Rizos, H. Mitogen-activated protein kinase dependency in BRAF/RAS wild-type melanoma: A rationale for combination inhibitors. Pigment. Cell Melanoma Res. 2020, 33, 345–357. [Google Scholar] [CrossRef]
- Craig, S.; Earnshaw, C.H.; Virós, A. Ultraviolet light and melanoma. J. Pathol. 2018, 244, 578–585. [Google Scholar] [CrossRef]
- O’Neill, C.H.; Scoggins, C.R. Melanoma. J. Surg. Oncol. 2019, 120, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Imbernón-Moya, A.; Podlipnik, S.; Malvehy, J.; Puig, S. Initial Evaluation of Patients with Pigmented Skin Lesions. Actas Dermosifiliogr. 2016, 107, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Horrell, E.M.W.; Wilson, K.; D’Orazio, J.A. Melanoma—Epidemiology, Risk Factors, and the Role of Adaptive Pigmentation. In Melanoma—Current Clinical Management and Future Therapeutics; Murph, M., Ed.; IntechOpen: London, UK, 2015; ISBN 978-953-51-7237-6. [Google Scholar]
- Coricovac, D.; Dehelean, C.; Moaca, E.A.; Pinzaru, I.; Bratu, T.; Navolan, D.; Boruga, O. Cutaneous melanoma-a long road from experimental models to clinical outcome: A review. Int. J. Mol. Sci. 2018, 19, 1566. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, L.H.; Szasz, A.M.; Kuras, M.; Rodriguez Murillo, J.; Sugihara, Y.; Pla, I.; Horvath, Z.; Pawłowski, K.; Rezeli, M.; Miharada, K.; et al. The Hidden Story of Heterogeneous B-raf V600E Mutation Quantitative Protein Expression in Metastatic Melanoma—Association with Clinical Outcome and Tumor Phenotypes. Cancers 2019, 11, 1981. [Google Scholar] [CrossRef]
- Wagstaff, W.; Mwamba, R.N.; Grullon, K.; Armstrong, M.; Zhao, P.; Hendren-Santiago, B.; Qin, K.H.; Li, A.J.; Hu, D.A.; Youssef, A.; et al. Melanoma: Molecular genetics, metastasis, targeted therapies, immunotherapies, and therapeutic resistance. Genes Dis. 2022, 9, 1608–1623. [Google Scholar] [CrossRef]
- Šitum, M.; Buljan, M.; Kolić, M.; Vučić, M. Melanoma-Clinical, Dermatoscopical, and Histopathological Morphological Characteristics. Acta Dermatovenerol. Croat. 2014, 22, 1–12. [Google Scholar]
- Duncan, L.M.D. The Classification of Cutaneous Melanoma. Hematol. Oncol. Clin. N. Am. 2009, 23, 501–513. [Google Scholar] [CrossRef]
- Ammirati, C.T.; Hruza, G.J. Clinical presentations of cutaneous melanoma. Facial Plast. Surg. Clin. N. Am. 2005, 13, 33–46. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fujisawa, Y. Diagnosis and Management of Acral Lentiginous Melanoma. Curr. Treat. Options Oncol. 2018, 19, 1–11. [Google Scholar] [CrossRef]
- Goydos, J.S.; Shoen, S.L. Acral lentiginous melanoma. In Cancer Treatment and Research; Kaufman, H.L., Mehnert, J.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2016; Volume 167, pp. 321–329. ISBN 978-3-319-22539-5. [Google Scholar]
- Cabrera, R.; Recule, F. Unusual Clinical Presentations of Malignant Melanoma: A Review of Clinical and Histologic Features with Special Emphasis on Dermatoscopic Findings. Am. J. Clin. Dermatol. 2018, 19, 15–23. [Google Scholar] [CrossRef]
- Cormier, J.; Voss, R.; Woods, T.; Cromwell, K.; Nelson, K. Improving outcomes in patients with melanoma: Strategies to ensure an early diagnosis. Patient Relat. Outcome Meas. 2015, 6, 229. [Google Scholar] [CrossRef] [PubMed]
- Greinert, R.; Boniol, M. Skin cancer—Primary and secondary prevention (information campaigns and screening)—With a focus on children & sunbeds. Prog. Biophys. Mol. Biol. 2011, 107, 473–476. [Google Scholar] [PubMed]
- Fontanillas, P.; Alipanahi, B.; Furlotte, N.A.; Johnson, M.; Wilson, C.H.; Pitts, S.J.; Gentleman, R.; Auton, A. Disease risk scores for skin cancers. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Berwick, M.; Erdei, E.; Hay, J. Melanoma epidemiology and public health. Dermatol. Clin. 2009, 27, 205–214. [Google Scholar] [CrossRef]
- Abbasi, N.R.; Shaw, H.M.; Rigel, D.S.; Friedman, R.J.; McCarthy, W.H.; Osman, I.; Kopf, A.W.; Polsky, D. Early Diagnosis of Cutaneous Melanoma: Revisiting the ABCD Criteria. JAMA 2004, 292, 2771–2776. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Grainge, M.J.; Chuchu, N.; Ferrante di Ruffano, L.; Matin, R.N.; Thomson, D.R.; Wong, K.Y.; Aldridge, R.B.; Abbott, R.; et al. Visual inspection for diagnosing cutaneous melanoma in adults. Cochrane Database Syst. Rev. 2018, 12, CD013194. [Google Scholar] [CrossRef]
- Quaglino, P.; Fava, P.; Broganelli, P.; Burzi, L.; Marra, E.; Ribero, S.; Fierro, M.T. Epidemiology, Prevention and Clinical Diagnosis of Melanoma. In Current Management of Melanoma; Cafiero, F., De Cian, F., Eds.; Springer: Cham, Switzerland, 2021; pp. 7–16. ISBN 978-3-030-45347-3. [Google Scholar]
- Ward, W.H.; Lambreton, F.; Goel, N.; Yu, J.Q.; Farma, J.M. Clinical Presentation and Staging of Melanoma. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017; pp. 79–89. ISBN 978-0-9944381-4-0. [Google Scholar]
- Dorrell, D.N.; Strowd, L.C. Skin Cancer Detection Technology. Dermatol. Clin. 2019, 37, 527–536. [Google Scholar] [CrossRef]
- Gualco, M. Histopathological Examination: The Keystone of Treatment of Melanoma. In Current Management of Melanoma; Cafiero, F., De Cian, F., Eds.; Springer: Cham, Switzerland, 2021; pp. 27–37. ISBN 978-3-030-45347-3. [Google Scholar]
- Osella-Abate, S.; Bertero, L.; Senetta, R.; Mariani, S.; Lisa, F.; Coppola, V.; Metovic, J.; Pasini, B.; Puig, S.S.; Fierro, M.T.; et al. TERT Promoter Mutations are Associated with Visceral Spreading in Melanoma of the Trunk. Cancers 2019, 11, 452. [Google Scholar] [CrossRef]
- Dalmasso, B.; Vanni, I.; Bruno, W.; Andreotti, V.; Pastorino, L.; Spagnolo, F.; Ghiorzo, P. Molecular Assessment in Patients with Melanoma: When and Why? In Current Management of Melanoma; Cafiero, F., De Cian, F., Eds.; Springer: Cham, Switzerland, 2021; pp. 39–45. [Google Scholar]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Hartman, R.I.; Lin, J.Y. Cutaneous Melanoma—A Review in Detection, Staging, and Management. Hematol. Oncol. Clin. N. Am. 2019, 33, 25–38. [Google Scholar] [CrossRef]
- Meng, X.; Chen, J.; Zhang, Z.; Li, K.; Li, J.; Yu, Z.; Zhang, Y. Non-invasive optical methods for melanoma diagnosis. Photodiagnosis Photodyn. Ther. 2021, 34, 102266. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, F.; Boutros, A.; Croce, E.; Tanda, E.; Cecchi, F.; Queirolo, P. New Melanoma Staging: Prognostic Factors. In Current Management of Melanoma; Cafiero, F., De Cian, F., Eds.; Springer: Cham, Switzerland, 2021; pp. 47–53. ISBN 978-3-030-45347-3. [Google Scholar]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017, 67, 472. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef] [PubMed]
- Eddy, K.; Chen, S. Overcoming Immune Evasion in Melanoma. Int. J. Mol. Sci. 2020, 21, 8984. [Google Scholar] [CrossRef] [PubMed]
- Merlino, G.; Herlyn, M.; Fisher, D.E.; Bastian, B.C.; Flaherty, K.T.; Davies, M.A.; Wargo, J.A.; Curiel-Lewandrowski, C.; Weber, M.J.; Leachman, S.A.; et al. The state of melanoma: Challenges and opportunities. Pigment. Cell Melanoma Res. 2016, 29, 404–416. [Google Scholar] [CrossRef]
- Michielin, O.; Atkins, M.B.; Koon, H.B.; Dummer, R.; Ascierto, P.A. Evolving impact of long-term survival results on metastatic melanoma treatment. J. Immunother. Cancer 2020, 8, e000948. [Google Scholar] [CrossRef]
- Switzer, B.; Puzanov, I.; Skitzki, J.J.; Hamad, L.; Ernstoff, M.S. Managing Metastatic Melanoma in 2022: A Clinical Review. JCO Oncol. Pract. 2022, 18, 335–351. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Paskal, W.; Włodarski, P.K. Intratumor and Intertumor Heterogeneity in Melanoma. Transl. Oncol. 2017, 10, 956. [Google Scholar] [CrossRef]
- Ng, M.F.; Simmons, J.L.; Boyle, G.M. Heterogeneity in Melanoma. Cancers 2022, 14, 3030. [Google Scholar] [CrossRef]
- Food and Drug Administration. Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 10 August 2022).
- European Medicines Agency. Medicines Database. Available online: https://www.ema.europa.eu/en/medicines (accessed on 10 August 2022).
- Henriques, V.; Martins, T.; Link, W.; Ferreira, B.I. The Emerging Therapeutic Landscape of Advanced Melanoma. Curr. Pharm. Des. 2018, 24, 549–558. [Google Scholar] [CrossRef]
- Kozovska, Z.; Gabrisova, V.; Kucerova, L. Malignant melanoma: Diagnosis, treatment and cancer stem cells. Neoplasma 2016, 63, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2019. Eur. J. Cancer 2020, 126, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.M. Surgical Management of Melanoma. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017; pp. 91–100. ISBN 978-0-9944381-4-0. [Google Scholar]
- Friedman, E.B.; Thompson, J.F. Continuing and new roles for surgery in the management of patients with stage IV melanoma. Melanoma Manag. 2018, 5, MMT03. [Google Scholar] [CrossRef] [PubMed]
- Bello, D.M. Indications for the surgical resection of stage IV disease. J. Surg. Oncol. 2019, 119, 249–261. [Google Scholar] [CrossRef]
- Enomoto, L.M.; Levine, E.A.; Shen, P.; Votanopoulos, K.I. Role of Surgery for Metastatic Melanoma. Curr. Manag. Melanoma 2021, 100, 127–139. [Google Scholar] [CrossRef]
- Spagnolo, F.; Tanda, E.; Boutros, A.; Cecchi, F.; Queirolo, P. Medical Treatment of Melanoma: Adjuvant and Neoadjuvant Therapies. In Current Management of Melanoma; Cafiero, F., De Cian, F., Eds.; Springer: Cham, Switzerland, 2021; pp. 157–165. [Google Scholar]
- Spagnolo, F.; Boutros, A.; Tanda, E.; Cecchi, F.; Queirolo, P. Systemic Treatment in Advanced Melanoma. In Current Management of Melanoma; Cafiero, F., De Cian, F., Eds.; Springer: Cham, Switzerland, 2021; pp. 167–174. ISBN 978-3-030-45347-3. [Google Scholar]
- Pinho, J.O.; Matias, M.; Gaspar, M.M. Emergent nanotechnological strategies for systemic chemotherapy against melanoma. Nanomaterials 2019, 9, 1455. [Google Scholar] [CrossRef]
- Shi, W. Radiation Therapy for Melanoma. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017; pp. 101–120. ISBN 978-0-9944381-4-0. [Google Scholar]
- Sayan, A.; Plant, R.; Eccles, B.; Davies, C.; Ilankovan, V. Recent advances in the management of cutaneous malignant melanoma: Our case cohort. Br. J. Oral Maxillofac. Surg. 2021, 59, 534–545. [Google Scholar] [CrossRef]
- United States National Institutes of Health. ClinicalTrials.gov Database. Available online: https://clinicaltrials.gov/ (accessed on 10 August 2022).
- Wilson, M.A.; Schuchter, L.M. Chemotherapy for Melanoma. In Melanoma. Cancer Treatment and Research; Kaufman, H., Mehnert, J., Eds.; Springer: Cham, Switzerland, 2016; Volume 167, pp. 209–229. ISBN 978-3-319-22539-5. [Google Scholar]
- Gupta, A.; Gomes, F.; Lorigan, P. The role for chemotherapy in the modern management of melanoma. Melanoma Manag. 2017, 4, 125. [Google Scholar] [CrossRef]
- Luke, J.J.; Schwartz, G.K. Chemotherapy in the Management of Advanced Cutaneous Malignant Melanoma. Clin. Dermatol. 2013, 31, 290–297. [Google Scholar] [CrossRef]
- Middleton, M.R.; Grob, J.J.; Aaronson, N.; Fierlbeck, G.; Tilgen, W.; Seiter, S.; Gore, M.; Aamdal, S.; Cebon, J.; Coates, A.; et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol. 2000, 18, 158–166. [Google Scholar] [CrossRef]
- Gogas, H.J.; Kirkwood, J.M.; Sondak, V.K. Chemotherapy for metastatic melanoma. Cancer 2007, 109, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.S.; Chapman, P.B. The History and Future of Chemotherapy for Melanoma. Hematol. Oncol. Clin. N. Am. 2009, 23, 583. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Bertinato, L.; Staniszewska, M.; Pancer, K.; Wieczorek, M.; Salmaso, S.; Caliceti, P.; Garofalo, M. From Conventional Therapies to Immunotherapy: Melanoma Treatment in Review. Cancers 2020, 12, 3057. [Google Scholar] [CrossRef]
- Queirolo, P.; Boutros, A.; Tanda, E.; Spagnolo, F.; Quaglino, P. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: A model of cancer immunotherapy. Semin. Cancer Biol. 2019, 59, 290–297. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Approves Opdualag for Unresectable or Metastatic Melanoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-opdualag-unresectable-or-metastatic-melanoma (accessed on 11 August 2022).
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Wittig, J.A. Principles of Immunotherapy in Melanoma. Surg. Clin. N. Am. 2020, 100, 161–173. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernández-Rubio, L.; Morente, P.; Fernández-Hinojal, G.; Echaide, M.; Garnica, M.; et al. Understanding LAG-3 Signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar] [CrossRef]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.M.; Okazaki, T. LAG-3: From molecular functions to clinical applications. J. Immunother. Cancer 2020, 8, e001014. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Vukadin, S.; Khaznadar, F.; Kizivat, T.; Vcev, A.; Smolic, M. Molecular Mechanisms of Resistance to Immune Checkpoint Inhibitors in Melanoma Treatment: An Update. Biomedicines 2021, 9, 835. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Botticelli, A.; Visconti, I.C.; Angeletti, D.; Fiore, M.; Marchetti, P.; Lambiase, A.; de Vincentiis, M.; Greco, A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020, 2020, 9235638. [Google Scholar] [CrossRef]
- Huang, A.C.; Zappasodi, R. A decade of checkpoint blockade immunotherapy in melanoma: Understanding the molecular basis for immune sensitivity and resistance. Nat. Immunol. 2022, 23, 660–670. [Google Scholar] [CrossRef]
- Simeone, E.; Grimaldi, A.M.; Festino, L.; Trojaniello, C.; Vitale, M.G.; Vanella, V.; Palla, M.; Ascierto, P.A. Immunotherapy in metastatic melanoma: A novel scenario of new toxicities and their management. Melanoma Manag. 2019, 6, MMT30. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Patel, A.; Hossain, S.; Kaufman, H.L. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am. J. Clin. Dermatol. 2017, 18, 1–15. [Google Scholar] [CrossRef]
- Larocca, C.A.; LeBoeuf, N.R.; Silk, A.W.; Kaufman, H.L. An Update on the Role of Talimogene Laherparepvec (T-VEC) in the Treatment of Melanoma: Best Practices and Future Directions. Am. J. Clin. Dermatol. 2020, 21, 821–832. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.M.; Soares, P.; Pópulo, H. Melanoma treatment in review. Immuno Targets Ther. 2018, 7, 35. [Google Scholar] [CrossRef]
- Tanda, E.T.; Vanni, I.; Boutros, A.; Andreotti, V.; Bruno, W.; Ghiorzo, P.; Spagnolo, F. Current State of Target Treatment in BRAF Mutated Melanoma. Front. Mol. Biosci. 2020, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Amaral, T.; Sinnberg, T.; Meier, F.; Krepler, C.; Levesque, M.; Niessner, H.; Garbe, C. The mitogen-activated protein kinase pathway in melanoma part I—Activation and primary resistance mechanisms to BRAF inhibition. Eur. J. Cancer 2017, 73, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Bartnik, E.; Fiedorowicz, M.; Rutkowski, P. Targeted Therapy in Melanoma and Mechanisms of Resistance. Int. J. Mol. Sci. 2020, 21, 4576. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, J.; Mackiewicz, A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Contemp. Oncol. 2018, 22, 68. [Google Scholar] [CrossRef]
- Kasakovski, D.; Skrygan, M.; Gambichler, T.; Susok, L. Advances in Targeting Cutaneous Melanoma. Cancers 2021, 13, 2090. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef]
- Munhoz, R.R.; Postow, M.A. Combinatorial Approaches to the Treatment of Advanced Melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 145–158. [Google Scholar] [CrossRef]
- Haas, L.; Elewaut, A.; Gerard, C.L.; Umkehrer, C.; Leiendecker, L.; Pedersen, M.; Krecioch, I.; Hoffmann, D.; Novatchkova, M.; Kuttke, M.; et al. Acquired resistance to anti-MAPK targeted therapy confers an immune-evasive tumor microenvironment and cross-resistance to immunotherapy in melanoma. Nat. Cancer 2021, 2, 693–708. [Google Scholar] [CrossRef]
- Yu, C.; Liu, X.; Yang, J.; Zhang, M.; Jin, H.; Ma, X.; Shi, H. Combination of Immunotherapy with Targeted Therapy: Theory and Practice in Metastatic Melanoma. Front. Immunol. 2019, 10, 990. [Google Scholar] [CrossRef]
- Villani, A.; Potestio, L.; Fabbrocini, G.; Troncone, G.; Malapelle, U.; Scalvenzi, M. The Treatment of Advanced Melanoma: Therapeutic Update. Int. J. Mol. Sci. 2022, 23, 6388. [Google Scholar] [CrossRef]
- U.S. National Institutes of Health. Recruiting, Not Yet Recruiting, Active, Not Recruiting, Enrolling by Invitation Studies|Interventional Studies|Melanoma. Available online: https://clinicaltrials.gov/ct2/results?cond=Melanoma&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=Intr&rslt=&Search=Apply (accessed on 4 September 2022).
- Pinho, J.O.; Amaral, J.D.; Castro, R.E.; Rodrigues, C.M.; Casini, A.; Soveral, G.; Gaspar, M.M. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine 2019, 14, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine 2016, 11, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Merino, M.; Contreras, A.; Casares, N.; Troconiz, I.F.; ten Hagen, T.L.; Berraondo, P.; Zalba, S.; Garrido, M.J. A new immune-nanoplatform for promoting adaptive antitumor immune response. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, W.; Park, H.B.; Kwak, M.; Oh, J.; Lee, P.C.W.; Jin, J.O. Indocyanine green and poly I:C containing thermo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J. Immunother. Cancer 2019, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goto, P.L.; Siqueira-Moura, M.P.; Tedesco, A.C. Application of aluminum chloride phthalocyanine-loaded solid lipid nanoparticles for photodynamic inactivation of melanoma cells. Int. J. Pharm. 2017, 518, 228–241. [Google Scholar] [CrossRef]
- Clemente, N.; Ferrara, B.; Gigliotti, C.L.; Boggio, E.; Capucchio, M.T.; Biasibetti, E.; Schiffer, D.; Mellai, M.; Annovazzi, L.; Cangemi, L.; et al. Solid Lipid Nanoparticles Carrying Temozolomide for Melanoma Treatment. Preliminary in Vitro and in Vivo Studies. Int. J. Mol. Sci. 2018, 19, 255. [Google Scholar] [CrossRef]
- Malta, R.; Loureiro, J.B.; Costa, P.; Sousa, E.; Pinto, M.; Saraiva, L.; Amaral, M.H. Development of lipid nanoparticles containing the xanthone LEM2 for topical treatment of melanoma. J. Drug Deliv. Sci. Technol. 2021, 61, 102226. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B. Biointerfaces 2011, 85, 262–269. [Google Scholar] [CrossRef]
- Ferraz, L.S.; Watashi, C.M.; Colturato-Kido, C.; Pelegrino, M.T.; Paredes-Gamero, E.J.; Weller, R.B.; Seabra, A.B.; Rodrigues, T. Antitumor Potential of S-Nitrosothiol-Containing Polymeric Nanoparticles against Melanoma. Mol. Pharm. 2018, 15, 1160–1168. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Zhang, Z.; Han, J.; Yang, X.; Wang, A.; Zhang, X. Apatinib-loaded nanoparticles inhibit tumor growth and angiogenesis in a model of melanoma. Biochem. Biophys. Res. Commun. 2020, 521, 296–302. [Google Scholar] [CrossRef]
- Ledezma, D.K.; Balakrishnan, P.B.; Cano-Mejia, J.; Sweeney, E.E.; Hadley, M.; Bollard, C.M.; Villagra, A.; Fernandes, R. Indocyanine Green-Nexturastat A-PLGA Nanoparticles Combine Photothermal and Epigenetic Therapy for Melanoma. Nanomaterials 2020, 10, 161. [Google Scholar] [CrossRef]
- Pandesh, S.; Haghjooy Javanmard, S.; Shakeri-Zadeh, A.; Shokrani, P. Targeted Photothermal Therapy of Melanoma in C57BL/6 Mice using Fe3O4@Au Core-shell Nanoparticles and Near-infrared Laser. J. Biomed. Phys. Eng. 2021, 11, 29. [Google Scholar] [CrossRef]
- Zhang, X.; Teodoro, J.G.; Nadeau, J.L. Intratumoral gold-doxorubicin is effective in treating melanoma in mice. Nanomedicine 2015, 11, 1365–1375. [Google Scholar] [CrossRef]
- Lopes, J.; Ferreira-Gonçalves, T.; Figueiredo, I.V.; Rodrigues, C.M.P.; Ferreira, H.; Ferreira, D.; Viana, A.S.; Faísca, P.; Gaspar, M.M.; Coelho, J.M.P.; et al. Proof-of-Concept Study of Multifunctional Hybrid Nanoparticle System Combined with NIR Laser Irradiation for the Treatment of Melanoma. Biomolecules 2021, 11, 511. [Google Scholar] [CrossRef]
- Matos, C.P.; Albino, M.; Lopes, J.; Viana, A.S.; Côrte-Real, L.; Mendes, F.; Pessoa, J.C.; Tomaz, A.I.; Reis, C.P.; Gaspar, M.M.; et al. New iron(III) anti-cancer aminobisphenolate/phenanthroline complexes: Enhancing their therapeutic potential using nanoliposomes. Int. J. Pharm. 2022, 623, 121925. [Google Scholar] [CrossRef]
- Cruz, N.; Pinho, J.O.; Soveral, G.; Ascensão, L.; Matela, N.; Reis, C.; Gaspar, M.M. A Novel Hybrid Nanosystem Integrating Cytotoxic and Magnetic Properties as a Tool to Potentiate Melanoma Therapy. Nanomaterials 2020, 10, 693. [Google Scholar] [CrossRef]
- Lopes, J.; Coelho, J.M.P.; Vieira, P.M.C.; Viana, A.S.; Gaspar, M.M.; Reis, C. Preliminary assays towards melanoma cells using phototherapy with gold-based nanomaterials. Nanomaterials 2020, 10, 1536. [Google Scholar] [CrossRef]
- Hersh, E.M.; O’Day, S.J.; Ribas, A.; Samlowski, W.E.; Gordon, M.S.; Shechter, D.E.; Clawson, A.A.; Gonzalez, R. A phase 2 clinical trial of nab-paclitaxel in previously treated and chemotherapy-naive patients with metastatic melanoma. Cancer 2010, 116, 155–163. [Google Scholar]
- Kottschade, L.A.; Suman, V.J.; Amatruda, T.; McWilliams, R.R.; Mattar, B.I.; Nikcevich, D.A.; Behrens, R.; Fitch, T.R.; Jaslowski, A.J.; Markovic, S.N. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma. Cancer 2011, 117, 1704–1710. [Google Scholar] [CrossRef]
- Kottschade, L.A.; Suman, V.J.; Perez, D.G.; McWilliams, R.R.; Kaur, J.S.; Amatruda, T.T.; Geoffroy, F.J.; Gross, H.M.; Cohen, P.A.; Jaslowski, A.J.; et al. A randomized phase 2 study of temozolomide and bevacizumab or nab-paclitaxel, carboplatin, and bevacizumab in patients with unresectable stage IV melanoma. Cancer 2013, 119, 586–592. [Google Scholar] [CrossRef]
- U.S. National Institutes of Health. Nab-Paclitaxel and Bevacizumab or Ipilimumab as First-Line Therapy in Treating Patients with Stage IV Melanoma That Cannot Be Removed by Surgery—Study Results. Available online: https://clinicaltrials.gov/ct2/show/results/NCT02158520?term=NCT02158520&draw=2&rank=1 (accessed on 13 August 2022).
- U.S. National Institutes of Health. Abraxane and Avastin As Therapy for Patients with Malignant Melanoma, A Phase II Study—Study Results. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00462423?term=NCT00462423&draw=2&rank=1 (accessed on 13 August 2022).
- Hersh, E.M.; Del Vecchio, M.; Brown, M.P.; Kefford, R.; Loquai, C.; Testori, A.; Bhatia, S.; Gutzmer, R.; Conry, R.; Haydon, A.; et al. A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma. Ann. Oncol. 2015, 26, 2267. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Mita, M.M.; Ramanathan, R.K.; Weiss, G.J.; Mita, A.C.; Lorusso, P.M.; Burris, H.A.; Hart, L.L.; Low, S.C.; Parsons, D.M.; et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res. 2016, 22, 3157–3163. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Institutes of Health. Pharmacokinetic Study of Liposomal Vincristine in Patients with Malignant Melanoma & Hepatic Dysfunction—Study Results. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00145041?term=NCT00145041&draw=2&rank=1 (accessed on 13 August 2022).
- Bedikian, A.Y.; Silverman, J.A.; Papadopoulos, N.E.; Kim, K.B.; Hagey, A.E.; Vardeleon, A.; Hwu, W.J.; Homsi, J.; Davies, M.; Hwu, P. Pharmacokinetics and Safety of Marqibo (Vincristine Sulfate Liposomes Injection) in Cancer Patients with Impaired Liver Function. J. Clin. Pharmacol. 2011, 51, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Gargett, T.; Abbas, M.N.; Rolan, P.; Price, J.D.; Gosling, K.M.; Ferrante, A.; Ruszkiewicz, A.; Atmosukarto, I.I.C.; Altin, J.; Parish, C.R.; et al. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol. Immunother. 2018, 67, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
| Type of Treatment | Mechanism | Drug | FDA Approval Date | EMA Approval Date |
|---|---|---|---|---|
| Chemotherapy | ||||
| Alkylating agent | Dacarbazine | 1975 | 2002 | |
| Immunotherapy | ||||
| Antiviral | Interferon alpha-2b | 1996 | 2000 | |
| Peginterferon alpha-2b | 2011 | - | ||
| Interleukin | Interleukin-2 | 1998 | - | |
| Monoclonal antibody anti-CTLA4 | Ipilimumab | 2011 | 2011 | |
| Monoclonal antibody anti-PD-1 | Pembrolizumab | 2014 | 2015 | |
| Nivolumab | 2014 | 2015 | ||
| Monoclonal antibody anti-PD-L1 | Atezolizumab * | 2020 | - | |
| Monoclonal antibody anti-LAG-3 | Relatlimab-rmbw * | 2022 | - | |
| Oncolytic herpes virus | Talimogene laherparepvec | 2015 | 2015 | |
| Targeted therapy | ||||
| BRAF inhibitor | Vemurafenib | 2011 | 2012 | |
| Dabrafenib | 2013 | 2013 | ||
| Encorafenib * | 2018 | 2018 | ||
| MEK inhibitor | Trametinib | 2013 | 2014 | |
| Cobimetinib * | 2015 | 2015 | ||
| Binimetinib * | 2018 | 2018 | ||
| Type of Treatment | Name of Drugs | FDA Approval Date | EMA Approval Date | |
|---|---|---|---|---|
| Combinatorial approaches | Targeted therapy | Trametinib + Dabrafenib | 2014 | 2015 |
| Cobimetinib + Vemurafenib | 2015 | 2015 | ||
| Binimetinib + Encorafenib | 2018 | 2018 | ||
| Immunotherapy | Nivolumab + Ipilimumab | 2015 | 2016 | |
| Nivolumab + Relatlimab-rmbw | 2022 | - | ||
| Targeted therapy + Immunotherapy | Cobimetinib + Vemurafenib + Atezolizumab | 2020 | - | |
| Clinical Trial Phase | Clinical Trial Description | Melanoma Stage | Sponsor | Estimated Starting or Completion Date | Trial ID |
|---|---|---|---|---|---|
| 1 | Safety and efficacy of the combination of ipilimumab and imatinib mesylate. | IV | M.D. Anderson Cancer Center | 2013–2024 | NCT01738139 |
| Safety of the combination of panobinostat (histone deacetylase inhibitor) and ipilimumab. | III/IV | H. Lee Moffitt Cancer Center and Research Institute | 2014–2023 | NCT02032810 | |
| Safety and efficacy of the combination of imiquimod and pembrolizumab. | IIIB-IV | Mayo Clinic | 2017–2023 | NCT03276832 | |
| Efficacy of intermittent dosing in the combination of vemurafenib and cobimetinib in the treatment of advanced BRAF V600 mutant melanoma with elevated levels of LDH. | IIIC-IV | H. Lee Moffitt Cancer Center and Research Institute | 2018–2022 | NCT03543969 | |
| Safety of the administration of neoadjuvant atezolizumab treatment before surgery in non-metastatic resectable melanoma. | I/II | The Methodist Hospital Research Institute | 2020–2025 | NCT04020809 | |
| Safety and efficacy of the combination of PeptiCRAd-1 and pembrolizumab. | Inoperable or metastatic | Valo Therapeutics Oy | 2022–2024 | NCT05492682 | |
| 2 | Efficacy of the combination of T-VEC and pembrolizumab. | III/IV | National Cancer Institute | 2017–2023 | NCT02965716 |
| Safety and effectiveness of the combination of PD-L1 (atezolizumab) and anti-VEGF (bevacizumab) therapies. | III/IV | Elizabeth Buchbinder | 2020–2023 | NCT04356729 | |
| Efficacy of the combination of T-VEC and nivolumab. | IIIB/C/D/IVM1a | The Netherlands Cancer Institute | 2020–2023 | NCT04330430 | |
| Safety and efficacy of the combination of pembrolizumab and infliximab. | III/IV | Massachusetts General Hospital | 2022–2025 | NCT05034536 | |
| Safety and efficacy of the combination of PD-1 antibody tislelizumab and dacarbazine. | III/IV | Henan Cancer Hospital | 2022–2024 | NCT05466474 | |
| 3 | Efficacy of the immunization with natural dendritic cells as adjuvant treatment after complete radical lymph node dissection or sentinel node procedure. | IIIB/C | Radboud University Medical Center | 2016–2024 | NCT02993315 |
| Analysis of the safety, efficacy and pharmacokinetic between the combination of atezolizumab, cobimetinib and vemurafenib or combination of only cobimetinib and vemurafenib in previously untreated BRAF V600 mutant melanoma. | IIIC/IV | Hoffmann-La Roche | 2017–2023 | NCT02908672 | |
| Assessment of fixed-dose combination of relatlimab and nivolumab versus nivolumab monotherapy after complete resection. | III/IV | Bristol-Myers Squibb | 2021–2025 | NCT05002569 | |
| Safety and efficacy of the combination of encorafenib and binimetinib in comparison to placebo in BRAF V600E/K mutant melanoma. | IIB/C | Pierre Fabre Medicament | 2022–2035 | NCT05270044 | |
| 4 | Tolerability and long-term safety of dabrafenib and trametinib, alone or in combination. | Advanced or metastatic | Novartis Pharmaceuticals | 2017–2027 | NCT03340506 |
| Safety of pembrolizumab. | III/IV | Merck Sharp & Dohme LLC | 2019–2026 | NCT03715205 |
| Nanosystem | Main Clinical Trial Description | Melanoma Stage | Sponsor | Starting and Completion Date | Trial ID |
|---|---|---|---|---|---|
| Liposomes | Safety, efficacy and pharmacokinetic profile study of vincristine sulfate liposomes (Phase 1). | III/IV | Acrotech Biopharma LLC | 2005–2007 | NCT00145041 |
| Safety and immunogenicity of a dendritic cells targeted-liposomal vaccine (Phase 1). | IV | Lipotek Pty Ltd. | 2009–2012 | NCT01052142 | |
| Polymeric nanoparticles | Safety and efficacy of nanoparticle albumin-bound paclitaxel (Phase 2). | Unresectable or metastatic | Jonsson Comprehensive Cancer Center | 2004–2010 | NCT00081042 |
| Safety and efficacy of the combination of nanoparticle albumin-bound paclitaxel with carboplatin (Phase 2). | IV | Alliance for Clinical Trials in Oncology | 2006–2010 | NCT00404235 | |
| Safety and efficacy of the combination of nanoparticle albumin-bound paclitaxel with avastin (Phase 2). | III/IV | Lynn E. Spitler, MD | 2007–2012 | NCT00462423 | |
| Comparison of the safety and efficacy of the combination of bevacizumab, carboplatin and nanoparticle albumin-bound paclitaxel with the combination of bevacizumab and temozolomide (Phase 2). | IV | Alliance for Clinical Trials in Oncology | 2008–2012 | NCT00626405 | |
| Comparison of the safety and efficacy of nanoparticle albumin-bound paclitaxel versus dacarbazine (Phase 3). | IV | Celgene | 2009–2014 | NCT00864253 | |
| Safety and pharmacokinetic and pharmacodynamic profile of PSMA-targeted PLA/PEG docetaxel nanoparticles (Phase 1). | Advanced or metastatic | BIND Therapeutics | 2011–2016 | NCT01300533 | |
| Comparison of the safety and efficacy of the combination of nanoparticle albumin-bound paclitaxel with bevacizumab versus ipilimumab alone (Phase 2). | IV | Academic and Community Cancer Research United | 2013–2019 | NCT02158520 |
| Nanosystem | Main Clinical Trial Description | Melanoma Stage | Sponsor | Estimated Starting or Completion Date | Trial ID |
|---|---|---|---|---|---|
| Liposomes | Safety and tolerability of a liposomal tetravalent RNA-drug products vaccine (Phase 1). | IIIB/C/IV | BioNTech SE | 2015–2023 | NCT02410733 |
| Safety and feasibility of a tumor mRNA-loaded liposomal vaccine (Phase 1). | IIB-IV | University of Florida | 2022–2027 | NCT05264974 | |
| Lipid nanoparticles | Safety and efficacy of a lipid nanoparticle encapsulating mRNAs encoding a human T-cell co-stimulator and pro-inflammatory cytokines as monotherapy or in combination with durvalumab (Phase 1). | Advanced or metastatic | ModernaTX, Inc. | 2018–2023 | NCT03739931 |
| Polymeric nanoparticles | Safety and efficacy of the combination of nanoparticle albumin-bound paclitaxel with bevacizumab (Phase 1). | IV | Mayo Clinic | 2014–2025 | NCT02020707 |
| Comparison of the safety and efficacy of the combination of nanoparticle albumin-bound paclitaxel and carboplatin with and without endostatin (Phase 2). | Advanced | Peking University Cancer Hospital & Institute | 2019–2022 | NCT03917069 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers 2022, 14, 4652. https://doi.org/10.3390/cancers14194652
Lopes J, Rodrigues CMP, Gaspar MM, Reis CP. Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers. 2022; 14(19):4652. https://doi.org/10.3390/cancers14194652
Chicago/Turabian StyleLopes, Joana, Cecília M. P. Rodrigues, Maria Manuela Gaspar, and Catarina Pinto Reis. 2022. "Melanoma Management: From Epidemiology to Treatment and Latest Advances" Cancers 14, no. 19: 4652. https://doi.org/10.3390/cancers14194652
APA StyleLopes, J., Rodrigues, C. M. P., Gaspar, M. M., & Reis, C. P. (2022). Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers, 14(19), 4652. https://doi.org/10.3390/cancers14194652