Prognostic Value of the Serum HER2 Extracellular Domain Level in Breast Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
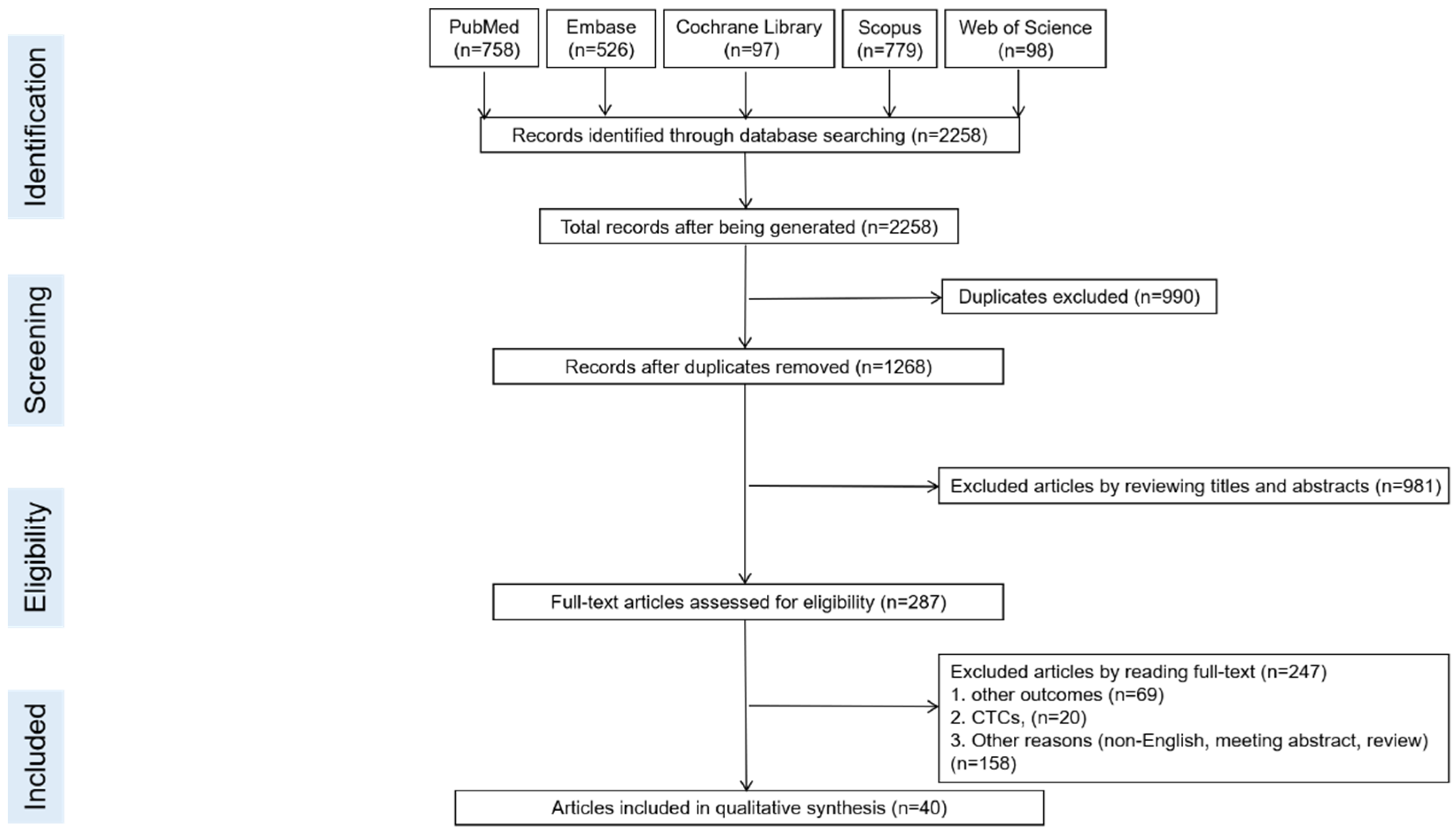
3. Results
3.1. Eligible Studies
3.2. Study Characteristics
3.3. Progression-Free Survival
3.4. Disease-Free Survival
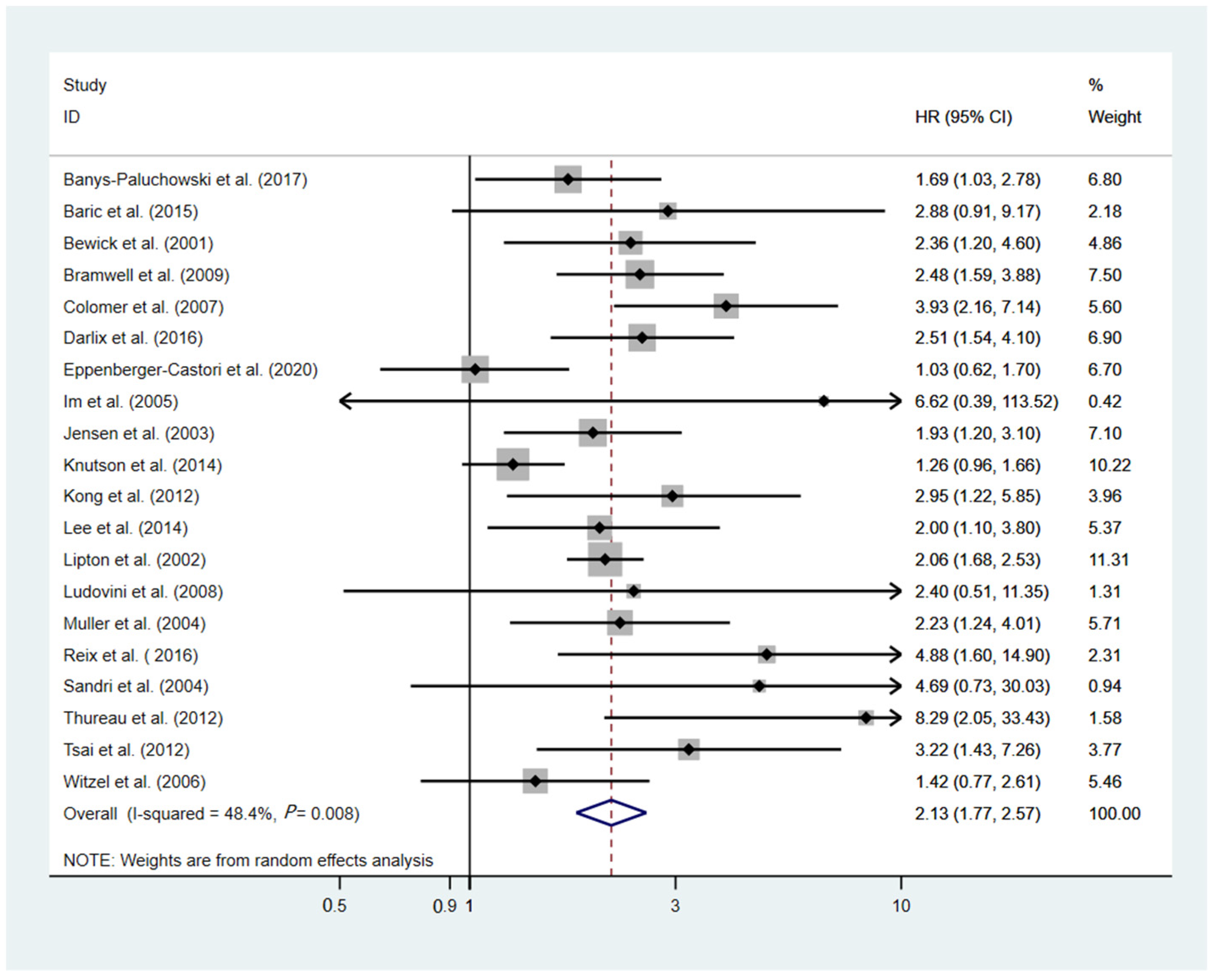
3.5. Overall Survival
3.6. Objective Response Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 31 July 2020).
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef]
- Perrier, A.; Gligorov, J.; Lefèvre, G.; Boissan, M. The extracellular domain of Her2 in serum as a biomarker of breast cancer. Lab. Investig. 2018, 98, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.L.; Sliwkowski, M.X.; Osborne, C.K.; Perez, E.A.; Puglisi, F.; Gianni, L. Treatment of HER2-positive breast cancer: Current status and future perspectives. Nat. Rev. Clin. Oncol. 2011, 9, 16–32. [Google Scholar] [CrossRef]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch. Pathol. Lab. Med. 2014, 138, 241–256. [Google Scholar] [CrossRef]
- Moreno-Aspitia, A.; Hillman, D.W.; Dyar, S.H.; Tenner, K.S.; Gralow, J.; Kaufman, P.A.; Davidson, N.E.; Lafky, J.M.; Reinholz, M.M.; Lingle, W.L.; et al. Soluble human epidermal growth factor receptor 2 (HER2) levels in patients with HER2-positive breast cancer receiving chemotherapy with or without trastuzumab: Results from North Central Cancer Treatment Group adjuvant trial N9831. Cancer 2013, 119, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A.; Leitzel, K.; Ali, S.M.; Carney, W.; Platek, G.; Steplewski, K.; Westlund, R.; Gagnon, R.; Martin, A.M.; Maltzman, J. Human epidermal growth factor receptor 2 (HER2) extracellular domain levels are associated with progression-free survival in patients with HER2-positive metastatic breast cancer receiving lapatinib monotherapy. Cancer 2011, 117, 5013–5020. [Google Scholar] [CrossRef]
- Carney, W.P.; Neumann, R.; Lipton, A.; Leitzel, K.; Ali, S.; Price, C.P. Potential clinical utility of serum HER-2/neu oncoprotein concentrations in patients with breast cancer. Clin. Chem. 2003, 49, 1579–1598. [Google Scholar] [CrossRef]
- Christianson, T.A.; Doherty, J.K.; Lin, Y.J.; Ramsey, E.E.; Holmes, R.; Keenan, E.J.; Clinton, G.M. NH2-terminally truncated HER-2/neu protein: Relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998, 58, 5123–5129. [Google Scholar]
- Reix, N.; Malina, C.; Chenard, M.P.; Bellocq, J.P.; Delpous, S.; Moliere, S.; Sevrin, A.; Neuberger, K.; Tomasetto, C.; Mathelin, C. A prospective study to assess the clinical utility of serum HER2 extracellular domain in breast cancer with HER2 overexpression. Breast Cancer Res. Treat. 2016, 160, 249–259. [Google Scholar] [CrossRef]
- Tsé, C.; Gauchez, A.S.; Jacot, W.; Lamy, P.J. HER2 shedding and serum HER2 extracellular domain: Biology and clinical utility in breast cancer. Cancer Treat. Rev. 2012, 38, 133–142. [Google Scholar] [CrossRef]
- Leyland-Jones, B.; Smith, B.R. Serum HER2 testing in patients with HER2-positive breast cancer: The death knell tolls. Lancet Oncol. 2011, 12, 286–295. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, J.; Zhang, S.; Bian, L.; Hu, H.; Xu, C.; Hao, X.; Liu, B.; Ye, Q.; Liu, Y.; et al. Meaningful interpretation of serum HER2 ECD levels requires clear patient clinical background, and serves several functions in the efficient management of breast cancer patients. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 458, 23–29. [Google Scholar] [CrossRef]
- Darlix, A.; Lamy, P.J.; Lopez-Crapez, E.; Braccini, A.L.; Firmin, N.; Romieu, G.; Thezenas, S.; Jacot, W. Serum HER2 extra-cellular domain, S100ß and CA 15-3 levels are independent prognostic factors in metastatic breast cancer patients. BMC Cancer 2016, 16, 428. [Google Scholar] [CrossRef]
- Leary, A.F.; Hanna, W.M.; van de Vijver, M.J.; Penault-Llorca, F.; Rüschoff, J.; Osamura, R.Y.; Bilous, M.; Dowsett, M. Value and limitations of measuring HER-2 extracellular domain in the serum of breast cancer patients. J. Clin. Oncol. 2009, 27, 1694–1705. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, C.; Fan, H.; Xiang, Q.; Xu, L.; Liu, Q.; Zhou, S.; Xie, Q.; Chen, S.; Mu, G.; et al. Prognostic value of baseline serum HER2 extracellular domain level with a cut-off value of 15 ng/mL in patients with breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2018, 172, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Carney, W.P.; Bernhardt, D.; Jasani, B. Circulating HER2 Extracellular Domain: A Specific and Quantitative Biomarker of Prognostic Value in all Breast Cancer Patients? Biomark. Cancer 2013, 5, 31–39. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Wells, G. The Newcaastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 17 August 2022).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 276–284. [Google Scholar] [CrossRef]
- Colomer, R.; Llombart-Cussac, A.; Lloveras, B.; Ramos, M.; Mayordomo, J.I.; Fernandez, R.; Tusquets, I.; Gil, M.; Barnadas, A.; Constenla, M.; et al. High circulating HER2 extracellular domain levels correlate with reduced efficacy of an aromatase inhibitor in hormone receptor-positive metastatic breast cancer: A confirmatory prospective study. Cancer 2007, 110, 2178–2185. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Witzel, I.; Riethdorf, S.; Rack, B.; Janni, W.; Fasching, P.A.; Solomayer, E.F.; Aktas, B.; Kasimir-Bauer, S.; Pantel, K.; et al. Clinical Relevance of Serum HER2 and Circulating Tumor Cell Detection in Metastatic Breast Cancer Patients. Anticancer Res. 2017, 37, 3117–3128. [Google Scholar] [PubMed]
- Lipton, A.; Ali, S.M.; Leitzel, K.; Demers, L.; Harvey, H.A.; Chaudri-Ross, H.A.; Brady, C.; Wyld, P.; Carney, W. Serum HER-2/neu and response to the aromatase inhibitor letrozole versus tamoxifen. J. Clin. Oncol. 2003, 21, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.T.; Johansson, H.; Colleoni, M.; Zorzino, L.; Passerini, R.; Orlando, L.; Viale, G. Serum levels of HER2 ECD can determine the response rate to low dose oral cyclophosphamide and methotrexate in patients with advanced stage breast carcinoma. Anticancer Res. 2004, 24, 1261–1266. [Google Scholar] [PubMed]
- Tchou, J.; Lam, L.; Li, Y.R.; Edwards, C.; Ky, B.; Zhang, H. Monitoring serum HER2 levels in breast cancer patients. SpringerPlus 2015, 4, 237. [Google Scholar] [CrossRef]
- Baric, M.; Kulic, A.; Sirotkovic-Skerlev, M.; Dedic, P.N.; Vidovic, M.; Horvatic-Herceg, G.; Vrbanec, D. Circulating Her-2/neu extracellular domain in breast cancer patients-correlation with prognosis and clinicopathological parameters including steroid receptor, HER-2/neu receptor coexpression. Pathol. Oncol. Res. 2015, 21, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wang, X.; Xu, X.; Feng, J.; Han, M.; Zhang, H.; Chen, Z.H.; Wang, S.; Zang, Y.M.; Huang, P.; et al. Outcome prediction values of soluble human epidermal growth factor receptor-2 extracellular domain in metastatic breast cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 1108–1113. [Google Scholar] [PubMed]
- Eppenberger-Castori, S.; Klingbiel, D.; Ruhstaller, T.; Dietrich, D.; Rufle, D.A.; Rothgiesser, K.; Pagani, O.; Thurlimann, B. Plasma HER2ECD a promising test for patient prognosis and prediction of response in HER2 positive breast cancer: Results of a randomized study—SAKK 22/99. BMC Cancer 2020, 20, 114. [Google Scholar] [CrossRef]
- Jensen, B.V.; Johansen, J.S.; Price, P.A. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin. Cancer Res. 2003, 9, 4423–4434. [Google Scholar]
- Kostler, W.J.; Schwab, B.; Singer, C.F.; Neumann, R.; Rucklinger, E.; Brodowicz, T.; Tomek, S.; Niedermayr, M.; Hejna, M.; Steger, G.G.; et al. Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin. Cancer Res. 2004, 10, 1618–1624. [Google Scholar] [CrossRef]
- Kontani, K.; Kuroda, N.; Hashimoto, S.; Murazawa, C.; Norimura, S.; Tanaka, H.; Ohtani, M.; Fujiwara-Honjo, N.; Kushida, Y.; Date, M.; et al. Clinical usefulness of human epidermal growth factor receptor-2 extracellular domain as a biomarker for monitoring cancer status and predicting the therapeutic efficacy in breast cancer. Cancer Biol. Ther. 2013, 14, 20–28. [Google Scholar] [CrossRef]
- Fornier, M.N.; Seidman, A.D.; Schwartz, M.K.; Ghani, F.; Thiel, R.; Norton, L.; Hudis, C. Serum HER2 extracellular domain in metastatic breast cancer patients treated with weekly trastuzumab and paclitaxel: Association with HER2 status by immunohistochemistry and fluorescence in situ hybridization and with response rate. Ann. Oncol. 2005, 16, 234–239. [Google Scholar] [CrossRef]
- Molina, R.; Auge, J.M.; Escudero, J.M.; Filella, X.; Zanon, G.; Pahisa, J.; Farrus, B.; Munoz, M.; Velasco, M. Evaluation of tumor markers (HER-2/neu oncoprotein, CEA, and CA 15.3) in patients with locoregional breast cancer: Prognostic value. Tumour Biol. 2010, 31, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ludovini, V.; Gori, S.; Colozza, M.; Pistola, L.; Rulli, E.; Floriani, I.; Pacifico, E.; Tofanetti, F.R.; Sidoni, A.; Basurto, C.; et al. Evaluation of serum HER2 extracellular domain in early breast cancer patients: Correlation with clinicopathological parameters and survival. Ann. Oncol. 2008, 19, 883–890. [Google Scholar] [CrossRef]
- Witzel, I.; Thomssen, C.; Krenkel, S.; Wilczak, W.; Bubenheim, M.; Pantel, K.; Neumann, R.; Janicke, F.; Muller, V. Clinical utility of determination of HER-2/neu and EGFR fragments in serum of patients with metastatic breast cancer. Int. J. Biol. Mark. 2006, 21, 131–140. [Google Scholar] [CrossRef]
- Im, S.A.; Kim, S.B.; Lee, M.H.; Im, Y.H.; Lee, K.H.; Song, H.S.; Lee, M.A.; Lee, J.; Lee, N.S.; Ham, H.S.; et al. Docetaxel plus epirubicin as first-line chemotherapy in MBC (KCSG 01-10-05): Phase II trial and the predictive values of circulating HER2 extracellular domain and vascular endothelial growth factor. Oncol. Rep. 2005, 14, 481–487. [Google Scholar]
- Darlix, A.; Lamy, P.J.; Lopez-Crapez, E.; Braccini, A.L.; Firmin, N.; Romieu, G.; Thezenas, S.; Jacot, W. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res. Treat. 2016, 157, 307–318. [Google Scholar]
- Colomer, R.; Llombart-Cussac, A.; Tusquets, I.; Rifa, J.; Mayordomo, J.I.; Ojeda, B.; Ciruelos, E.; Hornedo, J.; Vicente, D.; Cortes-Funes, H. Biweekly gemcitabine plus vinorelbine in first-line metastatic breast cancer: Efficacy and correlation with HER2 extracellular domain. Clin. Transl. Oncol. 2006, 8, 896–902. [Google Scholar] [CrossRef]
- Zuo, W.J.; He, M.; Zheng, H.; Liu, Y.; Liu, X.Y.; Jiang, Y.Z.; Wang, Z.H.; Lu, R.Q.; Shao, Z.M. Serum HER2 levels predict treatment efficacy and prognosis in patients with HER2-positive breast cancer undergoing neoadjuvant treatment. Gland Surg. 2021, 10, 1300–1314. [Google Scholar] [CrossRef]
- Lipton, A.; Ali, S.M.; Leitzel, K.; Demers, L.; Chinchilli, V.; Engle, L.; Harvey, H.A.; Brady, C.; Nalin, C.M.; Dugan, M.; et al. Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J. Clin. Oncol. 2002, 20, 1467–1472. [Google Scholar] [CrossRef]
- Cameron, D.; Casey, M.; Press, M.; Lindquist, D.; Pienkowski, T.; Romieu, C.G.; Chan, S.; Jagiello-Gruszfeld, A.; Kaufman, B.; Crown, J.; et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 2008, 112, 533–543. [Google Scholar] [CrossRef]
- Kong, Y.; Dai, S.; Xie, X.; Xiao, X.; Lv, N.; Guo, J.; Li, L.; Jia, W.; Zhang, Y.; Liu, W.; et al. High serum HER2 extracellular domain levels: Correlation with a worse disease-free survival and overall survival in primary operable breast cancer patients. J. Cancer Res. Clin. Oncol. 2012, 138, 275–284. [Google Scholar] [CrossRef]
- Esteva, F.J.; Valero, V.; Booser, D.; Guerra, L.T.; Murray, J.L.; Pusztai, L.; Cristofanilli, M.; Arun, B.; Esmaeli, B.; Fritsche, H.A.; et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2002, 20, 1800–1808. [Google Scholar] [CrossRef]
- Finn, R.S.; Gagnon, R.; Di Leo, A.; Press, M.F.; Arbushites, M.; Koehler, M. Prognostic and predictive value of HER2 extracellular domain in metastatic breast cancer treated with lapatinib and paclitaxel in a randomized phase III study. J. Clin. Oncol. 2009, 27, 5552–5558. [Google Scholar] [CrossRef]
- Muller, V.; Witzel, I.; Luck, H.J.; Kohler, G.; Minckwitz, G.; Mobus, V.; Sattler, D.; Wilczak, W.; Loning, T.; Janicke, F.; et al. Prognostic and predictive impact of the HER-2/ neu extracellular domain (ECD) in the serum of patients treated with chemotherapy for metastatic breast cancer. Breast Cancer Res. Treat. 2004, 86, 9–18. [Google Scholar] [CrossRef]
- Ryu, D.W.; Lee, C.H. Impact of Serum HER2 Levels on Survival and Its Correlation with Clinicopathological Parameters in Women with Breast Cancer. J. Breast Cancer 2012, 15, 71–78. [Google Scholar] [CrossRef]
- Thureau, S.; Clatot, F.; Laberge-Le-Couteulx, S.; Baron, M.; Basuyau, J.P.; Blot, E. Elevated HER2 extracellular domain level in primary breast cancer with HER2 overexpression predicts early failure of adjuvant trastuzumab. Anticancer Res. 2012, 32, 1429–1433. [Google Scholar]
- Colomer, R.; Llombart-Cussac, A.; Lluch, A.; Barnadas, A.; Ojeda, B.; Caranana, V.; Fernandez, Y.; Garcia-Conde, J.; Alonso, S.; Montero, S.; et al. Biweekly paclitaxel plus gemcitabine in advanced breast cancer: Phase II trial and predictive value of HER2 extracellular domain. Ann. Oncol. 2004, 15, 201–206. [Google Scholar] [CrossRef]
- Lüftner, D.; Henschke, P.; Flath, B.; Akrivakis, C.; Schnabel, S.; Prinz, B.; Geppert, R.; Wernecke, K.D.; Possinger, K. Serum HER-2/neu as a Prediction and Monitoring Parameter in a Phase II Study with Weekly Paclitaxel in Metastatic Breast Cancer. Anticancer Res. 2004, 24, 895–906. [Google Scholar]
- Knutson, K.L.; Clynes, R.; Shreeder, B.; Yeramian, P.; Kemp, K.P.; Ballman, K.; Tenner, K.S.; Erskine, C.L.; Norton, N.; Northfelt, D.; et al. Improved Survival of HER2+ Breast Cancer Patients Treated with Trastuzumab and Chemotherapy Is Associated with Host Antibody Immunity against the HER2 Intracellular Domain. Cancer Res. 2016, 76, 3702–3710. [Google Scholar] [CrossRef]
- Tsai, H.-P.; Chen, S.-C.; Chien, H.-T.; Jan, Y.-Y.; Chao, T.-C.; Chen, M.-F.; Hsieh, L.-L. Relationships between serum HER2 ECD, TIMP-1 and clinical outcomes in Taiwanese breast cancer. World J. Surg. Oncol. 2012, 10, 42. [Google Scholar] [CrossRef]
- Lee, M.H.; Jung, S.Y.; Kang, S.H.; Song, E.J.; Park, I.H.; Kong, S.Y.; Kwon, Y.M.; Lee, K.S.; Kang, H.S.; Lee, E.S. The Significance of Serum HER2 Levels at Diagnosis on Intrinsic Subtype-Specific Outcome of Operable Breast Cancer Patients. PLoS ONE 2016, 11, 163370. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, J.W.; Yu, J.H.; Ko, B.S.; Kim, H.J.; Son, B.H.; Gong, G.; Lee, H.J.; Kim, S.B.; Jung, K.H.; et al. Preoperative serum HER2 extracellular domain levels in primary invasive breast cancer. BMC Cancer 2014, 14, 929. [Google Scholar] [CrossRef]
- Bramwell, V.H.; Doig, G.S.; Tuck, A.B.; Wilson, S.M.; Tonkin, K.S.; Tomiak, A.; Perera, F.; Vandenberg, T.A.; Chambers, A.F. Changes over time of extracellular domain of HER2 (ECD/HER2) serum levels have prognostic value in metastatic breast cancer. Breast Cancer Res. Treat. 2009, 114, 503–511. [Google Scholar] [CrossRef]
- Bewick, M.; Conlon, M.; Gerard, S.; Lee, H.; Parissenti, A.M.; Zhang, L.; Gluck, S.; Lafrenie, R.M. HER-2 expression is a prognostic factor in patients with metastatic breast cancer treated with a combination of high-dose cyclophosphamide, mitoxantrone, paclitaxel and autologous blood stem cell support. Bone Marrow Transplant. 2001, 27, 847–853. [Google Scholar] [CrossRef]
- Di Gioia, D.; Dresse, M.; Mayr, D.; Nagel, D.; Heinemann, V.; Stieber, P. Serum HER2 in combination with CA 15-3 as a parameter for prognosis in patients with early breast cancer. Clin. Chim. Acta 2015, 440, 16–22. [Google Scholar] [CrossRef]
- Colomer, R.; Montero, S.; Lluch, A.; Ojeda, B.; Barnadas, A.; Casado, A.; Massuti, B.; Cortes-Funes, H.; Lloveras, B. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin. Cancer Res. 2000, 6, 2356–2362. [Google Scholar]
- Lipton, A.; Leitzel, K.; Ali, S.; Carney, W.; Platek, G.; O’Rourke, L.; Martin, A.M.; Maltzman, J. Decrease in serum extracellular domain of HER2 at 4 and 8 weeks is associated with prolonged progression-free survival on lapatinib monotherapy. Cancer Res. 2009, 69, 244s. [Google Scholar] [CrossRef]
- Lee, C.K.; Davies, L.; Gebski, V.J.; Lord, S.J.; Di Leo, A.; Johnston, S.; Geyer, C., Jr.; Cameron, D.; Press, M.F.; Ellis, C.; et al. Serum Human Epidermal Growth Factor 2 Extracellular Domain as a Predictive Biomarker for Lapatinib Treatment Efficacy in Patients With Advanced Breast Cancer. J. Clin. Oncol. 2016, 34, 936–944. [Google Scholar] [CrossRef]


| Study | Year | Sample Size | Elevated (%) | Ethnicity | Study Design | Disease Status | Treatment | Outcomes | Cutoff of ECD | Exposure Assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Banys et al. [26] | 2017 | 25 | 47.04 | Caucasian | Prospective | Metastatic | NA | OS | 15 ng/mL | ELISA |
| Baric et al. [30] | 2015 | 79 | 44.30 | Caucasian | Retrospective | Adjuvant | Adjuvant treatment | OS | 15.86 ng/mL | ELISA |
| Bewick et al. [59] | 2001 | 46 | 43.48 | Caucasian | Retrospective | Metastatic | Chemotherapy | PFS, OS | 2500 U/ml | ELISA |
| Bramwell et al. [58] | 2009 | 158 | 21.52 | Caucasian | Prospective | Metastatic | NA | OS | 15 ng/mL | ELISA |
| Colomer et al. [25] | 2007 | 226 | 18.58 | Caucasian | Prospective | Metastatic | Endocrine therapy | PFS, ORR, OS | 20 ng/mL | ELISA |
| Colomer et al. [52] | 2004 | 42 | 28.57 | Caucasian | Prospective | Metastatic | Chemotherapy | ORR | 30 ng/mL | ELISA |
| Colomer et al. [42] | 2006 | 48 | 29.17 | Caucasian | Prospective | Metastatic | Chemotherapy | ORR | 50 ng/mL | ELISA |
| David et.al. [45] | 2008 | 198 | 25.00 | Caucasian | Prospective | Metastatic | TKIs | PFS | 82 ng/mL | ELISA |
| Darlix et al. [41] | 2016 | 250 | 25.20 | Caucasian | Retrospective | Metastatic | NA | OS | 30 ng/ml | ELISA |
| Gioia et al. [60] | 2015 | 241 | 12.03 | Caucasian | Retrospective | Adjuvant | Chemotherapy + Trastuzumab | DFS | 15 ng/mL | Chemiluminescence |
| Eppenberger et al. [32] | 2020 | 131 | 67.18 | Caucasian | Prospective | Metastatic | Chemotherapy + Trastuzumab | OS, PFS | 15 ng/mL | Chemiluminescence |
| Esteva et al. [47] | 2002 | 30 | 70.00 | Caucasian | Prospective | Metastatic | Chemotherapy + Trastuzumab | ORR | 14.9 ng/mL | ELISA |
| Finn et al. [48] | 2009 | 579 | 16.58 | Caucasian | Prospective | Metastatic | TKIs/Chemotherapy | ORR, PFS | 16 ng/ml | ELISA |
| Fornier et al. [36] | 2005 | 55 | 69.09 | Caucasian | Retrospective | Metastatic | Chemotherapy + Trastuzumab | ORR | 15 ng/mL | Chemiluminescence |
| Im et al. [40] | 2005 | 27 | 14.81 | Asian | Prospective | Metastatic | Chemotherapy | ORR, OS | 15 ng/ml | ELISA |
| Jensen et al. [33] | 2003 | 100 | 32.00 | Caucasian | Prospective | Metastatic | Chemotherapy | OS, PFS | 15 ng/ml | ELISA |
| Knutson et al. [54] | 2014 | 54 | 55.56 | Caucasian | Prospective | Metastatic | Chemotherapy + Trastuzumab | PFS, OS | 15 ng/mL | ELISA |
| Kong et al. [46] | 2012 | 252 | 15.08 | Asian | Prospective | Adjuvant | Adjuvant treatment | DFS, OS | 15 ng/mL | Chemiluminescence |
| Kontani et al. [35] | 2013 | 19 | 63.16 | Asian | Prospective | Metastatic | Chemotherapy + Trastuzumab | ORR | 15.2 ng/mL | Chemiluminescence |
| Kostler et al. [34] | 2004 | 55 | 72.73 | Caucasian | Prospective | Metastatic | Chemotherapy + Trastuzumab | ORR | 15 ng/mL | ELISA |
| Lee et al. [56] | 2016 | 436 | 11.93 | Asian | Retrospective | Adjuvant | Adjuvant treatment | DFS | 15 ng/mL | Chemiluminescence |
| Lee et al. [57] | 2014 | 2862 | 4.40 | Asian | Retrospective | Adjuvant | Adjuvant treatment | DFS, OS | 15.2 ng/mL | Chemiluminescence |
| Lipton et al. [44] | 2002 | 719 | 30.46 | Caucasian | Retrospective | Metastatic | Endocrine therapy | ORR, OS, PFS | 15 ng/mL | ELISA |
| Lipton et al. [27] | 2003 | 562 | 29.18 | Caucasian | Prospective | Metastatic | Endocrine therapy | ORR, PFS | 15 ng/mL | ELISA |
| Lipton et al. [10] | 2011 | 138 | 78.99 | Caucasian | Prospective | Metastatic | TKIs | PFS | 15 ng/mL | ELISA |
| Ludovini et al. [38] | 2008 | 256 | 8.98 | Caucasian | Prospective | Adjuvant | Adjuvant treatment | OS, DFS | 15 ng/mL | Chemiluminescence/ELISA |
| Luftner et al. [53] | 2004 | 35 | 62.86 | Caucasian | Prospective | Metastatic | Chemotherapy | ORR, PFS | 15 ng/mL | ELISA |
| Molina et al. [37] | 2010 | 275 | 14.91 | Caucasian | Prospective | Adjuvant | Adjuvant treatment | DFS | 15 ng/mL | ELISA |
| Moreno-Aspitia et al. [9] | 2013 | 2318 | 37.41 | Caucasian | Retrospective | Adjuvant | Chemotherapy ± Trastuzumab | DFS | 15 ng/mL | Chemiluminescence |
| Muller et al. [49] | 2004 | 103 | 35.92 | Caucasian | Retrospective | Metastatic | Chemotherapy | ORR, PFS, OS | 15 ng/mL | ELISA |
| Reix et al. [13] | 2016 | 334 | 15.27 | Caucasian | Prospective | (Neo)adjuvant/Metastatic | Chemotherapy + Trastuzumab | DFS, PFS, OS | 15 ng/mL | ELISA |
| Ryu et al. [50] | 2012 | 200 | 7.00 | Asian | Prospective | Adjuvant | Adjuvant treatment | DFS | 15 ng/mL | Chemiluminescence |
| Sandri et al. [28] | 2004 | 39 | 10.26 | Caucasian | Prospective | Metastatic | Chemotherapy | PFS, OS | 15 ng/mL | Chemiluminescence |
| Shao et al. [31] | 2014 | 62 | 41.94 | Asian | Prospective | Metastatic | Chemotherapy + Trastuzumab | PFS, ORR | 15 ng/mL | Chemiluminescence |
| Tchou et al. [29] | 2015 | 118 | 22.88 | Caucasian | Prospective | Adjuvant | Adjuvant treatment | DFS | 7 ng/mL | ELISA |
| Thureau et al. [51] | 2012 | 65 | 10.77 | Caucasian | Retrospective | Adjuvant | Chemotherapy + Trastuzumab | DFS, OS | 15 ng/mL | ELISA |
| Tsai et al. [55] | 2012 | 185 | 12.43 | Asian | Prospective | Adjuvant | Adjuvant treatment | DFS, OS | 8.9 ng/mL | ELISA |
| Wang et al. [16] | 2016 | 546 | 43.22 | Asian | Prospective | Metastatic | NA | PFS | 15 ng/mL | Chemiluminescence |
| Witzel et al. [39] | 2006 | 76 | 39.47 | Caucasian | Prospective | Metastatic | NA | PFS, OS | NA | NA |
| Zuo et al. [43] | 2021 | 309 | 53.07 | Asian | Retrospective | Neoadjuvant | Chemotherapy + Trastuzumab | DFS | 15 ng/mL | Chemiluminescence |
| Categories | PFS | DFS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | No. of Patients | Pooled HR (95% CI) | Heterogeneity | No. of Studies | No. of Patients | Pooled HR (95% CI) | Heterogeneity | |||||
| Random | p-Value | I² (%) | p-Value | Random | p-Value | I² (%) | p-Value | |||||
| All patients | 17 | 3662 | 1.74 (1.40–1.72) | <0.001 | 91.2 | <0.001 | 13 | 7599 | 2.31 (1.94–2.75) | <0.001 | 25.5 | 0.187 |
| Treatment | ||||||||||||
| Chemotherapy | 6 | 611 | 1.81 (1.37–2.39) | <0.001 | 1.8 | 0.366 | 1 | 795 | 1.81 (1.26–2.61) | NA | NA | |
| Endocrine therapy | 3 | 1507 | 1.91 (1.57–2.32) | <0.001 | 59.2 | 0.086 | ||||||
| TKIs | 3 | 627 | 1.44 (0.85–2.43) | 0.17 | 82.2 | 0.004 | ||||||
| Trastuzumab + Chemotherapy | 4 | 295 | 1.74 (1.31–2.30) | <0.001 | 24.3 | 0.265 | 5 | 2318 | 3.25 (1.98–5.31) | 57.4 | 0.038 | |
| Adjuvant therapy | 8 | 4332 | 2.60 (2/02–3.36) | 0 | 0.967 | |||||||
| Cutoff value | ||||||||||||
| 15 ng/mL | 12 | 2537 | 1.64 (1.27–2.11) | <0.001 | 92.2 | <0.001 | 12 | 7260 | 2.68 (2.11–3.41) | <0.001 | 27.3 | 0.176 |
| 5–10 ng/mL | 1 | 185 | 3.58 (1.65–7.76) | 0.001 | - | - | ||||||
| 15–30 ng/mL | 3 | 805 | 2.65 (1.97–3.56) | <0.001 | 0 | 0.928 | ||||||
| 82 ng/mL | 1 | 198 | 1.50 (0.92–2.45) | 0.105 | - | - | ||||||
| 2500 U/mL | 1 | 46 | 2.33 (1.22–4.46) | 0.011 | - | - | ||||||
| Disease status | ||||||||||||
| Metastatic | 18 | 3662 | 1.74 (1.40–2.17) | <0.001 | 91.2 | <0.001 | ||||||
| (Neo)adjuvant | 13 | 7445 | 2.73 (2.17–3.42) | <0.001 | 25.5 | 0.187 | ||||||
| Ethnicity | ||||||||||||
| Caucasian | 16 | 3054 | 1.71 (1.36–2.16) | <0.001 | 91.2 | <0.001 | 7 | 3317 | 2.59 (1.88–3.57) | <0.001 | 29.3 | 0.205 |
| Asian | 2 | 608 | 1.93 (1.18–3.17) | 0.009 | 43.8 | 0.182 | 6 | 4128 | 2.95 (2.13–4.07) | <0.001 | 13.8 | 0.326 |
| Categories | OS | ORR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | No. of Patients | Pooled HR (95% CI) | Heterogeneity | No. of Studies | No. of Patients | Pooled or (95% CI) | Heterogeneity | |||||
| Random | p-Value | I2 (%) | p-Value | Random | p-Value | I2 (%) | p-Value | |||||
| All patients | 20 | 5963 | 2.13 (1.77–2.57) | <0.001 | 48.4% | 0.008 | 14 | 2274 | 0.80 (0.49–1.31) | 0.381 | 73 | < 0.001 |
| Treatment | ||||||||||||
| Chemotherapy | 5 | 315 | 2.19 (1.60–3.01) | < 0.001 | 0 | 0.817 | 7 | 605 | 0.69 (0.38–1.26) | 0.225 | 43.6 | 0.1 |
| Endocrine therapy | 2 | 945 | 2.67 (1.44–4.97) | 0.002 | 75.1 | 0.045 | 3 | 1507 | 0.37 (0.28–0.49) | < 0.001 | 0 | 0.988 |
| TKIs | 1 | 291 | 1.03 (0.50–2.13) | 0.931 | NA | NA | ||||||
| Trastuzumab + Chemotherapy | 4 | 584 | 2.00 (1.02–1.95) | 0.045 | 76.8 | 0.005 | 4 | 159 | 5.50 (1.15–26.21) | 0.033 | 0.035 | 65 |
| Adjuvant therapy | 5 | 3382 | 2.56 (1.75–3.74) | < 0.001 | 0 | 0.896 | ||||||
| Cutoff value | ||||||||||||
| 15 ng/mL | 14 | 5101 | 1.98 (1.59–2.48) | < 0.001 | 51.1 | 0.014 | 10 | 1667 | 1.02 (0.53–1.97) | 0.985 | 76.4 | < 0.001 |
| 5–10 ng/mL | 1 | 185 | 3.22 (1.43–7.26) | 0.005 | NA | NA | ||||||
| 15–30 ng/mL | 3 | 555 | 2.99 (2.09–4.29) | < 0.001 | 0 | 0.523 | 3 | 847 | 0.46 (0.16–1.29) | 0.138 | 73.7 | 0.022 |
| 50 ng/mL | 1 | 48 | 1.06 (0.30–3.71) | 0.924 | NA | NA | ||||||
| 2500 U/ml | 1 | 46 | 2.36 (1.21–4.62) | 0.012 | NA | NA | ||||||
| Disease status | ||||||||||||
| Metastatic | 13 | 2182 | 1.94 (1.58–2.38) | < 0.001 | 54.8 | 0.009 | 13 | 2562 | 0.80 (0.49–1.31) | 0.381 | 73 | < 0.001 |
| (Neo)adjuvant | 6 | 3447 | 2.78(1.92–4.01) | < 0.001 | 0 | 0.605 | ||||||
| Metastatic and (Neo) adjuvant | 1 | 334 | 4.88 (1.6–14.89) | 0.005 | NA | NA | ||||||
| Ethnicity | ||||||||||||
| Caucasian | 16 | 2889 | 2.07 (1.68–2.55) | < 0.001 | 55.2 | 0.004 | 11 | 2166 | 0.74 (0.46–1.20) | 0.220 | 71.0 | < 0.001 |
| Asian | 4 | 3074 | 2.58 (1.71–3.90) | < 0.001 | 0 | 0.689 | 3 | 108 | 2.63 (0.11–63.22) | 0.522 | 85.2 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, L.; Zhang, D.; Ma, F. Prognostic Value of the Serum HER2 Extracellular Domain Level in Breast Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4551. https://doi.org/10.3390/cancers14194551
Wu Y, Li L, Zhang D, Ma F. Prognostic Value of the Serum HER2 Extracellular Domain Level in Breast Cancer: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(19):4551. https://doi.org/10.3390/cancers14194551
Chicago/Turabian StyleWu, Yun, Lixi Li, Di Zhang, and Fei Ma. 2022. "Prognostic Value of the Serum HER2 Extracellular Domain Level in Breast Cancer: A Systematic Review and Meta-Analysis" Cancers 14, no. 19: 4551. https://doi.org/10.3390/cancers14194551
APA StyleWu, Y., Li, L., Zhang, D., & Ma, F. (2022). Prognostic Value of the Serum HER2 Extracellular Domain Level in Breast Cancer: A Systematic Review and Meta-Analysis. Cancers, 14(19), 4551. https://doi.org/10.3390/cancers14194551







