Contributions of Circulating microRNAs for Early Detection of Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. miRNA Isolation
2.3. TaqMan Assay
2.4. Statistical Analyses
3. Results
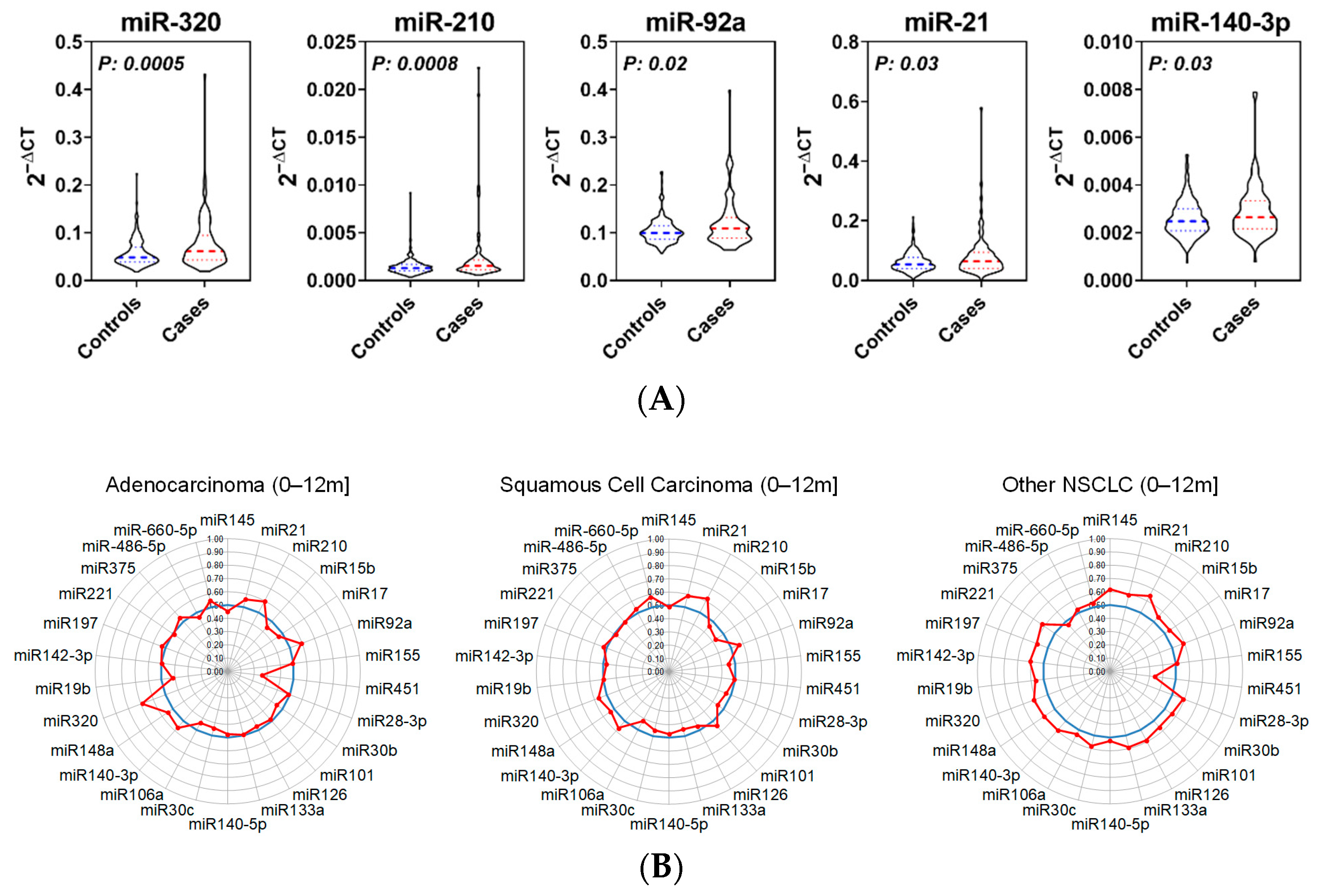
3.1. Candidate miRNA Selection
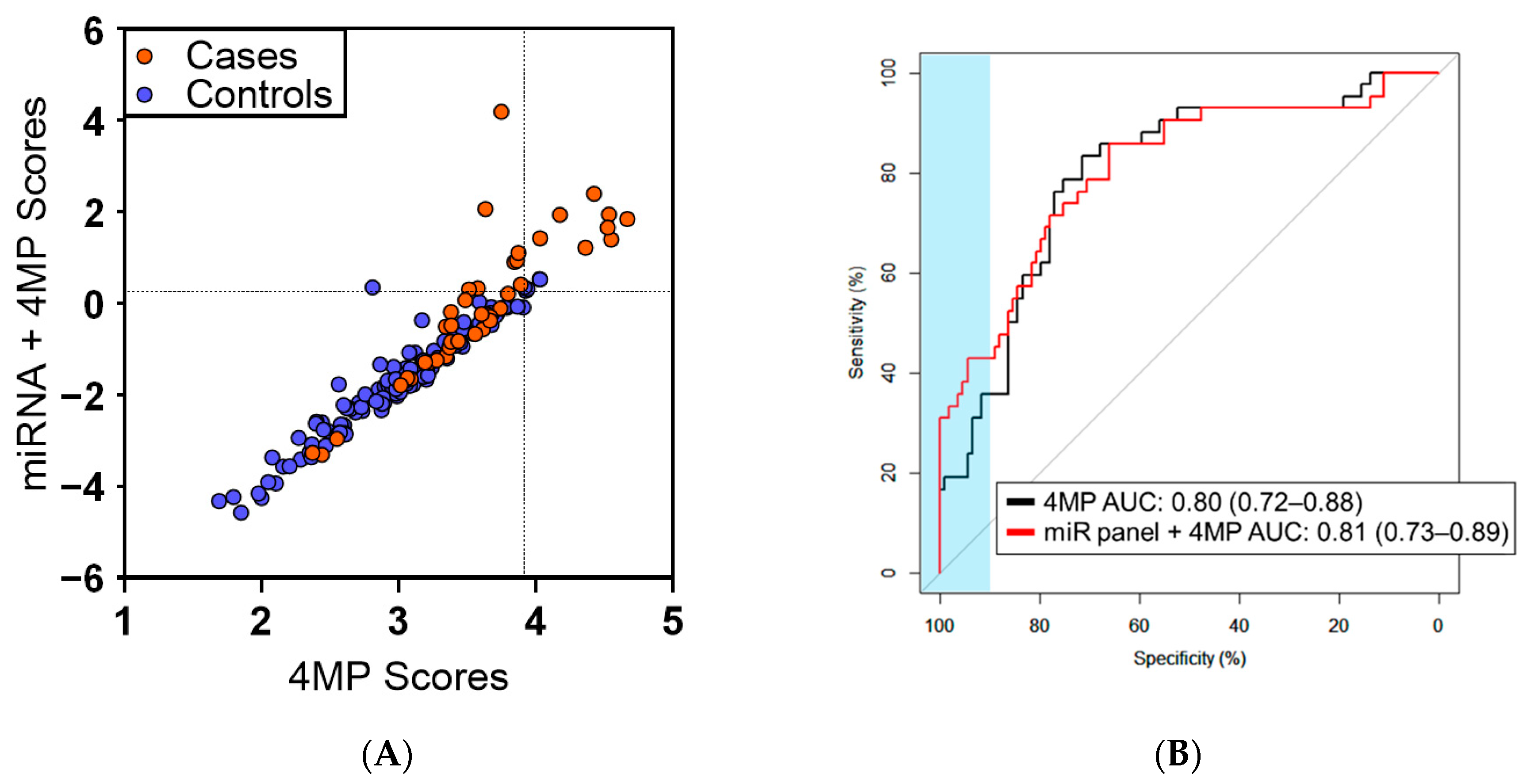
3.2. Predictive Performance of miRNAs for Early Detection of Lung Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- SEER*Explorer. Available online: https://seer.cancer.gov/explorer/index.html (accessed on 11 August 2021).
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Dama, E.; Colangelo, T.; Fina, E.; Cremonesi, M.; Kallikourdis, M.; Veronesi, G.; Bianchi, F. Biomarkers and Lung Cancer Early Detection: State of the Art. Cancers 2021, 13, 3919. [Google Scholar] [CrossRef] [PubMed]
- Vannini, I.; Fanini, F.; Fabbri, M. Emerging Roles of MicroRNAs in Cancer. Curr. Opin. Genet. Dev. 2018, 48, 128–133. [Google Scholar] [CrossRef]
- Anfossi, S.; Babayan, A.; Pantel, K.; Calin, G.A. Clinical Utility of Circulating Non-Coding RNAs—An Update. Nat. Rev. Clin. Oncol. 2018, 15, 541–563. [Google Scholar] [CrossRef]
- Zhong, S.; Golpon, H.; Zardo, P.; Borlak, J. MiRNAs in Lung Cancer. A Systematic Review Identifies Predictive and Prognostic MiRNA Candidates for Precision Medicine in Lung Cancer. Transl. Res. 2021, 230, 164–196. [Google Scholar] [CrossRef]
- Boeri, M.; Verri, C.; Conte, D.; Roz, L.; Modena, P.; Facchinetti, F.; Calabrò, E.; Croce, C.M.; Pastorino, U.; Sozzi, G. MicroRNA Signatures in Tissues and Plasma Predict Development and Prognosis of Computed Tomography Detected Lung Cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 3713–3718. [Google Scholar] [CrossRef]
- Sozzi, G.; Boeri, M.; Rossi, M.; Verri, C.; Suatoni, P.; Bravi, F.; Roz, L.; Conte, D.; Grassi, M.; Sverzellati, N.; et al. Clinical Utility of a Plasma-Based MiRNA Signature Classifier within Computed Tomography Lung Cancer Screening: A Correlative MILD Trial Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 768–773. [Google Scholar] [CrossRef]
- Bianchi, F.; Nicassio, F.; Marzi, M.; Belloni, E.; Dall’Olio, V.; Bernard, L.; Pelosi, G.; Maisonneuve, P.; Veronesi, G.; Di Fiore, P.P. A Serum Circulating MiRNA Diagnostic Test to Identify Asymptomatic High-Risk Individuals with Early Stage Lung Cancer. EMBO Mol. Med. 2011, 3, 495–503. [Google Scholar] [CrossRef]
- Montani, F.; Marzi, M.J.; Dezi, F.; Dama, E.; Carletti, R.M.; Bonizzi, G.; Bertolotti, R.; Bellomi, M.; Rampinelli, C.; Maisonneuve, P.; et al. MiR-Test: A Blood Test for Lung Cancer Early Detection. J. Natl. Cancer Inst. 2015, 107, djv063. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, Z.; Wang, W.; Ba, Y.; Ma, L.; Zhang, C.; Wang, C.; Ren, Z.; Zhao, Y.; Wu, S.; et al. Identification of Ten Serum MicroRNAs from a Genome-Wide Serum MicroRNA Expression Profile as Novel Noninvasive Biomarkers for Nonsmall Cell Lung Cancer Diagnosis. Int. J. Cancer 2012, 130, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Integrative Analysis of Lung Cancer Etiology and Risk (INTEGRAL) Consortium for Early Detection of Lung Cancer; Guida, F.; Sun, N.; Bantis, L.E.; Muller, D.C.; Li, P.; Taguchi, A.; Dhillon, D.; Kundnani, D.L.; Patel, N.J.; et al. Assessment of Lung Cancer Risk on the Basis of a Biomarker Panel of Circulating Proteins. JAMA Oncol. 2018, 4, e182078. [Google Scholar] [CrossRef]
- Ostrin, E.J.; Bantis, L.E.; Wilson, D.O.; Patel, N.; Wang, R.; Kundnani, D.; Adams-Haduch, J.; Dennison, J.B.; Fahrmann, J.F.; Chiu, H.T.; et al. Contribution of a Blood-Based Protein Biomarker Panel to the Classification of Indeterminate Pulmonary Nodules. J. Thorac. Oncol. 2021, 16, 228–236. [Google Scholar] [CrossRef]
- Fan, L.; Qi, H.; Teng, J.; Su, B.; Chen, H.; Wang, C.; Xia, Q. Identification of Serum MiRNAs by Nano-Quantum Dots Microarray as Diagnostic Biomarkers for Early Detection of Non-Small Cell Lung Cancer. Tumor Biol. 2016, 37, 7777–7784. [Google Scholar] [CrossRef]
- Raponi, M.; Dossey, L.; Jatkoe, T.; Wu, X.; Chen, G.; Fan, H.; Beer, D.G. MicroRNA Classifiers for Predicting Prognosis of Squamous Cell Lung Cancer. Cancer Res. 2009, 69, 5776–5783. [Google Scholar] [CrossRef]
- Qi, Z.; Yang, D.-Y.; Cao, J. Increased Micro-RNA 17, 21, and 192 Gene Expressions Improve Early Diagnosis in Non-Small Cell Lung Cancer. Med. Oncol. 2014, 31, 195. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yin, Y.; Li, S. Detection of Circulating Exosomal MiR-17-5p Serves as a Novel Non-Invasive Diagnostic Marker for Non-Small Cell Lung Cancer Patients. Pathol. Res. Pract. 2019, 215, 152466. [Google Scholar] [CrossRef]
- Cazzoli, R.; Buttitta, F.; Di Nicola, M.; Malatesta, S.; Marchetti, A.; Rom, W.N.; Pass, H.I. MicroRNAs Derived from Circulating Exosomes as Noninvasive Biomarkers for Screening and Diagnosing Lung Cancer. J. Thorac. Oncol. 2013, 8, 1156–1162. [Google Scholar] [CrossRef]
- Hetta, H.F.; Zahran, A.M.; El-Mahdy, R.I.; Nabil, E.E.; Esmaeel, H.M.; Elkady, O.A.; Elkady, A.; Mohareb, D.A.; Mahmoud Mostafa, M.; John, J. Assessment of Circulating MiRNA-17 and MiRNA-222 Expression Profiles as Non-Invasive Biomarkers in Egyptian Patients with Non-Small-Cell Lung Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 1927–1933. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Kong, H.; Hou, Y.; Ge, D.; Huang, W.; Ou, J.; Yang, D.; Zhang, L.; Wu, G.; Song, Y.; et al. Two Plasma MicroRNA Panels for Diagnosis and Subtype Discrimination of Lung Cancer. Lung Cancer 2018, 123, 44–51. [Google Scholar] [CrossRef]
- Xi, K.-X.; Zhang, X.-W.; Yu, X.-Y.; Wang, W.-D.; Xi, K.-X.; Chen, Y.-Q.; Wen, Y.-S.; Zhang, L.-J. The Role of Plasma MiRNAs in the Diagnosis of Pulmonary Nodules. J. Thorac. Dis. 2018, 10, 4032–4041. [Google Scholar] [CrossRef]
- Chen, Q.; Si, Q.; Xiao, S.; Xie, Q.; Lin, J.; Wang, C.; Chen, L.; Chen, Q.; Wang, L. Prognostic Significance of Serum MiR-17-5p in Lung Cancer. Med. Oncol. 2012, 30, 353. [Google Scholar] [CrossRef]
- Gallach, S.; Jantus-Lewintre, E.; Calabuig-Fariñas, S.; Montaner, D.; Alonso, S.; Sirera, R.; Blasco, A.; Usó, M.; Guijarro, R.; Martorell, M.; et al. MicroRNA Profiling Associated with Non-Small Cell Lung Cancer: Next Generation Sequencing Detection, Experimental Validation, and Prognostic Value. Oncotarget 2017, 8, 56143–56157. [Google Scholar] [CrossRef]
- Wu, C.; Cao, Y.; He, Z.; He, J.; Hu, C.; Duan, H.; Jiang, J. Serum Levels of MiR-19b and MiR-146a as Prognostic Biomarkers for Non-Small Cell Lung Cancer. Tohoku J. Exp. Med. 2014, 232, 85–95. [Google Scholar] [CrossRef]
- Bulgakova, O.; Zhabayeva, D.; Kussainova, A.; Pulliero, A.; Izzotti, A.; Bersimbaev, R. MiR-19 in Blood Plasma Reflects Lung Cancer Occurrence but Is Not Specifically Associated with Radon Exposure. Oncol. Lett. 2018, 15, 8816–8824. [Google Scholar] [CrossRef]
- Zaporozhchenko, I.A.; Morozkin, E.S.; Skvortsova, T.E.; Ponomaryova, A.A.; Rykova, E.Y.; Cherdyntseva, N.V.; Polovnikov, E.S.; Pashkovskaya, O.A.; Pokushalov, E.A.; Vlassov, V.V.; et al. Plasma MiR-19b and MiR-183 as Potential Biomarkers of Lung Cancer. PLoS ONE 2016, 11, e0165261. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, W.; Shan, X.; Zhu, W.; Xu, J.; Guo, R.; Cheng, W.; Wang, F.; Qi, L.-W.; Chen, Y.; et al. A Six-MicroRNA Panel in Plasma Was Identified as a Potential Biomarker for Lung Adenocarcinoma Diagnosis. Oncotarget 2016, 8, 6513–6525. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Zhan, M.; Mann, D.L.; Stass, S.A.; Jiang, F. Differential MiRNA Expressions in Peripheral Blood Mononuclear Cells for Diagnosis of Lung Cancer. Lab. Investig. 2015, 95, 1197–1206. [Google Scholar] [CrossRef]
- Pu, Q.; Huang, Y.; Lu, Y.; Peng, Y.; Zhang, J.; Feng, G.; Wang, C.; Liu, L.; Dai, Y. Tissue-Specific and Plasma MicroRNA Profiles Could Be Promising Biomarkers of Histological Classification and TNM Stage in Non-Small Cell Lung Cancer. Thorac. Cancer 2016, 7, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Begum, S.; Hayashi, M.; Ogawa, T.; Jabboure, F.J.; Brait, M.; Izumchenko, E.; Tabak, S.; Ahrendt, S.A.; Westra, W.H.; Koch, W.; et al. An Integrated Genome-Wide Approach to Discover Deregulated MicroRNAs in Non-Small Cell Lung Cancer: Clinical Significance of MIR-23b-3p Deregulation. Sci. Rep. 2015, 5, 13236. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yu, Y.; Cao, H.; Shen, H.; Li, X.; Pan, S.; Shu, Y. Deregulated Expression of MiR-21, MiR-143 and MiR-181a in Non Small Cell Lung Cancer Is Related to Clinicopathologic Characteristics or Patient Prognosis. Biomed. Pharmacother. 2010, 64, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yin, Y.; Liu, X.; Xi, X.; Xue, W.; Qu, Y. Non-Small Cell Lung Cancer Associated MicroRNA Expression Signature: Integrated Bioinformatics Analysis, Validation and Clinical Significance. Oncotarget 2017, 8, 24564–24578. [Google Scholar] [CrossRef] [PubMed]
- Solomides, C.C.; Evans, B.J.; Navenot, J.-M.; Vadigepalli, R.; Peiper, S.C.; Wang, Z. MicroRNA Profiling in Lung Cancer Reveals New Molecular Markers for Diagnosis. Acta Cytol. 2012, 56, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Li, R.; Chen, Z.; Shen, Y.; Lu, J.; Xie, X.; Ge, Q. Differentially Expressed MiRNAs in Tumor, Adjacent, and Normal Tissues of Lung Adenocarcinoma. BioMed Res. Int. 2016, 2016, 1428271. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shan, W.; Zhang, Y.; Lv, X.; Li, X.; Wei, C. Up-Regulation of MiR-21 Expression Predicate Advanced Clinicopathological Features and Poor Prognosis in Patients with Non-Small Cell Lung Cancer. Pathol. Oncol. Res. 2016, 22, 161–167. [Google Scholar] [CrossRef]
- Abd-El-Fattah, A.A.; Sadik, N.A.H.; Shaker, O.G.; Aboulftouh, M.L. Differential MicroRNAs Expression in Serum of Patients with Lung Cancer, Pulmonary Tuberculosis, and Pneumonia. Cell Biochem. Biophys. 2013, 67, 875–884. [Google Scholar] [CrossRef]
- Aiso, T.; Ohtsuka, K.; Ueda, M.; Karita, S.; Yokoyama, T.; Takata, S.; Matsuki, N.; Kondo, H.; Takizawa, H.; Okada, A.A.; et al. Serum Levels of Candidate MicroRNA Diagnostic Markers Differ among the Stages of Non-Small-Cell Lung Cancer. Oncol. Lett. 2018, 16, 6643–6651. [Google Scholar] [CrossRef]
- Gao, F.; Chang, J.; Wang, H.; Zhang, G. Potential Diagnostic Value of MiR-155 in Serum from Lung Adenocarcinoma Patients. Oncol. Rep. 2014, 31, 351–357. [Google Scholar] [CrossRef]
- Świtlik, W.Z.; Karbownik, M.S.; Suwalski, M.; Kozak, J.; Szemraj, J. Serum MiR-210-3p as a Potential Noninvasive Biomarker of Lung Adenocarcinoma: A Preliminary Study. Genet. Test. Mol. Biomark. 2019, 23, 353–358. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Z.; Shi, H.; Pan, C. Identification and Evaluation of 2 Circulating MicroRNAs for Non-Small Cell Lung Cancer Diagnosis. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-X.; Bian, H.-B.; Wang, J.-R.; Cheng, Z.-X.; Wang, K.-M.; De, W. Prognostic Significance of Serum MiRNA-21 Expression in Human Non-Small Cell Lung Cancer. J. Surg. Oncol. 2011, 104, 847–851. [Google Scholar] [CrossRef]
- Yang, J.; Li, B.; Lu, H.; Chen, Y.; Lu, C.; Zhu, R.; Liu, S.; Yi, Q.; Li, J.; Song, C. Serum MiR-152, MiR-148a, MiR-148b, and MiR-21 as Novel Biomarkers in Non-Small Cell Lung Cancer Screening. Tumor Biol. 2015, 36, 3035–3042. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, K.; Zhou, Y.; Hu, Z.; Chen, S.; Huang, Y. Application of Serum MicroRNA-9-5p, 21–25p, and 223-3p Combined with Tumor Markers in the Diagnosis of Non-Small-Cell Lung Cancer in Yunnan in Southwestern China. OncoTargets Ther. 2018, 11, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yang, W.; Wang, W.; Hu, W.; Zhu, C.; Yang, H. Serum MicroRNA-21 Is a Potential Diagnostic Marker for Earlier Lung Squamous Cell Carcinoma Detection. Int. J. Clin. Exp. Med. 2017, 10, 3352–3358. [Google Scholar]
- Zhao, W.; Zhao, J.-J.; Zhang, L.; Xu, Q.-F.; Zhao, Y.-M.; Shi, X.-Y.; Xu, A.-G. Serum MiR-21 Level: A Potential Diagnostic and Prognostic Biomarker for Non-Small Cell Lung Cancer. Int. J. Clin. Exp. Med. 2015, 8, 14759–14763. [Google Scholar] [PubMed]
- Abu-Duhier, F.M.; Javid, J.; Sughayer, M.A.; Mir, R.; Albalawi, T.; Alauddin, M.S. Clinical Significance of Circulatory MiRNA-21 as an Efficient Non-Invasive Biomarker for the Screening of Lung Cancer Patients. Asian Pac. J. Cancer Prev. 2018, 19, 2607–2611. [Google Scholar] [CrossRef]
- Geng, Q.; Fan, T.; Zhang, B.; Wang, W.; Xu, Y.; Hu, H. Five MicroRNAs in Plasma as Novel Biomarkers for Screening of Early-Stage Non-Small Cell Lung Cancer. Respir. Res. 2014, 15, 149. [Google Scholar] [CrossRef]
- Jin, X.; Chen, Y.; Chen, H.; Fei, S.; Chen, D.; Cai, X.; Liu, L.; Lin, B.; Su, H.; Zhao, L.; et al. Evaluation of Tumor-Derived Exosomal MiRNA as Potential Diagnostic Biomarkers for Early-Stage Non-Small Cell Lung Cancer Using next-Generation Sequencing. Clin. Cancer Res. 2017, 23, 5311–5319. [Google Scholar] [CrossRef]
- Lin, Y.; Leng, Q.; Jiang, Z.; Guarnera, M.A.; Zhou, Y.; Chen, X.; Wang, H.; Zhou, W.; Cai, L.; Fang, H.; et al. A Classifier Integrating Plasma Biomarkers and Radiological Characteristics for Distinguishing Malignant from Benign Pulmonary Nodules. Int. J. Cancer 2017, 141, 1240–1248. [Google Scholar] [CrossRef]
- Mozzoni, P.; Banda, I.; Goldoni, M.; Corradi, M.; Tiseo, M.; Acampa, O.; Balestra, V.; Ampollini, L.; Casalini, A.; Carbognani, P.; et al. Plasma and EBC MicroRNAs as Early Biomarkers of Non-Small-Cell Lung Cancer. Biomarkers 2013, 18, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Zhang, H.; Zhang, L.; Zhou, X.; Wang, T.; Zhang, J.; Shu, Y.; Zhu, W.; Wen, W.; Liu, P. Identification of Four Plasma MicroRNAs as Potential Biomarkers in the Diagnosis of Male Lung Squamous Cell Carcinoma Patients in China. Cancer Med. 2018, 7, 2370–2381. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, Z.; Todd, N.W.; Zhang, H.; Liao, J.; Yu, L.; Guarnera, M.A.; Li, R.; Cai, L.; Zhan, M.; et al. Diagnosis of Lung Cancer in Individuals with Solitary Pulmonary Nodules by Plasma MicroRNA Biomarkers. BMC Cancer 2011, 11, 374. [Google Scholar] [CrossRef]
- Shen, J.; Todd, N.W.; Zhang, H.; Yu, L.; Lingxiao, X.; Mei, Y.; Guarnera, M.; Liao, J.; Chou, A.; Lu, C.L.; et al. Plasma MicroRNAs as Potential Biomarkers for Non-Small-Cell Lung Cancer. Lab. Investig. 2011, 91, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mei, H.; Xu, C.; Tang, H.; Wei, W. Circulating MicroRNA-339-5p and -21 in Plasma as an Early Detection Predictors of Lung Adenocarcinoma. Pathol. Res. Pract. 2018, 214, 119–125. [Google Scholar] [CrossRef]
- Tang, D.; Shen, Y.; Wang, M.; Yang, R.; Wang, Z.; Sui, A.; Jiao, W.; Wang, Y. Identification of Plasma MicroRNAs as Novel Noninvasive Biomarkers for Early Detection of Lung Cancer. Eur. J. Cancer Prev. 2013, 22, 540–548. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Wang, Q.; Cui, Y.; Luo, S. Clinical Significance of the Expression of MiRNA-21, MiRNA-31 and MiRNA-Let7 in Patients with Lung Cancer. Saudi J. Biol. Sci. 2019, 26, 777–781. [Google Scholar] [CrossRef]
- Wei, J.; Gao, W.; Zhu, C.-J.; Liu, Y.-Q.; Mei, Z.; Cheng, T.; Shu, Y.-Q. Identification of Plasma MicroRNA-21 as a Biomarker for Early Detection and Chemosensitivity of Non-Small Cell Lung Cancer. Chin. J. Cancer 2011, 30, 407–414. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, F.; Shen, T.; Luo, Q.; Ding, Z.; Qian, L.; Huang, J. Plasma MiR-145, MiR-20a, MiR-21 and MiR-223 as Novel Biomarkers for Screening Early-stage Non-small Cell Lung Cancer. Oncol. Lett. 2017, 13, 669–676. [Google Scholar] [CrossRef]
- Zheng, D.; Haddadin, S.; Wang, Y.; Gu, L.-Q.; Perry, M.C.; Freter, C.E.; Wang, M.X. Plasma Micrornas as Novel Biomarkers for Early Detection of Lung Cancer. Int. J. Clin. Exp. Pathol. 2011, 4, 575–586. [Google Scholar]
- Azimi, S.A.; Sadegh Nia, H.R.; Mosavi Jarrahi, A.; Jamaati, H.R.; Kazempour Dizaji, M.; Dargahi, H.; Bahrami, N.; Pasdar, A.; Khosravi, A.; Bahrami, N.; et al. Ectopic Expression of MiRNA-21 and MiRNA-205 in Non-Small Cell Lung Cancer. Int. J. Cancer Manag. 2019, 12, e85456. [Google Scholar] [CrossRef]
- Abdollahi, A.; Rahmati, S.; Ghaderi, B.; Sigari, N.; Nikkhoo, B.; Sharifi, K.; Abdi, M. A Combined Panel of Circulating MicroRNA as a Diagnostic Tool for Detection of the Non-Small Cell Lung Cancer. QJM 2019, 112, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Ouyang, Q.; Hu, S.; Tang, J. Detection of Lung Cancer with Blood MicroRNA-21 Expression Levels in Chinese Population. Oncol. Lett. 2011, 2, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Gu, W.-G.; Li, C.; Nie, S.-L.; Yu, F. Analysis on Expression Level and Diagnostic Value of MiR-19 and MiR-21 in Peripheral Blood of Patients with Undifferentiated Lung Cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8367–8373. [Google Scholar] [CrossRef]
- Razzak, R.; Bédard, E.L.R.; Kim, J.O.; Gazala, S.; Guo, L.; Ghosh, S.; Joy, A.; Nijjar, T.; Wong, E.; Roa, W.H. MicroRNA Expression Profiling of Sputum for the Detection of Early and Locally Advanced Non-Small-Cell Lung Cancer: A Prospective Case–Control Study. Curr. Oncol. 2016, 23, 86–94. [Google Scholar] [CrossRef]
- Roa, W.H.; Kim, J.O.; Razzak, R.; Du, H.; Guo, L.; Singh, R.; Gazala, S.; Ghosh, S.; Wong, E.; Joy, A.A.; et al. Sputum MicroRNA Profiling: A Novel Approach for the Early Detection of Non-Small Cell Lung Cancer. Clin. Investig. Med. 2012, 35, E271–E281. [Google Scholar] [CrossRef]
- Shen, J.; Liao, J.; Guarnera, M.A.; Fang, H.; Cai, L.; Stass, S.A.; Jiang, F. Analysis of MicroRNAs in Sputum to Improve Computed Tomography for Lung Cancer Diagnosis. J. Thorac. Oncol. 2014, 9, 33–40. [Google Scholar] [CrossRef]
- Xie, Y.; Todd, N.W.; Liu, Z.; Zhan, M.; Fang, H.; Peng, H.; Alattar, M.; Deepak, J.; Stass, S.A.; Jiang, F. Altered MiRNA Expression in Sputum for Diagnosis of Non-Small Cell Lung Cancer. Lung Cancer 2010, 67, 170–176. [Google Scholar] [CrossRef]
- Xing, L.; Su, J.; Guarnera, M.A.; Zhang, H.; Cai, L.; Zhou, R.; Stass, S.A.; Jiang, F. Sputum MicroRNA Biomarkers for Identifying Lung Cancer in Indeterminate Solitary Pulmonary Nodules. Clin. Cancer Res. 2015, 21, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Todd, N.W.; Xing, L.; Xie, Y.; Zhang, H.; Liu, Z.; Fang, H.; Zhang, J.; Katz, R.L.; Jiang, F. Early Detection of Lung Adenocarcinoma in Sputum by a Panel of MicroRNA Markers. Int. J. Cancer 2010, 127, 2870–2878. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, P.; Hu, G.; Xiao, Z.; Xu, F.; Zhong, T.; Huang, F.; Kuang, H.; Zhang, W. Relative Expressions of MiR-205-5p, MiR-205-3p, and MiR-21 in Tissues and Serum of Non-Small Cell Lung Cancer Patients. Mol. Cell. Biochem. 2013, 383, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-G.; Zhu, W.-Y.; Huang, Y.-Y.; Ma, L.-N.; Zhou, S.-Q.; Wang, Y.-K.; Zeng, F.; Zhou, J.-H.; Zhang, Y.-K. High Expression of Serum MiR-21 and Tumor MiR-200c Associated with Poor Prognosis in Patients with Lung Cancer. Med. Oncol. 2012, 29, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Sourvinou, I.; Vorkas, P.A.; Yousef, G.M.; Lianidou, E. Clinical Evaluation of MicroRNA Expression Profiling in Non Small Cell Lung Cancer. Lung Cancer 2013, 81, 388–396. [Google Scholar] [CrossRef]
- Tian, F.; Shen, Y.; Chen, Z.; Li, R.; Lu, J.; Ge, Q. Aberrant MiR-181b-5p and MiR-486-5p Expression in Serum and Tissue of Non-Small Cell Lung Cancer. Gene 2016, 591, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, W.; Xu, J.; Yin, L.; Zhu, H.; Liu, J.; Wang, T.; He, X. MiR-21 Expression Significance in Non-Small Cell Lung Cancer Tissue and Plasma. Int. J. Clin. Exp. Med. 2017, 10, 2918–2924. [Google Scholar]
- Yang, X.; Su, W.; Chen, X.; Geng, Q.; Zhai, J.; Shan, H.; Guo, C.; Wang, Z.; Fu, H.; Jiang, H.; et al. Validation of a Serum 4-MicroRNA Signature for the Detection of Lung Cancer. Transl. Lung Cancer Res. 2019, 8, 636–648. [Google Scholar] [CrossRef]
- Rani, S.; Gately, K.; Crown, J.; O’Byrne, K.; O’Driscoll, L. Global Analysis of Serum MicroRNAs as Potential Biomarkers for Lung Adenocarcinoma. Cancer Biol. Ther. 2013, 14, 1104–1112. [Google Scholar] [CrossRef]
- Tan, X.; Qin, W.; Zhang, L.; Hang, J.; Li, B.; Zhang, C.; Wan, J.; Zhou, F.; Shao, K.; Sun, Y.; et al. A 5-MicroRNA Signature for Lung Squamous Cell Carcinoma Diagnosis and Hsa-MiR-31 for Prognosis. Clin. Cancer Res. 2011, 17, 6802–6811. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Ge, Q.; Wu, W.; Zhu, Q.; Luo, J.; Chen, L. Characterization of MicroRNA Transcriptome in Tumor, Adjacent, and Normal Tissues of Lung Squamous Cell Carcinoma. J. Thorac. Cardiovasc. Surg. 2015, 149, 1404–1414.e4. [Google Scholar] [CrossRef]
- Yang, M.; Xiao, L.; Zhang, Y.; Li, G.; Zhou, J. Clinical Significance of Serum MiR-31 as a Predictive Biomarker for Lung Adenocarcinoma. Int. J. Clin. Exp. Pathol. 2017, 10, 4668–4674. [Google Scholar]
- Yan, H.-J.; Ma, J.-Y.; Wang, L.; Gu, W. Expression and Significance of Circulating MicroRNA-31 in Lung Cancer Patients. Med. Sci. Monit. 2015, 21, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, J.; Guarnera, M.A.; Fang, H.; Cai, L.; Jiang, F. Digital PCR Quantification of MiRNAs in Sputum for Diagnosis of Lung Cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zuo, J.; Tan, Q.; Thin, K.Z.; Li, P.; Zhu, M.; Yu, M.; Fu, Z.; Liang, C.; Tu, J. Plasma MiR-92a-2 as a Biomarker for Small Cell Lung Cancer. Cancer Biomark. 2017, 18, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, T.; Liu, H.; Lv, T.; Yuan, D.; Yao, Y.; Lv, Y.; Song, Y. MiR-101 and Mcl-1 in Non-Small-Cell Lung Cancer: Expression Profile and Clinical Significance. Med. Oncol. 2012, 29, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shan, X.; Wang, J.; Zhu, J.; Huang, Z.; Zhang, H.; Zhou, X.; Cheng, W.; Shu, Y.; Zhu, W.; et al. A Three-MicroRNA Signature for Lung Squamous Cell Carcinoma Diagnosis in Chinese Male Patients. Oncotarget 2017, 8, 86897–86907. [Google Scholar] [CrossRef]
- Chen, S.-W.; Wang, T.-B.; Tian, Y.-H.; Zheng, Y.-G. Down-Regulation of MicroRNA-126 and MicroRNA-133b Acts as Novel Predictor Biomarkers in Progression and Metastasis of Non Small Cell Lung Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 14983–14988. [Google Scholar]
- Świtlik, W.; Karbownik, M.S.; Suwalski, M.; Kozak, J.; Szemraj, J. MiR-30a-5p Together with MiR-210-3p as a Promising Biomarker for Non-Small Cell Lung Cancer: A Preliminary Study. Cancer Biomark. 2018, 21, 479–488. [Google Scholar] [CrossRef]
- Tafsiri, E.; Darbouy, M.; Shadmehr, M.B.; Zagryazhskaya, A.; Alizadeh, J.; Karimipoor, M. Expression of MiRNAs in Non-Small-Cell Lung Carcinomas and Their Association with Clinicopathological Features. Tumor Biol. 2015, 36, 1603–1612. [Google Scholar] [CrossRef]
- Grimolizzi, F.; Monaco, F.; Leoni, F.; Bracci, M.; Staffolani, S.; Bersaglieri, C.; Gaetani, S.; Valentino, M.; Amati, M.; Rubini, C.; et al. Exosomal MiR-126 as a Circulating Biomarker in Non-Small-Cell Lung Cancer Regulating Cancer Progression. Sci. Rep. 2017, 7, 15277. [Google Scholar] [CrossRef]
- Lin, Q.; Mao, W.; Shu, Y.; Lin, F.; Liu, S.; Shen, H.; Gao, W.; Li, S.; Shen, D. A Cluster of Specified MicroRNAs in Peripheral Blood as Biomarkers for Metastatic Non-Small-Cell Lung Cancer by Stem-Loop RT-PCR. J. Cancer Res. Clin. Oncol. 2012, 138, 85–93. [Google Scholar] [CrossRef]
- Wang, P.; Yang, D.; Zhang, H.; Wei, X.; Ma, T.; Cheng, Z.; Hong, Q.; Hu, J.; Zhuo, H.; Song, Y.; et al. Early Detection of Lung Cancer in Serum by a Panel of MicroRNA Biomarkers. Clin. Lung Cancer 2015, 16, 313–319.e1. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, K.; Zha, Y.; Chen, D.; He, J.; Ma, H.; Liu, X.; Le, H.; Zhang, Y. Diagnostic Value of Serum MiR-182, MiR-183, MiR-210, and MiR-126 Levels in Patients with Early-Stage Non-Small Cell Lung Cancer. PLoS ONE 2016, 11, e0153046. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Chen, G.; Yan, G.; Zhang, X.; Wan, Y.; Li, Q.; Zhu, B.; Zhuo, W. Identification of a Serum MicroRNA Expression Signature for Detection of Lung Cancer, Involving MiR-23b, MiR-221, MiR-148b and MiR-423-3p. Lung Cancer 2017, 114, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Lin, Y.; Jiang, F.; Lee, C.-J.; Zhan, M.; Fang, H.; Wang, Y.; Jiang, F. A Plasma MiRNA Signature for Lung Cancer Early Detection. Oncotarget 2017, 8, 111902–111911. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fang, H.; Jiang, F.; Ning, Y. External Validation of a Panel of Plasma MicroRNA Biomarkers for Lung Cancer. Biomark. Med. 2019, 13, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Su, M.; Wu, Y.; Fu, L.; Kang, K.; Li, Q.; Li, L.; Hui, G.; Li, F.; Gou, D. Circulating Plasma MiRNAs as Potential Biomarkers of Non–Small Cell Lung Cancer Obtained by High-Throughput Real-Time PCR Profiling. Cancer Epidemiol. Prev. Biomark. 2019, 28, 327–336. [Google Scholar] [CrossRef]
- Bagheri, A.; Khorshid, H.R.K.; Tavallaie, M.; Mowla, S.J.; Sherafatian, M.; Rashidi, M.; Zargari, M.; Boroujeni, M.E.; Hosseini, S.M. A Panel of Noncoding RNAs in Non–Small-Cell Lung Cancer. J. Cell. Biochem. 2019, 120, 8280–8290. [Google Scholar] [CrossRef]
- Xing, L.; Todd, N.W.; Yu, L.; Fang, H.; Jiang, F. Early Detection of Squamous Cell Lung Cancer in Sputum by a Panel of MicroRNA Markers. Mod. Pathol. 2010, 23, 1157–1164. [Google Scholar] [CrossRef]
- Bjaanæs, M.M.; Halvorsen, A.R.; Solberg, S.; Jørgensen, L.; Dragani, T.A.; Galvan, A.; Colombo, F.; Anderlini, M.; Pastorino, U.; Kure, E.; et al. Unique MicroRNA-Profiles in EGFR-Mutated Lung Adenocarcinomas. Int. J. Cancer 2014, 135, 1812–1821. [Google Scholar] [CrossRef]
- Lan, D.; Zhang, X.; He, R.; Tang, R.; Li, P.; He, Q.; Chen, G. MiR-133a Is Downregulated in Non-Small Cell Lung Cancer: A Study of Clinical Significance. Eur. J. Med. Res. 2015, 20, 50. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Chen, H.; Mo, Y.; Ye, H.; Luo, Y.; Guo, K.; Mai, Z.; Zhang, Y.; Chen, B.; et al. Down-Regulation of MiR-133a as a Poor Prognosticator in Non-Small Cell Lung Cancer. Gene 2016, 591, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhou, L.; Li, L.; Liu, P.; Jiao, S. MicroRNA-140-3p Expression Level Is an Independent Prognostic Factor in NSCLC. Int. J. Clin. Exp. Pathol. 2016, 9, 8565–8569. [Google Scholar]
- Shen, H.; Shen, J.; Wang, L.; Shi, Z.; Wang, M.; Jiang, B.; Shu, Y. Low MiR-145 Expression Level Is Associated with Poor Pathological Differentiation and Poor Prognosis in Non-Small Cell Lung Cancer. Biomed. Pharmacother. 2015, 69, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-J.; Zheng, Y.-H.; Wang, P.; Zhang, J.-Z. Serum MiR-125a-5p, MiR-145 and MiR-146a as Diagnostic Biomarkers in Non-Small Cell Lung Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 765–771. [Google Scholar]
- Chen, Y.; Min, L.; Ren, C.; Xu, X.; Yang, J.; Sun, X.; Wang, T.; Wang, F.; Sun, C.; Zhang, X. MiRNA-148a Serves as a Prognostic Factor and Suppresses Migration and Invasion through Wnt1 in Non-Small Cell Lung Cancer. PLoS ONE 2017, 12, e0171751. [Google Scholar] [CrossRef]
- He, Y.; Yang, Y.; Kuang, P.; Ren, S.; Rozeboom, L.; Rivard, C.J.; Li, X.; Zhou, C.; Rhirsch, F. Seven-MicroRNA Panel for Lung Adenocarcinoma Early Diagnosis in Patients Presenting with Ground-Glass Nodules. OncoTargets Ther. 2017, 10, 5915–5926. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Mohamed, E.I.; El-Kaream, S.A.A.; Badawi, M.I.; Darwish, S.H. Underexpression of MiR-486-5p but Not Overexpression of MiR-155 Is Associated with Lung Cancer Stages. MicroRNA 2018, 7, 120–127. [Google Scholar] [CrossRef]
- Arife, Z.; Necdet, Ö.; Serdar, K.; Tuba, E.; Mehmet, T.K.; Kürşad, T.; Mustafa, T.; Leyla, T.; Mehmet, E.E. Diagnostic Value of MiR-125b as a Potential Biomarker for Stage I Lung Adenocarcinoma. Curr. Mol. Med. 2019, 19, 216–227. [Google Scholar]
- Cui, E.; Li, H.; Hua, F.; Wang, B.; Mao, W.; Feng, X.; Li, J.; Wang, X. Serum MicroRNA 125b as a Diagnostic or Prognostic Biomarker for Advanced NSCLC Patients Receiving Cisplatin-Based Chemotherapy. Acta Pharmacol. Sin. 2013, 34, 309–313. [Google Scholar] [CrossRef]
- Roth, C.; Kasimir-Bauer, S.; Pantel, K.; Schwarzenbach, H. Screening for Circulating Nucleic Acids and Caspase Activity in the Peripheral Blood as Potential Diagnostic Tools in Lung Cancer. Mol. Oncol. 2011, 5, 281–291. [Google Scholar] [CrossRef]
- Luo, J.; Shi, K.; Yin, S.; Tang, R.; Chen, W.; Huang, L.; Gan, T.; Cai, Z.; Chen, G. Clinical Value of MiR-182-5p in Lung Squamous Cell Carcinoma: A Study Combining Data from TCGA, GEO, and RT-QPCR Validation. World J. Surg. Oncol. 2018, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, X.; Liu, J.; Liu, H.; Du, X. Up-Regulation of MiR-182-5p Predicts Poor Prognosis in Patients with Lung Cancer and Associates with Tumor Cell Growth and Migration. Int. J. Clin. Exp. Pathol. 2017, 10, 3061–3068. [Google Scholar]
- Zou, J.-G.; Ma, L.-F.; Li, X.; Xu, F.-L.; Fei, X.-Z.; Liu, Q.; Bai, Q.-L.; Dong, Y.-L. Circulating MicroRNA Array (MiR-182, 200b and 205) for the Early Diagnosis and Poor Prognosis Predictor of Non-Small Cell Lung Cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, X.; He, J.; Chen, D.; Hunag, Y.; Zhang, Y.K. Overexpression of Members of the MicroRNA-183 Family Is a Risk Factor for Lung Cancer: A Case Control Study. BMC Cancer 2011, 11, 393. [Google Scholar] [CrossRef]
- Halvorsen, A.R.; Bjaanæs, M.; LeBlanc, M.; Holm, A.M.; Bolstad, N.; Rubio, L.; Peñalver, J.C.; Cervera, J.; Mojarrieta, J.C.; López-Guerrero, J.A.; et al. A Unique Set of 6 Circulating MicroRNAs for Early Detection of Non-Small Cell Lung Cancer. Oncotarget 2016, 7, 37250–37259. [Google Scholar] [CrossRef]
- Tang, R.; Zhong, T.; Dang, Y.; Zhang, X.; Li, P.; Chen, G. Association between Downexpression of MiR-203 and Poor Prognosis in Non-Small Cell Lung Cancer Patients. Clin. Transl. Oncol. 2016, 18, 360–368. [Google Scholar] [CrossRef]
- Võsa, U.; Vooder, T.; Kolde, R.; Fischer, K.; Välk, K.; Tõnisson, N.; Roosipuu, R.; Vilo, J.; Metspalu, A.; Annilo, T. Identification of MiR-374a as a Prognostic Marker for Survival in Patients with Early-Stage Nonsmall Cell Lung Cancer. Genes Chromosomes Cancer 2011, 50, 812–822. [Google Scholar] [CrossRef]
- Zhang, Y.; Sui, J.; Shen, X.; Li, C.; Yao, W.; Hong, W.; Peng, H.; Pu, Y.; Yin, L.; Liang, G. Differential Expression Profiles of MicroRNAs as Potential Biomarkers for the Early Diagnosis of Lung Cancer. Oncol. Rep. 2017, 37, 3543–3553. [Google Scholar] [CrossRef]
- Zhang, Y.-K.; Zhu, W.-Y.; He, J.-Y.; Chen, D.-D.; Huang, Y.-Y.; Le, H.-B.; Liu, X.-G. MiRNAs Expression Profiling to Distinguish Lung Squamous-Cell Carcinoma from Adenocarcinoma Subtypes. J. Cancer Res. Clin. Oncol. 2012, 138, 1641–1650. [Google Scholar] [CrossRef]
- Leng, Q.; Wang, Y.; Jiang, F. A Direct Plasma MiRNA Assay for Early Detection and Histological Classification of Lung Cancer. Transl. Oncol. 2018, 11, 883–889. [Google Scholar] [CrossRef]
- Sromek, M.; Glogowski, M.; Chechlinska, M.; Kulinczak, M.; Szafron, L.; Zakrzewska, K.; Owczarek, J.; Wisniewski, P.; Wlodarczyk, R.; Talarek, L.; et al. Changes in Plasma MiR-9, MiR-16, MiR-205 and MiR-486 Levels after Non-Small Cell Lung Cancer Resection. Cell. Oncol. 2017, 40, 529–536. [Google Scholar] [CrossRef]
- He, R.; Cen, W.; Cen, J.; Cen, W.; Li, J.; Li, M.; Gan, T.; Hu, X.; Chen, G. Clinical Significance of MiR-210 and Its Prospective Signaling Pathways in Non-Small Cell Lung Cancer: Evidence from Gene Expression Omnibus and the Cancer Genome Atlas Data Mining with 2763 Samples and Validation via Real-Time Quantitative PCR. Cell. Physiol. Biochem. 2018, 46, 925–952. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Zhang, H.; Yang, Z.-G.; Wen, G.-Q.; Cui, Y.-B.; Shao, G.-G. Prognostic Significance of Serum MicroRNA-210 Levels in Nonsmall-Cell Lung Cancer. J. Int. Med. Res. 2013, 41, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Liu, Y.; Meng, H.-H.; Wang, X.-W. Expression of MiR-210 in Senile COPD Complicating Primary Lung Cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 38–42. [Google Scholar] [PubMed]
- Wang, X.; Zhi, X.; Zhang, Y.; An, G.; Feng, G. Role of Plasma MicroRNAs in the Early Diagnosis of Non-Small-Cell Lung Cancers: A Case-Control Study. J. Thorac. Dis. 2016, 8, 1645–1652. [Google Scholar] [CrossRef]
- Ge, X.; Liu, S.; Liu, H.; Li, D.; Wang, C. Increased Levels of Tissue MicroRNA-221 in Human Non-Small Cell Lung Cancer and Its Clinical Significance. Int. J. Clin. Exp. Pathol. 2016, 9, 2003–2008. [Google Scholar]
- Lv, S.; Xue, J.; Wu, C.; Wang, L.; Wu, J.; Xu, S.; Liang, X.; Lou, J. Identification of A Panel of Serum MicroRNAs as Biomarkers for Early Detection of Lung Adenocarcinoma. J. Cancer 2017, 8, 48–56. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Zhao, Y.; Liu, W.; Wu, H.; Liu, J.; Mu, X.; Wu, H. Down-Regulated MicroRNA-375 Expression as a Predictive Biomarker in Non-Small Cell Lung Cancer Brain Metastasis and Its Prognostic Significance. Pathol. Res. Pract. 2017, 213, 882–888. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Y.; Zhang, J.; Huang, W.; Jiang, H.; Hou, Y.; Xu, C.; Zhai, C.; Gao, X.; Wang, S.; et al. The Expression of MiR-375 Is Associated with Carcinogenesis in Three Subtypes of Lung Cancer. PLoS ONE 2015, 10, e0144187. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, L.; Sun, C.; Guo, L.; Lin, M.; Huang, J.; Zhu, L. Decreased Circulating MiR-375: A Potential Biomarker for Patients with Non-Small-Cell Lung Cancer. Gene 2014, 534, 60–65. [Google Scholar] [CrossRef]
- Goto, A.; Tanaka, M.; Yoshida, M.; Umakoshi, M.; Nanjo, H.; Shiraishi, K.; Saito, M.; Kohno, T.; Kuriyama, S.; Konno, H.; et al. The Low Expression of MiR-451 Predicts a Worse Prognosis in Non-Small Cell Lung Cancer Cases. PLoS ONE 2017, 12, e0181270. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.-L.; Jiang, X.-Y.; Wang, Y.-Y.; Li, D.-G.; She, Y.; Chang, J.-H.; Meng, A.-M. The Expression and Function of MiRNA-451 in Non-Small Cell Lung Cancer. Cancer Lett. 2011, 311, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Qu, S.; Hu, R.; Gao, W.; Jin, S.; Liu, M.; Zhao, Q. A Panel of MiRNAs Derived from Plasma Extracellular Vesicles as Novel Diagnostic Biomarkers of Lung Adenocarcinoma. FEBS Open Bio 2019, 9, 2149–2158. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, X.; Yang, Y.; Zhao, Y.; Shen, Z.; Huang, Y. MicroRNA Expression Profiling of Lung Adenocarcinoma in Xuanwei, China: A Preliminary Study. Medicine 2019, 98, e15717. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, Y.; Xu, C.; Qin, H.; Lei, Z.; Shen, D.; Liu, Z.; Huang, J.-A. Expression Profile Analysis of MicroRNAs and Downregulated MiR-486-5p and MiR-30a-5p in Non-Small Cell Lung Cancer. Oncol. Rep. 2015, 34, 1779–1786. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhang, Q.; Tang, L.; Liu, X.; Dai, Y.; Xiao, L.; Huang, S.; Chen, L.; Guo, Z.; et al. MicroRNA-486 as a Biomarker for Early Diagnosis and Recurrence of Non-Small Cell Lung Cancer. PLoS ONE 2015, 10, e0134220. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Z.; Dong, J.; Li, J.; Shi, X.; Sun, N.; Luo, M.; Zhou, F.; Tan, F.; He, J. Combination of Serum MiRNAs with Cyfra21-1 for the Diagnosis of Non-Small Cell Lung Cancer. Cancer Lett. 2015, 367, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L., Jr.; Omenn, G.S.; Valanis, B.; Williams, J.H., Jr. The Beta-Carotene and Retinol Efficacy Trial: Incidence of Lung Cancer and Cardiovascular Disease Mortality During 6-Year Follow-up After Stopping β-Carotene and Retinol Supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Inoue, J.; Inazawa, J. Cancer-Associated MiRNAs and Their Therapeutic Potential. J. Hum. Genet. 2021, 66, 937–945. [Google Scholar] [CrossRef]
- Van Roosbroeck, K.; Calin, G.A. Chapter Four—Cancer Hallmarks and MicroRNAs: The Therapeutic Connection. In Advances in Cancer Research; Croce, C.M., Fisher, P.B., Eds.; miRNA and Cancer; Academic Press: Cambridge, MA, USA, 2017; Volume 135, pp. 119–149. [Google Scholar]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for MicroRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.Y.; Calin, G.A. MicroRNAs as Therapeutic Targets in Human Cancers. WIREs RNA 2014, 5, 537–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Cases | Controls |
|---|---|---|
| N | 102 | 212 |
| Age, mean ± StDev | 65 ± 6 | 65 ± 6 |
| Sex, N (%) | ||
| Female | 32 (31%) | 63 (30%) |
| Male | 70 (69%) | 149 (70%) |
| Smoking Status, N (%) | ||
| Current | 70 (69%) | 143 (67%) |
| Former | 32 (31%) | 69 (33%) |
| Smoking Pack Years (PYs), mean ± StDev | 53.5 ± 21.4 | 48.7 ± 20.5 |
| Histology, N (%) | ||
| Adenocarcinoma | 37 (36%) | - |
| Squamous Cell Carcinoma | 27 (26%) | - |
| Other Non-Small-Cell Lung Carcinoma | 38 (37%) | - |
| Hsa-miRNA | Alternate ID(s) | Validated Reports of Dysregulation in Lung Cancer Clinical Specimens | TaqMan Assay ID | References | |
|---|---|---|---|---|---|
| Tissue | Biofluid | ||||
| miR-15b-5p | miR-15b | 3 | 390 | [9,10,16] | |
| miR-17-5p | miR-17 | 2 | 11 | 2308 | [9,10,11,16,17,18,19,20,21,22,23,24] |
| miR-19b-3p | miR-19b; miR-19b-1 | 1 | 9 | 396 | [9,10,22,25,26,27,28,29,30,31] |
| miR-21-5p | miR-21 | 7 | 45 | 397 | [9,10,18,23,25,28,29,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76] |
| miR-28-3p | 2 | 2446 | [9,10] | ||
| miR-30b-5p | miR-30b | 5 | 602 | [9,10,11,12,77] | |
| miR-30c-5p | 5 | 419 | [9,10,11,12,78] | ||
| miR-31-5p | miR-31 | 5 | 6 | 2279 | [25,32,34,58,68,70,79,80,81,82,83] |
| miR-92a-3p | miR-92a-2 | 6 | 431 | [9,10,11,12,16,84] | |
| miR-101-3p | miR-101 | 1 | 1 | 2253 | [10,85] |
| miR-106a-5p | miR-106a | 4 | 2169 | [9,10,53,86] | |
| miR-126-3p | miR-126 | 6 | 21 | 2228 | [9,10,11,25,28,32,34,41,51,55,68,70,71,74,87,88,89,90,91,92,93,94,95,96,97,98,99] |
| miR-133a-3p | miR-133a | 3 | 1 | 2246 | [10,100,101,102] |
| miR-140-3p | 2 | 2 | 2234 | [9,10,79,103] | |
| miR-140-5p | 4 | 1187 | [9,10,11,12] | ||
| miR-142-3p | 3 | 464 | [9,10,11] | ||
| miR-145-5p | miR-145 | 2 | 13 | 2278 | [10,13,23,32,39,49,51,57,60,71,95,96,98,104,105] |
| miR-148a-3p | miR-148; miR-148a | 2 | 5 | 470 | [10,11,12,44,63,106,107] |
| miR-155-5p | miR-155 | 2 | 9 | 2623 | [38,40,49,57,61,67,70,108,109,110,111] |
| miR-182-5p | miR-182 | 6 | 10 | 2334 | [17,23,25,34,38,55,61,68,70,71,79,93,112,113,114,115] |
| miR-197-3p | miR-197 | 5 | 497 | [9,10,31,38,61] | |
| miR-203a-3p | miR-203 | 2 | 1 | 507 | [17,116,117] |
| miR-205-5p | miR-205 | 8 | 15 | 509 | [23,28,32,34,35,51,62,68,70,72,77,80,88,95,96,99,114,116,118,119,120,121,122] |
| miR-210-3p | miR-210 | 7 | 20 | 512 | [9,17,23,34,41,51,54,55,66,67,68,70,79,83,88,89,93,95,96,98,99,107,121,123,124,125,126] |
| miR-221-3p | miR-221 | 2 | 7 | 524 | [9,10,13,23,29,94,120,127,128] |
| miR-320a-3p | miR-320 | 3 | 2277 | [9,10,13] | |
| miR-375 | 2 | 5 | 564 | [22,68,70,71,129,130,131] | |
| miR-451a | miR-451 | 5 | 7 | 1141 | [9,10,25,31,34,35,74,97,132,133,134] |
| miR-486-5p | miR-486 | 7 | 16 | 1278 | [9,10,11,34,35,41,50,52,54,55,68,70,71,75,79,88,108,122,126,134,135,136,137] |
| miR-660-5p | miR-660 | 3 | 1515 | [9,10,138] | |
| Case:Ctrl | |||
|---|---|---|---|
| Hsa-miRNA | Fold-Change | AUC | p |
| miR-451a | 0.88 | 0.37 | 0.0002 |
| miR-320a-3p | 1.36 | 0.62 | 0.0005 |
| miR-210-3p | 1.63 | 0.62 | 0.0008 |
| miR-92a-3p | 1.16 | 0.58 | 0.0217 |
| mir-21-5p | 1.33 | 0.58 | 0.0258 |
| miR-140-3p | 1.11 | 0.57 | 0.0318 |
| miR-148a-3p | 1.14 | 0.56 | 0.1135 |
| miR-660-5p | 1.24 | 0.55 | 0.1264 |
| miR-106a-5p | 0.97 | 0.46 | 0.2274 |
| miR-197-3p | 1.23 | 0.54 | 0.2274 |
| miR-17-5p | 1.01 | 0.47 | 0.3960 |
| miR-101-3p | 1.05 | 0.53 | 0.4178 |
| miR-221-3p | 1.04 | 0.52 | 0.4789 |
| miR-15b-5p | 0.98 | 0.48 | 0.4880 |
| miR-30b-5p | 1.02 | 0.48 | 0.5065 |
| miR-155-5p | 1.02 | 0.48 | 0.5686 |
| miR-142-3p | 1.06 | 0.52 | 0.6024 |
| miR-30c-5p | 1.02 | 0.48 | 0.6238 |
| miR-19b-3p | 1 | 0.49 | 0.6705 |
| miR-140-5p | 0.96 | 0.49 | 0.6988 |
| miR-145-5p | 1.07 | 0.51 | 0.7983 |
| miR-375 | 1.11 | 0.51 | 0.8771 |
| miR-28-3p | 1.07 | 0.5 | 0.8949 |
| miR-486-5p | 1.08 | 0.5 | 0.9118 |
| miR-126-3p | 1.05 | 0.5 | 0.9762 |
| miR-133a-3p | 1.1 | 0.5 | 0.9815 |
| Specificity | 4MP Sensitivity | 4MP + 3 miRNA Sensitivity | Δ (95% CI) | p |
|---|---|---|---|---|
| 99% | 19.0% | 31.0% | 11.9% (2.38, 23.9) | 0.031 |
| 98% | 19.0% | 33.3% | 14.3% (4.8, 26.2) | 0.019 |
| 95% | 19.0% | 38.1% | 19.1% (0.0, 28.6) | 0.006 |
| 90% | 35.7% | 42.9% | 7.1% (-4.8, 26.2) | 0.369 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vykoukal, J.; Fahrmann, J.F.; Patel, N.; Shimizu, M.; Ostrin, E.J.; Dennison, J.B.; Ivan, C.; Goodman, G.E.; Thornquist, M.D.; Barnett, M.J.; et al. Contributions of Circulating microRNAs for Early Detection of Lung Cancer. Cancers 2022, 14, 4221. https://doi.org/10.3390/cancers14174221
Vykoukal J, Fahrmann JF, Patel N, Shimizu M, Ostrin EJ, Dennison JB, Ivan C, Goodman GE, Thornquist MD, Barnett MJ, et al. Contributions of Circulating microRNAs for Early Detection of Lung Cancer. Cancers. 2022; 14(17):4221. https://doi.org/10.3390/cancers14174221
Chicago/Turabian StyleVykoukal, Jody, Johannes F. Fahrmann, Nikul Patel, Masayoshi Shimizu, Edwin J. Ostrin, Jennifer B. Dennison, Cristina Ivan, Gary E. Goodman, Mark D. Thornquist, Matt J. Barnett, and et al. 2022. "Contributions of Circulating microRNAs for Early Detection of Lung Cancer" Cancers 14, no. 17: 4221. https://doi.org/10.3390/cancers14174221
APA StyleVykoukal, J., Fahrmann, J. F., Patel, N., Shimizu, M., Ostrin, E. J., Dennison, J. B., Ivan, C., Goodman, G. E., Thornquist, M. D., Barnett, M. J., Feng, Z., Calin, G. A., & Hanash, S. M. (2022). Contributions of Circulating microRNAs for Early Detection of Lung Cancer. Cancers, 14(17), 4221. https://doi.org/10.3390/cancers14174221








