Metformin with Temozolomide for Newly Diagnosed Glioblastoma: Results of Phase I Study and a Brief Review of Relevant Studies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Treatment
2.2. Patients
2.3. Genetic Analysis
2.4. Study Endpoints
2.5. Dose-Limiting Toxicity
2.6. Statistical Analyses
3. Results
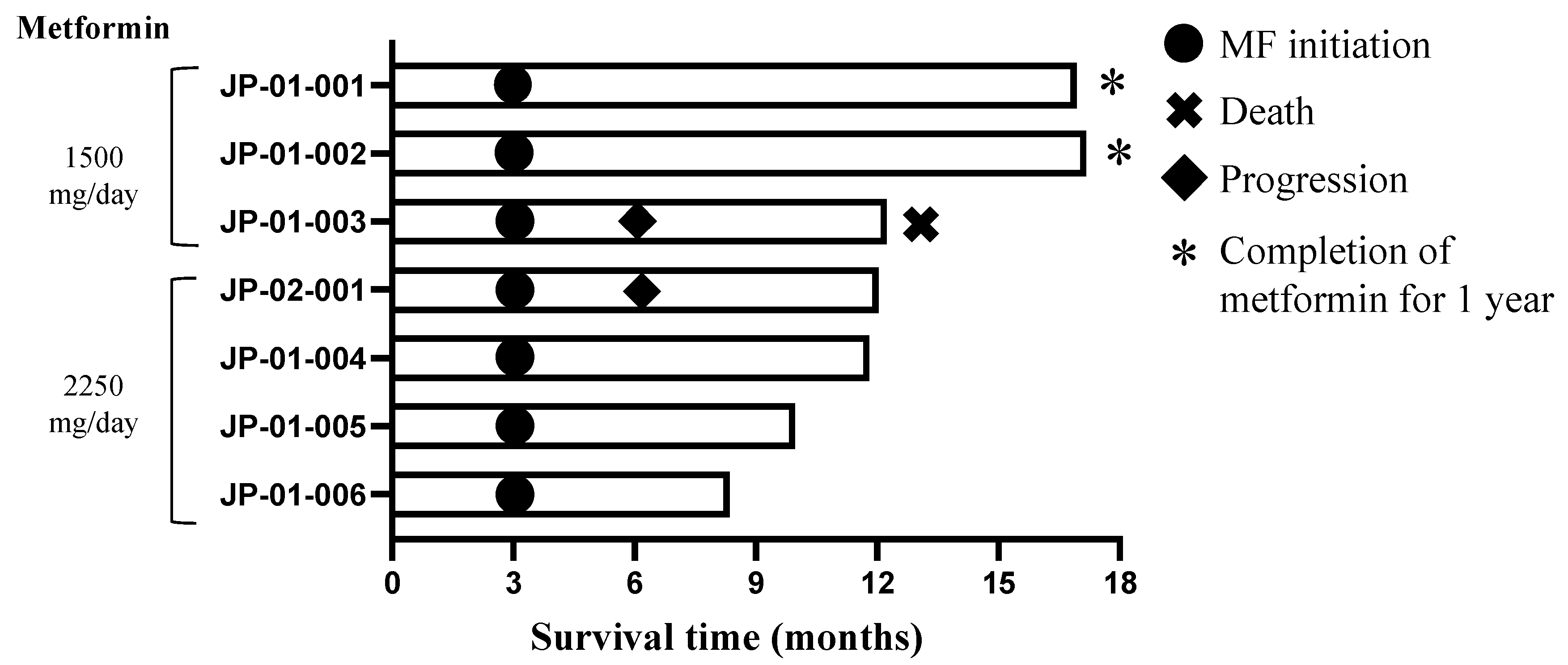
3.1. Patient Characteristics
3.2. Safety Data
3.3. Efficacy
4. Discussion
4.1. Safety
4.2. MF Dose
4.3. MF Suppresses Tumorigenicity at a High Dose
4.4. Anti-Cancer Mechanism of MF for Glioma Stem Cells
4.5. The Effect of MF Depends on Extracellular Glucose Concentration
4.6. MF Has Limited Suppressive Effects on Massive Tumors or Progressively Growing Tumors
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, K.; Yun, S.; Moon, S.; Park, Y.; Han, J.H.; Kim, C.Y.; Lee, H.S.; Choe, G. Prognostic relevance of programmed cell death ligand 1 expression in glioblastoma. J. Neuro-Oncol. 2018, 136, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Committee of Brain Tumor Registry of Japan. Report of Brain Tumor Registry of Japan (2005–2008) 14th Edition. Neurol. Med. Chir. 2017, 57 (Suppl. S1), 9–102. [Google Scholar] [CrossRef]
- Sato, A.; Sunayama, J.; Okada, M.; Watanabe, E.; Seino, S.; Shibuya, K.; Suzuki, K.; Narita, Y.; Shibui, S.; Kayama, T.; et al. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl. Med. 2012, 1, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Suva, M.L.; Tirosh, I. The Glioma Stem Cell Model in the Era of Single-Cell Genomics. Cancer Cell 2020, 37, 630–636. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Yu, T.S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef]
- Sunayama, J.; Matsuda, K.; Sato, A.; Tachibana, K.; Suzuki, K.; Narita, Y.; Shibui, S.; Sakurada, K.; Kayama, T.; Tomiyama, A.; et al. Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stem-like cells. Stem Cells 2010, 28, 1930–1939. [Google Scholar] [CrossRef]
- Sunayama, J.; Sato, A.; Matsuda, K.; Tachibana, K.; Suzuki, K.; Narita, Y.; Shibui, S.; Sakurada, K.; Kayama, T.; Tomiyama, A.; et al. Dual blocking of mTor and PI3K elicits a prodifferentiation effect on glioblastoma stem-like cells. Neuro-Oncol. 2010, 12, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Sunayama, J.; Sato, A.; Matsuda, K.; Tachibana, K.; Watanabe, E.; Seino, S.; Suzuki, K.; Narita, Y.; Shibui, S.; Sakurada, K.; et al. FoxO3a functions as a key integrator of cellular signals that control glioblastoma stem-like cell differentiation and tumorigenicity. Stem Cells 2011, 29, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Tsai, H.L.; Kung, Y.T.; Yeh, Y.S.; Huang, C.W.; Ma, C.J.; Chiu, H.C.; Wang, J.Y. Dose-Dependent Relationship Between Metformin and Colorectal Cancer Occurrence Among Patients with Type 2 Diabetes—A Nationwide Cohort Study. Transl. Oncol. 2018, 11, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Metformin Use and Gastric Cancer Risk in Diabetic Patients After Helicobacter pylori Eradication. J. Natl. Cancer Inst. 2019, 111, 484–489. [Google Scholar] [CrossRef]
- Kordes, S.; Pollak, M.N.; Zwinderman, A.H.; Mathot, R.A.; Weterman, M.J.; Beeker, A.; Punt, C.J.; Richel, D.J.; Wilmink, J.W. Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015, 16, 839–847. [Google Scholar] [CrossRef]
- Lin, H.C.; Kachingwe, B.H.; Lin, H.L.; Cheng, H.W.; Uang, Y.S.; Wang, L.H. Effects of metformin dose on cancer risk reduction in patients with type 2 diabetes mellitus: A 6-year follow-up study. Pharmacotherapy 2014, 34, 36–45. [Google Scholar] [CrossRef]
- Tsai, M.J.; Yang, C.J.; Kung, Y.T.; Sheu, C.C.; Shen, Y.T.; Chang, P.Y.; Huang, M.S.; Chiu, H.C. Metformin decreases lung cancer risk in diabetic patients in a dose-dependent manner. Lung Cancer 2014, 86, 137–143. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K. WHO Classification of Tumours of the Central Nervous System, Revised, 4th ed.; IARC Press: Lyon, France, 2016. [Google Scholar]
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours WHO Classification of Tumours, 5th ed.; IARC Press: Lyon, France, 2021. [Google Scholar]
- Arita, H.; Narita, Y.; Matsushita, Y.; Fukushima, S.; Yoshida, A.; Takami, H.; Miyakita, Y.; Ohno, M.; Shibui, S.; Ichimura, K. Development of a robust and sensitive pyrosequencing assay for the detection of IDH1/2 mutations in gliomas. Brain Tumor Pathol. 2015, 32, 22–30. [Google Scholar] [CrossRef]
- Arita, H.; Yamasaki, K.; Matsushita, Y.; Nakamura, T.; Shimokawa, A.; Takami, H.; Tanaka, S.; Mukasa, A.; Shirahata, M.; Shimizu, S.; et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol. Commun. 2016, 4, 79. [Google Scholar] [CrossRef]
- Arita, H.; Narita, Y.; Fukushima, S.; Tateishi, K.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Collins, V.P.; Kawahara, N.; et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013, 126, 267–276. [Google Scholar] [CrossRef]
- Ohno, M.; Miyakita, Y.; Takahashi, M.; Igaki, H.; Matsushita, Y.; Ichimura, K.; Narita, Y. Survival benefits of hypofractionated radiotherapy combined with temozolomide or temozolomide plus bevacizumab in elderly patients with glioblastoma aged ≥ 75 years. Radiat. Oncol. 2019, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Chan, D.K.; Shank, J.J.; Griffith, K.A.; Fan, H.; Szulawski, R.; Yang, K.; Reynolds, R.K.; Johnston, C.; McLean, K.; et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight 2020, 5, e133247. [Google Scholar] [CrossRef] [PubMed]
- Fenn, K.; Maurer, M.; Lee, S.M.; Crew, K.D.; Trivedi, M.S.; Accordino, M.K.; Hershman, D.L.; Kalinsky, K. Phase 1 Study of Erlotinib and Metformin in Metastatic Triple-Negative Breast Cancer. Clin. Breast Cancer 2020, 20, 80–86. [Google Scholar] [CrossRef]
- Omar, A.I.; Mason, W.P. Temozolomide: The evidence for its therapeutic efficacy in malignant astrocytomas. Core Evid. 2010, 4, 93–111. [Google Scholar] [CrossRef]
- Broekman, K.E.; Hof, M.A.J.; Touw, D.J.; Gietema, J.A.; Nijman, H.W.; Lefrandt, J.D.; Reyners, A.K.L.; Jalving, M. Phase I study of metformin in combination with carboplatin/paclitaxel chemotherapy in patients with advanced epithelial ovarian cancer. Investig. New Drugs 2020, 38, 1454–1462. [Google Scholar] [CrossRef]
- Gulati, S.; Desai, J.; Palackdharry, S.M.; Morris, J.C.; Zhu, Z.; Jandarov, R.; Riaz, M.K.; Takiar, V.; Mierzwa, M.; Gutkind, J.S.; et al. Phase 1 dose-finding study of metformin in combination with concurrent cisplatin and radiotherapy in patients with locally advanced head and neck squamous cell cancer. Cancer 2020, 126, 354–362. [Google Scholar] [CrossRef]
- Khawaja, M.R.; Nick, A.M.; Madhusudanannair, V.; Fu, S.; Hong, D.; McQuinn, L.M.; Ng, C.S.; Piha-Paul, S.A.; Janku, F.; Subbiah, V.; et al. Phase I dose escalation study of temsirolimus in combination with metformin in patients with advanced/refractory cancers. Cancer Chemother. Pharmacol. 2016, 77, 973–977. [Google Scholar] [CrossRef]
- Maraka, S.; Groves, M.D.; Mammoser, A.G.; Melguizo-Gavilanes, I.; Conrad, C.A.; Tremont-Lukats, I.W.; Loghin, M.E.; O’Brien, B.J.; Puduvalli, V.K.; Sulman, E.P.; et al. Phase 1 lead-in to a phase 2 factorial study of temozolomide plus memantine, mefloquine, and metformin as postradiation adjuvant therapy for newly diagnosed glioblastoma. Cancer 2019, 125, 424–433. [Google Scholar] [CrossRef]
- Molenaar, R.J.; van de Venne, T.; Weterman, M.J.; Mathot, R.A.; Klumpen, H.J.; Richel, D.J.; Wilmink, J.W. A phase Ib study of everolimus combined with metformin for patients with advanced cancer. Investig. New Drugs 2018, 36, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Porper, K.; Shpatz, Y.; Plotkin, L.; Pechthold, R.G.; Talianski, A.; Champ, C.E.; Furman, O.; Shimoni-Sebag, A.; Symon, Z.; Amit, U.; et al. A Phase I clinical trial of dose-escalated metabolic therapy combined with concomitant radiation therapy in high-grade glioma. J. Neuro-Oncol. 2021, 153, 487–496. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Decensi, A.; Puntoni, M.; Goodwin, P.; Cazzaniga, M.; Gennari, A.; Bonanni, B.; Gandini, S. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. 2010, 3, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Libby, G.; Donnelly, L.A.; Donnan, P.T.; Alessi, D.R.; Morris, A.D.; Evans, J.M. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care 2009, 32, 1620–1625. [Google Scholar] [CrossRef]
- Mazurek, M.; Litak, J.; Kamieniak, P.; Kulesza, B.; Jonak, K.; Baj, J.; Grochowski, C. Metformin as Potential Therapy for High-Grade Glioma. Cancers 2020, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Zheng, Z.J.; Kan, H.; Song, Y.; Cui, W.; Zhao, G.; Kip, K.E. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: A meta-analysis. Diabetes Care 2011, 34, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, L.; Navone, S.E.; Masseroli, M.M.; Balsamo, M.; Caroli, M.; Valtorta, S.; Moresco, R.M.; Campanella, R.; Schisano, L.; Fiore, G.; et al. Effects of Metformin as Add-On Therapy against Glioblastoma: An Old Medicine for Novel Oncology Therapeutics. Cancers 2022, 14, 1412. [Google Scholar] [CrossRef]
- Seliger, C.; Luber, C.; Gerken, M.; Schaertl, J.; Proescholdt, M.; Riemenschneider, M.J.; Meier, C.R.; Bogdahn, U.; Leitzmann, M.F.; Klinkhammer-Schalke, M.; et al. Use of metformin and survival of patients with high-grade glioma. Int. J. Cancer 2019, 144, 273–280. [Google Scholar] [CrossRef]
- Seliger, C.; Genbrugge, E.; Gorlia, T.; Chinot, O.; Stupp, R.; Nabors, B.; Weller, M.; Hau, P.; EORTC Brain Tumor Group. Use of metformin and outcome of patients with newly diagnosed glioblastoma: Pooled analysis. Int. J. Cancer 2020, 146, 803–809. [Google Scholar] [CrossRef]
- Dallaglio, K.; Bruno, A.; Cantelmo, A.R.; Esposito, A.I.; Ruggiero, L.; Orecchioni, S.; Calleri, A.; Bertolini, F.; Pfeffer, U.; Noonan, D.M.; et al. Paradoxic effects of metformin on endothelial cells and angiogenesis. Carcinogenesis 2014, 35, 1055–1066. [Google Scholar] [CrossRef]
- Valtorta, S.; Lo Dico, A.; Raccagni, I.; Martelli, C.; Pieri, V.; Rainone, P.; Todde, S.; Zinnhardt, B.; De Bernardi, E.; Coliva, A.; et al. Imaging Metformin Efficacy as Add-On Therapy in Cells and Mouse Models of Human EGFR Glioblastoma. Front. Oncol. 2021, 11, 664149. [Google Scholar] [CrossRef]
- Wurth, R.; Pattarozzi, A.; Gatti, M.; Bajetto, A.; Corsaro, A.; Parodi, A.; Sirito, R.; Massollo, M.; Marini, C.; Zona, G.; et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: A role for metformin-induced inhibition of Akt. Cell Cycle 2013, 12, 145–156. [Google Scholar] [CrossRef]
- Labuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopien, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Marcus, H.J.; Carpenter, K.L.; Price, S.J.; Hutchinson, P.J. In vivo assessment of high-grade glioma biochemistry using microdialysis: A study of energy-related molecules, growth factors and cytokines. J. Neuro-Oncol. 2010, 97, 11–23. [Google Scholar] [CrossRef]
- Marrone, K.A.; Zhou, X.; Forde, P.M.; Purtell, M.; Brahmer, J.R.; Hann, C.L.; Kelly, R.J.; Coleman, B.; Gabrielson, E.; Rosner, G.L.; et al. A Randomized Phase II Study of Metformin plus Paclitaxel/Carboplatin/Bevacizumab in Patients with Chemotherapy-Naive Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer. Oncologist 2018, 23, 859–865. [Google Scholar] [CrossRef]
- Miranda, V.C.; Braghiroli, M.I.; Faria, L.D.; Bariani, G.; Alex, A.; Bezerra Neto, J.E.; Capareli, F.C.; Sabbaga, J.; Lobo Dos Santos, J.F.; Hoff, P.M.; et al. Phase 2 Trial of Metformin Combined with 5-Fluorouracil in Patients with Refractory Metastatic Colorectal Cancer. Clin. Color. Cancer 2016, 15, 321–328.e1. [Google Scholar] [CrossRef]
- Nanni, O.; Amadori, D.; De Censi, A.; Rocca, A.; Freschi, A.; Bologna, A.; Gianni, L.; Rosetti, F.; Amaducci, L.; Cavanna, L.; et al. Metformin plus chemotherapy versus chemotherapy alone in the first-line treatment of HER2-negative metastatic breast cancer. The MYME randomized, phase 2 clinical trial. Breast Cancer Res. Treat. 2019, 174, 433–442. [Google Scholar] [CrossRef]
- Parikh, A.B.; Kozuch, P.; Rohs, N.; Becker, D.J.; Levy, B.P. Metformin as a repurposed therapy in advanced non-small cell lung cancer (NSCLC): Results of a phase II trial. Investig. New Drugs 2017, 35, 813–819. [Google Scholar] [CrossRef]
- Pujalte Martin, M.; Borchiellini, D.; Thamphya, B.; Guillot, A.; Paoli, J.B.; Besson, D.; Hilgers, W.; Priou, F.; El Kouri, C.; Hoch, B.; et al. TAXOMET: A French Prospective Multicentric Randomized Phase II Study of Docetaxel Plus Metformin Versus Docetaxel Plus Placebo in Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2021, 19, 501–509. [Google Scholar] [CrossRef]
- Skinner, H.; Hu, C.; Tsakiridis, T.; Santana-Davila, R.; Lu, B.; Erasmus, J.J.; Doemer, A.J.; Videtic, G.M.M.; Coster, J.; Yang, A.X.; et al. Addition of Metformin to Concurrent Chemoradiation in Patients with Locally Advanced Non-Small Cell Lung Cancer: The NRG-LU001 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1324–1332. [Google Scholar] [CrossRef]
- Tsakiridis, T.; Pond, G.R.; Wright, J.; Ellis, P.M.; Ahmed, N.; Abdulkarim, B.; Roa, W.; Robinson, A.; Swaminath, A.; Okawara, G.; et al. Metformin in Combination with Chemoradiotherapy in Locally Advanced Non-Small Cell Lung Cancer: The OCOG-ALMERA Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.; Esteva, F.J.; Patel, M.M.; Raghavendra, A.S.; Ueno, N.T.; Moulder, S.L.; Hess, K.R.; Shroff, G.S.; Hodge, S.; Koenig, K.H.; et al. Efficacy and safety of the combination of metformin, everolimus and exemestane in overweight and obese postmenopausal patients with metastatic, hormone receptor-positive, HER2-negative breast cancer: A phase II study. Investig. New Drugs 2019, 37, 345–351. [Google Scholar] [CrossRef] [PubMed]
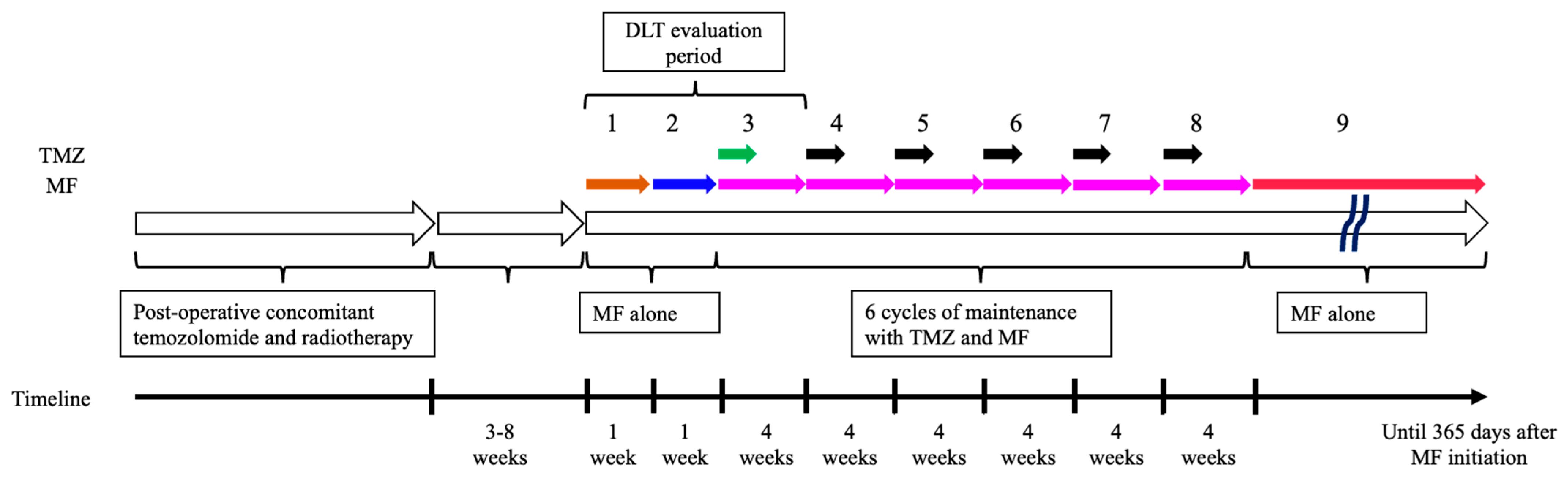
| Dose Level | MF Dose | ||
|---|---|---|---|
| Step 1 | Step 2 | Step 3 | |
| Level 2 | 500 mg/day | 1500 mg/day | 2250 mg/day |
| Level 1 | 500 mg/day | 1000 mg day | 1500 mg/day |
| Level −1 | 500 mg/day | 750 mg/day | 1000 mg/day |
| Dose Level | Patient | Sex | Age | KPS | Diagnosis (WHO2016) | Diagnosis (WHO2021) | Measurable Residual Tumor | IDH1/2 | MGMT | TERT | H3F3A | BRAF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level 1 | JP-01-001 | Male | 32 | 90 | Glioblastoma, IDH-mutant | Astrocytoma, IDH-mutant, WHO grade 4 | No | Mutant | Unmethylated | Wild type | Wild type | Wild type |
| Level 1 | JP-01-002 | Female | 67 | 80 | Glioblastoma, IDH-wildtype | Glioblastoma, IDH-wildtype, WHO grade 4 | No | Wild-type | Unmethylated | Mutant | Wild type | Wild type |
| Level 1 | JP-01-003 | Male | 34 | 70 | Glioblastoma, IDH-wildtype | Glioblastoma, IDH-wildtype, WHO grade 4 | No | Wild-type | Unmethylated | Wild type | Wild type | Wild type |
| Level 2 | JP-02-001 | Male | 46 | 100 | Glioblastoma, IDH-wildtype | Glioblastoma, IDH-wildtype, WHO grade 4 | Yes | Wild-type | Unmethylated | Wild type | Wild type | Wild type |
| Level 2 | JP-01-004 | Male | 40 | 90 | Glioblastoma, IDH-mutant | Astrocytoma, IDH-mutant, WHO grade 4 | Yes | Mutant | Methylated | Wild type | Wild type | Wild type |
| Level 2 | JP-01-005 | Female | 41 | 80 | Glioblastoma, IDH-mutant | Astrocytoma, IDH-mutant, WHO grade 4 | Yes | Mutant | Methylated | Wild type | Wild type | Wild type |
| Level 2 | JP-01-006 | Male | 46 | 90 | Glioblastoma, IDH-wildtype | Glioblastoma, IDH-wildtype, WHO grade 4 | No | Wild-type | Unmethylated | Mutant | Wild type | Wild type |
| Category | All (n = 7) | Level 1: MF 1500 mg/Day (n = 3) | Level 2: MF 2250 mg/Day (n = 4) | |||||
|---|---|---|---|---|---|---|---|---|
| All Grades | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
| Hematologic | ||||||||
| Leukopenia | 2 (28.6%) | 0 | 1 (33.3%) | 0 | 0 | 0 | 1 (25.0%) | 0 |
| Neutropenia | 2 (28.6%) | 0 | 0 | 1 (33.3%) | 0 | 0 | 1 (25.0%) | 0 |
| Lymphocytopenia | 1 (14.3%) | 0 | 0 | 0 | 0 | 0 | 1 (25.0%) | 0 |
| Thrombocytopenia | 1 (14.3%) | 0 | 0 | 0 | 0 | 1 (25.0%) | 0 | 0 |
| Anemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatic | ||||||||
| Aspartate transaminase | 1 (14.3%) | 0 | 1 (33.3%) | 0 | 0 | 0 | 0 | 0 |
| Alanine transaminase | 2 (28.6%) | 0 | 0 | 1 (33.3%) | 0 | 1 (25.0%) | 0 | 0 |
| Appetite loss | 3 (42.9%) | 0 | 0 | 0 | 0 | 2 (50.0%) | 1 (25.0%) | 0 |
| Nausea | 3 (42.9%) | 0 | 0 | 0 | 0 | 3 (75.0%) | 0 | 0 |
| Vomiting | 1 (14.3%) | 0 | 0 | 0 | 0 | 1 (25.0%) | 0 | 0 |
| Constipation | 2 (28.6%) | 0 | 1 (33.3%) | 0 | 0 | 1 (25.0%) | 0 | 0 |
| Fatigue | 1 (14.3%) | 0 | 1 (33.3%) | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 3 (42.9%) | 0 | 2 (66.7%) | 0 | 0 | 0 | 1 (25.0%) | 0 |
| Abdominal pain | 1 (14.3%) | 0 | 0 | 0 | 0 | 0 | 1 (25.0%) | 0 |
| Seizure | 1 (14.3%) | 1 (14.3%) | 0 | 0 | 0 | 0 | 0 | 1 (25.0%) |
| Somnolence | 1 (14.3%) | 0 | 0 | 0 | 0 | 1 (25.0%) | 0 | 0 |
| Intracranial hemorrhage | 1 (14.3%) | 0 | 0 | 0 | 0 | 1 (25.0%) | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohno, M.; Kitanaka, C.; Miyakita, Y.; Tanaka, S.; Sonoda, Y.; Mishima, K.; Ishikawa, E.; Takahashi, M.; Yanagisawa, S.; Ohashi, K.; et al. Metformin with Temozolomide for Newly Diagnosed Glioblastoma: Results of Phase I Study and a Brief Review of Relevant Studies. Cancers 2022, 14, 4222. https://doi.org/10.3390/cancers14174222
Ohno M, Kitanaka C, Miyakita Y, Tanaka S, Sonoda Y, Mishima K, Ishikawa E, Takahashi M, Yanagisawa S, Ohashi K, et al. Metformin with Temozolomide for Newly Diagnosed Glioblastoma: Results of Phase I Study and a Brief Review of Relevant Studies. Cancers. 2022; 14(17):4222. https://doi.org/10.3390/cancers14174222
Chicago/Turabian StyleOhno, Makoto, Chifumi Kitanaka, Yasuji Miyakita, Shota Tanaka, Yukihiko Sonoda, Kazuhiko Mishima, Eiichi Ishikawa, Masamichi Takahashi, Shunsuke Yanagisawa, Ken Ohashi, and et al. 2022. "Metformin with Temozolomide for Newly Diagnosed Glioblastoma: Results of Phase I Study and a Brief Review of Relevant Studies" Cancers 14, no. 17: 4222. https://doi.org/10.3390/cancers14174222
APA StyleOhno, M., Kitanaka, C., Miyakita, Y., Tanaka, S., Sonoda, Y., Mishima, K., Ishikawa, E., Takahashi, M., Yanagisawa, S., Ohashi, K., Nagane, M., & Narita, Y. (2022). Metformin with Temozolomide for Newly Diagnosed Glioblastoma: Results of Phase I Study and a Brief Review of Relevant Studies. Cancers, 14(17), 4222. https://doi.org/10.3390/cancers14174222








