Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment
Abstract
:Simple Summary
Abstract
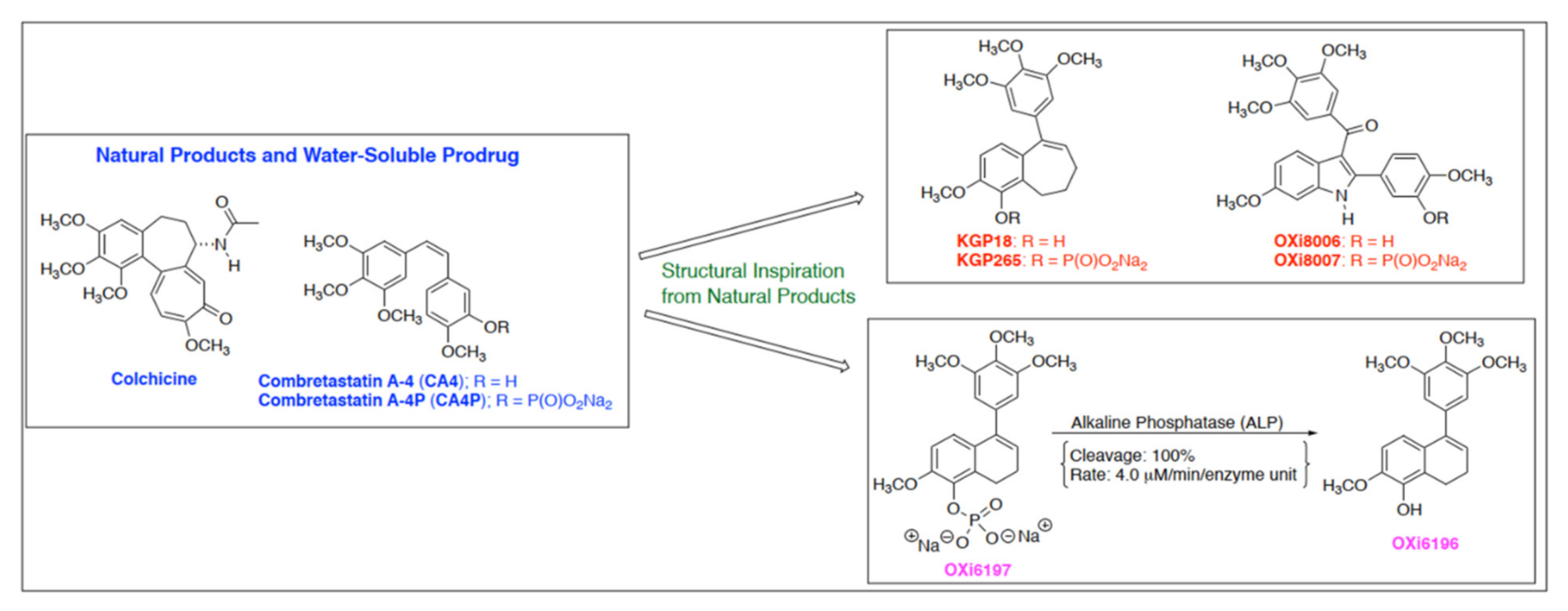
1. Introduction
2. Materials and Methods
2.1. Cell Studies
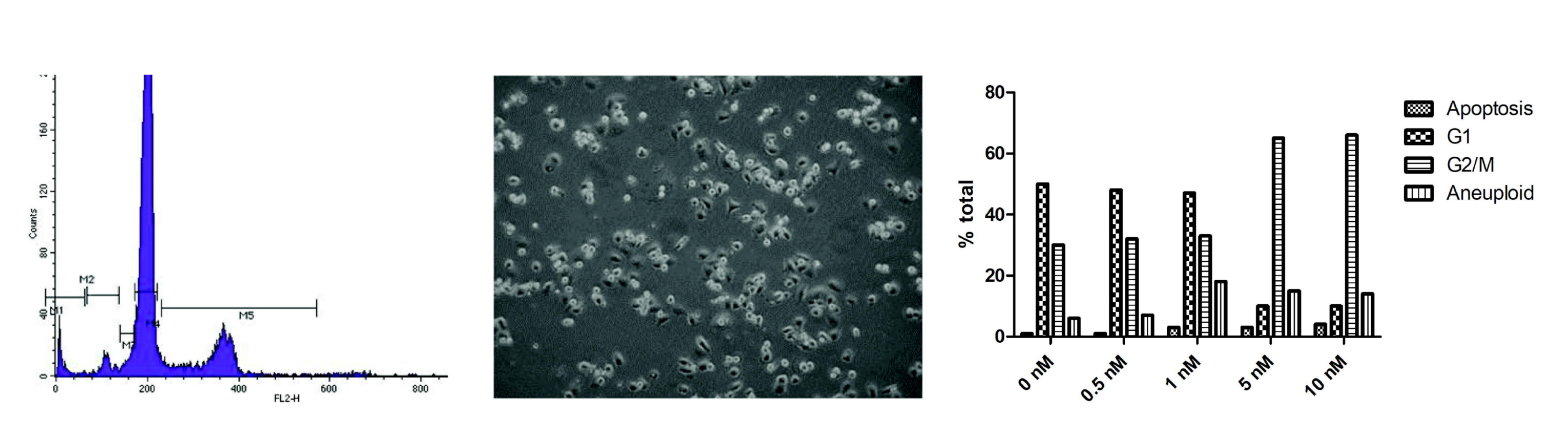
2.1.1. Cell Cycle Analysis
2.1.2. Western Blotting
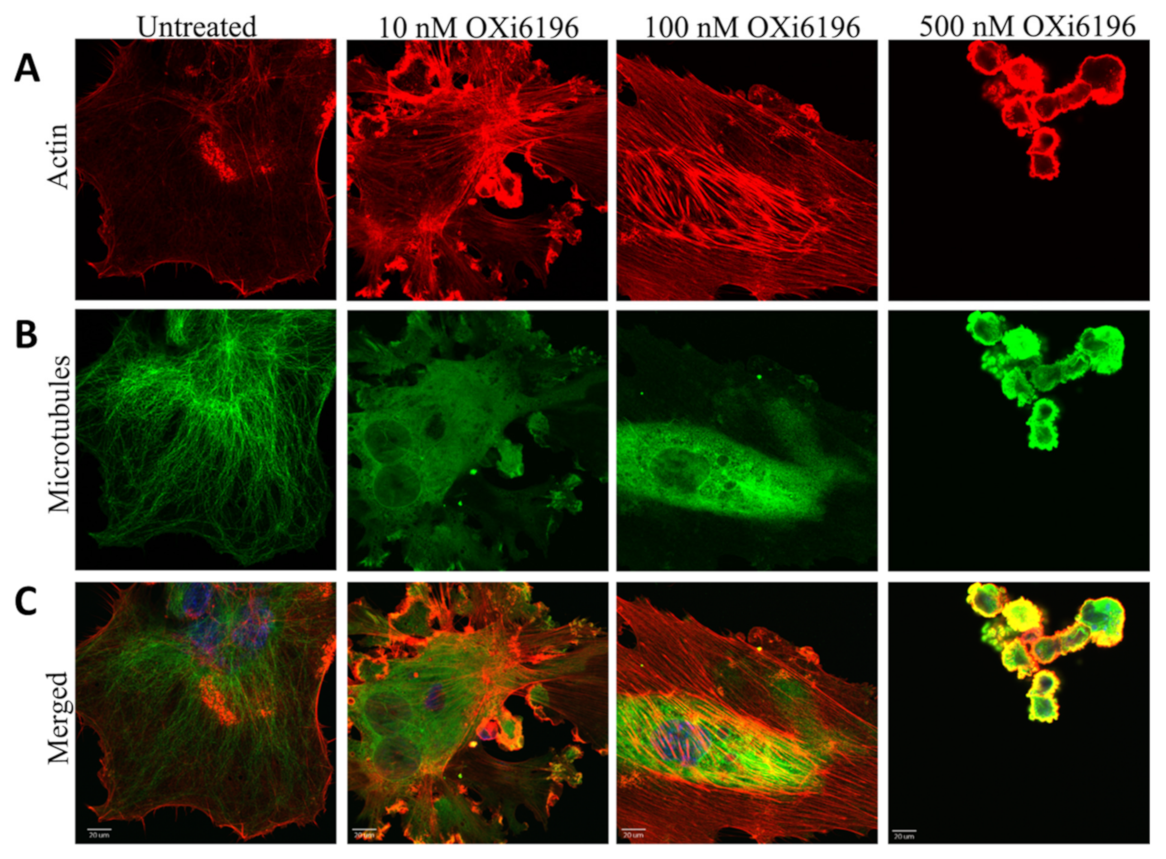
2.1.3. Fluorescence Imaging of Endothelial Cells
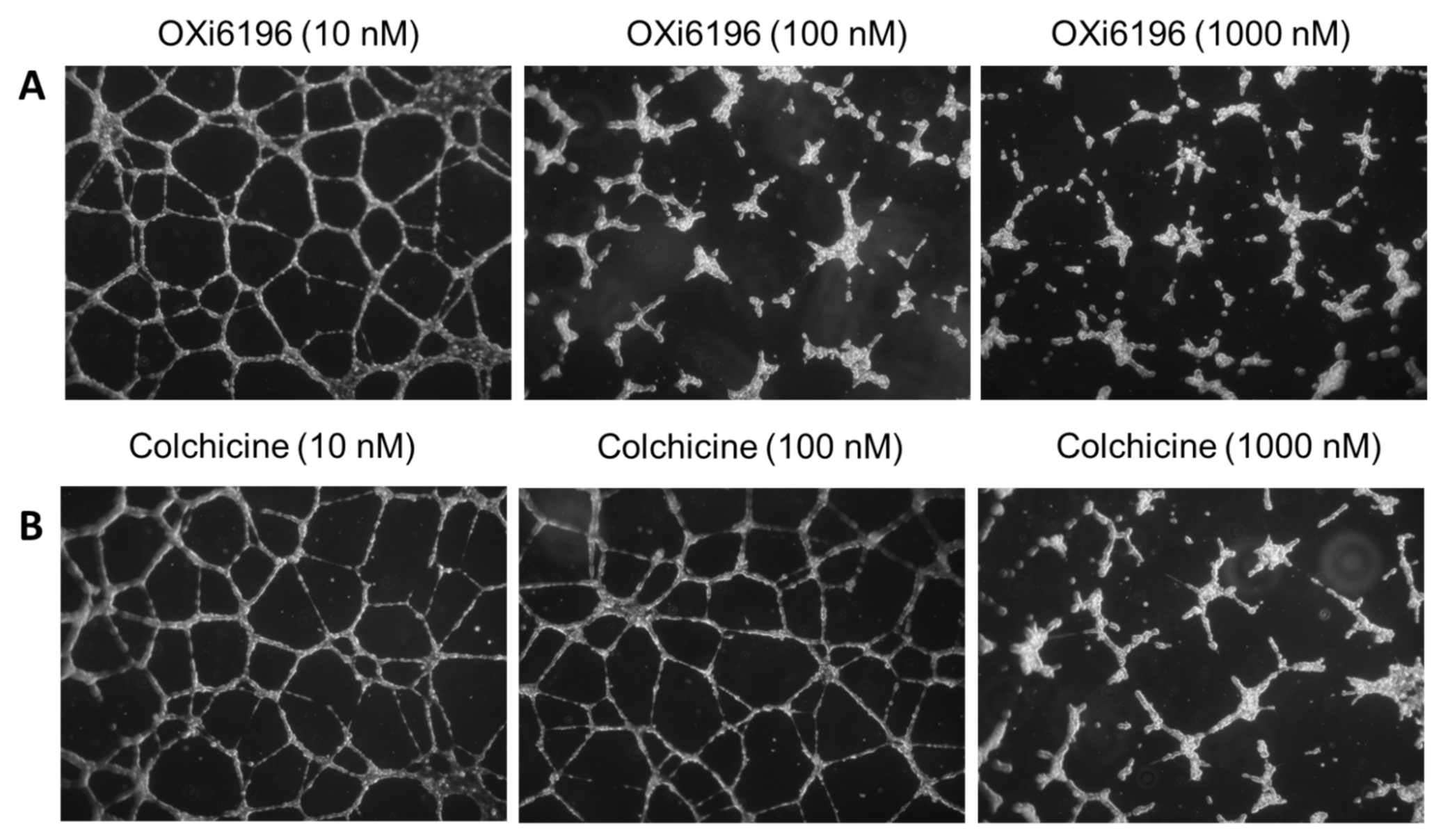
2.1.4. Endothelial Tube Disruption Assay
2.1.5. Cancer Cell Culture
2.2. Animal Models
2.3. Bioluminescence Imaging (BLI)
2.4. Therapy
2.5. Histology
3. Results
3.1. Cell Culture Observations
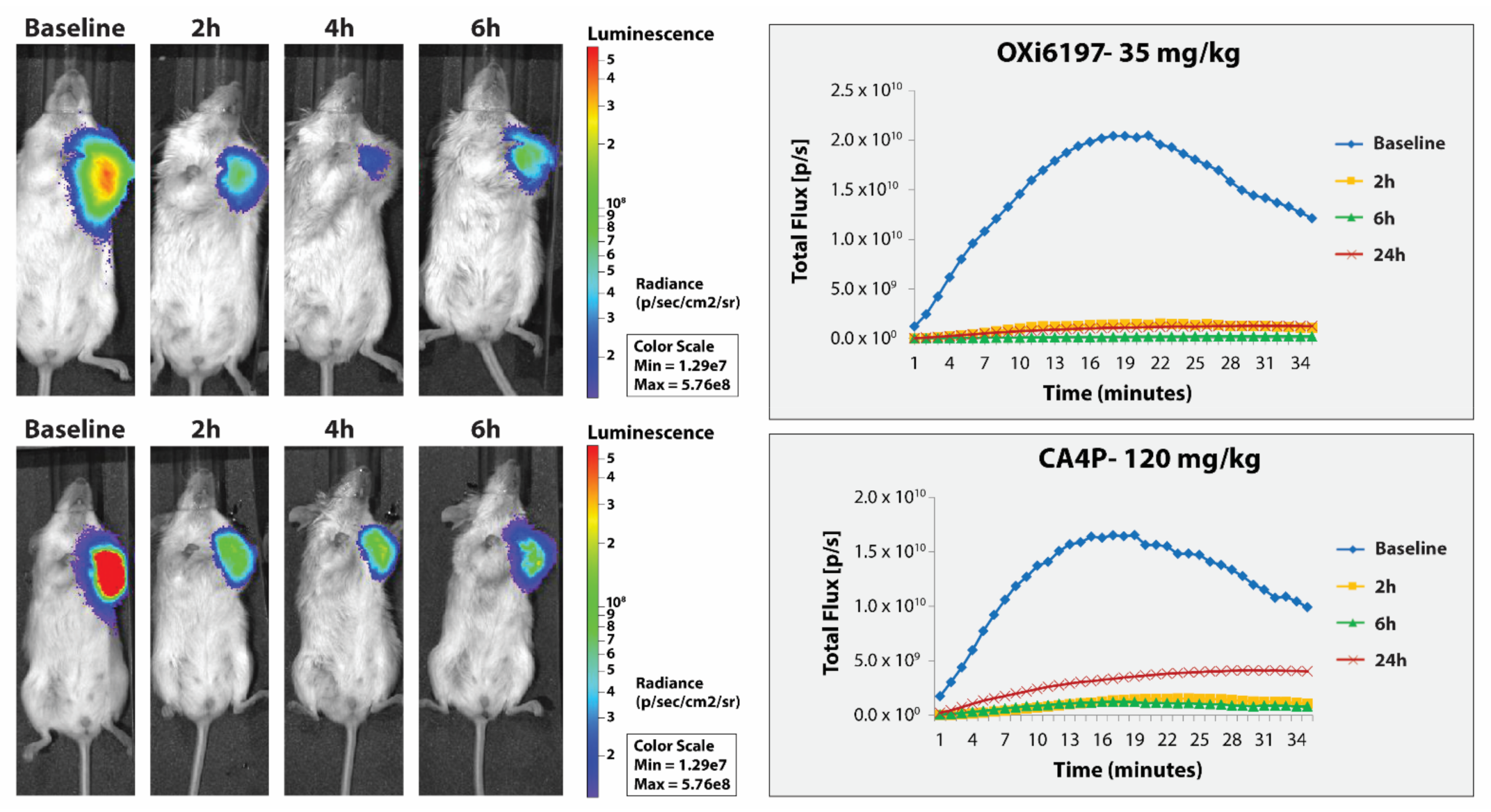
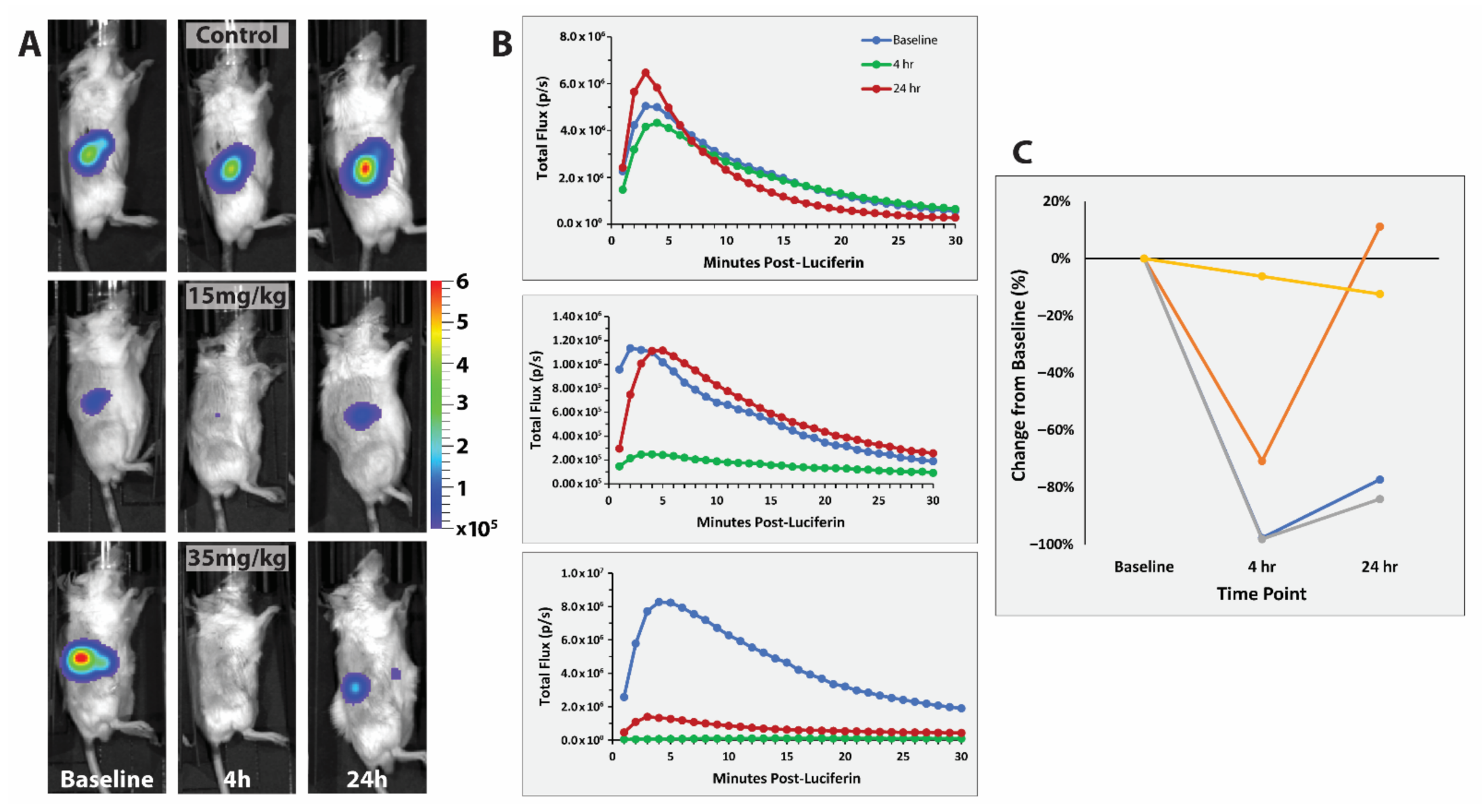
3.2. MDA-MB-231 Breast Tumors
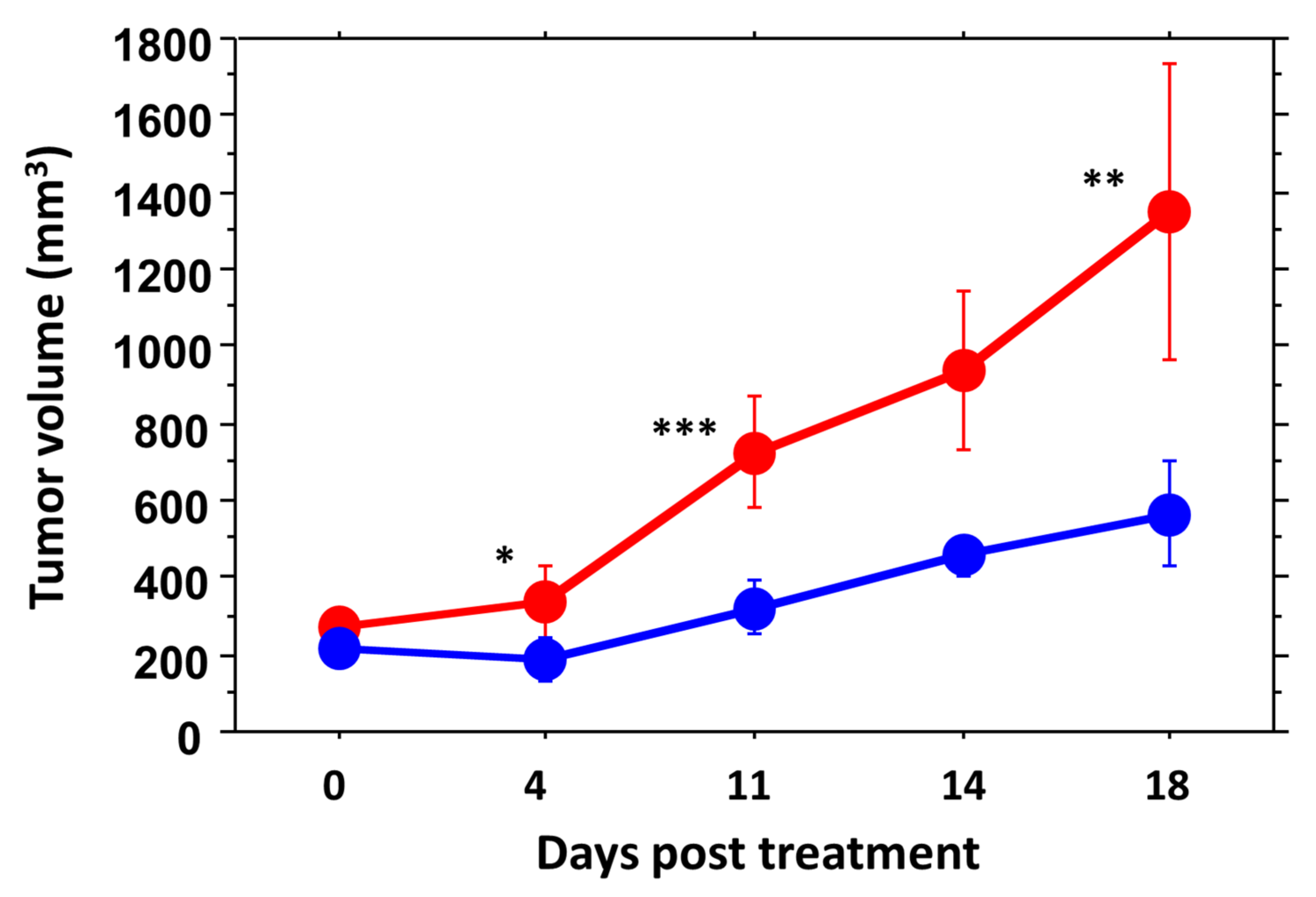
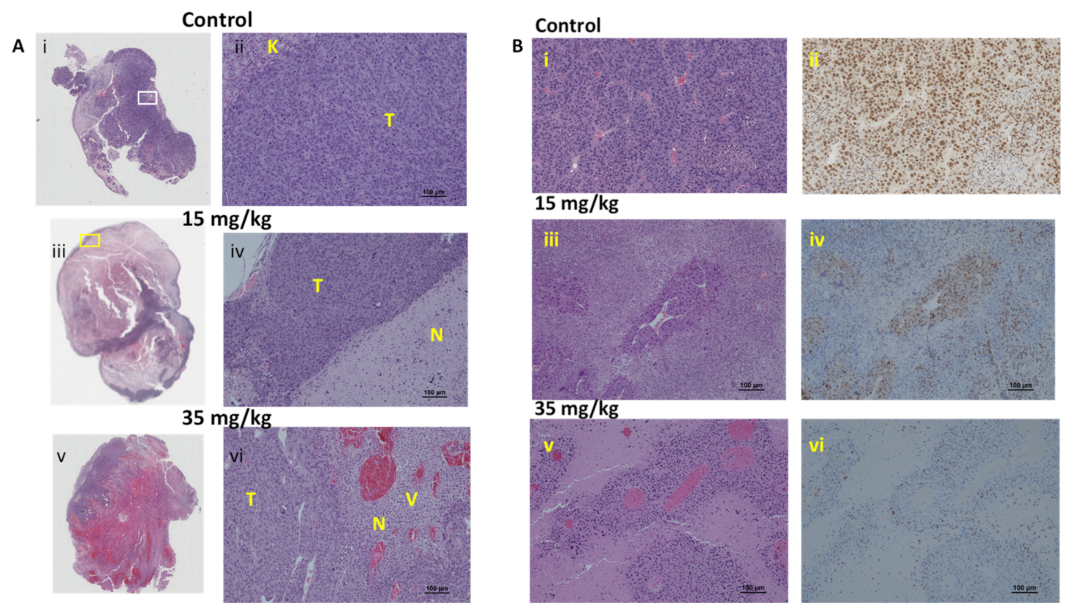
3.3. RENCA Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siemann, D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, P.; Eskens, F.A. Vascular disrupting agents in clinical development. Br. J. Cancer 2007, 96, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Ojha, R.; Lee, H.Y.; Liou, J.P. Early investigational tubulin inhibitors as novel cancer therapeutics. Expert Opin. Investig. Drugs 2016, 25, 917–936. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W.; Chaplin, D.J.; Horsman, M.R. Realizing the Potential of Vascular Targeted Therapy: The Rationale for Combining Vascular Disrupting Agents and Anti-Angiogenic Agents to Treat Cancer. Cancer Investig. 2017, 35, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Denekamp, J. Vascular Attack as a Therapeutic Strategy for Cancer. Cancer Metastasis Rev. 1990, 9, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Sosa, J.A.; Elisei, R.; Jarzab, B.; Bal, C.S.; Koussis, H.; Gramza, A.W.; Ben-Yosef, R.; Gitlitz, B.J.; Haugen, B.; Karandikar, S.M.; et al. A randomized phase II/III trial of a tumor vascular disrupting agent fosbretabulin tromethamine (CA4P) with carboplatin (C) and paclitaxel (P) in anaplastic thyroid cancer (ATC): Final survival analysis for the FACT trial. J. Clin. Oncol. 2011, 29, 5502. [Google Scholar] [CrossRef]
- Blay, J.Y.; Papai, Z.; Tolcher, A.W.; Italiano, A.; Cupissol, D.; Lopez-Pousa, A.; Chawla, S.P.; Bompas, E.; Babovic, N.; Penel, N.; et al. Ombrabulin plus cisplatin versus placebo plus cisplatin in patients with advanced soft-tissue sarcomas after failure of anthracycline and ifosfamide chemotherapy: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 531–540. [Google Scholar] [CrossRef]
- Pal, S.; Azad, A.; Bhatia, S.; Drabkin, H.; Costello, B.; Sarantopoulos, J.; Kanesvaran, R.; Lauer, R.; Starodub, A.; Hauke, R.; et al. A Phase I/II Trial of BNC105P with Everolimus in Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2015, 21, 3420–3427. [Google Scholar] [CrossRef]
- Clemenson, C.; Chargari, C.; Deutsch, E. Combination of vascular disrupting agents and ionizing radiation. Crit. Rev. Oncol. Hematol. 2013, 86, 143–160. [Google Scholar] [CrossRef]
- Horsman, M.R. Enhancing the radiation response of tumors but not early or late responding normal tissues using a vascular disrupting agent. Acta Oncol. 2017, 56, 1634–1638. [Google Scholar] [CrossRef] [Green Version]
- Pedley, R.B.; Hill, S.A.; Boxer, G.M.; Flynn, A.A.; Boden, R.; Watson, R.; Dearling, J.; Chaplin, D.J.; Begent, R.H. Eradication of colorectal xenografts by combined radioimmunotherapy and combretastatin a-4 3-O-phosphate. Cancer Res. 2001, 61, 4716–4722. [Google Scholar]
- Uckun, F.M.; Cogle, C.R.; Lin, T.L.; Qazi, S.; Trieu, V.N.; Schiller, G.; Watts, J.M. A Phase 1B Clinical Study of Combretastatin A1 Diphosphate (OXi4503) and Cytarabine (ARA-C) in Combination (OXA) for Patients with Relapsed or Refractory Acute Myeloid Leukemia. Cancers 2020, 12, 74. [Google Scholar] [CrossRef]
- Horsman, M.R.; Wittenborn, T.R.; Nielsen, P.S.; Elming, P.B. Tumors Resistant to Checkpoint Inhibitors Can Become Sensitive after Treatment with Vascular Disrupting Agents. Int. J. Mol. Sci. 2020, 21, 4778. [Google Scholar] [CrossRef]
- Drzyzga, A.; Cichoń, T.; Czapla, J.; Jarosz-Biej, M.; Pilny, E.; Matuszczak, S.; Wojcieszek, P.; Urbaś, Z.; Smolarczyk, R. The Proper Administration Sequence of Radiotherapy and Anti-Vascular Agent—DMXAA Is Essential to Inhibit the Growth of Melanoma Tumors. Cancers 2021, 13, 3924. [Google Scholar] [CrossRef]
- Zhao, D.; Chang, C.-H.; Kim, J.G.; Liu, H.; Mason, R.P. In vivo near-infrared spectroscopy and MRI monitoring of tumor response to Combretastatin A4 phosphate correlated with therapeutic outcome. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 574–581. [Google Scholar] [CrossRef]
- Hori, K.; Furumoto, S.; Kubota, K. Tumor blood flow interruption after radiotherapy strongly inhibits tumor regrowth. Cancer Sci. 2008, 99, 1485–1491. [Google Scholar] [CrossRef]
- Murata, R.; Siemann, D.W.; Overgaard, J.; Horsman, M.R. Improved tumor response by combining radiation and the vascular-damaging drug 5,6-dimethylxanthenone-4-acetic acid. Radiat Res. 2001, 156, 503–509. [Google Scholar] [CrossRef]
- Liu, L.; O’Kelly, D.; Schuetze, R.; Carlson, G.; Zhou, H.; Trawick, M.L.; Pinney, K.G.; Mason, R.P. Non-Invasive Evaluation of Acute Effects of Tubulin Binding Agents: A Review of Imaging Vascular Disruption in Tumors. Molecules 2021, 26, 2551. [Google Scholar] [CrossRef]
- Smolarczyk, R.; Czapla, J.; Jarosz-Biej, M.; Czerwinski, K.; Cichon, T. Vascular disrupting agents in cancer therapy. Eur. J. Pharmacol. 2021, 891, 173692. [Google Scholar] [CrossRef]
- Nainwal, L.M.; Alam, M.M.; Shaquiquzzaman, M.; Marella, A.; Kamal, A. Combretastatin-based compounds with therapeutic characteristics: A patent review. Expert Opin. Ther. Pat. 2019, 29, 703–731. [Google Scholar] [CrossRef]
- Seddigi, Z.S.; Malik, M.S.; Saraswati, A.P.; Ahmed, S.A.; Babalghith, A.O.; Lamfon, H.A.; Kamal, A. Recent advances in combretastatin based derivatives and prodrugs as antimitotic agents. Medchemcomm 2017, 8, 1592–1603. [Google Scholar] [CrossRef] [PubMed]
- Nofal, A.E.; Shatla, I.M.; Abdelhafeez, D.A.; Mustafa, M.; Aly, O.M. OMA1520 and OMA1774, novel 1,2,4-triazole bearing analogs of combretastatin A-4, inhibit hepatocellular carcinoma: Histological and immunohistochemical studies. Biomed. Pharmacother. 2021, 138, 111417. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, G.; Gill, R.K.; Soni, R.; Bariwal, J. Recent developments in tubulin polymerization inhibitors: An overview. Eur. J. Med. Chem. 2014, 87, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Sherbet, G.V. Combretastatin analogues in cancer biology: A prospective view. J. Cell. Biochem. 2020, 121, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- MacDonough, M.T.; Strecker, T.E.; Hamel, E.; Hall, J.J.; Chaplin, D.J.; Trawick, M.L.; Pinney, K.G. Synthesis and biological evaluation of indole-based, anti-cancer agents inspired by the vascular disrupting agent 2-(3’-hydroxy-4’-methoxyphenyl)-3-(3″, 4″, 5″-trimethoxybenzoyl)-6-methoxyindole (OXi8006). Bioorg. Med. Chem. 2013, 21, 6831–6843. [Google Scholar] [CrossRef] [PubMed]
- Maguire, C.J.; Chen, Z.; Mocharla, V.P.; Sriram, M.; Strecker, T.E.; Hamel, E.; Zhou, H.; Lopez, R.; Wang, Y.; Mason, R.P.; et al. Synthesis of dihydronaphthalene analogues inspired by combretastatin A-4 and their biological evaluation as anticancer agents. Medchemcomm 2018, 9, 1649–1662. [Google Scholar] [CrossRef]
- Niu, H.C.; Strecker, T.E.; Gerberich, J.L.; Campbell, J.W.; Saha, D.; Mondal, D.; Hamel, E.; Chaplin, D.J.; Mason, R.P.; Trawick, M.L.; et al. Structure Guided Design, Synthesis, and Biological Evaluation of Novel Benzosuberene Analogues as Inhibitors of Tubulin Polymerization. J. Med. Chem. 2019, 62, 5594–5615. [Google Scholar] [CrossRef]
- Pinney, K.; Mocharla, V.; Chen, Z.; Hadimani, M.; Kessler, J.; Dorsey, J.; Edvardsen, K.; Chaplin, D.; Prezioso, J.; Ghatak, A. Tubulin Binding Agents and Corresponding Prodrug Constructs. U.S. Patent US7001926B2, 21 February 2006. [Google Scholar]
- Sriram, M.; Hall, J.J.; Grohmann, N.C.; Strecker, T.E.; Wootton, T.; Franken, A.; Trawick, M.L.; Pinney, K.G. Design, synthesis and biological evaluation of dihydronaphthalene and benzosuberene analogs of the combretastatins as inhibitors of tubulin polymerization in cancer chemotherapy. Bioorg. Med. Chem. 2008, 16, 8161–8171. [Google Scholar] [CrossRef]
- Strecker, T.E.; Odutola, S.O.; Lopez, R.; Cooper, M.S.; Tidmore, J.K.; Charlton-Sevcik, A.K.; Li, L.; MacDonough, M.T.; Hadimani, M.B.; Ghatak, A.; et al. The vascular disrupting activity of OXi8006 in endothelial cells and its phosphate prodrug OXi8007 in breast tumor xenografts. Cancer Lett. 2015, 369, 229–241. [Google Scholar] [CrossRef]
- Tanpure, R.P.; George, C.S.; Strecker, T.E.; Devkota, L.; Tidmore, J.K.; Lin, C.M.; Herdman, C.A.; Macdonough, M.T.; Sriram, M.; Chaplin, D.J.; et al. Synthesis of structurally diverse benzosuberene analogues and their biological evaluation as anti-cancer agents. Bioorg. Med. Chem. 2013, 21, 8019–8032. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wang, H.; Gerberich, J.L.; Odutola, S.O.; Charlton-Sevcik, A.K.; Li, M.; Tanpure, R.P.; Tidmore, J.K.; Trawick, M.L.; Pinney, K.G.; et al. Imaging-Guided Evaluation of the Novel Small-Molecule Benzosuberene Tubulin-Binding Agent KGP265 as a Potential Therapeutic Agent for Cancer Treatment. Cancers 2021, 13, 4769. [Google Scholar] [CrossRef]
- Hadimani, M.B.; Macdonough, M.T.; Ghatak, A.; Strecker, T.E.; Lopez, R.; Sriram, M.; Nguyen, B.L.; Hall, J.J.; Kessler, R.J.; Shirali, A.R.; et al. Synthesis of a 2-aryl-3-aroyl indole salt (OXi8007) resembling combretastatin A-4 with application as a vascular disrupting agent. J. Nat. Prod. 2013, 76, 1668–1678. [Google Scholar] [CrossRef]
- Sato, M.; Saitoh, I.; Kiyokawa, Y.; Iwase, Y.; Kubota, N.; Ibano, N.; Noguchi, H.; Yamasaki, Y.; Inada, E. Tissue-Nonspecific Alkaline Phosphatase, a Possible Mediator of Cell Maturation: Towards a New Paradigm. Cells 2021, 10, 3338. [Google Scholar] [CrossRef]
- Le-Vinh, B.; Akkus-Dagdeviren, Z.B.; Le, N.M.N.; Nazir, I.; Bernkop-Schnurch, A. Alkaline Phosphatase: A Reliable Endogenous Partner for Drug Delivery and Diagnostics. Adv. Ther. 2022, 5, 2100219. [Google Scholar] [CrossRef]
- Jung, J.H.; Hong, C.M.; Jo, I.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Reliability of Alkaline Phosphatase for Differentiating Flare Phenomenon from Disease Progression with Bone Scintigraphy. Cancers 2022, 14, 254. [Google Scholar] [CrossRef]
- Mou, H.C.; Wang, Z.; Zhang, W.K.; Li, G.Q.; Zhou, H.; Yinwang, E.; Wang, F.Q.; Sun, H.X.; Xue, Y.C.; Wang, Z.N.; et al. Clinical Features and Serological Markers Risk Model Predicts Overall Survival in Patients Undergoing Breast Cancer and Bone Metastasis Surgeries. Front. Oncol. 2021, 11, 3436. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Singh, S.K.; Kakkar, N.; Prasad, R. Reduced L/B/K alkaline phosphatase gene expression in renal cell carcinoma: Plausible role in tumorigenesis. Biochimie 2014, 104, 27–35. [Google Scholar] [CrossRef]
- Pinney, K.; Mocharla, V.; Chen, Z.; Garner, C.; Ghatak, A.; Hadimani, M.; Kessler, J.; Dorsey, J.; Edvardsen, K.; Chaplin, D.; et al. Tubulin Binding Agents and Corresponding Prodrug Constructs. U.S. Patent US20040043969A1, 4 March 2004. [Google Scholar]
- Rasolofonjatovo, E.; Provot, O.; Hamze, A.; Rodrigo, J.; Bignon, J.; Wdzieczak-Bakala, J.; Desravines, D.; Dubois, J.; Brion, J.D.; Alami, M. Conformationnally restricted naphthalene derivatives type isocombretastatin A-4 and isoerianin analogues: Synthesis, cytotoxicity and antitubulin activity. Eur. J. Med. Chem. 2012, 52, 22–32. [Google Scholar] [CrossRef]
- Pavia-Jimenez, A.; Tcheuyap, V.T.; Brugarolas, J. Establishing a human renal cell carcinoma tumorgraft platform for preclinical drug testing. Nat. Protoc. 2014, 9, 1848–1859. [Google Scholar] [CrossRef]
- Serkova, N.J.; Glunde, K.; Haney, C.R.; Farhoud, M.; De Lille, A.; Redente, E.F.; Simberg, D.; Westerly, D.C.; Griffin, L.; Mason, R.P. Preclinical Applications of Multi-Platform Imaging in Animal Models of Cancer. Cancer Res. 2021, 81, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Inglis, D.J.; Lavranos, T.C.; Beaumont, D.M.; Leske, A.F.; Brown, C.K.; Hall, A.J.; Kremmidiotis, G. The vascular disrupting agent BNC105 potentiates the efficacy of VEGF and mTOR inhibitors in renal and breast cancer. Cancer Biol. Ther. 2014, 15, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Beck, H.; Wang, X.; Hsieh, H.P.; Mason, R.P.; Liu, X. Tubulin-destabilizing agent BPR0L075 induces vascular-disruption in human breast cancer mammary fat pad xenografts. PLoS ONE 2012, 7, e43314. [Google Scholar] [CrossRef]
- Blakey, D.C.; Westwood, F.R.; Walker, M.; Hughes, G.D.; Davis, P.D.; Ashton, S.E.; Ryan, A.J. Antitumor activity of the novel vascular targeting agent ZD6126 in a panel of tumor models. Clin. Cancer Res. 2002, 8, 1974–1983. [Google Scholar] [PubMed]
- Zhao, D.; Richer, E.; Antich, P.P.; Mason, R.P. Antivascular effects of combretastatin A4 phosphate in breast cancer xenograft assessed using dynamic bioluminescence imaging (BLI) and confirmed by magnetic resonance imaging (MRI). FASEB J. 2008, 22, 2445–2451. [Google Scholar] [CrossRef]
- Devkota, L.; Lin, C.M.; Strecker, T.E.; Wang, Y.; Tidmore, J.K.; Chen, Z.; Guddneppanavar, R.; Jelinek, C.J.; Lopez, R.; Liu, L.; et al. Design, synthesis, and biological evaluation of water-soluble amino acid prodrug conjugates derived from combretastatin, dihydronaphthalene, and benzosuberene-based parent vascular disrupting agents. Bioorg. Med. Chem. 2016, 24, 938–956. [Google Scholar] [CrossRef]
- Fadhel, M.N.; Baskoy, S.A.; Wang, Y.J.; Hysi, E.; Kolios, M.C. Use of photoacoustic imaging for monitoring vascular disrupting cancer treatments. J. Biophotonics 2020, e202000209. [Google Scholar] [CrossRef] [PubMed]
- Winn, B.A.; Devkota, L.; Kuch, B.; MacDonough, M.T.; Strecker, T.E.; Wang, Y.; Shi, Z.; Gerberich, J.L.; Mondal, D.; Ramirez, A.J.; et al. Bioreductively Activatable Prodrug Conjugates of Combretastatin A-1 and Combretastatin A-4 as Anticancer Agents Targeted toward Tumor-Associated Hypoxia. J. Nat. Prod. 2020, 83, 937–954. [Google Scholar] [CrossRef]
- Mirzavi, F.; Barati, M.; Vakili-Ghartavol, R.; Roshan, M.K.; Mashreghi, M.; Soukhtanloo, M.; Jaafari, M.R. Pegylated liposomal encapsulation improves the antitumor efficacy of combretastatin A4 in murine 4T1 triple-negative breast cancer model. Int. J. Pharm. 2022, 613, 121396. [Google Scholar] [CrossRef]
- Sobczuk, P.; Brodziak, A.; Khan, M.I.; Chhabra, S.; Fiedorowicz, M.; Wełniak-Kamińska, M.; Synoradzki, K.; Bartnik, E.; Cudnoch-Jędrzejewska, A.; Czarnecka, A.M. Choosing The Right Animal Model for Renal Cancer Research. Transl. Oncol. 2020, 13, 100745. [Google Scholar] [CrossRef]
- O’Shaughnessy, M.J.; Murray, K.S.; La Rosa, S.P.; Budhu, S.; Merghoub, T.; Somma, A.; Monette, S.; Kim, K.; Corradi, R.B.; Scherz, A.; et al. Systemic Antitumor Immunity by PD-1/PD-L1 Inhibition Is Potentiated by Vascular-Targeted Photodynamic Therapy of Primary Tumors. Clin. Cancer Res. 2018, 24, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.; Shah, P.; Hammers, H.; Lehet, K.; Sotomayor, P.; Azabdaftari, G.; Seshadri, M.; Pili, R. Vascular disruption in combination with mTOR inhibition in renal cell carcinoma. Mol. Cancer Ther. 2012, 11, 383–392. [Google Scholar] [CrossRef]
- Wu, F.T.H.; Xu, P.; Chow, A.; Man, S.; Kruger, J.; Khan, K.A.; Paez-Ribes, M.; Pham, E.; Kerbel, R.S. Pre- and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro- or macro-metastatic disease. Br. J. Cancer 2019, 120, 196–206. [Google Scholar] [CrossRef]
- Yuk, H.-D.; Lee, K.-H.; Lee, H.-S.; Jeong, S.-H.; Kho, Y.; Jeong, C.-W.; Kim, H.-H.; Ku, J.-H.; Kwak, C. PDLIM2 Suppression Inhibit Proliferation and Metastasis in Kidney Cancer. Cancers 2021, 13, 2991. [Google Scholar] [CrossRef]
- Devaud, C.; Westwood, J.A.; John, L.B.; Flynn, J.K.; Paquet-Fifield, S.; Duong, C.P.; Yong, C.S.; Pegram, H.J.; Stacker, S.A.; Achen, M.G.; et al. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol. Ther. 2014, 22, 18–27. [Google Scholar] [CrossRef]
- Murphy, G.P.; Hrushesky, W.J. A murine renal cell carcinoma. J. Natl. Cancer Inst. 1973, 50, 1013–1025. [Google Scholar] [CrossRef]
- Murphy, K.A.; James, B.R.; Wilber, A.; Griffith, T.S. A Syngeneic Mouse Model of Metastatic Renal Cell Carcinoma for Quantitative and Longitudinal Assessment of Preclinical Therapies. J. Vis. Exp. 2017, 122, e55080. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, M.E.; Kim, S.H.; Jang, W.S.; Ham, W.S. Development of a highly pulmonary metastatic orthotopic renal cell carcinoma murine model. Biol. Open 2021, 10, bio058566. [Google Scholar] [CrossRef]
- Ding, J.; Wang, C.; Chang, X. Establishment of a bioluminescent Renca cell line for renal carcinoma research. Int. Urol. Nephrol. 2018, 50, 55–61. [Google Scholar] [CrossRef]
- Danhier, P.; De Preter, G.; Magat, J.; Godechal, Q.; Porporato, P.E.; Jordan, B.F.; Feron, O.; Sonveaux, P.; Gallez, B. Multimodal cell tracking of a spontaneous metastasis model: Comparison between MRI, electron paramagnetic resonance and bioluminescence. Contrast Media Mol. Imaging 2014, 9, 143–153. [Google Scholar] [CrossRef]
- Alhasan, M.K.; Liu, L.; Lewis, M.A.; Magnusson, J.; Mason, R.P. Comparison of optical and power Doppler ultrasound imaging for non-invasive evaluation of arsenic trioxide as a vascular disrupting agent in tumors. PLoS ONE 2012, 7, e46106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folaron, M.; Seshadri, M. Bioluminescence and MR Imaging of the Safety and Efficacy of Vascular Disruption in Gliomas. Mol. Imaging Biol. MIB Off. Publ. Acad. Mol. Imaging 2016, 18, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Medinger, M.; Esser, N.; Soltau, J.; Lehmann, K.M.; Konerding, M.A.; Wolloscheck, T.; Ryan, A.J.; Drevs, J. Antitumor effect of the vascular-disrupting agent ZD6126 in a murine renal cell carcinoma model. Int. J. Oncol. 2011, 38, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.S.; Nassar, A.H.; Adib, E.; Jegede, O.A.; Alaiwi, S.A.; Manna, D.L.D.; Braun, D.A.; Zarei, M.; Du, H.; Pal, S.K.; et al. Gene Expression Signature Correlates with Outcomes in Metastatic Renal Cell Carcinoma Patients Treated with Everolimus Alone or with a Vascular Disrupting Agent. Mol. Cancer Ther. 2021, 20, 1454–1461. [Google Scholar] [CrossRef]
- West, C.M.; Price, P. Combretastatin A4 phosphate. Anticancer Drugs 2004, 15, 179–187. [Google Scholar] [CrossRef]
- Monk, K.A.; Siles, R.; Hadimani, M.B.; Mugabe, B.E.; Ackley, J.F.; Studerus, S.W.; Edvardsen, K.; Trawick, M.L.; Garner, C.M.; Rhodes, M.R.; et al. Design, synthesis, and biological evaluation of combretastatin nitrogen-containing derivatives as inhibitors of tubulin assembly and vascular disrupting agents. Bioorg. Med. Chem. 2006, 14, 3231–3244. [Google Scholar] [CrossRef]
- Gallego-Yerga, L.; de la Torre, C.; Sansone, F.; Casnati, A.; Mellet, C.O.; Fernandez, J.M.G.; Cena, V. Synthesis, self-assembly and anticancer drug encapsulation and delivery properties of cyclodextrin-based giant amphiphiles. Carbohydr. Polym. 2021, 252, 117135. [Google Scholar] [CrossRef]
- Calosi, M.; Guazzelli, E.; Braccini, S.; Lessi, M.; Bellina, F.; Galli, G.; Martinelli, E. Self-Assembled Amphiphilic Fluorinated Random Copolymers for the Encapsulation and Release of the Hydrophobic Combretastatin A-4 Drug. Polymers 2022, 14, 774. [Google Scholar] [CrossRef]
- Li, Y.H.; Lu, J.Q.; Deng, X.W.; Wang, X.; Jia, F.; Zhong, S.H.; Cui, X.Y.; Pan, Z.; Shao, L.H.; Wu, Y. Self-assembling combretastatin A4 incorporated protamine/nanodiamond hybrids for combined anti-angiogenesis and mild photothermal therapy in liver cancer. Nanotechnology 2021, 32, 465101. [Google Scholar] [CrossRef]
- Liu, Y.B.; Deng, F.; Zheng, R.R.; Chen, X.Y.; Zhao, L.P.; Yu, B.X.; Chen, A.; Jiang, X.Y.; Cheng, H.; Li, S.Y. Self-delivery nanomedicine for vascular disruption-supplemented chemo-photodynamic tumor therapy. J. Colloid Interface Sci. 2022, 612, 562–571. [Google Scholar] [CrossRef]
- Wei, Q.; Shen, N.; Yu, H.Y.; Wang, Y.; Tang, Z.H.; Chen, X.S. FXIIIa substrate peptide decorated BLZ945 nanoparticles for specifically remodeling tumor immunity. Biomater. Sci. 2020, 8, 5666–5676. [Google Scholar] [CrossRef]
- Thomson, P.; Naylor, M.A.; Everett, S.A.; Stratford, M.R.; Lewis, G.; Hill, S.; Patel, K.B.; Wardman, P.; Davis, P.D. Synthesis and biological properties of bioreductively targeted nitrothienyl prodrugs of combretastatin A-4. Mol. Cancer Ther. 2006, 5, 2886–2894. [Google Scholar] [CrossRef]
- Winn, B.A.; Shi, Z.; Carlson, G.J.; Wang, Y.; Nguyen, B.L.; Kelly, E.M.; Ross, R.D.t.; Hamel, E.; Chaplin, D.J.; Trawick, M.L.; et al. Bioreductively activatable prodrug conjugates of phenstatin designed to target tumor hypoxia. Bioorg. Med. Chem. Lett. 2017, 27, 636–641. [Google Scholar] [CrossRef]
- Siemann, D.W.; Shi, W. Dual targeting of tumor vasculature: Combining Avastin and vascular disrupting agents (CA4P or OXi4503). Anticancer Res. 2008, 28, 2027–2031. [Google Scholar]
- Siemann, D.W.; Horsman, M.R. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol. Ther. 2015, 153, 107–124. [Google Scholar] [CrossRef]
- Liang, W.; Ni, Y.; Chen, F. Tumor resistance to vascular disrupting agents: Mechanisms, imaging, and solutions. Oncotarget 2016, 7, 15444–15459. [Google Scholar] [CrossRef]
- Mason, R.P.; Zhao, D.W.; Liu, L.; Trawick, M.L.; Pinney, K.G. A perspective on vascular disrupting agents that interact with tubulin: Preclinical tumor imaging and biological assessment. Integr. Biol. 2011, 3, 375–387. [Google Scholar] [CrossRef]
- Karatoprak, G.; Küpeli Akkol, E.; Genç, Y.; Bardakci, H.; Yücel, Ç.; Sobarzo-Sánchez, E. Combretastatins: An Overview of Structure, Probable Mechanisms of Action and Potential Applications. Molecules 2020, 25, 2560. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Schuetze, R.; Gerberich, J.L.; Lopez, R.; Odutola, S.O.; Tanpure, R.P.; Charlton-Sevcik, A.K.; Tidmore, J.K.; Taylor, E.A.-S.; Kapur, P.; et al. Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment. Cancers 2022, 14, 4208. https://doi.org/10.3390/cancers14174208
Liu L, Schuetze R, Gerberich JL, Lopez R, Odutola SO, Tanpure RP, Charlton-Sevcik AK, Tidmore JK, Taylor EA-S, Kapur P, et al. Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment. Cancers. 2022; 14(17):4208. https://doi.org/10.3390/cancers14174208
Chicago/Turabian StyleLiu, Li, Regan Schuetze, Jeni L. Gerberich, Ramona Lopez, Samuel O. Odutola, Rajendra P. Tanpure, Amanda K. Charlton-Sevcik, Justin K. Tidmore, Emily A.-S. Taylor, Payal Kapur, and et al. 2022. "Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment" Cancers 14, no. 17: 4208. https://doi.org/10.3390/cancers14174208
APA StyleLiu, L., Schuetze, R., Gerberich, J. L., Lopez, R., Odutola, S. O., Tanpure, R. P., Charlton-Sevcik, A. K., Tidmore, J. K., Taylor, E. A.-S., Kapur, P., Hammers, H., Trawick, M. L., Pinney, K. G., & Mason, R. P. (2022). Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment. Cancers, 14(17), 4208. https://doi.org/10.3390/cancers14174208








