Detection and Characterization of Circulating Tumor Cells Using Imaging Flow Cytometry—A Perspective Study
Abstract
Simple Summary
Abstract
1. Liquid Biopsy
2. Flow Cytometry in Liquid Biopsy
3. Imaging Flow Cytometry—Pros and Cons in CTC Field
3.1. General Description
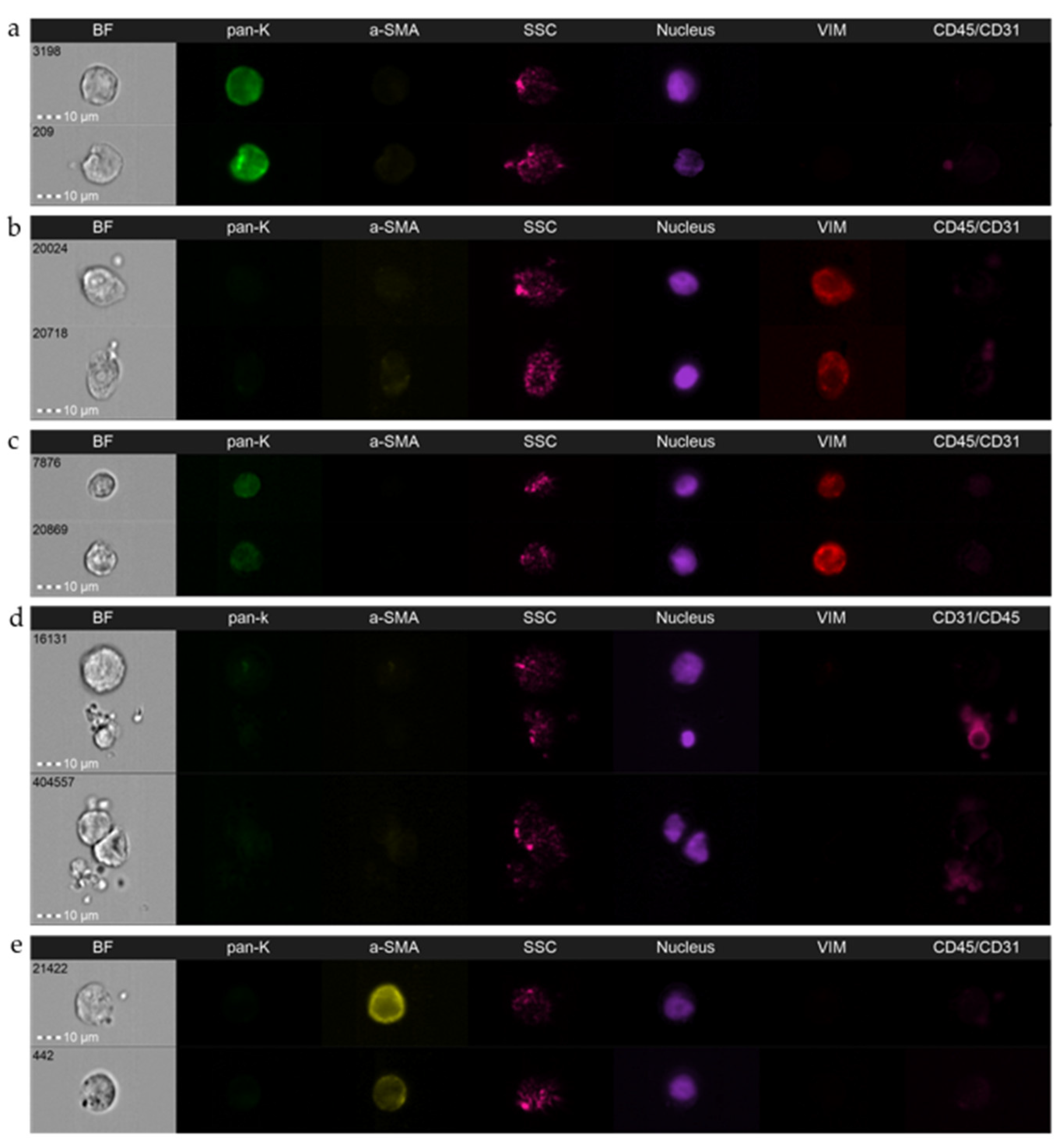
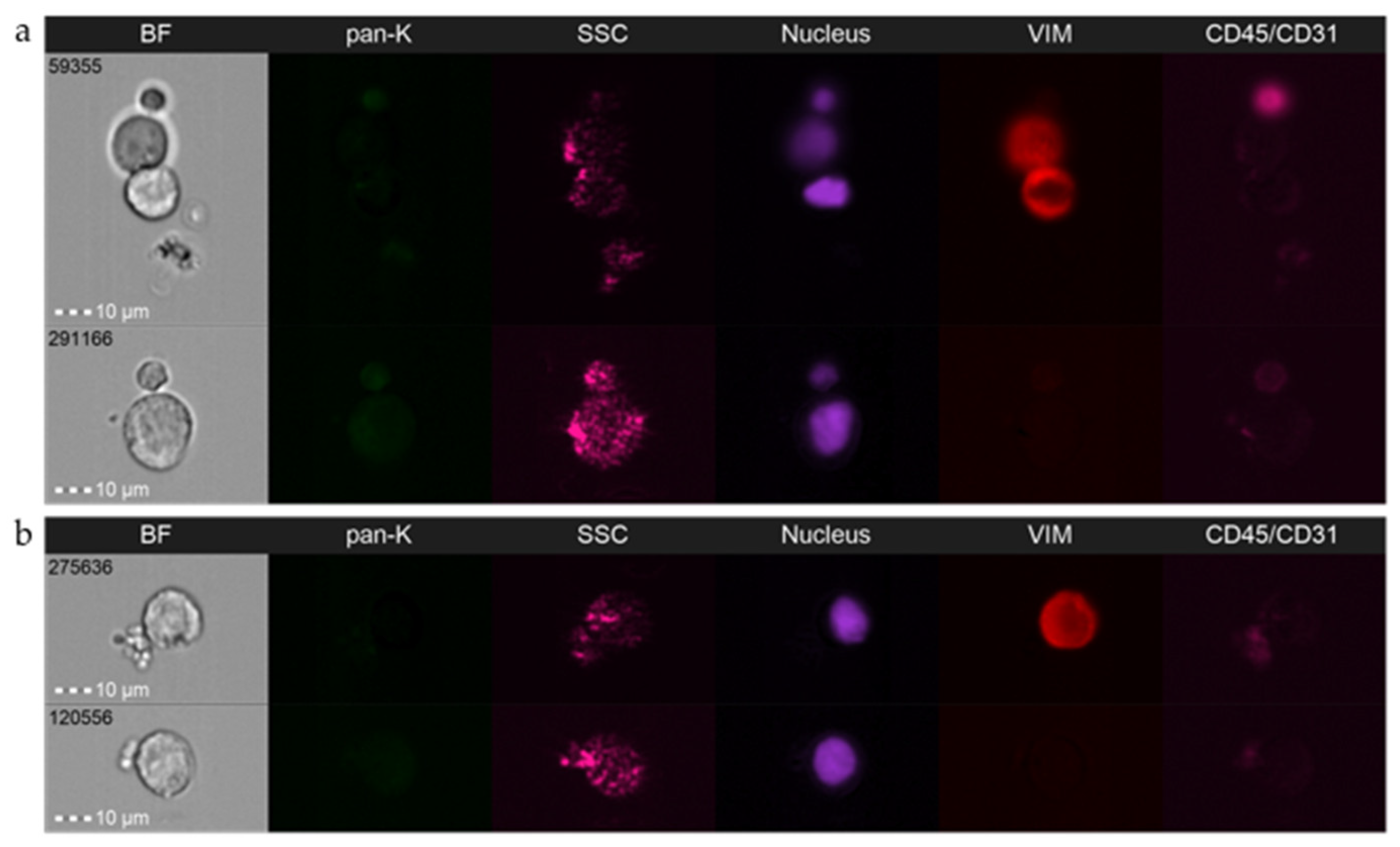
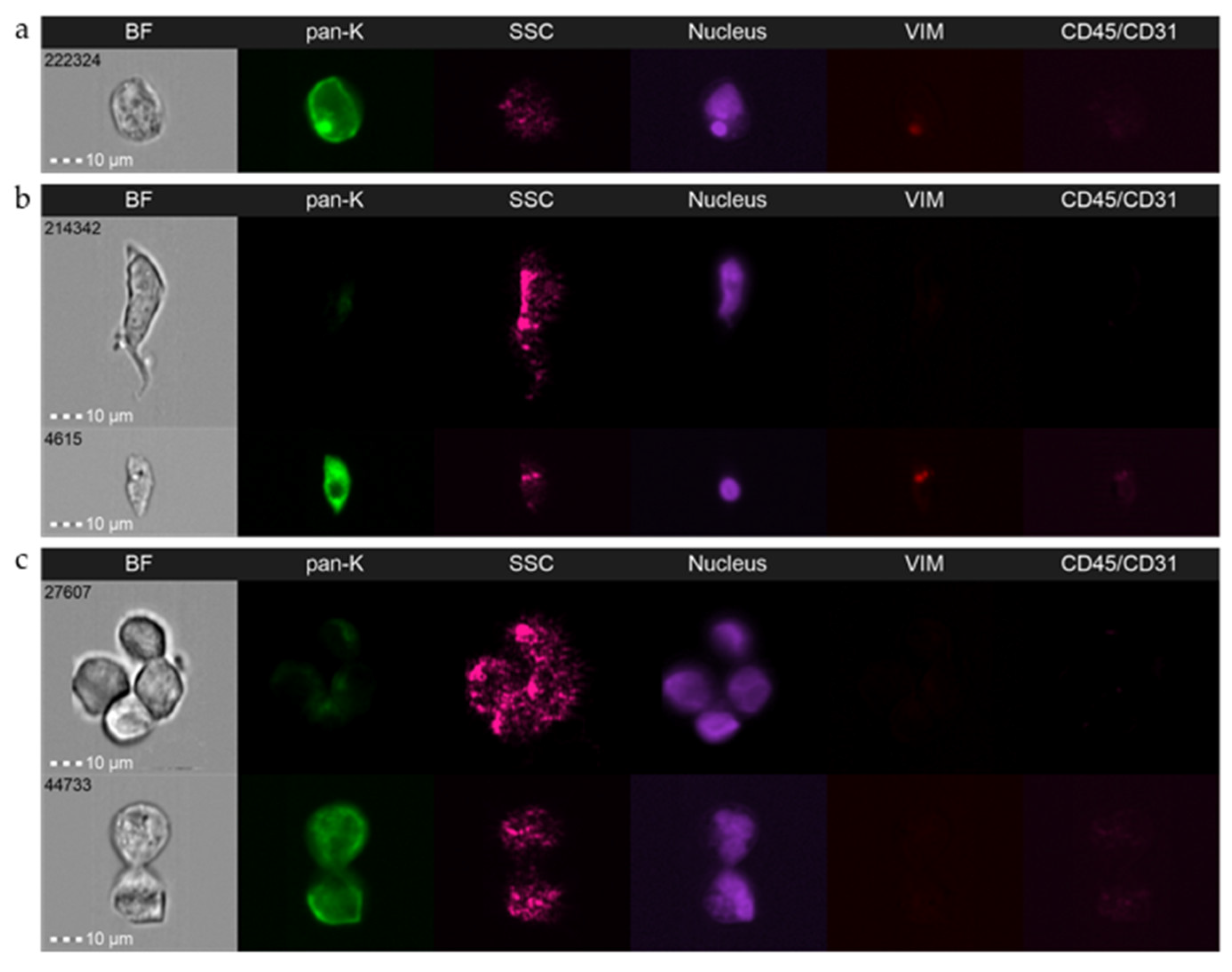
3.2. Detailed Analysis and Standardization
3.3. Caveats and Challenges
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pantel, K.; Alix-Panabières, C. Liquid Biopsy and Minimal Residual Disease—Latest Advances and Implications for Cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, T. A Case of Cancer in Which Cells Similar to Those in the Tumours Were Seen in the Blood after Death. Aust. Med. J. 1869, 14, 146–147. [Google Scholar]
- Bednarz-Knoll, N.; Alix-Panabières, C.; Pantel, K. Plasticity of Disseminating Cancer Cells in Patients with Epithelial Malignancies. Cancer Metastasis Rev. 2012, 31, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Topa, J.; Grešner, P.; Żaczek, A.J.; Markiewicz, A. Breast Cancer Circulating Tumor Cells with Mesenchymal Features—An Unreachable Target? Cell. Mol. Life Sci. 2022, 79, 81. [Google Scholar] [CrossRef]
- Beerepoot, L.V.; Mehra, N.; Vermaat, J.S.P.; Zonnenberg, B.A.; Gebbink, M.F.G.B.; Voest, E.E. Increased Levels of Viable Circulating Endothelial Cells Are an Indicator of Progressive Disease in Cancer Patients. Ann. Oncol. 2004, 15, 139–145. [Google Scholar] [CrossRef]
- Ao, Z.; Shah, S.H.; Machlin, L.M.; Parajuli, R.; Miller, P.C.; Rawal, S.; Williams, A.J.; Cote, R.J.; Lippman, M.E.; Datar, R.H.; et al. Identification of Cancer-Associated Fibroblasts in Circulating Blood from Patients with Metastatic Breast Cancer. Cancer Res. 2015, 75, 4681–4687. [Google Scholar] [CrossRef]
- Adams, D.L.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.-M.; et al. Circulating Giant Macrophages as a Potential Biomarker of Solid Tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef]
- De Wit, S.; van Dalum, G.; Terstappen, L.W.M.M. Detection of Circulating Tumor Cells. Scientifica 2014, 2014, 819362. [Google Scholar] [CrossRef]
- Hu, Y.; Fan, L.; Zheng, J.; Cui, R.; Liu, W.; He, Y.; Li, X.; Huang, S. Detection of Circulating Tumor Cells in Breast Cancer Patients Utilizing Multiparameter Flow Cytometry and Assessment of the Prognosis of Patients in Different CTCs Levels. Cytometry A 2010, 77, 213–219. [Google Scholar] [CrossRef]
- Al-Mawali, A.; Gillis, D.; Lewis, I. The Role of Multiparameter Flow Cytometry for Detection of Minimal Residual Disease in Acute Myeloid Leukemia. Am. J. Clin. Pathol. 2009, 131, 16–26. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Kennedy, B.; Evans, P.A.S.; Davies, F.E.; Richards, S.J.; Haynes, A.P.; Russell, N.H.; Hale, G.; Morgan, G.J.; Jack, A.S.; et al. Quantitation of Minimal Disease Levels in Chronic Lymphocytic Leukemia Using a Sensitive Flow Cytometric Assay Improves the Prediction of Outcome and Can Be Used to Optimize Therapy. Blood 2001, 98, 29–35. [Google Scholar] [CrossRef]
- Leers, M.P.G.; Schoffelen, R.H.M.G.; Hoop, J.G.M.; Theunissen, P.H.M.H.; Oosterhuis, J.W.A.; vd Bijl, H.; Rahmy, A.; Tan, W.; Nap, M. Multiparameter Flow Cytometry as a Tool for the Detection of Micrometastatic Tumour Cells in the Sentinel Lymph Node Procedure of Patients with Breast Cancer. J. Clin. Pathol. 2002, 55, 359–366. [Google Scholar] [CrossRef][Green Version]
- Kerst, G.; Kreyenberg, H.; Roth, C.; Well, C.; Dietz, K.; Coustan-Smith, E.; Campana, D.; Koscielniak, E.; Niemeyer, C.; Schlegel, P.G.; et al. Concurrent Detection of Minimal Residual Disease (MRD) in Childhood Acute Lymphoblastic Leukaemia by Flow Cytometry and Real-Time PCR. Br. J. Haematol. 2005, 128, 774–782. [Google Scholar] [CrossRef]
- Cruz, I.; Ciudad, J.; Cruz, J.J.; Ramos, M.; Gómez-Alonso, A.; Adansa, J.C.; Rodríguez, C.; Orfao, A. Evaluation of Multiparameter Flow Cytometry for the Detection of Breast Cancer Tumor Cells in Blood Samples. Am. J. Clin. Pathol. 2005, 123, 66–74. [Google Scholar] [CrossRef]
- Riethdorf, S.; Fritsche, H.; Müller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Jänicke, F.; et al. Detection of Circulating Tumor Cells in Peripheral Blood of Patients with Metastatic Breast Cancer: A Validation Study of the CellSearch System. Clin. Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef]
- Kallergi, G.; Politaki, E.; Alkahtani, S.; Stournaras, C.; Georgoulias, V. Evaluation of Isolation Methods for Circulating Tumor Cells (CTCs). Cell. Physiol. Biochem. 2016, 40, 411–419. [Google Scholar] [CrossRef]
- Maertens, Y.; Humberg, V.; Erlmeier, F.; Steffens, S.; Steinestel, J.; Bögemann, M.; Schrader, A.J.; Bernemann, C. Comparison of Isolation Platforms for Detection of Circulating Renal Cell Carcinoma Cells. Oncotarget 2017, 8, 87710–87717. [Google Scholar] [CrossRef]
- Bahnassy, A.A.; Salem, S.E.; Mohanad, M.; Abulezz, N.Z.; Abdellateif, M.S.; Hussein, M.; Zekri, C.A.N.; Zekri, A.-R.N.; Allahloubi, N.M.A. Prognostic Significance of Circulating Tumor Cells (CTCs) in Egyptian Non-Metastatic Colorectal Cancer Patients: A Comparative Study for Four Different Techniques of Detection (Flowcytometry, CellSearch, Quantitative Real-Time PCR and Cytomorphology). Exp. Mol. Pathol. 2019, 106, 90–101. [Google Scholar] [CrossRef]
- Pearl, M.L.; Dong, H.; Tulley, S.; Zhao, Q.; Golightly, M.; Zucker, S.; Chen, W.-T. Treatment Monitoring of Patients with Epithelial Ovarian Cancer Using Invasive Circulating Tumor Cells (ICTCs). Gynecol. Oncol. 2015, 137, 229–238. [Google Scholar] [CrossRef]
- Ntanovasilis, D.-A.; Apostolou, P.; Papasotiriou, I. Flow Cytometric Detection of Circulating Tumor Cells in Breast Cancer Patients: A Blinded Study. J. Cancer Ther. 2019, 10, 708–715. [Google Scholar] [CrossRef][Green Version]
- Wang, N.; Shi, L.; Li, H.; Hu, Y.; Du, W.; Liu, W.; Zheng, J.; Huang, S.; Qu, X. Detection of Circulating Tumor Cells and Tumor Stem Cells in Patients with Breast Cancer by Using Flow Cytometry: A Valuable Tool for Diagnosis and Prognosis Evaluation. Tumor Biol. 2012, 33, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.-Y.; Yang, C.-Y.; Liang, S.-C.; Liu, R.-S.; Yang, S.-H.; Lin, J.-K.; Chen, Y.-M.; Wu, Y.-C.; Jiang, J.-K.; Lin, C.-H. Interleukin-17A Modulates Circulating Tumor Cells in Tumor Draining Vein of Colorectal Cancers and Affects Metastases. Clin. Cancer Res. 2014, 20, 2885–2897. [Google Scholar] [CrossRef]
- Lopresti, A.; Malergue, F.; Bertucci, F.; Liberatoscioli, M.L.; Garnier, S.; DaCosta, Q.; Finetti, P.; Gilabert, M.; Raoul, J.L.; Birnbaum, D.; et al. Sensitive and Easy Screening for Circulating Tumor Cells by Flow Cytometry. JCI Insight 2019, 4, 128180. [Google Scholar] [CrossRef] [PubMed]
- Galizia, G.; Gemei, M.; Orditura, M.; Romano, C.; Zamboli, A.; Castellano, P.; Mabilia, A.; Auricchio, A.; De Vita, F.; Del Vecchio, L.; et al. Postoperative Detection of Circulating Tumor Cells Predicts Tumor Recurrence in Colorectal Cancer Patients. J. Gastrointest. Surg. 2013, 17, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Mușină, A.-M.; Zlei, M.; Mentel, M.; Scripcariu, D.-V.; Ștefan, M.; Aniţei, M.-G.; Filip, B.; Radu, I.; Gavrilescu, M.-M.; Panuţa, A.; et al. Evaluation of Circulating Tumor Cells in Colorectal Cancer Using Flow Cytometry. J. Int. Med. Res. 2021, 49, 0300060520980215. [Google Scholar] [CrossRef] [PubMed]
- Tralhão, J.G.; Hoti, E.; Serôdio, M.; Laranjeiro, P.; Paiva, A.; Abrantes, A.M.; Pais, M.L.; Botelho, M.F.; Castro Sousa, F. Perioperative Tumor Cell Dissemination in Patients with Primary or Metastatic Colorectal Cancer. Eur. J. Surg. Oncol. 2010, 36, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Alpaugh, R.K.; Gross, S.; O’Hara, S.M.; Smirnov, D.A.; Terstappen, L.W.M.M.; Allard, W.J.; Bilbee, M.; Cheng, J.D.; Hoffman, J.P.; et al. Isolation and Characterization of Circulating Tumor Cells in Patients with Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2006, 6, 125–132. [Google Scholar] [CrossRef]
- Fang, C.; Fan, C.; Wang, C.; Huang, Q.; Meng, W.; Yu, Y.; Yang, L.; Hu, J.; Li, Y.; Mo, X.; et al. Prognostic Value of CD133 + CD54 + CD44 + Circulating Tumor Cells in Colorectal Cancer with Liver Metastasis. Cancer Med. 2017, 6, 2850–2857. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Zappavigna, S.; Crocetto, F.; Giuliano, M.; Ribera, D.; Morra, R.; Scafuri, L.; Verde, A.; Bruzzese, D.; Iaccarino, S.; et al. Assessment of Total, PTEN–, and AR-V7+ Circulating Tumor Cell Count by Flow Cytometry in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Enzalutamide. Clin. Genitourin. Cancer 2021, 19, e286–e298. [Google Scholar] [CrossRef]
- Gening, S.O.; Abakumova, T.V.; Gafurbaeva, D.U.; Rizvanov, A.A.; Antoneeva, I.I.; Miftakhova, R.R.; Peskov, A.B.; Gening, T.P. The Detection of Stem-Like Circulating Tumor Cells Could Increase the Clinical Applicability of Liquid Biopsy in Ovarian Cancer. Life 2021, 11, 815. [Google Scholar] [CrossRef]
- Doan, M.; Vorobjev, I.; Rees, P.; Filby, A.; Wolkenhauer, O.; Goldfeld, A.E.; Lieberman, J.; Barteneva, N.; Carpenter, A.E.; Hennig, H. Diagnostic Potential of Imaging Flow Cytometry. Trends Biotechnol. 2018, 36, 649–652. [Google Scholar] [CrossRef]
- Görgens, A.; Bremer, M.; Ferrer-Tur, R.; Murke, F.; Tertel, T.; Horn, P.A.; Thalmann, S.; Welsh, J.A.; Probst, C.; Guerin, C.; et al. Optimisation of Imaging Flow Cytometry for the Analysis of Single Extracellular Vesicles by Using Fluorescence-Tagged Vesicles as Biological Reference Material. J. Extracell. Vesicles 2019, 8, 1587567. [Google Scholar] [CrossRef]
- Mastoridis, S.; Bertolino, G.M.; Whitehouse, G.; Dazzi, F.; Sanchez-Fueyo, A.; Martinez-Llordella, M. Multiparametric Analysis of Circulating Exosomes and Other Small Extracellular Vesicles by Advanced Imaging Flow Cytometry. Front. Immunol. 2018, 9, 1583. [Google Scholar] [CrossRef]
- Tertel, T.; Görgens, A.; Giebel, B. Analysis of Individual Extracellular Vesicles by Imaging Flow Cytometry. Methods Enzymol. 2020, 645, 55–78. [Google Scholar]
- López-Riquelme, N.; Minguela, A.; Villar-Permuy, F.; Ciprian, D.; Castillejo, A.; Álvarez-López, M.-R.; Soto, J.-L. Imaging Cytometry for Counting Circulating Tumor Cells: Comparative Analysis of the CellSearch vs. ImageStream Systems. APMIS 2013, 121, 1139–1143. [Google Scholar] [CrossRef]
- Mourtzikou, A.; Stamouli, M.; Karakitsos, P.; Athanasas, A. Colorectal Cancer CTCs Detection Using Two FCM Protocol Approaches. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef]
- Merugu, S.; Chen, L.; Gavens, E.; Gabra, H.; Brougham, M.; Makin, G.; Ng, A.; Murphy, D.; Gabriel, A.S.; Robinson, M.L.; et al. Detection of Circulating and Disseminated Neuroblastoma Cells Using the ImageStream Flow Cytometer for Use as Predictive and Pharmacodynamic Biomarkers. Clin. Cancer Res. 2020, 26, 122–134. [Google Scholar] [CrossRef]
- Carter, T.J.; Jeyaneethi, J.; Kumar, J.; Karteris, E.; Glynne-Jones, R.; Hall, M. Identification of Cancer-Associated Circulating Cells in Anal Cancer Patients. Cancers 2020, 12, 2229. [Google Scholar] [CrossRef]
- Semaan, A.; Bernard, V.; Kim, D.U.; Lee, J.J.; Huang, J.; Kamyabi, N.; Stephens, B.M.; Qiao, W.; Varadhachary, G.R.; Katz, M.H.; et al. Characterisation of Circulating Tumour Cell Phenotypes Identifies a Partial-EMT Sub-Population for Clinical Stratification of Pancreatic Cancer. Br. J. Cancer 2021, 124, 1970–1977. [Google Scholar] [CrossRef]
- Stavrakis, S.; Holzner, G.; Choo, J.; deMello, A. High-Throughput Microfluidic Imaging Flow Cytometry. Curr. Opin. Biotechnol. 2019, 55, 36–43. [Google Scholar] [CrossRef]
- Andree, K.C.; van Dalum, G.; Terstappen, L.W.M.M. Challenges in Circulating Tumor Cell Detection by the CellSearch System. Mol. Oncol. 2016, 10, 395–407. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, A.J.; Molina-Vallejo, M.P.; Aznar-Peralta, I.; González Puga, C.; Cañas García, I.; González, E.; Lorente, J.A.; Serrano, M.J.; Garrido-Navas, M.C. Deep Phenotypic Characterisation of CTCs by Combination of Microfluidic Isolation (IsoFlux) and Imaging Flow Cytometry (ImageStream). Cancers 2021, 13, 6386. [Google Scholar] [CrossRef]
- Chudasama, D.; Katopodis, P.; Stone, N.; Haskell, J.; Sheridan, H.; Gardner, B.; Urnovitz, H.; Schuetz, E.; Beck, J.; Hall, M.; et al. Liquid Biopsies in Lung Cancer: Four Emerging Technologies and Potential Clinical Applications. Cancers 2019, 11, 331. [Google Scholar] [CrossRef]
- Staudte, S.; Klinghammer, K.; Jurmeister, P.S.; Jank, P.; Blohmer, J.-U.; Liebs, S.; Rhein, P.; Hauser, A.E.; Tinhofer, I. Multiparametric Phenotyping of Circulating Tumor Cells for Analysis of Therapeutic Targets, Oncogenic Signaling Pathways and DNA Repair Markers. Cancers 2022, 14, 2810. [Google Scholar] [CrossRef]
- Dent, B.M.; Ogle, L.F.; O’Donnell, R.L.; Hayes, N.; Malik, U.; Curtin, N.J.; Boddy, A.V.; Plummer, E.R.; Edmondson, R.J.; Reeves, H.L.; et al. High-resolution Imaging for the Detection and Characterisation of Circulating Tumour Cells from Patients with Oesophageal, Hepatocellular, Thyroid and Ovarian Cancers. Int. J. Cancer 2016, 138, 206–216. [Google Scholar] [CrossRef]
- Jones, M.L.; Siddiqui, J.; Pienta, K.J.; Getzenberg, R.H. Circulating Fibroblast-like Cells in Men with Metastatic Prostate Cancer. Prostate 2013, 73, 176–181. [Google Scholar] [CrossRef]
- Wills, J.W.; Verma, J.R.; Rees, B.J.; Harte, D.S.G.; Haxhiraj, Q.; Barnes, C.M.; Barnes, R.; Rodrigues, M.A.; Doan, M.; Filby, A.; et al. Inter-Laboratory Automation of the in Vitro Micronucleus Assay Using Imaging Flow Cytometry and Deep Learning. Arch. Toxicol. 2021, 95, 3101–3115. [Google Scholar] [CrossRef] [PubMed]
- Ligthart, S.T.; Coumans, F.A.W.; Bidard, F.-C.; Simkens, L.H.J.; Punt, C.J.A.; de Groot, M.R.; Attard, G.; de Bono, J.S.; Pierga, J.-Y.; Terstappen, L.W.M.M. Circulating Tumor Cells Count and Morphological Features in Breast, Colorectal and Prostate Cancer. PLoS ONE 2013, 8, e67148. [Google Scholar] [CrossRef]
- Mendelaar, P.A.J.; Kraan, J.; Van, M.; Zeune, L.L.; Terstappen, L.W.M.M.; Oomen-de Hoop, E.; Martens, J.W.M.; Sleijfer, S. Defining the Dimensions of Circulating Tumor Cells in a Large Series of Breast, Prostate, Colon, and Bladder Cancer Patients. Mol. Oncol. 2021, 15, 116–125. [Google Scholar] [CrossRef]
- Bünger, S.; Zimmermann, M.; Habermann, J.K. Diversity of Assessing Circulating Tumor Cells (CTCs) Emphasizes Need for Standardization: A CTC Guide to Design and Report Trials. Cancer Metastasis Rev. 2015, 34, 527–545. [Google Scholar] [CrossRef]
- Reduzzi, C.; Vismara, M.; Gerratana, L.; Silvestri, M.; De Braud, F.; Raspagliesi, F.; Verzoni, E.; Di Cosimo, S.; Locati, L.D.; Cristofanilli, M.; et al. The Curious Phenomenon of Dual-Positive Circulating Cells: Longtime Overlooked Tumor Cells. Semin. Cancer Biol. 2020, 60, 344–350. [Google Scholar] [CrossRef] [PubMed]
- de Wit, S.; Zeune, L.; Hiltermann, T.; Groen, H.; Dalum, G.; Terstappen, L. Classification of Cells in CTC-Enriched Samples by Advanced Image Analysis. Cancers 2018, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.; Acquaviva, C.; Boudin, L.; Finetti, P.; Garnier, S.; Aulas, A.; Liberatoscioli, M.L.; Cabaud, O.; Guille, A.; de Nonneville, A.; et al. Identification of Atypical Circulating Tumor Cells with Prognostic Value in Metastatic Breast Cancer Patients. Cancers 2022, 14, 932. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Ho, H.; Lichterman, J.; Lu, Y.-T.; Zhang, Y.; Garcia, M.A.; Chen, S.-F.; Liang, A.-J.; Hodara, E.; Zhau, H.E.; et al. Subclassification of Prostate Cancer Circulating Tumor Cells by Nuclear Size Reveals Very Small Nuclear Circulating Tumor Cells in Patients with Visceral Metastases: VsnCTCs and Visceral Metastases in PC. Cancer 2015, 121, 3240–3251. [Google Scholar] [CrossRef]
- Luo, S.; Shi, Y.; Chin, L.K.; Hutchinson, P.E.; Zhang, Y.; Chierchia, G.; Talbot, H.; Jiang, X.; Bourouina, T.; Liu, A.-Q. Machine-Learning-Assisted Intelligent Imaging Flow Cytometry: A Review. Adv. Intell. Syst. 2021, 3, 2100073. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Liu, Y. Machine Learning-Driven Multiobjective Optimization: An Opportunity of Microfluidic Platforms Applied in Cancer Research. Cells 2022, 11, 905. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Qin, X.; Nair, S.; Huang, X.; Liu, Y. Label-Free Detection of Rare Circulating Tumor Cells by Image Analysis and Machine Learning. Sci. Rep. 2020, 10, 12226. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Y.; Jin, W.; Cheng, J.; Peng, H.; Xu, Y.; Zhou, K.; Chen, L.; Huang, K.; Wu, S.; et al. Circulating Tumor Cell Detection and Single-cell Analysis Using an Integrated Workflow Based on ChimeraX®-i120 Platform: A Prospective Study. Mol. Oncol. 2021, 15, 2345–2362. [Google Scholar] [CrossRef]
- Lannin, T.B.; Thege, F.I.; Kirby, B.J. Comparison and Optimization of Machine Learning Methods for Automated Classification of Circulating Tumor Cells: Automated Circulating Tumor Cell Image Classifiers. Cytometry A 2016, 89, 922–931. [Google Scholar] [CrossRef]
- Bednarz, N.; Eltze, E.; Semjonow, A.; Rink, M.; Andreas, A.; Mulder, L.; Hannemann, J.; Fisch, M.; Pantel, K.; Weier, H.-U.G.; et al. BRCA1 Loss Preexisting in Small Subpopulations of Prostate Cancer Is Associated with Advanced Disease and Metastatic Spread to Lymph Nodes and Peripheral Blood. Clin. Cancer Res. 2010, 16, 3340–3348. [Google Scholar] [CrossRef]
- Nastały, P.; Ruf, C.; Becker, P.; Bednarz-Knoll, N.; Stoupiec, M.; Kavsur, R.; Isbarn, H.; Matthies, C.; Wagner, W.; Höppner, D.; et al. Circulating Tumor Cells in Patients with Testicular Germ Cell Tumors. Clin. Cancer Res. 2014, 20, 3830–3841. [Google Scholar] [CrossRef]
- Obermayr, E.; Bednarz-Knoll, N.; Orsetti, B.; Weier, H.-U.; Lambrechts, S.; Castillo-Tong, D.C.; Reinthaller, A.; Braicu, I.; Mahner, S.; Sehouli, J.; et al. Circulating Tumor Cells: Potential Markers of Minimal Residual Disease in Ovarian Cancer? A Study of the OVCAD Consortium. Oncotarget 2017, 8, 106415–106428. [Google Scholar] [CrossRef]
- Hui, H.Y.L.; Clarke, K.M.; Fuller, K.A.; Stanley, J.; Chuah, H.H.; Ng, T.F.; Cheah, C.; McQuillan, A.; Erber, W.N. “Immuno-flowFISH” for the Assessment of Cytogenetic Abnormalities in Chronic Lymphocytic Leukemia. Cytometry A 2019, 95, 521–533. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muchlińska, A.; Smentoch, J.; Żaczek, A.J.; Bednarz-Knoll, N. Detection and Characterization of Circulating Tumor Cells Using Imaging Flow Cytometry—A Perspective Study. Cancers 2022, 14, 4178. https://doi.org/10.3390/cancers14174178
Muchlińska A, Smentoch J, Żaczek AJ, Bednarz-Knoll N. Detection and Characterization of Circulating Tumor Cells Using Imaging Flow Cytometry—A Perspective Study. Cancers. 2022; 14(17):4178. https://doi.org/10.3390/cancers14174178
Chicago/Turabian StyleMuchlińska, Anna, Julia Smentoch, Anna J. Żaczek, and Natalia Bednarz-Knoll. 2022. "Detection and Characterization of Circulating Tumor Cells Using Imaging Flow Cytometry—A Perspective Study" Cancers 14, no. 17: 4178. https://doi.org/10.3390/cancers14174178
APA StyleMuchlińska, A., Smentoch, J., Żaczek, A. J., & Bednarz-Knoll, N. (2022). Detection and Characterization of Circulating Tumor Cells Using Imaging Flow Cytometry—A Perspective Study. Cancers, 14(17), 4178. https://doi.org/10.3390/cancers14174178





