Characterizing Circulating Tumor Cells and Tumor-Derived Extracellular Vesicles in Metastatic Castration-Naive and Castration-Resistant Prostate Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
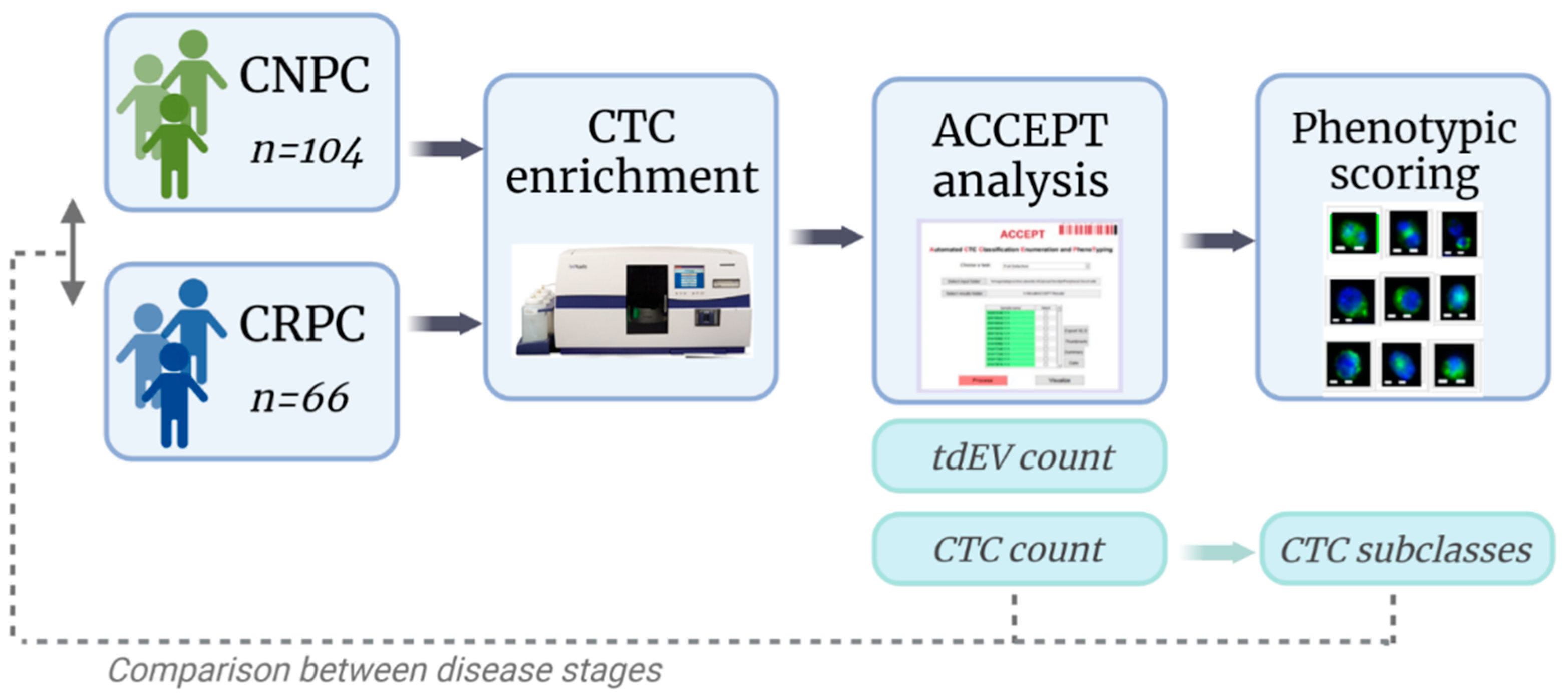
2.1. Patient Inclusion
2.2. Sample Preparation and Image Acquisition
2.3. CTC and tdEV Enumeration with ACCEPT
2.4. Subclassification and Scoring of ACCEPT Identified CTCs
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics and Screening
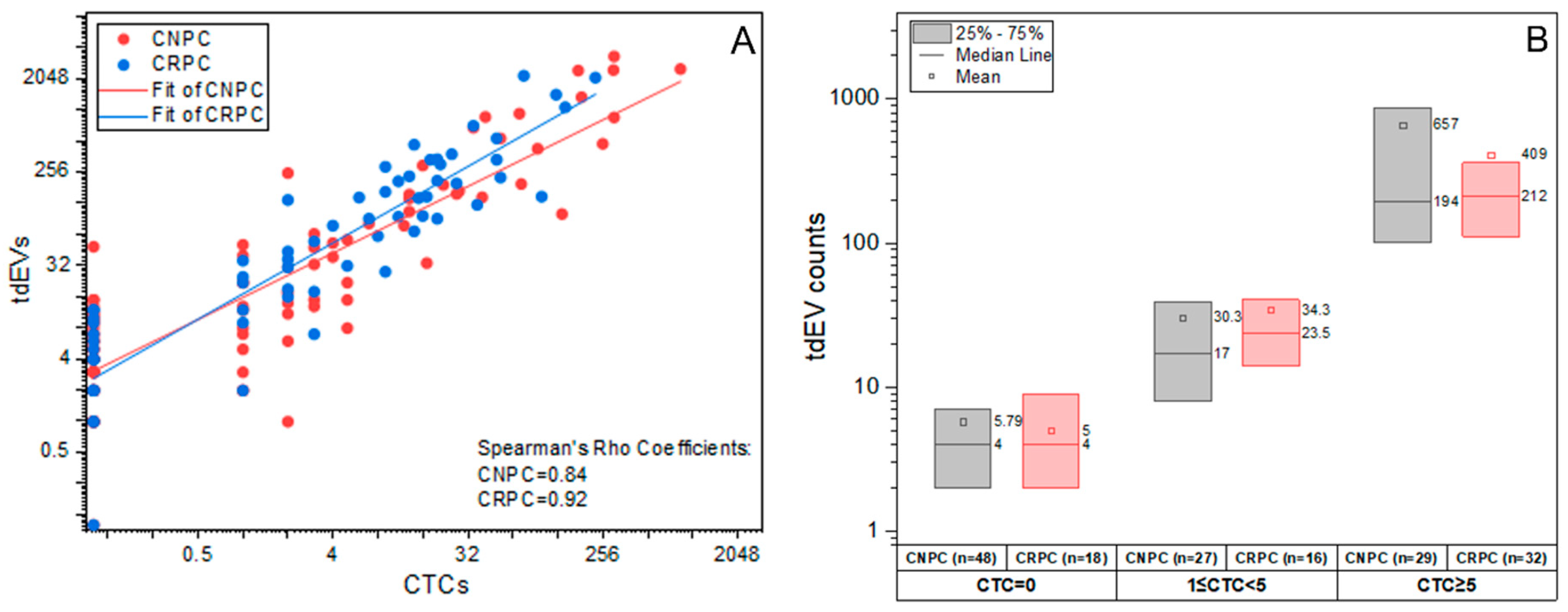
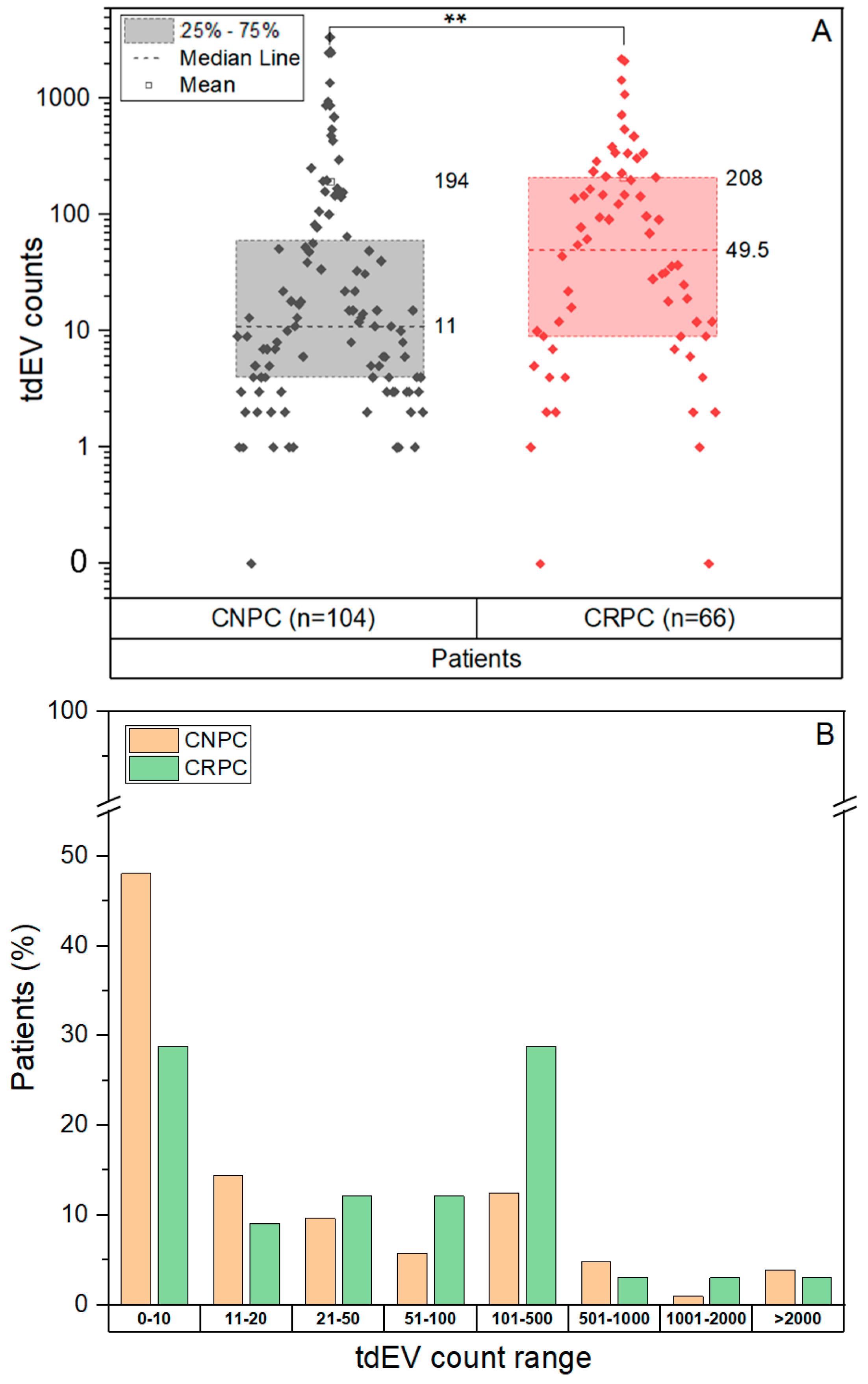
3.2. CTC and tdEV Enumeration Using CellSearch and ACCEPT
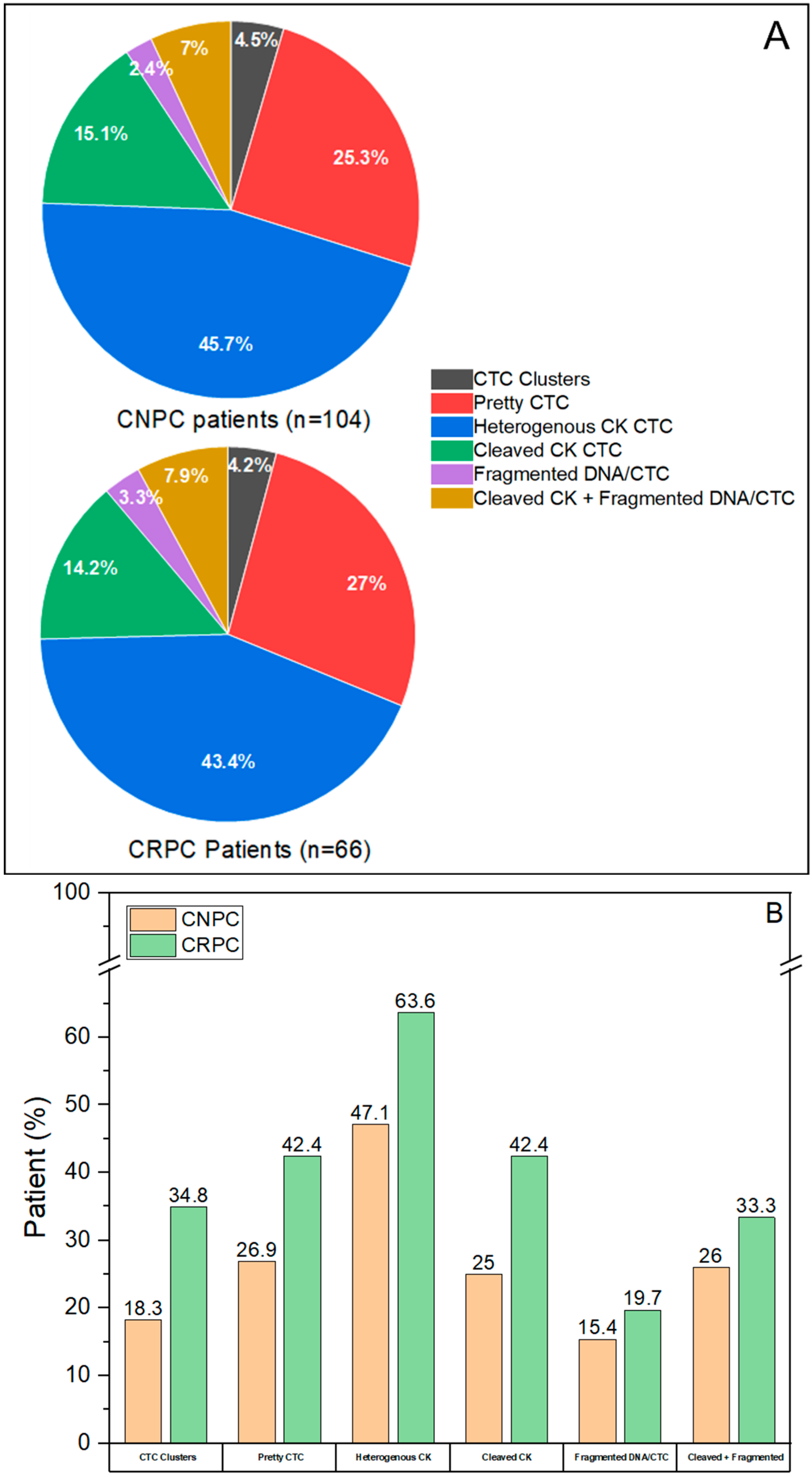
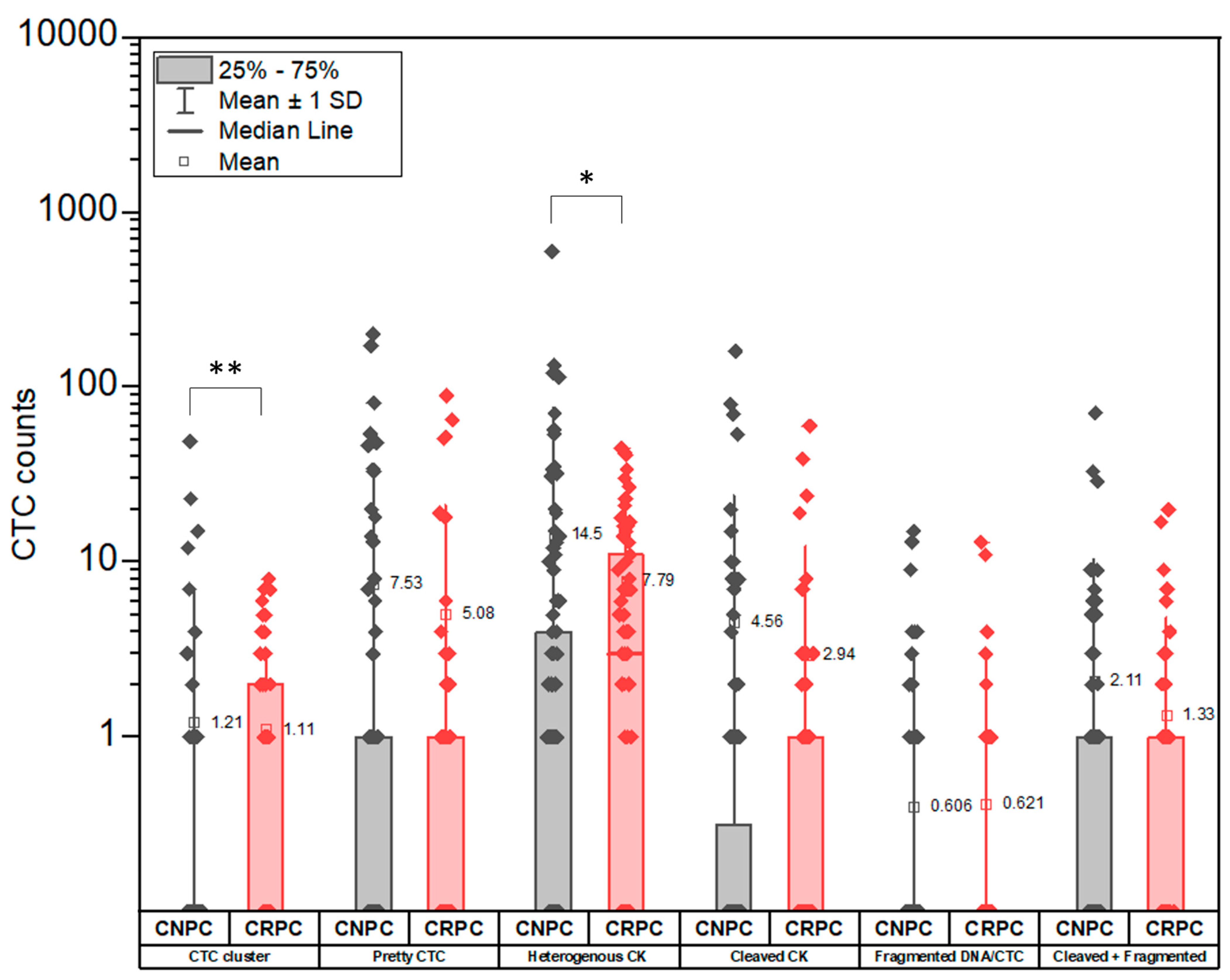
3.3. CTC Subclassification
3.4. Distribution of CTC Subclasses in CNPC and CRPC
3.5. Presence of CTC and tdEV Pre- and Post-Therapy in CNPC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyriakopoulos, C.E.; Chen, Y.-H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.-K.; Oh, W.K. First-Line Treatment of Hormone-Sensitive Metastatic Prostate Cancer: Is There a Single Standard of Care? J. Clin. Oncol. 2018, 36, 1060–1061. [Google Scholar] [CrossRef] [PubMed]
- Brady, L.; Kriner, M.; Coleman, I.; Morrissey, C.; Roudier, M.; True, L.D.; Gulati, R.; Plymate, S.R.; Zhou, Z.; Birditt, B.; et al. Inter- and intra-tumor heterogeneity of metastatic prostate cancer determined by digital spatial gene expression profiling. Nat. Commun. 2021, 12, 1426. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Hayes, D.F.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Reuben, J.M.; Doyle, G.V.; Matera, J.; Allard, W.J.; Miller, M.C.; et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol 2005, 23, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.A.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [PubMed]
- Nanou, A.; Miller, M.C.; Zeune, L.L.; De Wit, S.; Punt, C.J.A.; Groen, H.J.; Hayes, D.F.; De Bono, J.S.; Terstappen, L.W.M.M. Tumour-derived extracellular vesicles in blood of metastatic cancer patients associate with overall survival. Br. J. Cancer 2020, 122, 801–811. [Google Scholar] [CrossRef]
- Zeune, L.L.; de Wit, S.; Berghuis, A.S.; Ijzerman, M.; Terstappen, L.; Brune, C. How to Agree on a CTC: Evaluating the Consensus in Circulating Tumor Cell Scoring. Cytom. A 2018, 93, 1202–1206. [Google Scholar] [CrossRef]
- Nanou, A.; Zeune, L.L.; Bidard, F.-C.; Pierga, J.-Y.; Terstappen, L.W.M.M. HER2 expression on tumor-derived extracellular vesicles and circulating tumor cells in metastatic breast cancer. Breast Cancer Res. 2020, 22, 86. [Google Scholar] [CrossRef]
- Nanou, A.; Coumans, F.A.; van Dalum, G.; Zeune, L.L.; Dolling, D.; Onstenk, W.; Crespo, M.; Fontes, M.S.; Rescigno, P.; Fowler, G.; et al. Circulating tumor cells, tumor-derived extracellular vesicles and plasma cytokeratins in castration-resistant prostate cancer patients. Oncotarget 2018, 9, 19283–19293. [Google Scholar] [CrossRef] [Green Version]
- Kanker.nl. PICTURES—Study (Prostate Cancer). Available online: https://www.kanker.nl/trials/1309-pictures---studie-prostaatkanker- (accessed on 28 July 2022).
- WHO. Probing Intercellular Heterogeneity in Circulating Tumor Cells of De Novo Metastatic Hormone Sensitive Prostate Cancer Patients. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=NL8549 (accessed on 28 July 2022).
- Park, S.; Ang, R.R.; Duffy, S.P.; Bazov, J.; Chi, K.N.; Black, P.C.; Ma, H. Morphological Differences between Circulating Tumor Cells from Prostate Cancer Patients and Cultured Prostate Cancer Cells. PLoS ONE 2014, 9, e85264. [Google Scholar]
- Lorente, D.; Olmos, D.; Mateo, J.; Dolling, D.; Bianchini, D.; Seed, G.; Flohr, P.; Crespo, M.; Figueiredo, I.; Miranda, S.; et al. Circulating tumour cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann. Oncol. 2018, 29, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.G.; Miller, M.C.; Gross, S.; Allard, W.J.; Gomella, L.G.; Terstappen, L.W. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology 2005, 65, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Danila, D.C.; Fleisher, M.; Scher, H.I. Circulating tumor cells as biomarkers in prostate cancer. Clin. Cancer Res. 2011, 17, 3903–3912. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.; Jia, X.; de Bono, J.S.; Fleisher, M.; Pienta, K.J.; Raghavan, D.; Heller, G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10, 233–239. [Google Scholar] [CrossRef]
- Mout, L.; van Dessel, L.F.; Kraan, J.; de Jong, A.C.; Neves, R.P.; Erkens-Schulze, S.; Beaufort, C.M.; Sieuwerts, A.M.; van Riet, J.; Woo, T.L.; et al. Generating human prostate cancer organoids from leukapheresis enriched circulating tumour cells. Eur. J. Cancer 2021, 150, 179–189. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.J.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.J.; Uhr, J.W.; Terstappen, L.W.M.M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef]
- Coumans, F.; Terstappen, L. Detection and Characterization of Circulating Tumor Cells by the CellSearch Approach. Methods Mol. Biol. 2015, 1347, 263–278. [Google Scholar]
- Jansson, S.; Bendahl, P.-O.; Larsson, A.-M.; Aaltonen, K.E.; Rydén, L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 2016, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.J.; Moreno, J.G.; Pienta, K.J.; Gross, S.; Repollet, M.; O’Hara, S.M.; Russell, T.; Terstappen, L.W.M.M. Apoptosis of circulating tumor cells in prostate cancer patients. Cytom. A 2004, 62, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, R.; Martínez-Pena, I.; López-López, R. Relevance of CTC Clusters in Breast Cancer Metastasis. Adv. Exp. Med. Biol. 2020, 1220, 93–115. [Google Scholar] [PubMed]
- Herath, S.; Bazaz, S.R.; Monkman, J.; Warkiani, M.E.; Richard, D.; O’Byrne, K.; Kulasinghe, A. Circulating tumor cell clusters: Insights into tumour dissemination and metastasis. Expert. Rev. Mol. Diagn. 2020, 20, 1139–1147. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, A.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.D.S.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef]
- James, N.D.; De Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Zielinski, R.R.; Thomson, A.; Tan, T.H.; Sandhu, S.; Reaume, M.N.; Pook, D.W.; Parnis, F.; North, S.A.; et al. Updated overall survival outcomes in ENZAMET (ANZUP 1304), an international, cooperative group trial of enzalutamide in metastatic hormone-sensitive prostate cancer (mHSPC). J. Clin. Oncol. 2022, 40 (Suppl. 17), LBA5004. [Google Scholar] [CrossRef]



| Parameters | CTCs | tdEVs |
|---|---|---|
| Mean intensity CD45 | ≤5 AU | ≤5 AU |
| Mean intensity DAPI | >45 AU | ≤5 AU |
| Mean intensity CK | >60 AU | >60 AU |
| max intensity CK | - | >90 AU |
| Area CK | - | ≤150 μm2 |
| Eccentricity CK | - | ≤0.8 AU |
| Perimeter to area CK | - | ≤1 AU |
| Perimeter CK | - | >5 pixels |
| CK size | >16 μm2 | - |
| CK size | ≤400 μm2 | ≤150 μm2 |
| CK overlay with DAPI | >0.2 AU | - |
| Mean intensity marker 1 (PE) | ≤5 AU | ≤5 AU |
| Mean intensity marker 2 (FITC) | ≤5 AU | ≤5 AU |
| CNPC | CRPC | ||
|---|---|---|---|
| Characteristic | (N = 104) | (N = 66) | |
| Age at registration | p = 0.499 | ||
| Median (range)—years | 70 (49–83) | 69 (49–82) | |
| WHO PS at registration—no. (%) | p ≤ 0.001 *** | ||
| - 0 | 65 (62.5%) | 16 (24.2%) | |
| - 1 | 35(33.7%) | 50 (75.8%) | |
| - 2 | 4 (3.8%) | ||
| Initial PSA at primary diagnosis, µg/L | p = 0.176 | ||
| Mean ± SD | 647.3 ± 1665.8 | 334.5 ± 900.4 | |
| Median (range) | 107 (3.0–11,098.0) | 38 (3.0–4786.0) | |
| Hemoglobin—g/L | p = 0.032 * | ||
| Mean ± SD | 8.4 ± 1.3 | 7.9 ± 1.0 | |
| Median (range) | 8.5 (4.3–10.6) | 8 (5.5–9.7) | |
| Alkaline phosphatase—IU/L | p = 0.192 | ||
| Mean ± SD | 392.7 ± 1157.2 | 156.8 ± 144.1 | |
| Median (range) | 116(45.0–8854.0) | 104 (58.0–894.0) | |
| Lactate dehydrogenase—IU/L | p = 0.203 | ||
| Mean ± SD | 243.6 ± 89.6 | 313.3 ± 408.4 | |
| Median (range) | 223.5 (139.0–644.0) | 202.5 (153.0–2718.0) | |
| Albumin—g/L | p = 0.156 | ||
| Mean ± SD | 41.6 ± 5 | 43.1 ± 3.5 | |
| Median (range) | 42.5 (27.0–51.0) | 43 (35.0–54.0) | |
| Gleason score by diagnosis | p = 0.165 | ||
| Median (range) | 8 (6.0–10.0) | 9 (5.0–10.0) | |
| M-stage at diagnosis | p ≤ 0.001 *** | ||
| - M0 | 5 (4.8%) | 20 (30.3%) | |
| - M1a, M1b, M1c | 88 (91.4%) | 30 (50%) | |
| - Mx | 4 (3.8%) | 13(19.7%) | |
| Type of prior therapy | |||
| Docetaxel | 35 | ||
| Cabazitaxel | 8 | ||
| Enzalutamide | 27 | ||
| Abiraterone | 6 | ||
| Other systemic therapy | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isebia, K.T.; Dathathri, E.; Verschoor, N.; Nanou, A.; De Jong, A.C.; Coumans, F.A.W.; Terstappen, L.W.M.M.; Kraan, J.; Martens, J.W.M.; Bansal, R.; et al. Characterizing Circulating Tumor Cells and Tumor-Derived Extracellular Vesicles in Metastatic Castration-Naive and Castration-Resistant Prostate Cancer Patients. Cancers 2022, 14, 4404. https://doi.org/10.3390/cancers14184404
Isebia KT, Dathathri E, Verschoor N, Nanou A, De Jong AC, Coumans FAW, Terstappen LWMM, Kraan J, Martens JWM, Bansal R, et al. Characterizing Circulating Tumor Cells and Tumor-Derived Extracellular Vesicles in Metastatic Castration-Naive and Castration-Resistant Prostate Cancer Patients. Cancers. 2022; 14(18):4404. https://doi.org/10.3390/cancers14184404
Chicago/Turabian StyleIsebia, Khrystany T., Eshwari Dathathri, Noortje Verschoor, Afroditi Nanou, Anouk C. De Jong, Frank A. W. Coumans, Leon W. M. M. Terstappen, Jaco Kraan, John W. M. Martens, Ruchi Bansal, and et al. 2022. "Characterizing Circulating Tumor Cells and Tumor-Derived Extracellular Vesicles in Metastatic Castration-Naive and Castration-Resistant Prostate Cancer Patients" Cancers 14, no. 18: 4404. https://doi.org/10.3390/cancers14184404
APA StyleIsebia, K. T., Dathathri, E., Verschoor, N., Nanou, A., De Jong, A. C., Coumans, F. A. W., Terstappen, L. W. M. M., Kraan, J., Martens, J. W. M., Bansal, R., & Lolkema, M. P. (2022). Characterizing Circulating Tumor Cells and Tumor-Derived Extracellular Vesicles in Metastatic Castration-Naive and Castration-Resistant Prostate Cancer Patients. Cancers, 14(18), 4404. https://doi.org/10.3390/cancers14184404









