BRAF Mutations in Colorectal Liver Metastases: Prognostic Implications and Potential Therapeutic Strategies
Abstract
Simple Summary
Abstract
1. Introduction
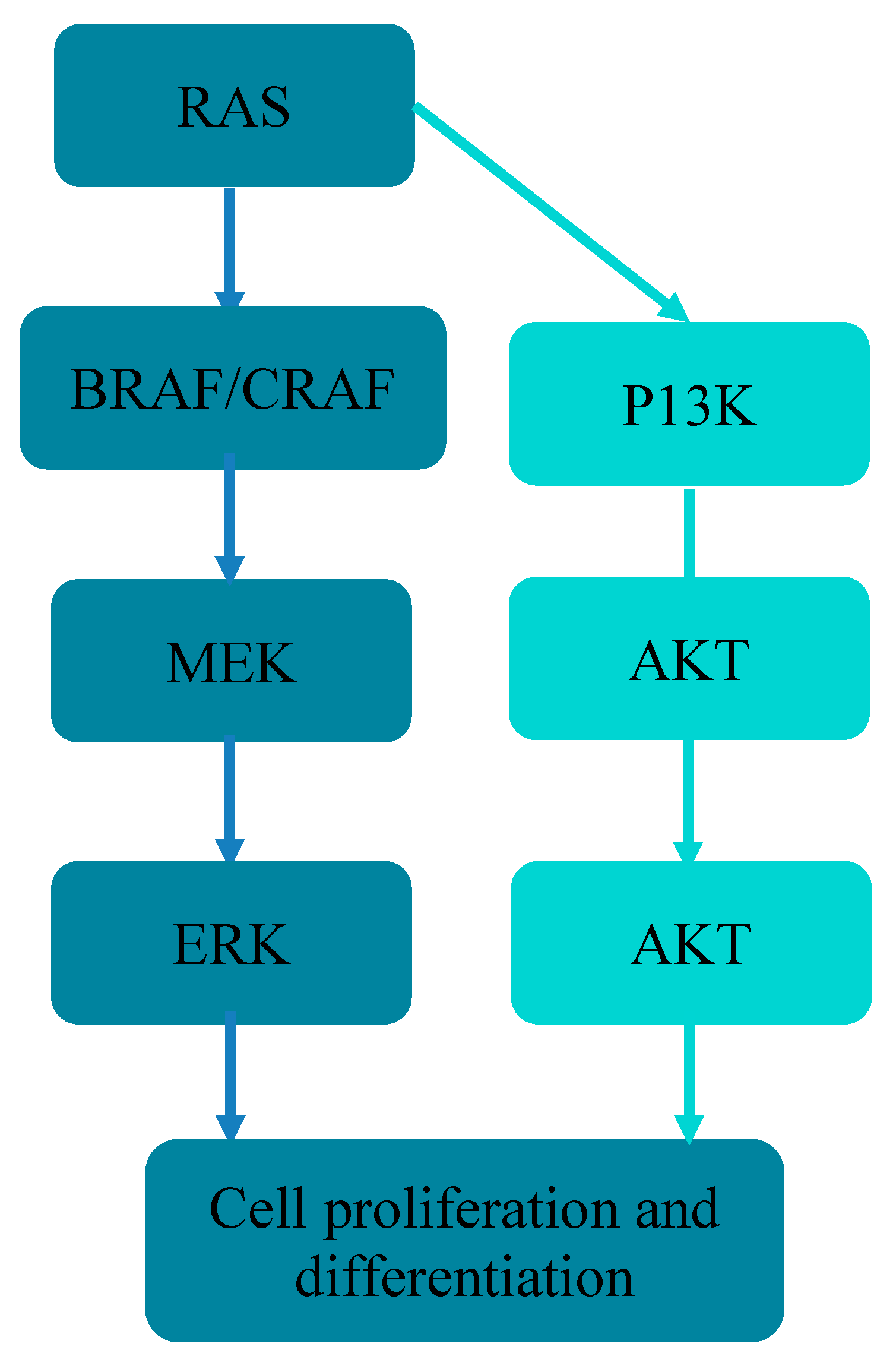
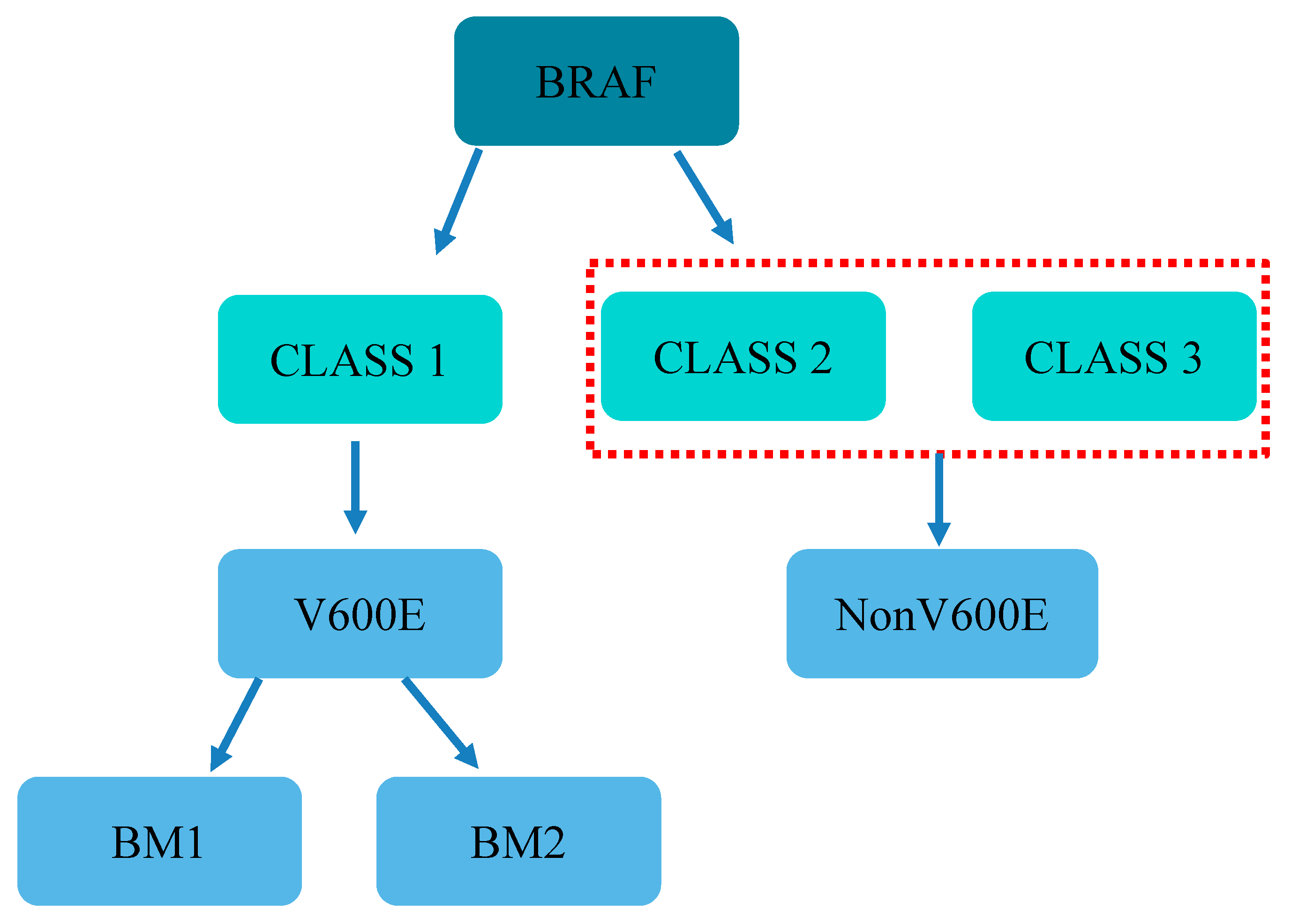
2. Molecular Mechanism
3. Clinicopathological Features
4. Clinical Implication
4.1. BRAF Mutation and Prognosis
4.2. Prognostic Risk Factors in Patients with BRAF-Mutated Tumors
4.3. Effect of Surgical Treatment on Prognosis
4.4. Recurrence Rates of Patients Who Underwent Surgical Intervention of CRLM
5. BRAF Inhibitors and Potential Targeted Therapies
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Wisneski, A.D.; Jin, C.; Huang, C.Y.; Warren, R.; Hirose, K.; Nakakura, E.K.; Corvera, C.U. Synchronous Versus Metachronous Colorectal Liver Metastasis Yields Similar Survival in Modern Era. J. Surg. Res. 2020, 256, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Adam, R.; Baba, H. How to increase the resectability of initially unresectable colorectal liver metastases: A surgical perspective. Ann. Gastroenterol. Surg. 2019, 3, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.; Salminen, T.; Soveri, L.M.; Kallio, R.; Kellokumpu, I.; Lamminmäki, A.; Halonen, P.; Ristamäki, R.; Lantto, E.; Uutela, A.; et al. Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): A nationwide prospective intervention study. Lancet Reg. Health Eur. 2021, 3, 100049. [Google Scholar] [CrossRef]
- Lévi, F.A.; Boige, V.; Hebbar, M.; Smith, D.; Lepère, C.; Focan, C.; Karaboué, A.; Guimbaud, R.; Carvalho, C.; Tumolo, S.; et al. Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann. Oncol. 2016, 27, 267–274. [Google Scholar] [CrossRef]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef]
- Rhaiem, R.; Rached, L.; Tashkandi, A.; Bouché, O.; Kianmanesh, R. Implications of RAS Mutations on Oncological Outcomes of Surgical Resection and Thermal Ablation Techniques in the Treatment of Colorectal Liver Metastases. Cancers 2022, 14, 816. [Google Scholar] [CrossRef]
- Winder, T.; Lenz, H.J. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology 2010, 138, 2163–2176. [Google Scholar] [CrossRef]
- Yamashita, S.; Chun, Y.S.; Kopetz, S.E.; Vauthey, J.N. Biomarkers in colorectal liver metastases. Br. J. Surg. 2018, 105, 618–627. [Google Scholar] [CrossRef]
- Sanz-Garcia, E.; Argiles, G.; Elez, E.; Tabernero, J. BRAF mutant colorectal cancer: Prognosis, treatment, and new perspectives. Ann. Oncol. 2017, 28, 2648–2657. [Google Scholar] [CrossRef]
- Chambard, J.C.; Lefloch, R.; Pouysségur, J.; Lenormand, P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta 2007, 1773, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Sartore-Bianchi, A.; Di Nicolantonio, F.; Zanon, C.; Moroni, M.; Veronese, S.; Siena, S.; Bardelli, A. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007, 67, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Kim, Y.; Wagner, D.; Sasaki, K.; Beer, A.; Schwarz, C.; Løes, I.M.; Smolle, M.; et al. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg. 2018, 153, e180996. [Google Scholar] [CrossRef]
- Schirripa, M.; Bergamo, F.; Cremolini, C.; Casagrande, M.; Lonardi, S.; Aprile, G.; Yang, D.; Marmorino, F.; Pasquini, G.; Sensi, E.; et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br. J. Cancer 2015, 112, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Umeda, Y.; Nagasaka, T.; Mori, Y.; Sadamori, H.; Sun, D.S.; Shinoura, S.; Yoshida, R.; Satoh, D.; Nobuoka, D.; Utsumi, M.; et al. Poor prognosis of KRAS or BRAF mutant colorectal liver metastasis without microsatellite instability. J. Hepatobiliary Pancreat. Sci. 2013, 20, 223–233. [Google Scholar] [CrossRef]
- Teng, H.W.; Huang, Y.C.; Lin, J.K.; Chen, W.S.; Lin, T.C.; Jiang, J.K.; Yen, C.C.; Li, A.F.; Wang, H.W.; Chang, S.C.; et al. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J. Surg. Oncol. 2012, 106, 123–129. [Google Scholar] [CrossRef]
- Mark, G.E.; Rapp, U.R. Primary structure of v-raf: Relatedness to the src family of oncogenes. Science 1984, 224, 285–289. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.M.; Freeman, A.K.; Morrison, D.K. Complexity in KSR function revealed by Raf inhibitor and KSR structure studies. Small GTPases 2011, 2, 276–281. [Google Scholar] [CrossRef][Green Version]
- Odabaei, G.; Chatterjee, D.; Jazirehi, A.R.; Goodglick, L.; Yeung, K.; Bonavida, B. Raf-1 kinase inhibitor protein: Structure, function, regulation of cell signaling, and pivotal role in apoptosis. Adv. Cancer Res. 2004, 91, 169–200. [Google Scholar] [CrossRef]
- Rebocho, A.P.; Marais, R. ARAF acts as a scaffold to stabilize BRAF:CRAF heterodimers. Oncogene 2013, 32, 3207–3212. [Google Scholar] [CrossRef]
- Zaman, A.; Wu, W.; Bivona, T.G. Targeting Oncogenic BRAF: Past, Present, and Future. Cancers 2019, 11, 1197. [Google Scholar] [CrossRef] [PubMed]
- Bivona, T.G. Dampening oncogenic RAS signaling. Science 2019, 363, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.J.; Haderk, F.; Stahlhut, C.; Schulze, C.J.; Hemmati, G.; Wildes, D.; Tzitzilonis, C.; Mordec, K.; Marquez, A.; Romero, J.; et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat. Cell Biol. 2018, 20, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Wu, C.; Bekaii-Saab, T. Targeting BRAF in metastatic colorectal cancer: Maximizing molecular approaches. Cancer Treat. Rev. 2017, 60, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Barras, D.; Missiaglia, E.; Wirapati, P.; Sieber, O.M.; Jorissen, R.N.; Love, C.; Molloy, P.L.; Jones, I.T.; McLaughlin, S.; Gibbs, P.; et al. BRAF V600E Mutant Colorectal Cancer Subtypes Based on Gene Expression. Clin. Cancer Res. 2017, 23, 104–115. [Google Scholar] [CrossRef]
- Loo, E.; Khalili, P.; Beuhler, K.; Siddiqi, I.; Vasef, M.A. BRAF V600E Mutation Across Multiple Tumor Types: Correlation Between DNA-based Sequencing and Mutation-specific Immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, C.P.; Zobell, S.D.; Furtado, L.V.; Baker, C.L.; Samowitz, W.S. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer 2011, 50, 307–312. [Google Scholar] [CrossRef]
- Ye, Z.L.; Qiu, M.Z.; Tang, T.; Wang, F.; Zhou, Y.X.; Lei, M.J.; Guan, W.L.; He, C.Y. Gene mutation profiling in Chinese colorectal cancer patients and its association with clinicopathological characteristics and prognosis. Cancer Med. 2020, 9, 745–756. [Google Scholar] [CrossRef]
- Yaeger, R.; Cercek, A.; Chou, J.F.; Sylvester, B.E.; Kemeny, N.E.; Hechtman, J.F.; Ladanyi, M.; Rosen, N.; Weiser, M.R.; Capanu, M.; et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer 2014, 120, 2316–2324. [Google Scholar] [CrossRef]
- Dijkstra, M.; Nieuwenhuizen, S.; Puijk, R.S.; Timmer, F.E.F.; Geboers, B.; Schouten, E.A.C.; Opperman, J.; Scheffer, H.J.; de Vries, J.J.J.; Versteeg, K.S.; et al. Primary Tumor Sidedness, RAS and BRAF Mutations and MSI Status as Prognostic Factors in Patients with Colorectal Liver Metastases Treated with Surgery and Thermal Ablation: Results from the Amsterdam Colorectal Liver Met Registry (AmCORE). Biomedicines 2021, 9, 962. [Google Scholar] [CrossRef]
- Gau, L.; Ribeiro, M.; Pereira, B.; Poirot, K.; Dupré, A.; Pezet, D.; Gagnière, J. Impact of BRAF mutations on clinical outcomes following liver surgery for colorectal liver metastases: An updated meta-analysis. Eur. J. Surg. Oncol. 2021, 47, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J. Hyperplastic and serrated polyps of the colorectum. Gastroenterol. Clin. N. Am. 2007, 36, 947–968. [Google Scholar] [CrossRef] [PubMed]
- Samowitz, W.S.; Sweeney, C.; Herrick, J.; Albertsen, H.; Levin, T.R.; Murtaugh, M.A.; Wolff, R.K.; Slattery, M.L. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005, 65, 6063–6069. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Shima, K.; Meyerhardt, J.A.; McCleary, N.J.; Ng, K.; Hollis, D.; Saltz, L.B.; Mayer, R.J.; Schaefer, P.; Whittom, R.; et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: Results from intergroup trial CALGB 89803. Clin. Cancer Res. 2012, 18, 890–900. [Google Scholar] [CrossRef]
- Løes, I.M.; Immervoll, H.; Sorbye, H.; Angelsen, J.H.; Horn, A.; Knappskog, S.; Lønning, P.E. Impact of KRAS, BRAF, PIK3CA, TP53 status and intraindividual mutation heterogeneity on outcome after liver resection for colorectal cancer metastases. Int. J. Cancer 2016, 139, 647–656. [Google Scholar] [CrossRef]
- Gagnière, J.; Dupré, A.; Gholami, S.S.; Pezet, D.; Boerner, T.; Gönen, M.; Kingham, T.P.; Allen, P.J.; Balachandran, V.P.; De Matteo, R.P.; et al. Is Hepatectomy Justified for BRAF Mutant Colorectal Liver Metastases?: A Multi-institutional Analysis of 1497 Patients. Ann. Surg. 2020, 271, 147–154. [Google Scholar] [CrossRef]
- Bachet, J.B.; Moreno-Lopez, N.; Vigano, L.; Marchese, U.; Gelli, M.; Raoux, L.; Truant, S.; Laurent, C.; Herrero, A.; Le Roy, B.; et al. BRAF mutation is not associated with an increased risk of recurrence in patients undergoing resection of colorectal liver metastases. Br. J. Surg. 2019, 106, 1237–1247. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takahashi, S.; Takahashi, N.; Masuishi, T.; Shoji, H.; Shinozaki, E.; Yamaguchi, T.; Kojima, M.; Gotohda, N.; Nomura, S.; et al. Survival Outcomes of Resected BRAF V600E Mutant Colorectal Liver Metastases: A Multicenter Retrospective Cohort Study in Japan. Ann. Surg. Oncol. 2020, 27, 3307–3315. [Google Scholar] [CrossRef]
- Lan, Y.T.; Chang, S.C.; Lin, P.C.; Lin, C.C.; Lin, H.H.; Huang, S.C.; Lin, C.H.; Liang, W.Y.; Chen, W.S.; Jiang, J.K.; et al. Clinicopathological and molecular features between synchronous and metachronous metastases in colorectal cancer. Am. J. Cancer Res. 2021, 11, 1646–1658. [Google Scholar]
- Han, L.; Xue, J.; Wu, X.; Liang, G.; Wu, Y.; Guo, F.; Gao, S.; Sun, G. Multidisciplinary approach to the diagnosis and treatment of patients with potentially resectable colorectal cancer liver metastasis: Results of a multicenter study. Ann. Palliat. Med. 2022, 11, 717–729. [Google Scholar] [CrossRef]
- Pikouli, A.; Papaconstantinou, D.; Wang, J.; Kavezou, F.; Pararas, N.; Nastos, C.; Pikoulis, E.; Margonis, G.A. Reevaluating the prognostic role of BRAF mutation in colorectal cancer liver metastases. Am. J. Surg. 2021, 223, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Takahashi, S.; Nomura, S.; Kojima, M.; Kudo, M.; Sugimoto, M.; Konishi, M.; Gotohda, N.; Taniguchi, H.; Yoshino, T. BRAF V600E potentially determines “Oncological Resectability” for “Technically Resectable” colorectal liver metastases. Cancer Med. 2021, 10, 6998–7011. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Benoist, S.; Devos, P.; Truant, S.; Guimbaud, R.; Lièvre, A.; Sefrioui, D.; Cohen, R.; Artru, P.; Dupré, A.; et al. Prognostic factors of BRAF V600E colorectal cancer with liver metastases: A retrospective multicentric study. World J. Surg. Oncol. 2022, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Sasaki, K.; Poultsides, G.; Imai, K.; Morioka, D.; Cameron, J.L.; Endo, I.; Baba, H.; et al. Demystifying BRAF mutation status in colorectal lover metastases: A multi-institutional, collaborative approach to 7 open clinical questions. In Proceedings of the 121st Annual Congress of Japan Surgical Society, Chiba, Japan, 2–10 April 2021. [Google Scholar]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef]
- Brose, M.S.; Cabanillas, M.E.; Cohen, E.E.; Wirth, L.J.; Riehl, T.; Yue, H.; Sherman, S.I.; Sherman, E.J. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: A non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1272–1282. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C.; et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J. Clin. Oncol. 2015, 33, 42–50. [Google Scholar] [CrossRef]
- Ponsioen, B.; Post, J.B.; Buissant des Amorie, J.R.; Laskaris, D.; van Ineveld, R.L.; Kersten, S.; Bertotti, A.; Sassi, F.; Sipieter, F.; Cappe, B.; et al. Quantifying single-cell ERK dynamics in colorectal cancer organoids reveals EGFR as an amplifier of oncogenic MAPK pathway signalling. Nat. Cell Biol. 2021, 23, 377–390. [Google Scholar] [CrossRef]
- Seymour, M.T.; Brown, S.R.; Middleton, G.; Maughan, T.; Richman, S.; Gwyther, S.; Lowe, C.; Seligmann, J.F.; Wadsley, J.; Maisey, N.; et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): A prospectively stratified randomised trial. Lancet Oncol. 2013, 14, 749–759. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- van Geel, R.; Tabernero, J.; Elez, E.; Bendell, J.C.; Spreafico, A.; Schuler, M.; Yoshino, T.; Delord, J.P.; Yamada, Y.; Lolkema, M.P.; et al. A Phase Ib Dose-Escalation Study of Encorafenib and Cetuximab with or without Alpelisib in Metastatic BRAF-Mutant Colorectal Cancer. Cancer Discov. 2017, 7, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B.; André, T.; Atreya, C.E.; Schellens, J.H.M.; Yoshino, T.; Bendell, J.C.; Hollebecque, A.; McRee, A.J.; Siena, S.; Middleton, G.; et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAF(V600E)-Mutant Colorectal Cancer. Cancer Discov. 2018, 8, 428–443. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A.; et al. Affimer Tagged Cubosomes: Targeting of Carcinoembryonic Antigen Expressing Colorectal Cancer Cells Using In Vitro and In Vivo Models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef]
- Yoshino, K.; Osumi, H.; Ito, H.; Yamaguchi, K.; Shinozaki, E. ASO Author Reflections: The Role of CEA Optimizing Perioperative Treatments for Colorectal Cancer with Liver Metastases. Ann. Surg. Oncol. 2022. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Wainberg, Z.A.; Patel, A.B.; Anadkat, M.J.; Stemmer, S.M.; Shacham-Shmueli, E.; Medina, E.; Zelinger, G.; Shelach, N.; Ribas, A. Reducing Skin Toxicities from EGFR Inhibitors with Topical BRAF Inhibitor Therapy. Cancer Discov. 2021, 11, 2158–2167. [Google Scholar] [CrossRef]
- Kopetz, S.; Guthrie, K.A.; Morris, V.K.; Lenz, H.J.; Magliocco, A.M.; Maru, D.; Yan, Y.; Lanman, R.; Manyam, G.; Hong, D.S.; et al. Randomized Trial of Irinotecan and Cetuximab With or Without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG S1406). J. Clin. Oncol. 2021, 39, 285–294. [Google Scholar] [CrossRef]
- Yeh, J.H.; Tsai, H.L.; Chen, Y.C.; Li, C.C.; Huang, C.W.; Chang, T.K.; Su, W.C.; Chen, P.J.; Liu, Y.P.; Wang, J.Y. BRAF, MEK, and EGFR Triplet Inhibitors as Salvage Therapy in BRAF-Mutated Metastatic Colorectal Cancer-A Case Series Study Target Therapy of BRAF-Mutated mCRC. Medicina 2021, 57, 1339. [Google Scholar] [CrossRef]
- Shen, C.; Hu, H.; Cai, Y.; Ling, J.; Zhang, J.; Wu, Z.; Xie, X.; Huang, M.; Wang, H.; Kang, L.; et al. mFOLFOXIRI with or without bevacizumab for conversion therapy of RAS/BRAF/PIK3CA mutant unresectable colorectal liver metastases: The FORBES non-randomized phase II trial. Ann. Transl. Med. 2022, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; García-Alfonso, P.; Viéitez, J.M.; Cano, M.T.; Rivera, F.; Reina-Zoilo, J.J.; Salud-Salvia, A.; Quintero, G.; Robles-Díaz, L.; Safont, M.J.; et al. Influence of BRAF and PIK3CA mutations on the efficacy of FOLFIRI plus bevacizumab or cetuximab as first-line therapy in patients with RAS wild-type metastatic colorectal carcinoma and <3 baseline circulating tumour cells: The randomised phase II VISNÚ-2 study. ESMO Open 2021, 6, 100062. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Kotani, D.; Mondaca, S.; Parikh, A.R.; Bando, H.; Van Seventer, E.E.; Taniguchi, H.; Zhao, H.; Thant, C.N.; de Stanchina, E.; et al. Response to Anti-EGFR Therapy in Patients with BRAF non-V600-Mutant Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 7089–7097. [Google Scholar] [CrossRef] [PubMed]
| Study | N * | Research Type | BRAF Mutation (%) | Mutant Subtype | Overall Survival (OS) | Recurrence/Disease-Free Survival (RFS/DFS) |
|---|---|---|---|---|---|---|
| HR (95%CI); p-Value | HR (95% CI) p-Value | |||||
| Margonis et al., 2018 [13] | 853 | multicenter cohort study | 41 (5.1%) | Yes | 2.76 (1.74–4.37); p < 0.001 | 2.04 (1.30–3.20); p = 0.002 |
| Bachet et al., 2019 [37] | 249 | case-matched study | 66 | No | NA; p = 0.004 | 1.16 (0.72–1.85); p = 0.547 |
| Gagniere et al., 2020 [36] | 1497 | multicenter cohort study | 35 (2%) | Yes | NA; p < 0.001 | NA; p < 0.001 |
| Yuan-Tzu et al., 2021 [39] | 492 | retrospective study | 25 (5.1%) | No | NA; p = 0.006 | NA |
| Shin et al., 2021 [42] | 172 | retrospective study | 5 (2.9%) | No | 27.6 (9.5–80.4); p < 0.001 | 12.5 (4.3–35.8); p < 0.001 |
| Targeted Therapeutics | Side Reactions | |||
|---|---|---|---|---|
| BRAF inhibitor | arthralgia | Rash/allergic reaction | fatigue | hair loss |
| MEK inhibitor | diarrhea | photosensitized reaction | fever | hemorrhage |
| EGFR inhibitor | acne-like rash | diarrhea | allergic reaction | constipation |
| VEGR inhibitor | gastrointestinal perforation | wound healing complications | hemorrhage | hypertension |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.-P.; Lin, C.; Wang, J.; Margonis, G.A.; Wu, B. BRAF Mutations in Colorectal Liver Metastases: Prognostic Implications and Potential Therapeutic Strategies. Cancers 2022, 14, 4067. https://doi.org/10.3390/cancers14174067
Wang P-P, Lin C, Wang J, Margonis GA, Wu B. BRAF Mutations in Colorectal Liver Metastases: Prognostic Implications and Potential Therapeutic Strategies. Cancers. 2022; 14(17):4067. https://doi.org/10.3390/cancers14174067
Chicago/Turabian StyleWang, Pei-Pei, Chen Lin, Jane Wang, Georgios Antonios Margonis, and Bin Wu. 2022. "BRAF Mutations in Colorectal Liver Metastases: Prognostic Implications and Potential Therapeutic Strategies" Cancers 14, no. 17: 4067. https://doi.org/10.3390/cancers14174067
APA StyleWang, P.-P., Lin, C., Wang, J., Margonis, G. A., & Wu, B. (2022). BRAF Mutations in Colorectal Liver Metastases: Prognostic Implications and Potential Therapeutic Strategies. Cancers, 14(17), 4067. https://doi.org/10.3390/cancers14174067







