An Overview of the Role of Multiparametric MRI in the Investigation of Testicular Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. mpMRI Protocol of the Scrotum
2.1. Normal Conventional MRI Findings
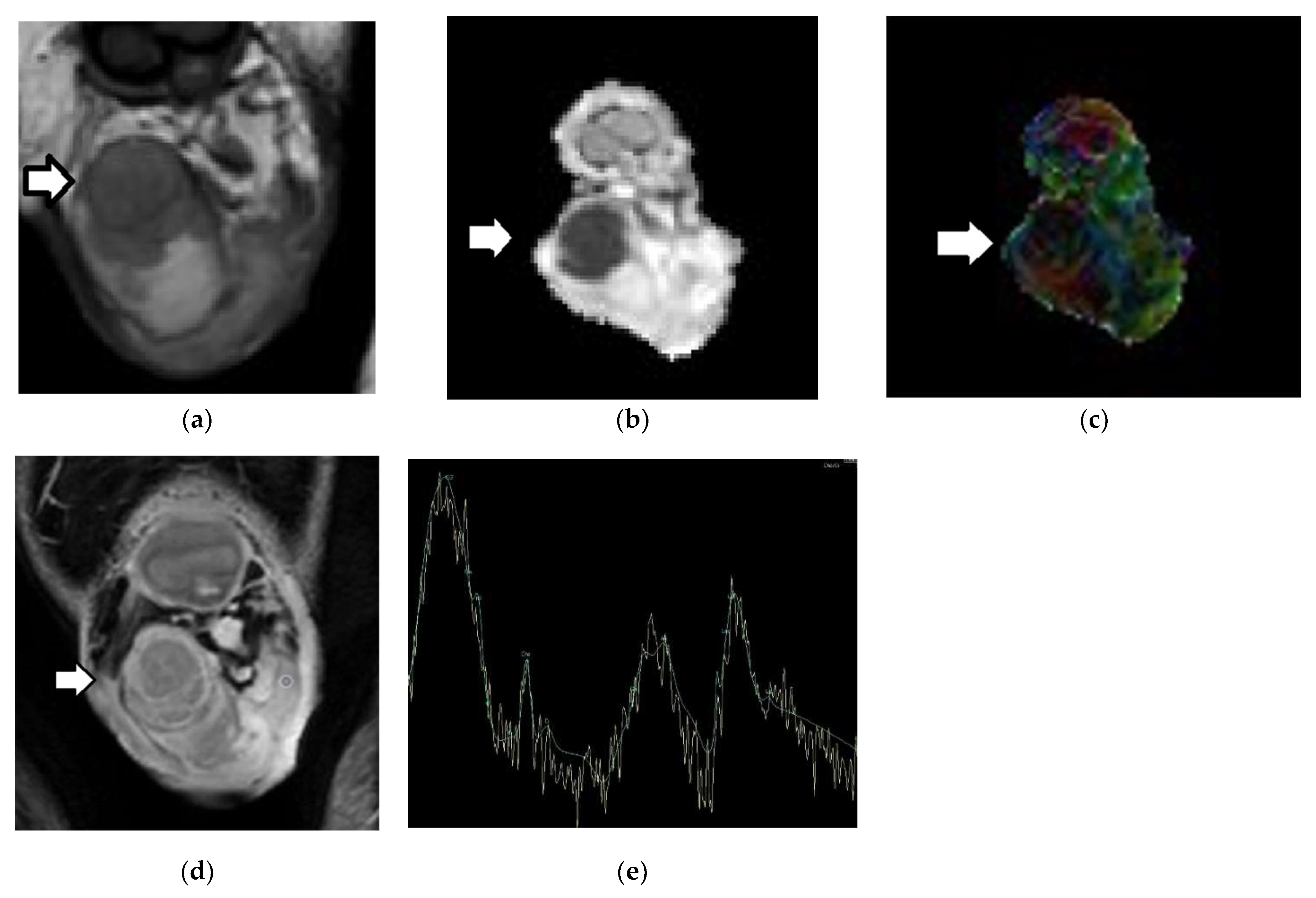
2.2. Diffusion-Weighted Imaging
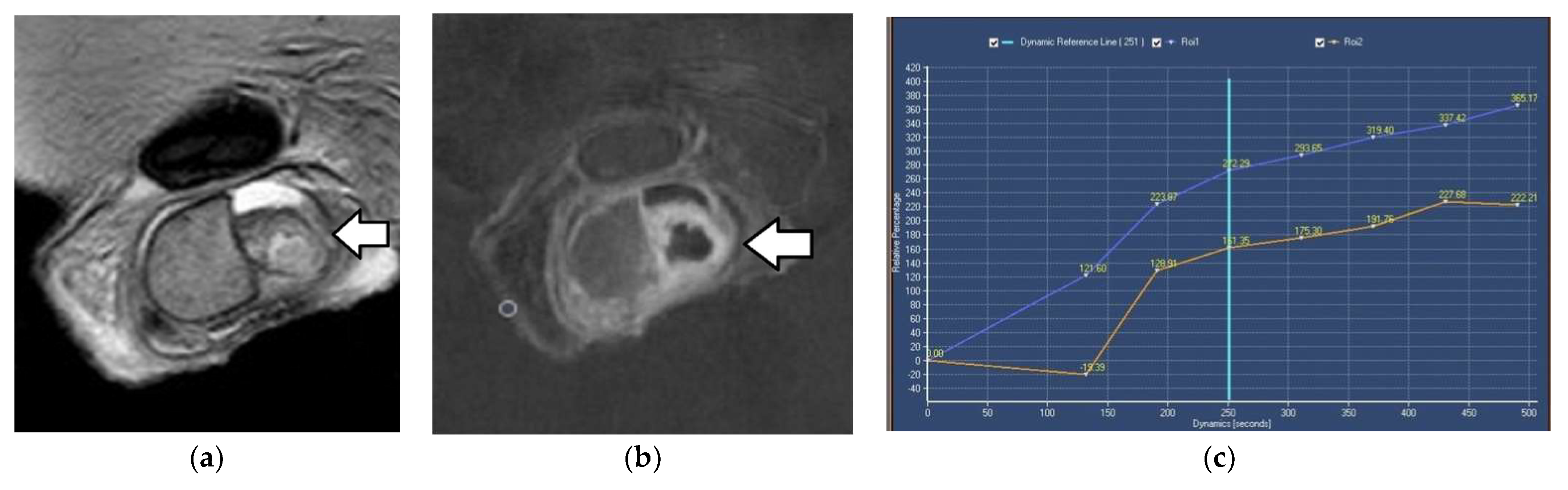
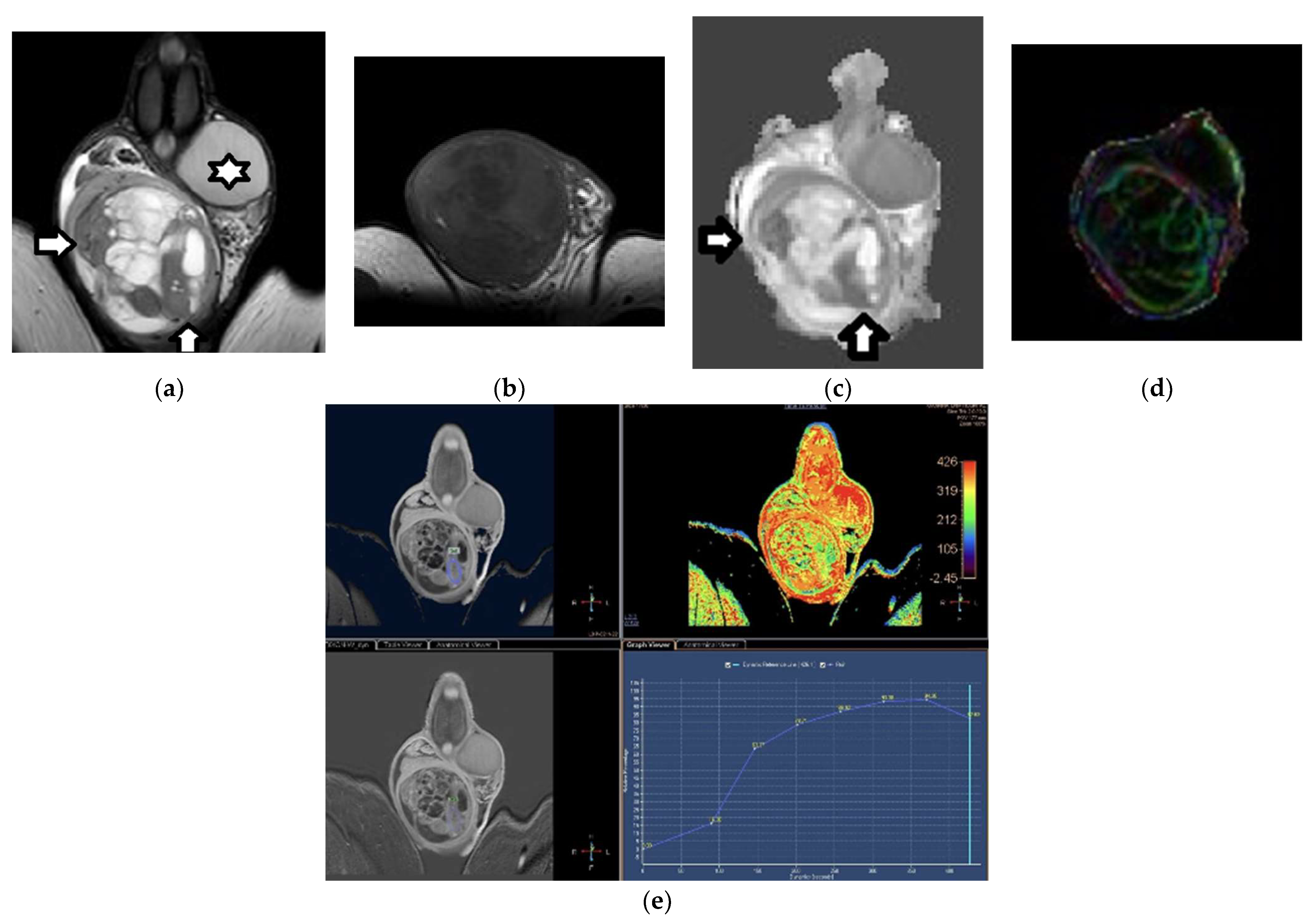
2.3. Dynamic Contrast-Enhanced MRI
2.4. New Advances
2.4.1. Diffusion Tensor Imaging
2.4.2. Magnetization Transfer Imaging
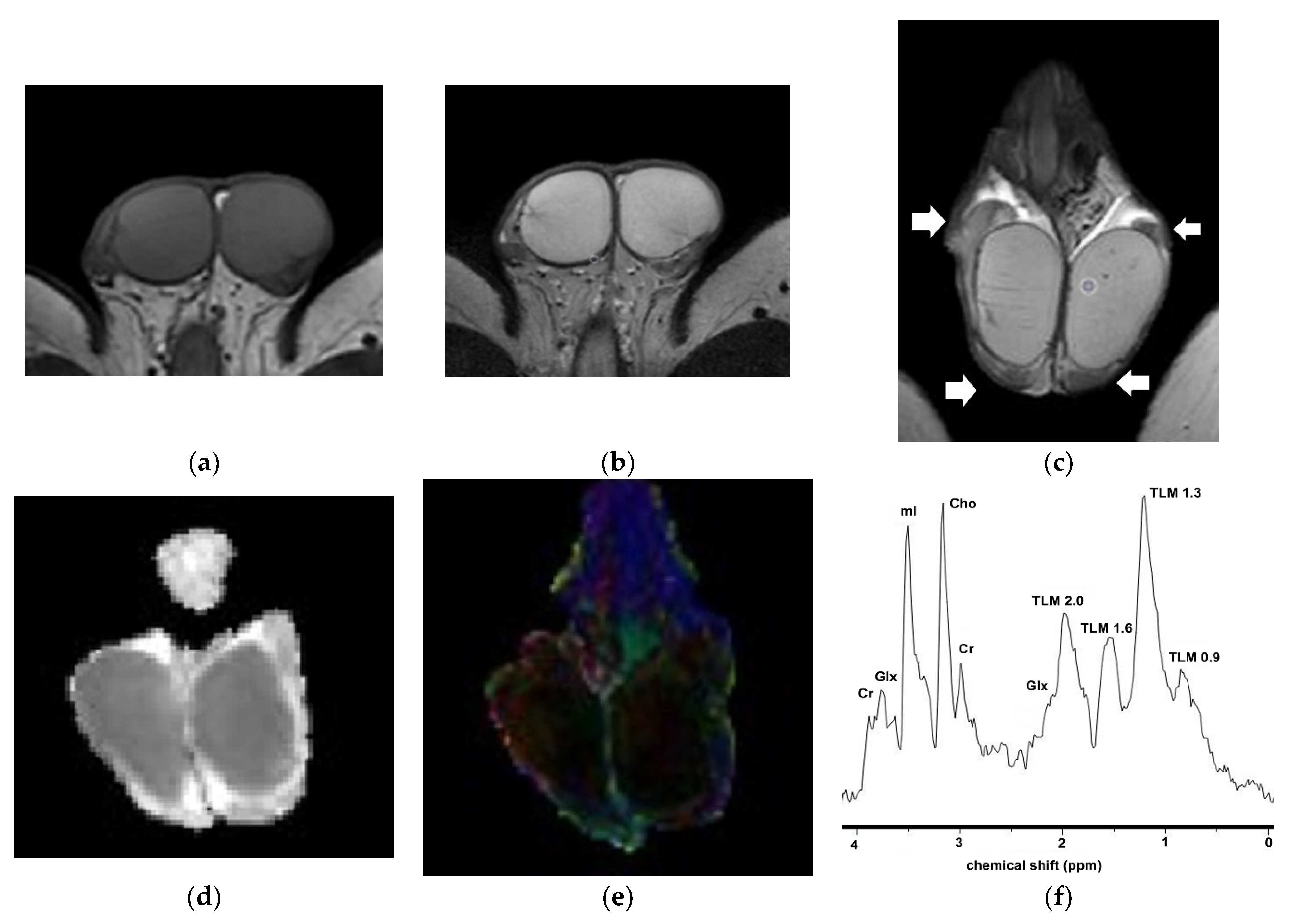
2.4.3. Proton Magnetic Resonance Spectroscopy
2.4.4. Volumetric ADC Histogram Analysis
2.4.5. MRI-Based Radiomics
3. Characterization of Testicular Masses: Benign versus Malignant
3.1. MRI Findings of TGCTs
3.2. Epidermoid Cysts
3.3. Testicular Lymphoma
4. Characterization of Testicular Tumors: Germ-Cell versus Sex-Cord Stromal Testicular Tumors
Sertoli-Cell Tumors
5. Histological Characterization of TGCTs
6. Local Staging of TGCTs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| US | Ultrasonography |
| SPIWG | Scrotal and Penile Imaging Working Group |
| TGCTs | Testicular germ-cell tumors |
| GCNIS | Germ-cell neoplasia in situ |
| NSGCTs | Nonseminomatous germ-cell tumors |
| BOT | Burned-out tumor |
| LCT | Leydig-cell tumor |
| SCT | Sertoli-cell tumor |
| mpMRI | Multiparametric MRI |
| T1WI | T1-weighted imaging |
| T2WI | T2-weighted imaging |
| DWI | Diffusion-weighted imaging |
| DCE | Dynamic contrast-enhanced |
| DTI | Diffusion tensor imaging |
| MTI | Magnetization transfer imaging |
| 1H-MRS | Proton MR spectroscopy |
| ADC | Apparent diffusion coefficient |
| ROI | Region of interest |
| TSI | Time–signal intensity |
| FA | Fractional anisotropy |
| MTR | Magnetization transfer ratio |
| VOI | Volume of interest |
| TSS | Testis-sparing surgery |
| EC | Epidermoid cyst |
| FSE | Frozen section examination |
| pT | Primary tumor |
References
- Dogra, V.S.; Gottlieb, R.H.; Oka, M.; Rubens, D.J. Sonography of the scrotum. Radiology 2003, 227, 18–36. [Google Scholar] [CrossRef]
- Tsili, A.C.; Bougia, C.K.; Pappa, O.; Argyropoulou, M.I. Ultrasonography of the scrotum: Revisiting a classic technique. Eur. J. Radiol. 2021, 145, 110000. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, V.; Marcher, C. Overview of Ultrasound Applications for Assessing Scrotal Disorders. J. Ultrasound Med. 2020, 39, 1047–1056. [Google Scholar] [CrossRef]
- Rebik, K.; Wagner, J.M.; Middleton, W. Scrotal Ultrasound. Radiol. Clin. N. Am. 2019, 57, 635–648. [Google Scholar] [CrossRef]
- Kühn, A.L.; Scortegagna, E.; Nowitzki, K.M.; Kim, Y.H. Ultrasonography of the Scrotum in Adults. Ultrasonography 2016, 35, 180–197. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, L.; Gaitini, D.; Dogra, V.S. Scrotal ultrasound in adults. Semin. Ultrasound CT MRI 2013, 34, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Sharbidre, K.G.; Lockhart, M.E. Imaging of scrotal masses. Abdom. Radiol. 2020, 45, 2087–2108. [Google Scholar] [CrossRef] [PubMed]
- Laguna, M.P.; Albers, P.; Algaba, F.; Bokemeyer, C.; Boormans, J.L.; di Nardo, D.; Fischer, S.; Fizazi, K.; Gremmels, H.; Leão, R.; et al. EAU guidelines on Testicular Cancer. Eur. Assoc. Urol. 2022. Available online: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Testicular-Cancer-2021-v1-1.pdf (accessed on 15 June 2022). [CrossRef]
- Woodward, P.J.; Sohaey, R.; O’Donoghue, M.J.; Green, D.E. From the archives of the AFIP: Tumors and tumor like lesions of the testis: Radiologic-pathologic correlation. Radiographics 2002, 22, 189–216. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, 2–44. [Google Scholar]
- Tenuta, M.; Sesti, F.; Bonaventura, I.; Mazzotta, P.; Pofi, R.; Gianfrilli, D.; Pozza, C. Use of contrast enhanced ultrasound in testicular diseases: A comprehensive review. Andrology 2021, 21, 1369–1382. [Google Scholar] [CrossRef]
- Cantisani, V.; DiLeo, N.; Bertolotto, M.; Fresilli, D.; Granata, A.; Polti, G.; Polito, E.; Pacini, P.; Guiban, O.; Del Gaudio, G.; et al. Role of multiparametric ultrasound in testicular focal lesions and diffuse pathology evaluation, with particular regard to elastography: Review of literature. Andrology 2021, 11, 1356–1368. [Google Scholar] [CrossRef]
- Saftoiu, A.; Gilja, O.H.; Sidhu, P.S.; Săftoiu, A.; Dietrich, C.F.; Cantisani, V.; Amy, D.; Bachmann-Nielsen, M.; Bob, F.; Bojunga, J.; et al. The EFSUMB guidelines and recommendations for the clinical practice of elastography in non-hepatic applications: Update 2018. Ultraschall Med. 2019, 40, 425–453. [Google Scholar] [CrossRef]
- Tsili, A.C.; Bertolotto, M.; Turgut, A.T.; Dogra, V.; Freeman, S.; Rocher, L.; Belfield, J.; Studniarek, M.; Ntorkou, A.; Derchi, L.E.; et al. MRI of the scrotum: Recommendations of the ESUR Scrotal and Penile Imaging Working Group. Eur. Radiol. 2018, 28, 31–43. [Google Scholar] [CrossRef]
- Wibmer, A.G.; Vargas, H.A. Imaging of Testicular and Scrotal Masses: The Essentials. In Diseases of the Abdomen and Pelvis 2018–2021 Diagnostic Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Cham, Switzerland, 2018; pp. 257–264. [Google Scholar]
- Al Ghamdi, M.; Al Yami, M.; Faqeeh, S.; Al Kubeyyer, B.; Al Shabyli, N.; Al Ayed, A. Beyond germ cell tumors, unusual testicular and extra-testicular masses and mass-like lesions: MRI and US pictorial review. Clin. Imaging 2021, 74, 106–122. [Google Scholar] [CrossRef]
- Tsili, A.C.; Argyropoulou, M.I.; Dolciami, M.; Ercolani, G.; Catalano, C.; Manganaro, L. When to ask for an MRI of the scrotum. Andrology 2021, 5, 1395–1409. [Google Scholar] [CrossRef]
- Patel, H.D.; Ramos, M.; Gupta, M.; Cheaib, J.G.; Sharma, R.; Zhang, A.; Bass, E.B.; Pierorazio, P.M. Magnetic resonance imaging to differentiate the histology of testicular masses: A systematic review of studies with pathologic confirmation. Urology 2020, 135, 4–10. [Google Scholar] [CrossRef]
- Marko, J.; Wolfman, D.J.; Aubin, A.L.; Sesterhenn, I.A. Testicular seminoma and its mimics: From the radiologic pathology archives. Radiographics 2017, 37, 1085–1098. [Google Scholar] [CrossRef]
- Liu, R.; Lei, Z.; Li, A.; Jiang, Y.; Ji, J. Differentiation of testicular seminoma and nonseminomatous germ cell tumor on magnetic resonance imaging. Medicine 2019, 98, 17937. [Google Scholar] [CrossRef]
- Mittal, P.K.; Abdalla, A.S.; Chatterjee, A.; Baumgarten, D.A.; Harri, P.A.; Patel, J.; Moreno, C.C.; Gabriel, H.; Miller, F.H. Spectrum of Extratesticular and Testicular Pathologic Conditions at Scrotal MR Imaging. Radiographics 2018, 38, 806–830. [Google Scholar] [CrossRef]
- Mathur, M.; Mills, I.; Spektor, M. Magnetic resonance imaging of the scrotum: Pictorial review with ultrasound correlation. Abdom. Radiol. 2017, 42, 1929–1955. [Google Scholar] [CrossRef] [PubMed]
- Coursey Moreno, C.; Small, W.C.; Camacho, J.C.; Master, V.; Kokabi, N.; Lewis, M.; Hartman, M.; Mittal, P.K. Testicular Tumors: What Radiologists Need to Know-Differential Diagnosis, Staging, and Management. RadioGraphics 2015, 35, 400–415. [Google Scholar] [CrossRef] [PubMed]
- Tsili, A.C.; Sofikitis, N.; Stiliara, E.; Argyropoulou, M.I. MRI of testicular malignancies. Abdom. Radiol. 2019, 44, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Parenti, G.C.; Feletti, F.; Carnevale, A.; Uccelli, L.; Giganti, M. Imaging of the scrotum: Beyond sonography. Insights Imaging 2018, 9, 137–148. [Google Scholar] [CrossRef]
- Tsili, A.C.; Bertolotto, M.; Rocher, L.; Turgut, A.T.; Dogra, V.; Seçil, M.; Freeman, S.; Belfield, J.; Studniarek, M.; Ntorkou, L.; et al. Sonographically indeterminate scrotal masses: How MRI helps in characterization. Diagn. Interv. Radiol. 2018, 24, 225–236. [Google Scholar] [CrossRef]
- Aganovic, L.; Cassidy, F. Imaging of the scrotum. Radiol. Clin. N. Am. 2012, 50, 1145–1165. [Google Scholar] [CrossRef]
- Cassidy, F.H.; Ishioka, K.M.; McMahon, C.J. MR imaging of scrotal tumours and pseudotumours. Radiographics 2010, 30, 665–683. [Google Scholar] [CrossRef]
- Tsili, A.C.; Giannakis, D.; Sylakos, A.; Ntorkou, A.; Sofikitis, N.; Argyropoulou, M.I. MR imaging of scrotum. Magn. Reson. Imaging Clin. N. Am. 2014, 22, 217–238. [Google Scholar] [CrossRef]
- Woldrich, J.M.; Im, R.D.; Hughes-Cassidy, F.M.; Aganovic, L.; Sakamoto, K. Magnetic resonance imaging for intratesticular and extratesticular scrotal lesions. Can. J. Urol. 2013, 20, 6855–6859. [Google Scholar]
- Park, S.B.; Lee, W.C.; Kim, J.K.; Choi, S.H.; Kang, B.S.; Moon, K.H.; Kim, Y.M.; Jeong, Y.K. Imaging features of benign solid testicular and paratesticular lesions. Eur. Radiol. 2011, 21, 2226–2234. [Google Scholar] [CrossRef]
- Mohrs, O.K.; Thoms, H.; Egner, T.; Brunier, A.; Eiers, M.; Kauczor, H.U.; Hallscheidt, P. MRI of patients with suspected scrotal or testicular lesions: Diagnostic value in daily practice. AJR Am. J. Roentgenol. 2012, 199, 609–615. [Google Scholar] [CrossRef]
- Gupta, R.; Alobaidi, M.; Jafri, S.Z.; Bis, K.; Amendola, M. Correlation of US and MRI Findings of Intratesticular and Paratesticular Lesions: From Infants to Adults. Curr. Prob. Diagn. Radiol. 2005, 34, 35–45. [Google Scholar] [CrossRef]
- Kim, W.; Rosen, M.A.; Langer, J.E.; Banner, M.P.; Siegelman, E.S.; Ramchandani, P. US MR imaging correlation in pathologic conditions of the scrotum. Radiographics 2007, 27, 1239–1253. [Google Scholar] [CrossRef]
- Andipa, E.; Liberopoulos, K.; Asvestis, C. Magnetic resonance imaging and ultrasound evaluation of penile and testicular masses. World J. Urol. 2004, 22, 382–391. [Google Scholar] [CrossRef]
- Serra, A.D.; Hricak, H.; Coakley, F.V.; Kim, B.; Dudley, A.; Morey, A.; Tschumper, B.; Carroll, P.R. Inconclusive clinical and ultrasound evaluation of the scrotum: Impact of magnetic resonance imaging on patient management and cost. Urology 1998, 51, 1018–1021. [Google Scholar] [CrossRef]
- Muglia, V.; Tucci, S., Jr.; Elias, J., Jr.; Trad, C.S.; Bilbey, I.; Cooperberg, P.L. Magnetic resonance imaging of scrotal diseases: When it makes the difference. Urology 2002, 59, 419–423. [Google Scholar] [CrossRef]
- Sica, G.T.; Teeger, S. MR imaging of scrotal, testicular, and penile diseases. Magn. Reson. Imaging Clin. N. Am. 1996, 4, 545–563. [Google Scholar] [CrossRef]
- Williamson, S.R.; Delahunt, B.; Magi-Galluzzi, C.; Algaba, F.; Egevad, L.; Ulbright, T.M.; Tickoo, S.K.; Srigley, J.R.; Epstein, J.I.; Berney, D.M. Members of the ISUP Testicular Tumour Panel. The World Health Organization, Classification of Testicular Germ Cell Tumours: A Review and Update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology 2017, 70, 335–346. [Google Scholar] [CrossRef]
- Idrees, M.T.; Ulbright, T.M.; Oliva, E.; Young, R.H.; Montironi, R.; Egevad, L.; Berney, D.; Srigley, J.R.; Epstein, J.I.; Tickoo, S.K.; et al. The World Health Organization 2016 classification of testicular non-germcell tumours:a review and update fromthe International Society of Urological Pathology Testis Consultation Panel. Histopathology 2017, 70, 513–521. [Google Scholar] [CrossRef]
- Zicherman, J.M.; Weissman, D.; Gribbin, C.; Epstein, R. Best cases from the AFIP: Primary diffuse large B-cell lymphoma of the epididymis and testis. Radiographics 2005, 25, 243–248. [Google Scholar] [CrossRef]
- Tsili, A.C.; Ntorkou, A.; Astrakas, L.G.; Boukali, E.; Giannakis, D.; Maliakas, V.; Sofikitis, N.; Argyropoulou, M.I. Magnetic resonance diffusion tensor imaging of the testis: Preliminary observations. Eur. J. Radiol. 2017, 95, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Tsili, A.C.; Ntorkou, A.; Baltogiannis, D.; Sylakos, A.; Stavrou, S.; Astrakas, L.; Maliakas, V.; Sofikitis, N.; Argyropoulou, M.I. Magnetization transfer imaging of normal and abnormal testis: Preliminary results. Eur. Radiol. 2016, 26, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Baleato-González, S.; García-Figueiras, R.; Santiago-Pérez, M.I.; Requejo-Isidro, I.; Vilanova, J.C. Usefulness of 1H magnetic resonance spectroscopy in human testes: Preliminary study. Clin. Radiol. 2015, 70, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Min, X.; Feng, Z.; Cai, W.; Li, B.; Zhang, P.; You, H.; Xie, J.; Wang, L. Discrimination between benign and malignant testicular lesions using volumetric apparent diffusion coefficient histogram analysis. Eur. J. Radiol. 2020, 126, 108939. [Google Scholar] [CrossRef]
- Min, X.; Feng, Z.; Wang, L.; Cai, J.; Yan, X.; Li, B.; Ke, Z.; Zhang, P.; You, H. Characterization of testicular germ cell tumors: Whole-lesion histogram analysis of the apparent diffusion coefficient at 3T. Eur. J. Radiol. 2018, 98, 25–31. [Google Scholar] [CrossRef]
- Zhang, P.; Min, X.; Feng, Z.; Kang, Z.; Li, B.; Cai, W.; Fan, C.; Yin, X.; Xie, J.; Lv, W.; et al. Value of Intra-Perinodular Textural Transition Features from MRI in Distinguishing between Benign and Malignant Testicular Lesions. Cancer Manag. Res. 2021, 13, 839–847. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, Z.; Cai, W.; You, H.; Fan, C.; Lv, W.; Min, X.; Wang, L. T2-weighted image-based radiomics signature for discriminating between seminomas and nonseminoma. Front. Oncol. 2019, 9, 1330. [Google Scholar] [CrossRef]
- Feliciani, G.; Mellini, L.; Carnevale, A.; Sarnelli, A.; Menghi, E.; Piccinini, F.; Scarpi, E.; Loi, E.; Galeotti, R.; Giganti, M.; et al. The potential role of MR based radiomic biomarkers in the characterization of focal testicular lesions. Sci. Rep. 2021, 11, 3456. [Google Scholar] [CrossRef]
- Tsili, A.C.; Argyropoulou, M.I.; Giannakis, D.; Tsampalas, S.; Sofikitis, N.; Tsampoulas, K. Diffusion-weighted MR imaging of normal and abnormal scrotum: Preliminary results. Asian J. Androl. 2012, 14, 649–654. [Google Scholar] [CrossRef]
- Tsili, A.C.; Giannakis, D.; Sylakos, A.; Ntorkou, A.; Astrakas, L.G.; Sofikitis, N.; Argyropoulou, M.I. Apparent diffusion coefficient values of normal testis and variations with age. Asian J. Androl. 2014, 16, 493–497. [Google Scholar] [CrossRef]
- Algebally, A.M.; Tantawy, H.I.; Yousef, R.R.; Szmigielski, W.; Darweesh, A. Advantage of Adding Diffusion Weighted Imaging to Routine MRI Examinations in the Diagnostics of Scrotal Lesions. Pol. J. Radiol. 2015, 80, 442–449. [Google Scholar] [CrossRef]
- Sonmez, G.; Sivrioglu, A.K.; Velioglu, M.; Incedayi, M.; Soydan, H.; Kara, K.; Ateş, F.; Saglam, M. Optimized imaging techniques for testicular masses: Fast and with high accuracy. Wien. Klin. Wochenschr. 2012, 124, 704–708. [Google Scholar] [CrossRef]
- Tsili, A.C.; Ntorkou, A.; Baltogiannis, D.; Goussia, A.; Astrakas, L.G.; Malamou-Mitsi, V.; Sofikitis, N.; Argyropoulou, M.I. The role of apparent diffusion coefficient values in detecting testicular intraepithelial neoplasia: Preliminary results. Eur. J. Radiol. 2015, 84, 828–833. [Google Scholar] [CrossRef]
- Pedersen, M.R.; Sloth Osther, P.J.; Nissen, H.D.; Vedsted, P.; Møller, H.; Rafaelsen, S. Elastography and diffusion-weighted MRI in patients with testicular microlithiasis, normal testicular tissue, and testicular cancer: An observational study. Acta Radiol. 2019, 60, 535–541. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Z.; Chen, Y.; Zhao, F.; Yu, H.; Guo, X.; Shi, K. Testicular tumors: Discriminative value of conventional MRI and diffusion weighted imaging. Medicine 2021, 100, e27799. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Jiang, Y.; Wu, Z.; Ji, J.; Li, A.; Wang, X.; Li, R. The utility of diffusion-weighted imaging and ADC values in the characterization of mumps orchitis and seminoma. Acta Radiol. 2022, 63, 416–423. [Google Scholar] [CrossRef]
- Essig, M.; Shiroishi, M.S.; Nguyen, T.B.; Saake, M.; Provenzale, J.M.; Enterline, D.; Anzalone, N.; Dörfler, A.; Rovira, A.; Wintermark, M.; et al. Perfusion MRI: The five most frequently asked technical questions. AJR Am. J. Roentgenol. 2013, 200, 24–34. [Google Scholar] [CrossRef]
- Reinges, M.H.; Kaiser, W.A.; Miersch, W.D.; Vogel, J.; Reiser, M. Dynamic MRI of benign and malignant testicular lesions: Preliminary observations. Eur. Radiol. 1995, 5, 615–622. [Google Scholar] [CrossRef]
- Reinges, M.H.; Kaiser, W.A.; Miersch, W.D.; Vogel, J. Dynamic magnetic resonance imaging of the contralateral testis in patients with malignant tumor of the testis. Urology 1994, 44, 540–547. [Google Scholar] [CrossRef]
- Watanabe, Y.; Dohke, M.; Ohkubo, K.; Ishimori, T.; Amoh, Y.; Okumura, A.; Oda, K.; Hayashi, T.; Dodo, Y.; Arai, Y. Scrotal disorders: Evaluation of testicular enhancement patterns at dynamic contrast-enhanced subtraction MR imaging. Radiology 2000, 217, 219–227. [Google Scholar] [CrossRef]
- Tsili, A.C.; Argyropoulou, M.I.; Astrakas, L.G.; Ntoulia, E.A.; Giannakis, D.; Sofikitis, N.; Tsampoulas, K. Dynamic contrast-enhanced subtraction MRI for characterizing intratesticular mass lesions. AJR Am. J. Roentgenol. 2013, 200, 578–585. [Google Scholar] [CrossRef]
- El Sanharawi, I.E.; Correas, J.M.; Glas, L.; Ferlicot, S.; Izard, V.; Ducot, B.; Bellin, M.F.; Benoît, G.; Rocher, L. Non-palpable incidentally found testicular tumors: Differentiation between benign, malignant, and burned-out tumors using dynamic contrast-enhanced MRI. Eur. J. Radiol. 2016, 85, 2072–2082. [Google Scholar] [CrossRef]
- Tsili, A.C.; Sylakos, A.; Ntorkou, A.; Stavrou, S.; Astrakas, L.G.; Sofikitis, N.; Argyropoulou, M.I. Apparent diffusion coefficient values and dynamic contrast enhancement patterns in differentiating seminomas from nonseminomatous testicular neoplasms. Eur. J. Radiol. 2015, 84, 1219–1226. [Google Scholar] [CrossRef]
- Choyke, P.L. Dynamic contrast-enhanced MR imaging of the scrotum: Reality check. Radiology 2000, 217, 14–15. [Google Scholar] [CrossRef]
- Huston, J.M.; Field, A.S. Clinical applications of diffusion tensor imaging. Magn. Reson. Imaging Clin. N. Am. 2013, 21, 279–298. [Google Scholar] [CrossRef]
- Lanzman, R.S.; Wittsack, H.J. Diffusion tensor imaging in abdominal organs. NMR Biomed. 2017, 30, 3. [Google Scholar] [CrossRef]
- Sled, J.G. Modelling and interpretation of magnetization transfer imaging in the brain. Neuroimage 2018, 182, 128–135. [Google Scholar] [CrossRef]
- Rapalino, O.; Ratai, E.M. Multiparametric Imaging Analysis: Magnetic Resonance Spectroscopy. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 671–686. [Google Scholar] [CrossRef]
- Martín Noguerol, T.; Sánchez-González, J.; Martínez Barbero, J.P.; García-Figueiras, R.; Baleato-González, S.; Luna, A. Clinical Imaging of Tumor Metabolism with ¹H Magnetic Resonance Spectroscopy. Magn. Reson. Imaging Clin. N. Am. 2016, 1, 57–86. [Google Scholar] [CrossRef]
- Tsili, A.C.; Astrakas, L.G.; Ntorkou, A.; Giannakis, D.; Stavrou, S.; Maliakas, V.; Sofikitis, N.; Argyropoulou, M.I. MR spectra of normal adult testes and variations with age: Preliminary observations. Eur. Radiol. 2016, 7, 2261–2267. [Google Scholar] [CrossRef]
- Just, N. Improving tumour heterogeneity MRI assessment with histograms. Br. J. Cancer 2014, 111, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Chong, I.; Ostrom, Q.; Khan, B.; Dandachi, D.; Garg, N.; Kotrotsou, A.; Colen, R.; Morón, F. Whole Tumor Histogram Analysis Using DW MRI in Primary Central Nervous System Lymphoma Correlates with Tumor Biomarkers and Outcome. Cancers 2019, 11, 1506. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Santinha, J.; Galvão, B.; Matos, C.; Couto, F.M.; Papanikolaou, N. Prediction of Prostate Cancer Disease Aggressiveness Using Bi-Parametric Mri Radiomics. Cancers 2021, 13, 6065. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Ottaiano, A.; Nasti, G.; Grassi, R.; Pilone, V.; et al. EOB—MR Based Radiomics Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1239. [Google Scholar] [CrossRef]
- Tsili, A.C.; Argyropoulou, M.I.; Giannakis, D.; Sofikitis, N.; Tsampoulas, K. MRI in the characterization and local staging of testicular neoplasms. AJR Am. J. Roentgenol. 2010, 194, 682–689. [Google Scholar] [CrossRef]
- Tsili, A.C.; Tsampoulas, C.; Giannakopoulos, X.; Stefanou, D.; Alamanos., Y.; Sofikitis., N.; Efremidis., S.C. MRI in the histologic characterization of testicular neoplasms. AJR Am. J. Roentgenol. 2007, 189, 331–337. [Google Scholar] [CrossRef]
- Johnson, J.O.; Mattrey, R.F.; Phillipson, J.; Johnson, J.O.; Mattrey, R.F.; Phillipson, J. Differentiation of seminomatous from nonseminomatous testicular tumors with MR imaging. AJR Am. J. Roentgenol. 1990, 154, 539–543. [Google Scholar] [CrossRef]
- Cho, J.H.; Chang, J.C.; Park, B.H.; Lee, J.G.; Son, C.H. Sonographic and MR imaging findings of testicular epidermoid cysts. AJR Am. J. Roentgenol. 2002, 178, 743–748. [Google Scholar] [CrossRef]
- Langer, J.E.; Ramchandani, P.; Siegelman, E.S.; Banner, M.P. Epidermoid cysts of the testicle: Sonographic and MR imaging features. AJR. Am. J. Roentgenol. 1999, 173, 1295–1299. [Google Scholar] [CrossRef]
- Anheuser, P.; Kranz, J.; Stolle, E.; Höflmayer, D.; Büscheck, F.; Mühlstädt, S.; Lock, G.; Dieckmann, K.P. Testicular epidermoid cysts: A reevaluation. BMC Urol. 2019, 19, 52. [Google Scholar] [CrossRef]
- Liu, R.; Lei, Z.; Chen, N.; Fang, J.; Zhang, Y. Imaging in testicular epidermoid cysts. Clin. Imaging 2018, 50, 211–215. [Google Scholar] [CrossRef]
- Taupin, T.; Rouvière, O.; Cuzin, B.; Mege-Lechevallier, F.; Boutier, R. Epidermoid cyst. Answer to the e-quid “A testicular mass”. Diagn. Interv. Imaging 2013, 94, 667–671. [Google Scholar] [CrossRef][Green Version]
- Tsili, A.C.; Argyropoulou, M.I.; Giannakis, D.; Sofikitis, N.; Tsampoulas, K. Primary diffuse large B-cell testicular lymphoma: Magnetic resonance imaging findings. Andrologia 2012, 44, 845–847. [Google Scholar] [CrossRef]
- Liu, K.L.; Chang, C.C.; Huang, K.H.; Tsang, Y.M.; Chen, S.J. Imaging diagnosis of testicular lymphoma. Abdom. Imaging 2006, 31, 610–612. [Google Scholar] [CrossRef]
- Rocher, L.; Ramchandani, P.; Belfield, J.; Bertolotto, M.; Derchi, L.E.; Correas, J.M.; Oyen, R.; Tsili, A.C.; Turgut, A.T.; Dogra, V.; et al. Incidentally detected non-palpable testicular tumours in adults at scrotal ultrasound: Impact of radiological findings on management Radiologic review and recommendations of the ESUR scrotal imaging subcommittee. Eur. Radiol. 2016, 26, 2268–2278. [Google Scholar] [CrossRef]
- Pozza, C.; Pofi, R.; Tenuta, M.; Tarsitano, M.G.; Sbardella, E.; Fattorini, G.; Cantisani, V.; Lenzi, A.; Isidori, A.M.; Gianfrilli, D. TESTIS UNIT. Clinical presentation, management and follow-up of 83 patients with Leydig cell tumors of the testis: A prospective case-cohort study. Hum. Reprod. 2019, 34, 1389–1403. [Google Scholar] [CrossRef]
- Tsitouridis, I.; Maskalidis, C.; Panagiotidou, D.; Kariki, E.P. Eleven patients with testicular Leydig cell tumors: Clinical, imaging, and pathologic correlation. J. Ultrasound Med. 2014, 33, 1855–1864. [Google Scholar] [CrossRef]
- Manganaro, L.; Vinci, V.; Pozza, C.; Saldari, M.; Gianfrilli, D.; Pofi, R.; Bernardo, S.; Cantisani, V.; Lenzi, A.; Scialpi, M.; et al. A prospective study on contrast-enhanced magnetic resonance imaging of testicular lesions: Distinctive features of Leydig cell tumours. Eur. Radiol. 2015, 25, 3586–3595. [Google Scholar] [CrossRef]
- Manganaro, L.; Saldari, M.; Pozza, C.; Vinci, V.; Gianfrilli, D.; Greco, E.; Franco, G.; Sergi, M.E.; Scialpi, M.; Catalano, C.; et al. Dynamic contrast-enhanced and diffusion-weighted MR imaging in the characterisation of small, non-palpable solid testicular tumours. Eur. Radiol. 2018, 28, 554–564. [Google Scholar] [CrossRef]
- Khanna, M.; Abualruz, A.R.; Yadav, S.K.; Mafraji, M.; Al-Rumaihi, K.; Al-Bozom, I.; Kumar, D.; Tsili, A.C.; Schieda, N. Diagnostic performance of multi-parametric MRI to differentiate benign sex cord stromal tumors from malignant (non-stromal and stromal) testicular neoplasms. Abdom. Radiol. 2021, 46, 319–330. [Google Scholar] [CrossRef]
- Rocher, L.; Glas, L.; Bellin, M.F.; Ferlicot, S.; Izard, V.; Benoit, G.; Albiges, L.; Fizazi, K.; Correas, J.M. Burned-out testis tumors in asymptomatic infertile men: Multiparametric sonography and MRI findings. J. Ultrasound Med. 2017, 36, 821–831. [Google Scholar] [CrossRef]
- Drevelengas, A.; Kalaitzoglou, I.; Destouni, E.; Skorsalaki, A.; Dimitriadis, A. Bilateral Sertoli cell tumor of the testis: MRI and sonographic appearance. Eur. Radiol. 1999, 9, 1934. [Google Scholar] [CrossRef]
- Loy, V.; Wigand, I.; Dieckmann, K.P. Incidence and distribution of carcinoma in situ in testes removed for germ cell tumour: Possible inadequacy of random testicular biopsy in detecting the condition. Histopathology 1990, 16, 198–200. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Skakkebaek, N.E. Carcinoma in situ of the testis: Review of biological and clinical features. Int. J. Cancer 1999, 83, 815–822. [Google Scholar] [CrossRef]
- Nason, G.J.; Aditya, I.; Leao, R.; Anson-Cartwright, L.; Jewett, M.A.S.; O’Malley, M.; Sweet, J.; Hamilton, R.J. Partial orchiectomy: The Princess Margaret cancer centre experience. Urol. Oncol. 2020, 38, 605.e19–605.e24. [Google Scholar] [CrossRef]
- Fankhauser, C.D.; Roth, L.; Kranzbühler, B.; Eberli, D.; Bode, P.; Moch, H.; Oliveira, P.; Ramani, V.; Beyer, J.; Hermanns, T. The Role of Frozen Section Examination During Inguinal Exploration in Men with Inconclusive Testicular Tumors: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2021, 7, 1400–1402. [Google Scholar] [CrossRef]
- Matei, D.V.; Vartolomei, M.D.; Renne, G.; Tringali, V.M.L.; Russo, A.; Bianchi, R.; Cozzi, G.; Bottero, D.; Musi, G.; Mazzarol, G.; et al. Reliability of Frozen Section Examination in a Large Cohort of Testicular Masses: What Did We Learn? Clin. Genitourin. Cancer 2017, 4, 689–696. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. The TNM Classification of Malignant Tumours 8th Edition 2016. Available online: https://www.uicc.org/resources/tnm-classification-malignant-tumours-8th-edition (accessed on 15 June 2022).
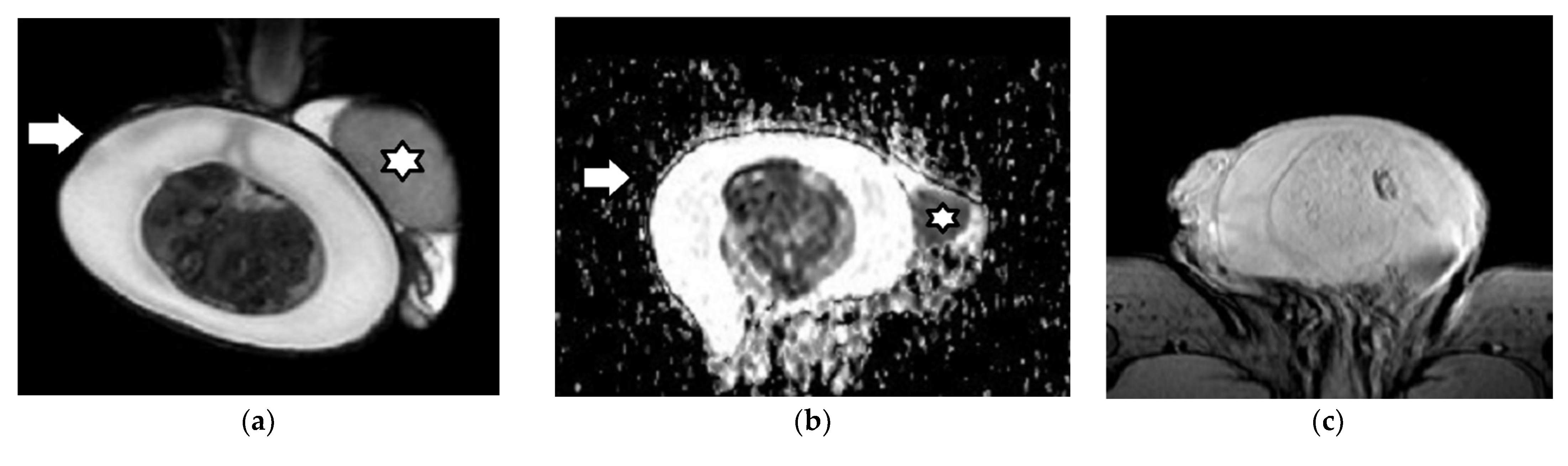
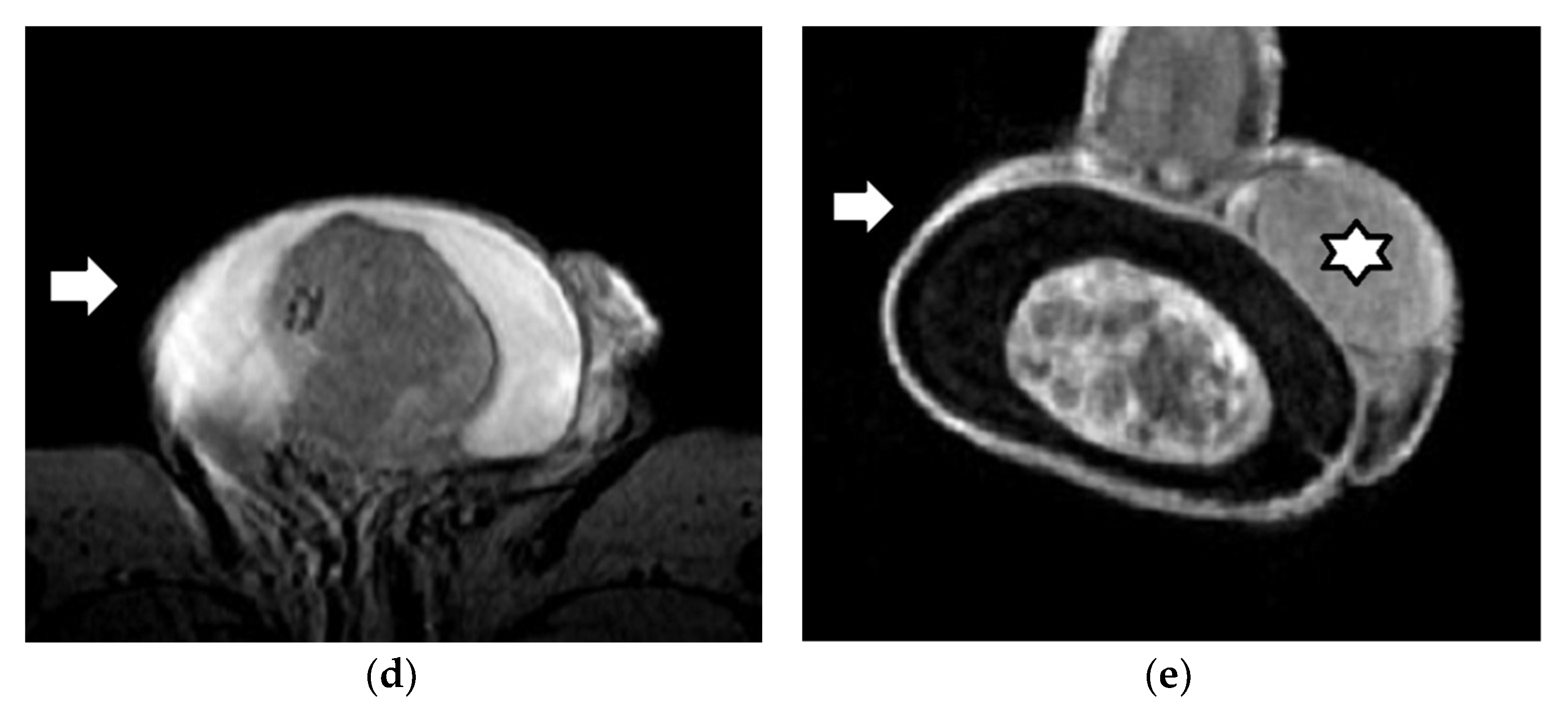
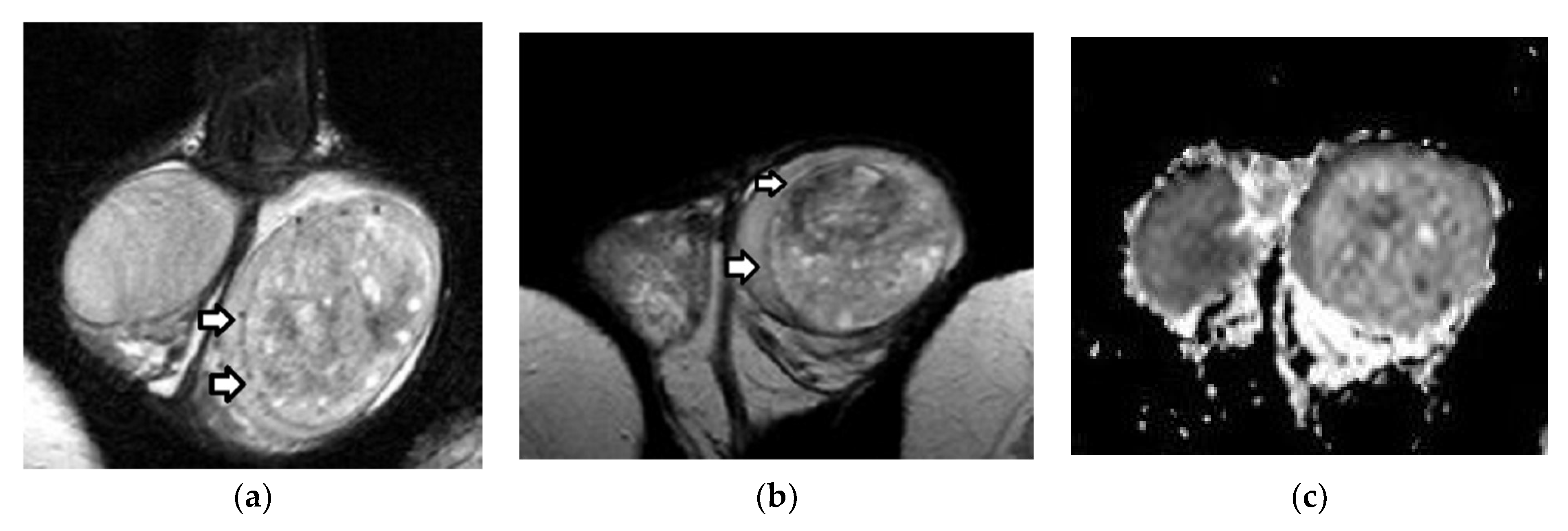
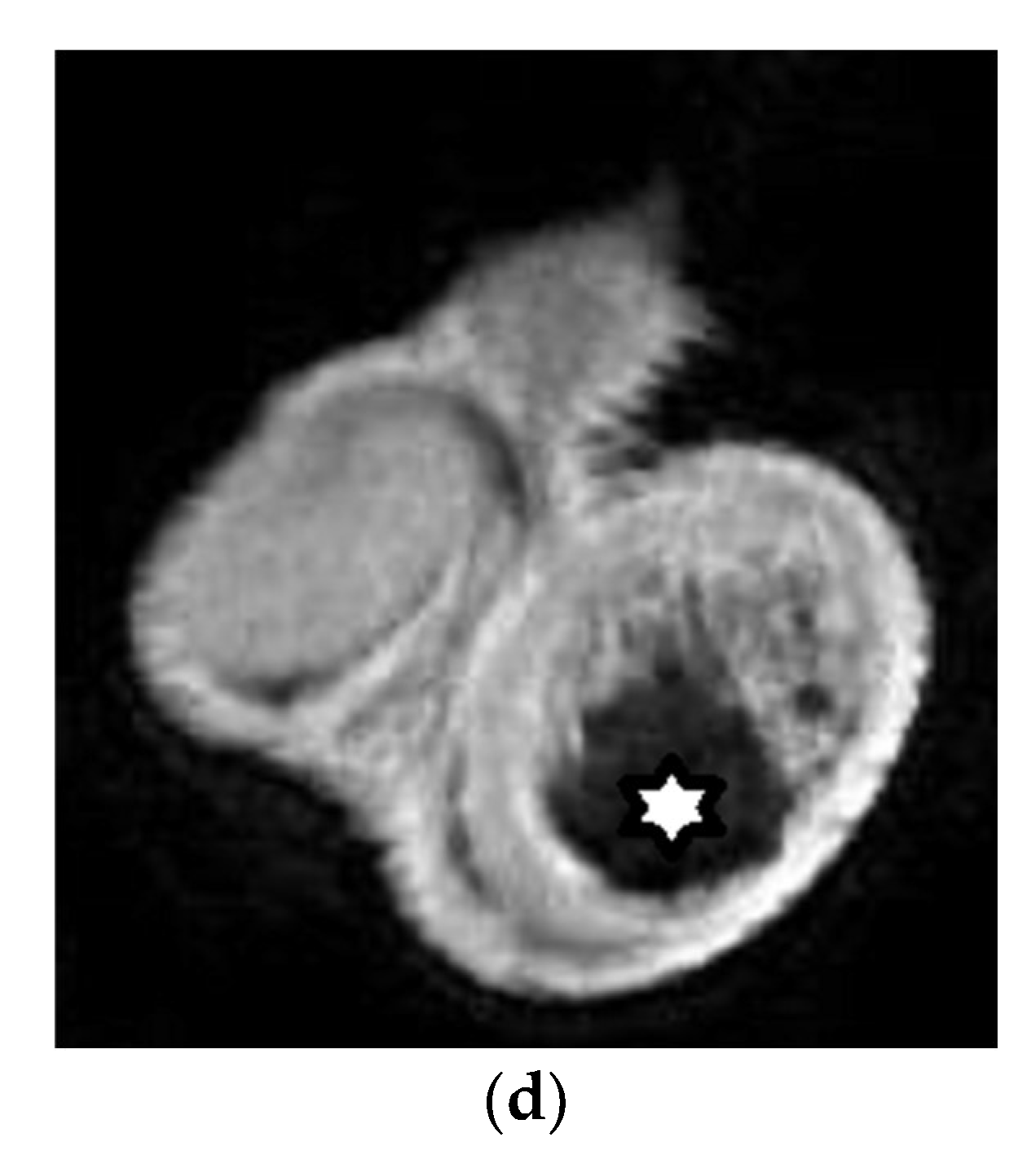
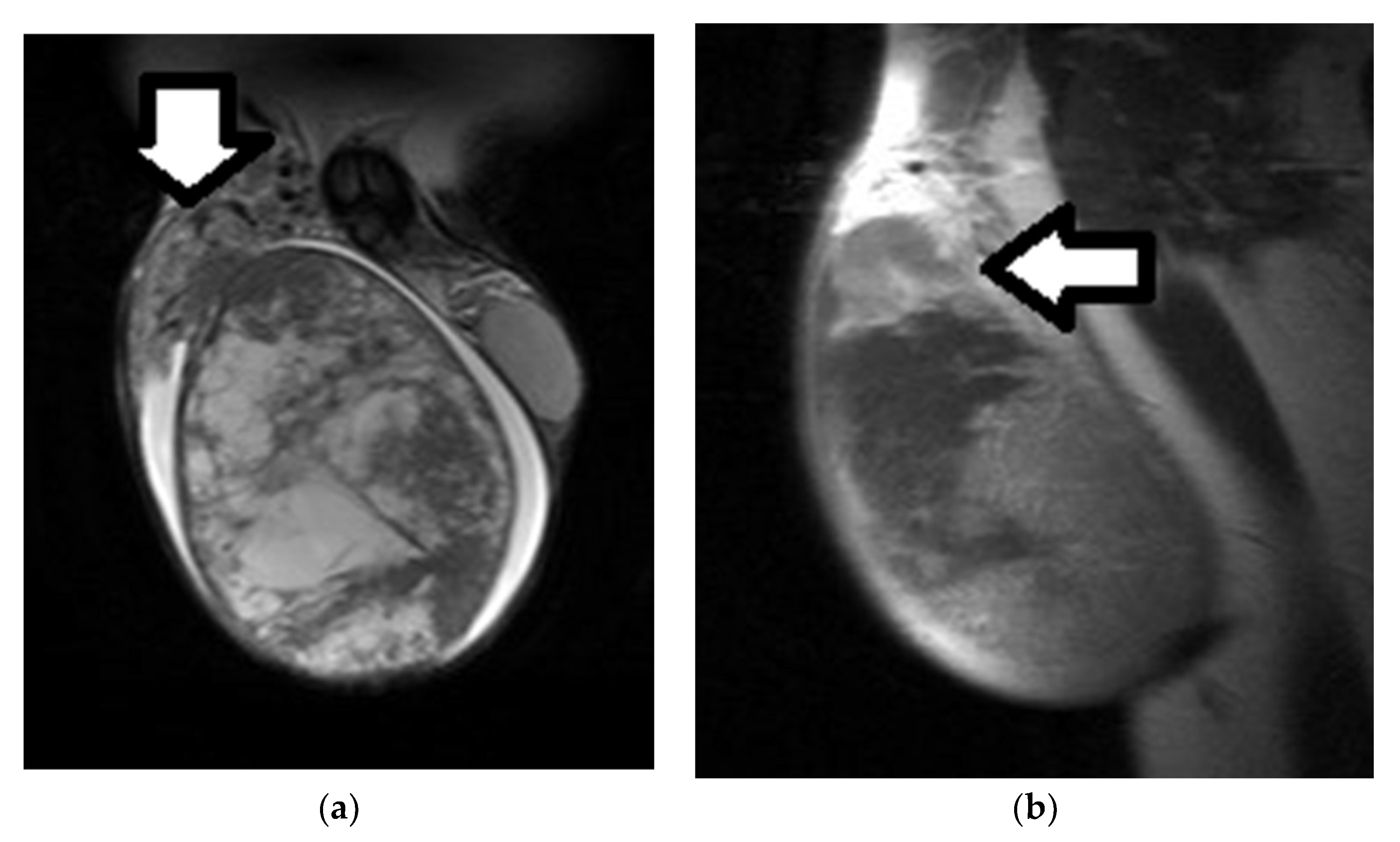
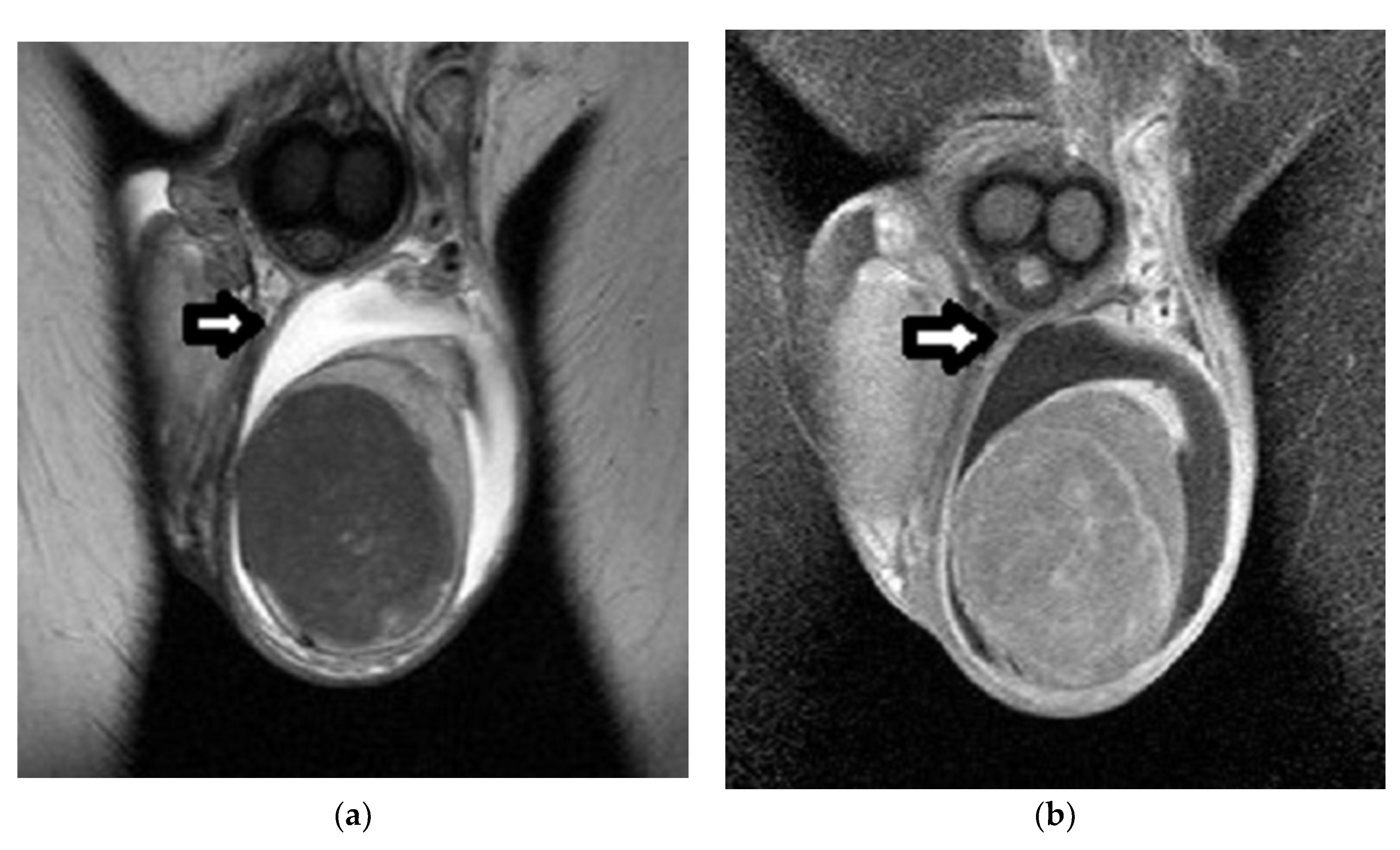

| First Author (Year) | Study Aims | MRI Scanner | b Values (s/mm2) | Main Findings | Study Limitations |
|---|---|---|---|---|---|
| Tsili et al. (2012) [50] | To evaluate the diagnostic performance of DWI in the characterization of scrotal lesions | 1.5 T | 0, 900 | Diagnostic performance of DWI alone: sensitivity, 85.7%; specificity 88.8%; PPV, 92.3%; NPV, 80%; accuracy, 87% Diagnostic accuracy of DWI + conventional imaging: 100% | Retrospective nature Small number of masses Consensus reading of MRI findings |
| Algebally et al. (2015) [52] | To assess the diagnostic value of adding DWI to conventional MRI in the characterization of scrotal lesions | 1.5 T | 400, 800 | Diagnostic accuracy of DWI + conventional imaging: 100% ADC cutoff ≤ 0.99 × 10−3 mm2/s in the characterization of intratesticular masses: sensitivity, 93.3%; specificity, 90%; PPV, 87.5%; NPV, 94.7% | Not all histologic types of intratesticular masses included |
| Somnez et al. (2012) [53] | To determine the effectiveness of DWI in the characterization of testicular masses | 1.5 T | 0, 500, 1000 | Diagnostic performance of lesion signal intensity on DWI: sensitivity, 90%; specificity, 60%; PPV, 81%, NPV, 75%; accuracy, 73% | Small number of masses |
| Wang et al. (2021) [56] | To assess the feasibility of conventional MRI features combined with ADC values for the differential diagnosis of testicular tumors | 3.0 T | 50, 1000 | Diagnostic performance of ADC: sensitivity, 70.6%; specificity, 71.7%; accuracy, 71.4% Diagnostic performance of ADC + conventional imaging: sensitivity, 94.1%; specificity, 89.1%; accuracy, 90.5% ADC < 0.90 × 10−3 mm2/s: diagnosis of malignancy | Retrospective nature Small number of masses Subjective assessment of conventional MRI findings |
| Liu et al. (2022) [57] | To characterize testicular lesions with low T2 signals using DWI (mumps orchitis versus seminoma) | 3.0 T | 0, 1000, 2000 | ADC cutoff for differentiating seminoma from mumps orchitis: 0.54 × 10−3 mm2/s Diagnosis of seminoma: sensitivity, 99%; specificity, 31% | Retrospective nature Small number of masses Limited histological types Low specificity of the cutoff value |
| First Author (Year) | Study Aims | MRI Scanner | Main Findings | Study Limitations |
|---|---|---|---|---|
| Reinges et al. (1995) [59] | To evaluate the possibility of a dynamic MRI technique for differentiating between benign and malignant testicular lesions | 1.5 T | Higher maximum increase in signal intensity after contrast injection in malignant tumors compared to normal testes and benign lesions | |
| Watanabe et al. (2000) [61] | To evaluate testicular enhancement patterns in various scrotal lesions on subtracted DCE-MRI | 1.5 T | TSI curve parameters (i.e., relative percentages of peak height and mean slope) helped in dividing testicular diseases into two groups: one group with no or decreased enhancement, and the other group with increased contrast enhancement, including malignant testicular tumors and acute mumps orchitis | |
| Tsili et al. (2012) [62] | To assess the value of DCE subtracted MRI in distinguishing between benign and malignant testicular lesions | 1.5 T | Strong association between the type of TSI curve and diagnosis: type I curve, 100% of normal testes; type II curve, 63.6% of benign intratesticular lesions; type III curve, 100% of malignant tumors; Relative maximum time to peak: independent predictor of malignancy | Retrospective nature Small number of masses Consensus reading of MRI findings |
| First Author (Year) | Study Aims | MRI Scanner | b Values (s/mm2) | Main Findings | Study Limitations |
|---|---|---|---|---|---|
| Tsili et al. (2012) [42] | To assess the efficacy of DTI in characterizing testicular pathology | 1.5 T | 0, 700 | Lower ADC in malignancies compared to normal testes and benign testicular lesions; Higher FA in both malignancies and benign testicular lesions compared to normal testes | Small number of cases Consensus reading of MRI findings |
| First Author (Year) | Study Aims | MRI Scanner | Main Findings | Study Limitations |
|---|---|---|---|---|
| Tsili et al. (2016) [43] | To assess the feasibility of MTR in characterizing various testicular lesions | 1.5 T | Higher MTR in testicular carcinomas compared to benign lesions and normal testes | Retrospective nature Small number of masses Consensus reading of MRI findings |
| First Author (Year) | Study Aims | MRI Scanner | b Values (s/mm2) | Main Findings | Study Limitations |
|---|---|---|---|---|---|
| Fan et al. (2020) [45] | To evaluate the role of volumetric ADC histogram analysis in discriminating between benign and malignant testicular masses | 3.0 T | 50, 100, 500, 1000 | Lower minimum ADC and 10th percentile ADC in testicular malignancies compared to benign lesions; Diagnostic performance of minimum ADC: sensitivity, 81.40%; specificity: 77.78% | Small number of masses |
| First Author (Year) | Study Aims | MRI Scanner | Main Findings | Study Limitations |
|---|---|---|---|---|
| Zhang et al. (2021) [47] | To compare the performance of histogram analysis and Ipris features in distinguishing between benign and malignant testicular lesions | 3.0 T | Differences in three histogram and nine Ipris features between benign and malignant lesions; Diagnostic performance of energy, total energy, and Ipris_shell1_id_std: sensitivity, 74.6%, 50.9%, and 65.5%, respectively; specificity: 72.0%, 96.0%, and 76.0%, respectively | Small number of masses Only T2WI data used Literature on Ipris limited to one study |
| First Author (Year) | Study Aims | MRI Scanner | b-Values (s/mm2) | Main Findings | Study Limitations |
|---|---|---|---|---|---|
| Mohrs et al. (2012) [32] | To evaluate the diagnostic value of MRI in the care of patients with suspected scrotal disorders | 1.5 T | Diagnostic performance of MRI in the classification of scrotal lesions: sensitivity, 95%; specificity: 92%; PPV, 97%; NPV, 91%; accuracy, 97% | Retrospective nature Heterogeneous data, not always histologically confirmed Consensus reading of MRI findings | |
| Serra et al. (1998) [36] | To determine the diagnostic utility and net cost of MRI in the management of clinically and sonographically inconclusive scrotal lesions | 1.5 T | Diagnostic accuracy of US versus MRI: 29% and 91%; Improvement of the management plan with the addition of MRI: 56% of cases for the general urologist and 50% of cases for the urologic oncologist; Overall net cost savings: USD 543 per patient for the urological oncologist; USD 730 per patient for the general urologist; USD 3833 per patient originally scheduled for surgery | Retrospective study, not randomized; unclear whether the results can be generalized to other clinics | |
| Muglia et al. (2002) [37] | To investigate the utility of MRI after inconclusive sonography in the evaluation of scrotal disease | 0.5 T and 1.5 T | MRI: provided additional and correct information (compared with US) in 82.1% of cases MRI: great concordance with the final diagnosis in cases of testicular malignancies | MRI not performed in all pathologies Absence of histological confirmation in all cases Various US machines Only conventional US used | |
| Wang et al. (2021) [56] | To explore the feasibility of conventional MRI features combined with ADC values for the differential diagnosis of testicular tumors | 3.0 T | 50, 1000 | Diagnostic performance of ADC alone: sensitivity, 70.6%; specificity, 71.7%; accuracy, 71.4% ADC cutoff < 0.90 × 10−3 mm2/s: diagnosis of malignancy Diagnostic performance of conventional MRI model alone: sensitivity, 94.1%; specificity, 79.3%; accuracy, 87.3% Diagnostic performance of ADC + conventional MRI model: sensitivity, 94.1%; specificity, 89.1%; accuracy, 90.5% | Retrospective nature Small number of masses Subjective assessment of conventional MRI features |
| Tsili et al. (2010) [76] | To assess the role of MRI in the preoperative characterization of testicular neoplasms | 1.5 T | Diagnostic performance of MRI in the characterization of malignant testicular tumors: sensitivity, 100%; specificity 87.5%; PPV, 96.5%; NPV, 100%; accuracy, 96.4% | Retrospective nature Small number of masses Consensus reading of MRI findings No direct comparison between sonographic and MRI findings |
| First Author (Year) | Study Aims | MRI Scanner | b Values (s/mm2) | Main Findings | Study Limitations |
|---|---|---|---|---|---|
| Feliciani et al. (2021) [49] | To assess the ability of MRI-based radiomics to differentiate between testicular germ-cell and non-germ-cell tumors | 1.5 T | Diagnostic performance of T2WI-based radiomics: accuracy, 89%; TPR in predicting testicular germ-cell tumor, 94%; TPR in predicting testicular non-germ-cell tumor, 75% | Retrospective nature Small number of cases Class imbalance Internal validation, no external dataset | |
| El Sanharawi et al. (2016) [63] | To evaluate DCE-MRI using qualitative, semi-quantitative, and quantitative parameters for the characterization of histologically proven, non-palpable, incidentally found intratesticular tumors | 1.5 T | Benign stromal tumors had higher maximal relative enhancement, shorter time to peak, higher initial enhancement slope, and higher transfer constants compared to malignancies and BOTs Diagnosis of malignancies and BOTs:
| Retrospective nature Not all histological types of testicular malignancies included No direct comparison between sonographic and MRI findings | |
| Pozza et al. (2019) [87] | To analyze the conventional MRI findings of LCTs over a 10-year period | 67.6% of LCTs had suggestive MRI findings | Selection bias | ||
| Manganaro et al. (2015) [89] | To evaluate the role of contrast-enhanced MRI in the identification of LCTs | 1.5 T | Diagnostic performance of MRI in characterizing LCTs: sensitivity, 89.47%; specificity, 96.65% Diagnostic performance of MRI in characterizing malignancies: sensitivity, 95.65%; specificity, 80.95%; accuracy, 93% | Lesions previously evaluated with CEUS Semi-quantitative analysis/ROI placement affected by small tumor size | |
| Manganaro et al. (2018) [90] | To explore the role of DCE-MRI using semi-quantitative and quantitative parameters and DWI in differentiating benign from malignant small, non-palpable, solid testicular tumors | 1.5 T | 0, 500, 1000 | Higher percentages of peak enhancement, wash-in-rate, volume transfer constant, rate constant, and initial area under the curve, and shorter time to peak, in benign lesions compared to malignancies Higher percentages of peak enhancement, wash-in-rate, volume transfer constant, rate constant, and initial area under the curve, and shorter time to peak, in LCTs compared to seminomas Best diagnostic cutoff for identification of seminomas: volume transfer constant, ≤0.135 min−1; rate constant, ≤0.45 min−1; initial area under the curve, ≤ 10.96; wash-in-rate, ≤1.11; percentage of peak enhancement, ≤96.72; time to peak, >99 s All tumors had similar ADC | Majority of lesions: LCTs and seminomas; no other histological types Palpable masses > 1.5 cm excluded Small tumor size may influence MRI measurements 50% of cases referred for infertility |
| Khanna et al. (2021) [91] | To assess the diagnostic performance of multiparametric MRI in differentiating benign testicular stromal tumors from malignant testicular neoplasms (non-stromal and stromal) | 1.5 T | 0, 400, 800 | Diagnostic performance of T2WI: sensitivity, 83%; specificity, 83%; PPV, 69%; NPV, 100%; accuracy, 85% Diagnostic performance of T2WI + DWI: sensitivity, 92%; specificity, 100%; PPV, 92%; NPV, 100%; accuracy, 95% Diagnostic performance of T2WI + DWI + T1PC: sensitivity, 92%; specificity, 100%; PPV, 93%; NPV, 100%; accuracy, 95% | Single-center study Retrospective nature Small number of masses |
| Rocher et al. (2017) [92] | Analysis of mpMRI findings in asymptomatic infertile men with pathologically confirmed BOTs | 1.5 T | 0, 800 | mpMRI findings of BOTs: well-delineated nodule, low T2 signal, high ADC, and lack of contrast enhancement | Small number of cases |
| First Author (Year) | Study Aims | MRI Scanner | b Values (s/mm2) | Main Findings | Study Limitations |
|---|---|---|---|---|---|
| Liu et al. (2019) [20] | To explore the utility of preoperative MRI for the differential diagnosis of testicular seminomas and nonseminomatous germ-cell tumors | 3.0 T | Diagnostic accuracy of MRI in preoperative diagnosis of seminomas: 95% | Small number of tumors | |
| Min et al. (2018) [46] | To assess the value of parameters derived from whole-lesion histograms of the ADC for the characterization of testicular germ-cell tumors | 3.0 T | 50, 1000 | Lower median of 10th, 25th, 50th, 75th, and 90th percentiles, and lower mean, minimum, and maximum ADC, in seminomas compared with nonseminomas; Higher median of kurtosis and skewness of ADC in seminomas compared with nonseminomas; Diagnostic performance of 10th percentile ADC: sensitivity, 100%; specificity, 92.86% | Retrospective nature Small number of tumors Consensus reading of MRI data No subgroup analysis for different histological subtypes of nonseminomas No comparison of the diagnostic performance of each histogram parameter Variations in MRI protocol |
| Zhang et al. (2021) [48] | To evaluate the performance of T2WI-based radiomics signatures for differentiating between seminomas and nonseminomas | 3.0 T | Diagnostic performance of T2WI-based radiomics signatures: sensitivity, 90%; specificity, 100% | Small sample size No independent validation cohort; internal validation instead Variations in MRI parameters (slice numbers and thickness) | |
| Feliciani et al. (2021) [49] | To assess the ability of MRI-based radiomics to differentiate between seminomas and non-seminomatous germ-cell tumors | 1.5 T | Diagnostic performance of T2WI-based radiomics: accuracy, 86%; TPR in predicting seminomas, 87%; TPR in predicting non-seminomatous germ-cell tumors, 86% | Retrospective nature Small number of cases Class imbalance Internal validation; no external dataset | |
| Wang et al. (2021) [56] | To explore the feasibility of conventional MRI features combined with ADC values for the differential diagnosis of testicular tumors | 3.0 T | 50, 1000 | ADC + cystic change: independent factors for differentiating testicular nonseminomas from seminomas; ADC + cystic change + T2 homogeneity: independent factors for differentiating nonseminomas from testicular lymphomas; ADC + intratumoral septa: independent factors for differentiating seminomas from lymphomas | Retrospective nature Small number of masses Subjective assessment of conventional MRI features |
| Tsili et al. (2015) [64] | To investigate the role of ADC values and DCE patterns in differentiating seminomas from nonseminomatous germ-cell tumors | 1.5 T | 0, 900 | Lower ADC in seminomas compared to nonseminomas; ADC cutoff point of 0.68 × 10−3 mm2/s: sensitivity, 63.6%; specificity, 100% No differences in DCE parameters between seminomas and nonseminomas | Retrospective nature Small number of tumors Consensus reading of MRI findings Inter-scanner and intra-scanner variability |
| Tsili et al. (2007) [77] | To evaluate the role of MRI in the preoperative characterization of the histological type of testicular tumors and, more specifically, to differentiate seminomatous from nonseminomatous testicular neoplasms | 1.5 T | Diagnostic accuracy of MRI: 91% | Retrospective nature Small number of tumors No direct comparison between sonographic and MRI findings Dynamic contrast-enhanced imaging not performed | |
| Johnson et al. (1990) [78] | To evaluate the role of MRI in the preoperative differentiation between seminomatous and nonseminomatous testicular neoplasms | 1.5 T | Diagnostic accuracy of MRI: 92.8% |
| First Author (Year) | Study Aima | MRI Scanner | Main Findings | Study Limitations |
|---|---|---|---|---|
| Tsili et al. (2010) [76] | To evaluate the role of MRI in the preoperative local staging of testicular neoplasms | 1.5 T | Diagnostic accuracy of MRI: 92.8% | Retrospective nature Small number of tumors Consensus reading of MRI findings No direct comparison between sonographic and MRI findings |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsili, A.C.; Sofikitis, N.; Pappa, O.; Bougia, C.K.; Argyropoulou, M.I. An Overview of the Role of Multiparametric MRI in the Investigation of Testicular Tumors. Cancers 2022, 14, 3912. https://doi.org/10.3390/cancers14163912
Tsili AC, Sofikitis N, Pappa O, Bougia CK, Argyropoulou MI. An Overview of the Role of Multiparametric MRI in the Investigation of Testicular Tumors. Cancers. 2022; 14(16):3912. https://doi.org/10.3390/cancers14163912
Chicago/Turabian StyleTsili, Athina C., Nikolaos Sofikitis, Ourania Pappa, Christina K. Bougia, and Maria I. Argyropoulou. 2022. "An Overview of the Role of Multiparametric MRI in the Investigation of Testicular Tumors" Cancers 14, no. 16: 3912. https://doi.org/10.3390/cancers14163912
APA StyleTsili, A. C., Sofikitis, N., Pappa, O., Bougia, C. K., & Argyropoulou, M. I. (2022). An Overview of the Role of Multiparametric MRI in the Investigation of Testicular Tumors. Cancers, 14(16), 3912. https://doi.org/10.3390/cancers14163912





