The Role of Ultrasound in the Evaluation of Inguinal Lymph Nodes in Patients with Vulvar Cancer: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Search Strategy
2.3. Eligibility Criteria and Selection Process
2.4. Data Extraction
2.5. Data Synthesis and Analysis
2.6. Quality Assessment
3. Results
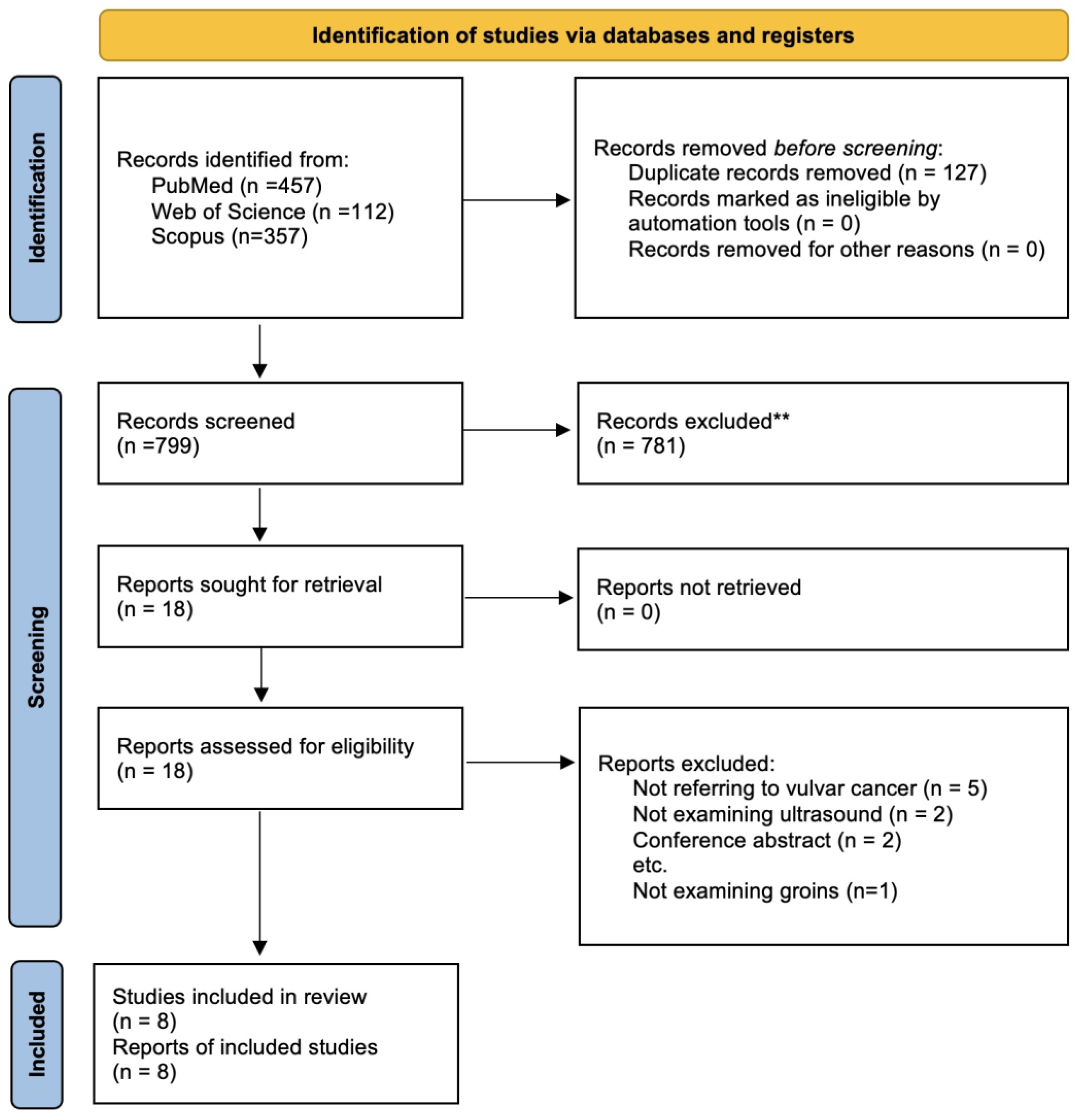
3.1. Search Strategy
3.2. Characteristics of the Included Studies
3.3. Quality Assessment
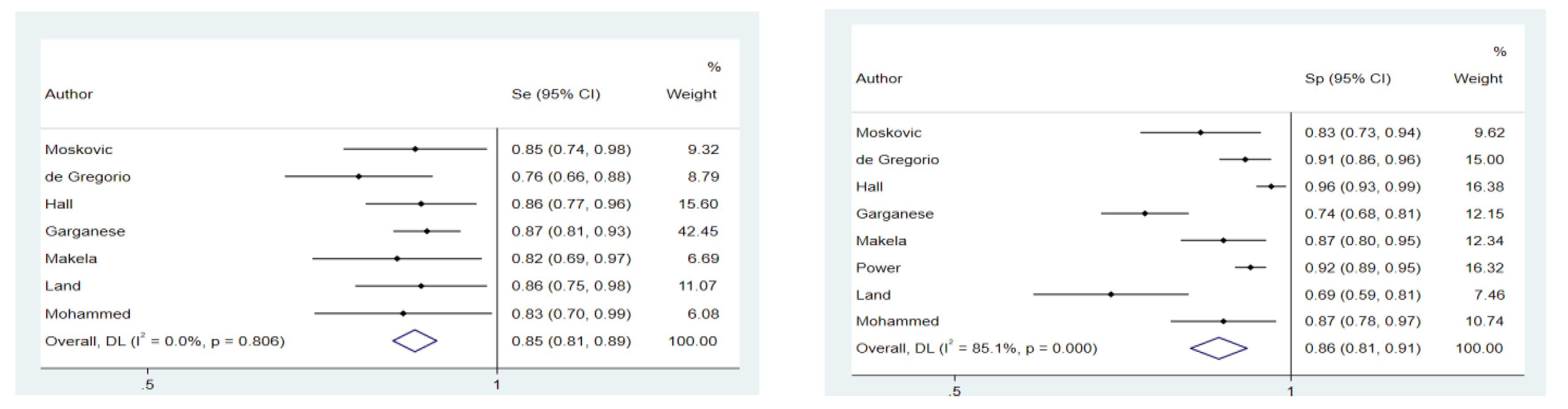
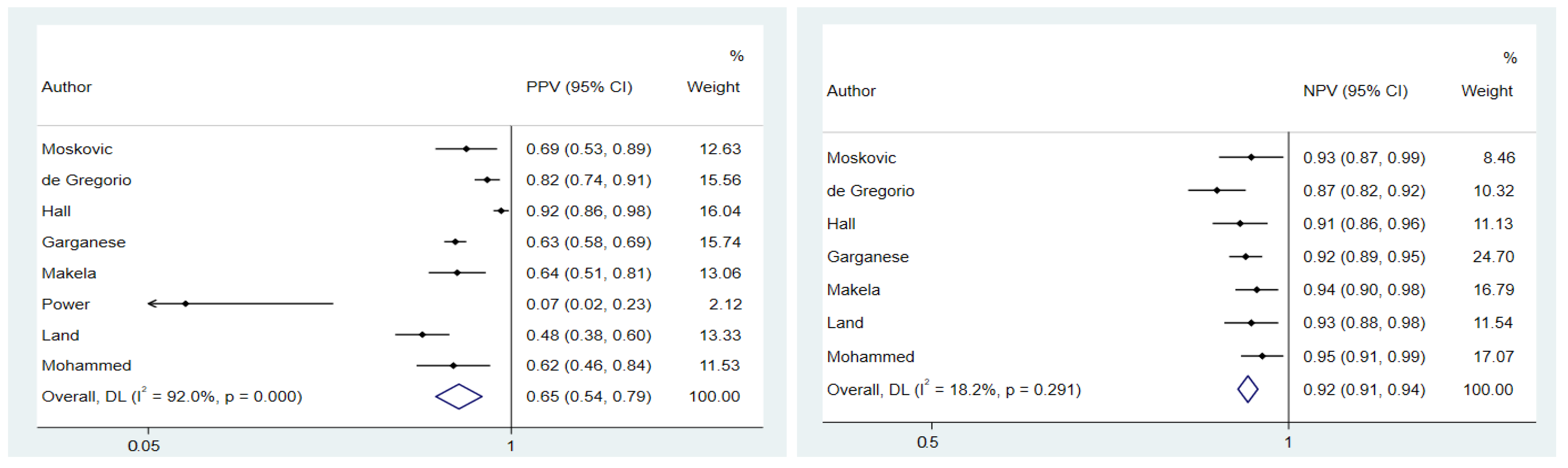
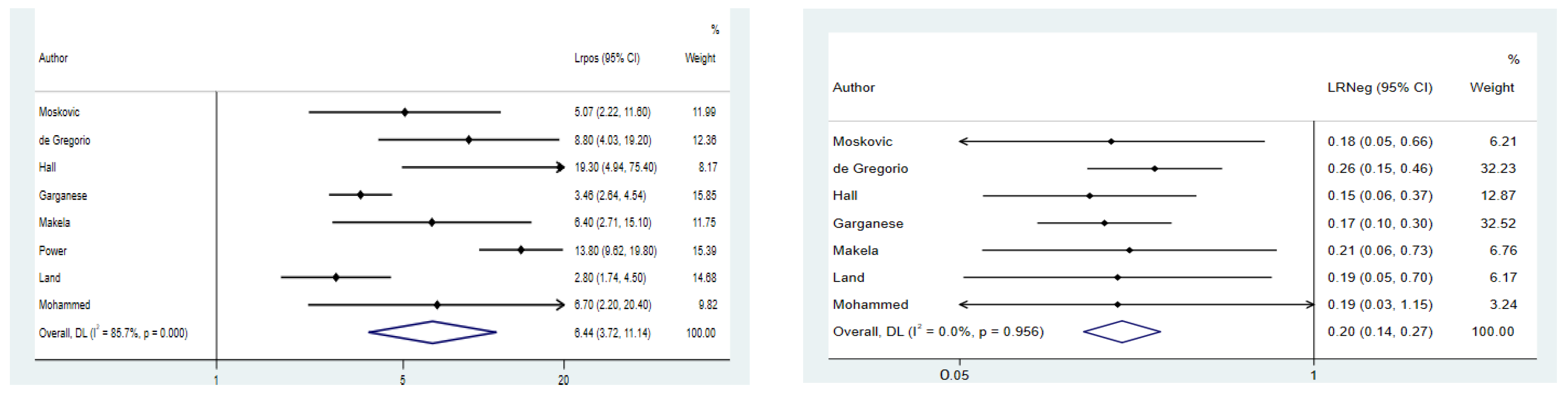
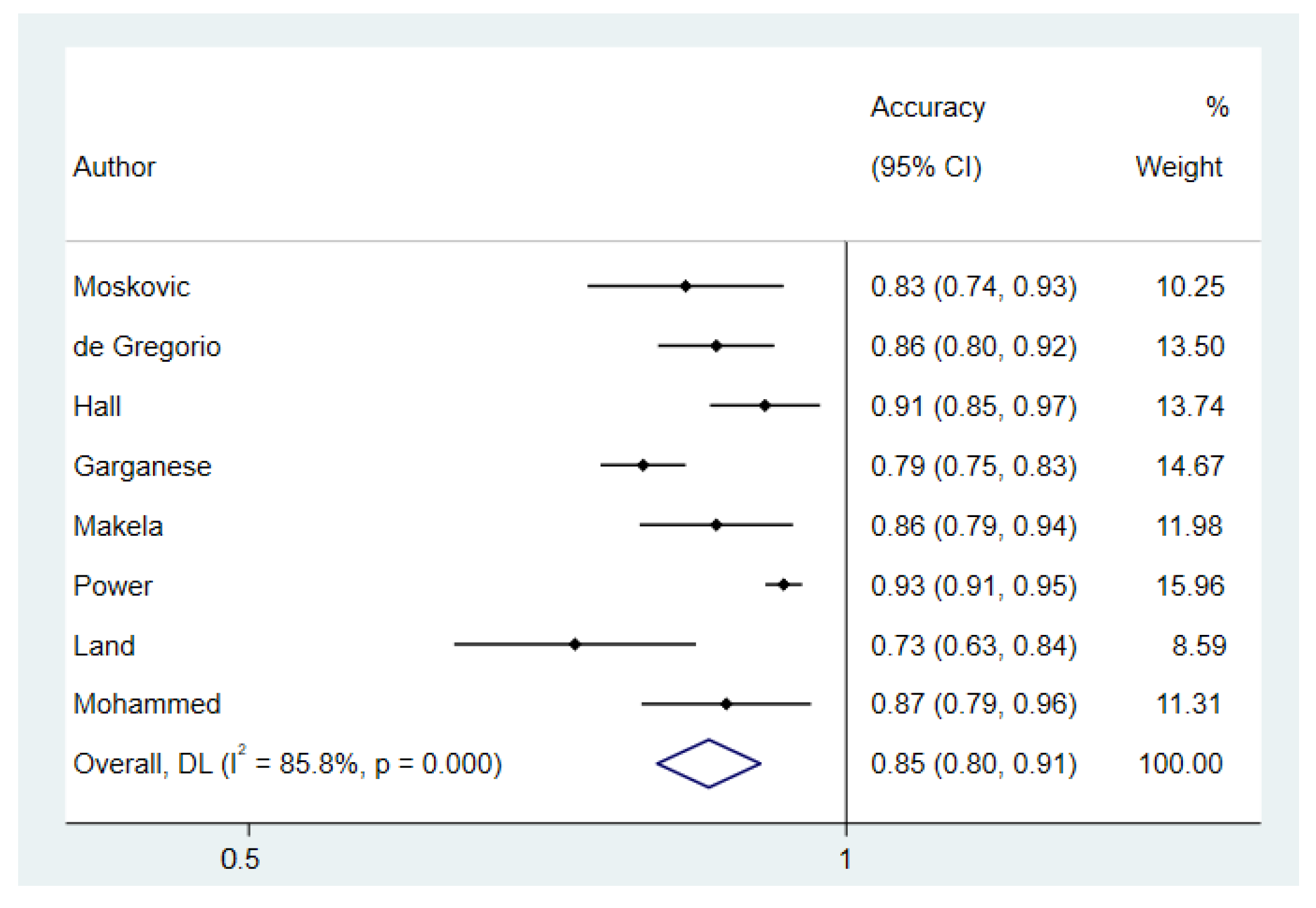
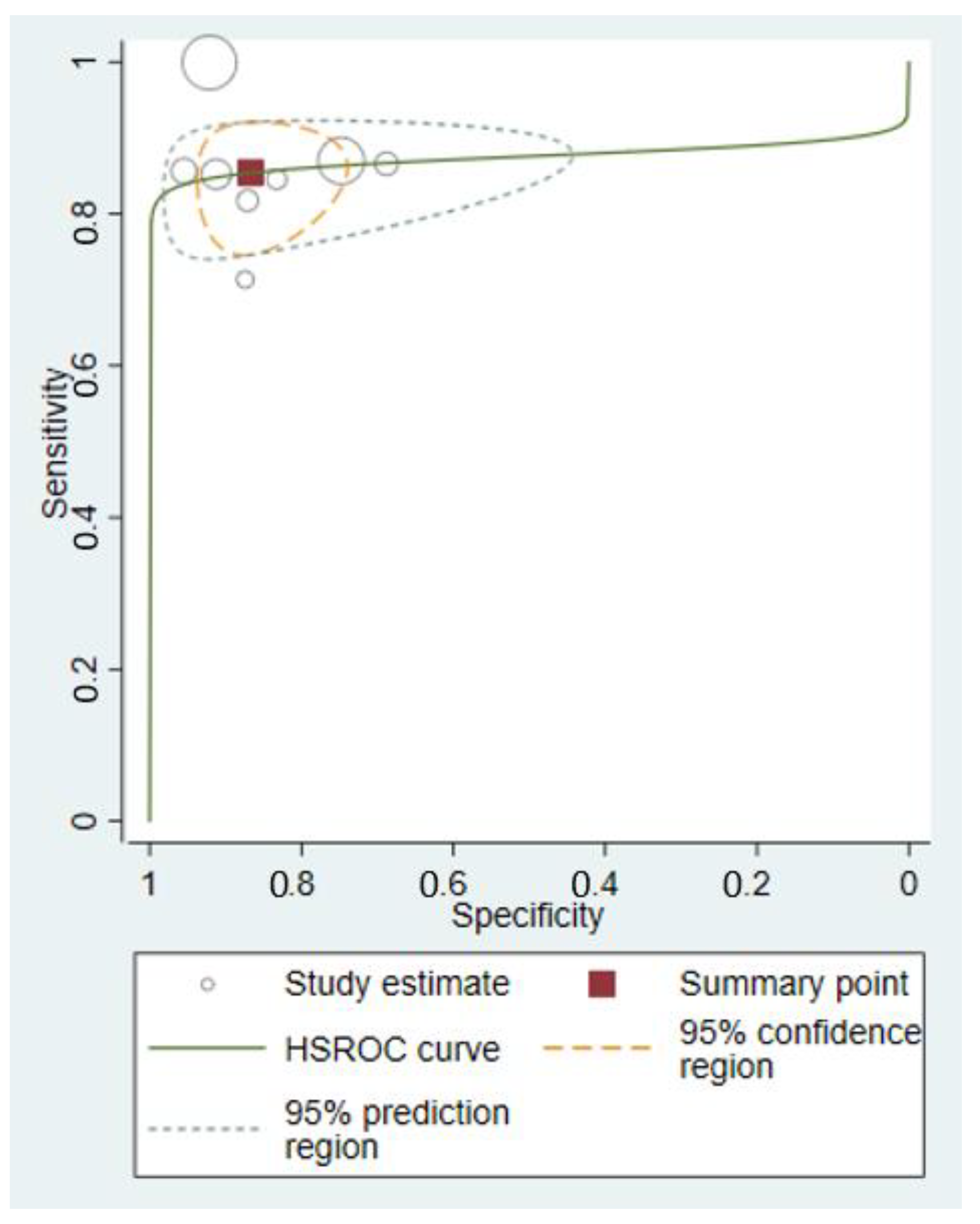
3.4. Meta-Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, Based on 2020 Submission Data (1999–2018): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Available online: www.cdc.gov/cancer/dataviz (accessed on 1 June 2021).
- The Global Cancer Observatory. Available online: https://gco.iarc.fr/today/home (accessed on 20 June 2022).
- Mancini, S.; Bucchi, L.; Baldacchini, F.; Giuliani, O.; Ravaioli, A.; Vattiato, R.; Preti, M.; Tumino, R.; Ferretti, S.; Biggeri, A.; et al. Incidence Trends of Vulvar Squamous Cell Carcinoma in Italy from 1990 to 2015. Gynecol. Oncol. 2020, 157, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Fanning, J.; Lambert, H.C.L.; Hale, T.M.; Morris, P.C.; Schuerch, C. Paget’s Disease of the Vulva: Prevalence of Associated Vulvar Adenocarcinoma, Invasive Paget’s Disease, and Recurrence after Surgical Excision. Am. J. Obstet. Gynecol. 1999, 180, 24–27. [Google Scholar] [CrossRef]
- Virarkar, M.; Vulasala, S.S.; Daoud, T.; Javadi, S.; Lall, C.; Bhosale, P. Vulvar Cancer: 2021 Revised FIGO Staging System and the Role of Imaging. Cancers 2022, 14, 2264. [Google Scholar] [CrossRef]
- Maggino, T.; Landoni, F.; Sartori, E.; Zola, P.; Gadducci, A.; Alessi, C.; Soldà, M.; Coscio, S.; Spinetti, G.; Maneo, A.; et al. Patterns of Recurrence in Patients with Squamous Cell Carcinoma of the Vulva. A Multicenter CTF Study. Cancer 2000, 89, 116–122. [Google Scholar] [CrossRef]
- Te Grootenhuis, N.C.; Pouwer, A.-F.W.; de Bock, G.H.; Hollema, H.; Bulten, J.; van der Zee, A.G.J.; de Hullu, J.A.; Oonk, M.H.M. Prognostic Factors for Local Recurrence of Squamous Cell Carcinoma of the Vulva: A Systematic Review. Gynecol. Oncol. 2018, 148, 622–631. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Cancer Stat Facts: Vulvar Cancer. Available online: https://seer.cancer.gov/statfacts/html/vulva.html (accessed on 10 June 2018).
- Pecorelli, S. Revised FIGO Staging for Carcinoma of the Vulva, Cervix, and Endometrium. Int. J. Gynecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Olawaiye, A.B.; Cotler, J.; Cuello, M.A.; Bhatla, N.; Okamoto, A.; Wilailak, S.; Purandare, C.N.; Lindeque, G.; Berek, J.S.; Kehoe, S.; et al. FIGO Staging for Carcinoma of the Vulva: 2021 Revision. Int. J. Gynecol. Obs. 2021, 155, 43–47. [Google Scholar] [CrossRef]
- Nomura, H.; Omi, M.; Netsu, S.; Aoki, Y.; Tanigawa, T.; Kurita, T.; Matoda, M.; Okamoto, S.; Omatsu, K.; Kanao, H. Positive Surgical Margin Is an Independent Predictor of Overall Survival of Patients with Vulvar Squamous Cell Carcinoma. J. Obs. Gynaecol. Res. 2021, 47, 3990–3997. [Google Scholar] [CrossRef]
- Gaarenstroom, K.N.; Kenter, G.G.; Trimbos, J.B.; Agous, I.; Amant, F.; Peters, A.A.W.; Vergote, I. Postoperative Complications after Vulvectomy and Inguinofemoral Lymphadenectomy Using Separate Groin Incisions. Int. J. Gynecol. Cancer 2003, 13, 522–527. [Google Scholar] [CrossRef]
- Koh, W.-J.; Greer, B.E.; Abu-Rustum, N.R.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Dizon, D.S.; et al. Vulvar Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017, 15, 92–120. [Google Scholar] [CrossRef] [Green Version]
- Van der Zee, A.G.J.; Oonk, M.H.; De Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; Van Dorst, E.B.; et al. Sentinel Node Dissection Is Safe in the Treatment of Early-Stage Vulvar Cancer. J. Clin. Oncol. 2008, 26, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.; van Hemel, B.M.; Hollema, H.; de Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; et al. Size of Sentinel-Node Metastasis and Chances of Non-Sentinel-Node Involvement and Survival in Early Stage Vulvar Cancer: Results from GROINSS-V, a Multicentre Observational Study. Lancet Oncol. 2010, 11, 646–652. [Google Scholar] [CrossRef]
- Collarino, A.; Fuoco, V.; Garganese, G.; Pereira Arias-Bouda, L.M.; Perotti, G.; Manca, G.; Vidal-Sicart, S.; Giammarile, F.; de Geus-Oei, L.-F.; Scambia, G.; et al. Lymphoscintigraphy and Sentinel Lymph Node Biopsy in Vulvar Carcinoma: Update from a European Expert Panel. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Garganese, G.; Collarino, A.; Fragomeni, S.M.; Rufini, V.; Perotti, G.; Gentileschi, S.; Evangelista, M.T.; Ieria, F.P.; Zagaria, L.; Bove, S.; et al. Groin Sentinel Node Biopsy and 18F-FDG PET/CT-Supported Preoperative Lymph Node Assessment in CN0 Patients with Vulvar Cancer Currently Unfit for Minimally Invasive Inguinal Surgery: The GroSNaPET Study. Eur. J. Surg. Oncol. 2017, 43, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Bai, Y.; Yue, Y. Safety and Benefit of Sentinel Lymph Nodes Biopsy Compared to Regional Lymph Node Dissection in Primary Vulvar Cancer Patients Without Distant Metastasis and Adjacent Organ Invasion: A Retrospective Population Study. Front. Oncol. 2021, 11, 676038. [Google Scholar] [CrossRef]
- Zach, D.; Kannisto, P.; Stenström Bohlin, K.; Moberg, L.; Kjölhede, P. Can We Extend the Indication for Sentinel Node Biopsy in Vulvar Cancer? A Nationwide Feasibility Study from Sweden. Int. J. Gynecol. Cancer 2020, 30, 402–405. [Google Scholar] [CrossRef]
- Iversen, T. The Value of Groin Palpation in Epidermoid Carcinoma of the Vulva. Gynecol. Oncol. 1981, 12, 291–295. [Google Scholar] [CrossRef]
- Piura, B.; Rabinovich, A.; Cohen, Y.; Friger, M.; Glezerman, M. Squamous Cell Carcinoma of the Vulva in the South of Israel: A Study of 50 Cases. J. Surg. Oncol. 1998, 67, 174–181. [Google Scholar] [CrossRef]
- Singh, K.; Orakwue, C.O.; Honest, H.; Balogun, M.; Lopez, C.; Luesley, D.M. Accuracy of Magnetic Resonance Imaging of Inguinofemoral Lymph Nodes in Vulval Cancer. Int. J. Gynecol. Cancer 2006, 16, 1179–1183. [Google Scholar] [CrossRef]
- Podratz, K.C.; Symmonds, R.E.; Taylor, W.F.; Williams, T.J. Carcinoma of the Vulva: Analysis of Treatment and Survival. Obs. Gynecol. 1983, 61, 63–74. [Google Scholar]
- Selman, T.J.; Luesley, D.M.; Acheson, N.; Khan, K.S.; Mann, C.H. A Systematic Review of the Accuracy of Diagnostic Tests for Inguinal Lymph Node Status in Vulvar Cancer. Gynecol. Oncol. 2005, 99, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.Y.; Sala, E.; Baldwin, P.; Reinhold, C.; Farhadi, A.; Hudolin, T.; Hricak, H. The Accuracy of Magnetic Resonance Imaging in Staging of Vulvar Cancer: A Retrospective Multi-Centre Study. Gynecol. Oncol. 2010, 117, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.E.; Dehdashti, F.; Gibb, R.K.; Mutch, D.G.; Rader, J.S.; Siegel, B.A.; Herzog, T.J. Prospective Evaluation of Positron Emission Tomography for the Detection of Groin Node Metastases from Vulvar Cancer. Gynecol. Oncol. 2002, 85, 179–184. [Google Scholar] [CrossRef] [PubMed]
- De Hullu, J.A.; Pruim, J.; Qué, T.H.; Aalders, J.G.; Boonstra, H.; Vaalburg, W.; Hollema, H.; Van Der Zee, A.G.J. Noninvasive Detection of Inguinofemoral Lymph Node Metastases in Squamous Cell Cancer of the Vulva by L-. Int. J. Gynecol. Cancer 1999, 9, 141–146. [Google Scholar] [CrossRef]
- Kamran, M.W.; O’Toole, F.; Meghen, K.; Wahab, A.N.; Saadeh, F.A.; Gleeson, N. Whole-Body [18F]Fluoro-2-Deoxyglucose Positron Emission Tomography Scan as Combined PET-CT Staging Prior to Planned Radical Vulvectomy and Inguinofemoral Lymphadenectomy for Squamous Vulvar Cancer: A Correlation with Groin Node Metastasis. Eur. J. Gynaecol. Oncol. 2014, 35, 230–235. [Google Scholar]
- Gui, B.; Persiani, S.; Miccò, M.; Pignatelli, V.; Rodolfino, E.; Avesani, G.; Di Paola, V.; Panico, C.; Russo, L.; Fragomeni, S.M.; et al. MRI Staging in Locally Advanced Vulvar Cancer: From Anatomy to Clinico-Radiological Findings. A Multidisciplinary VulCan Team Point of View. J. Pers. Med. 2021, 11, 1219. [Google Scholar] [CrossRef]
- Rufini, V.; Garganese, G.; Ieria, F.P.; Pasciuto, T.; Fragomeni, S.M.; Gui, B.; Florit, A.; Inzani, F.; Zannoni, G.F.; Scambia, G.; et al. Diagnostic Performance of Preoperative [18F]FDG-PET/CT for Lymph Node Staging in Vulvar Cancer: A Large Single-Centre Study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3303–3314. [Google Scholar] [CrossRef]
- Collarino, A.; Garganese, G.; Valdés Olmos, R.A.; Stefanelli, A.; Perotti, G.; Mirk, P.; Fragomeni, S.M.; Ieria, F.P.; Scambia, G.; Giordano, A.; et al. Evaluation of Dual-Timepoint 18F-FDG PET/CT Imaging for Lymph Node Staging in Vulvar Cancer. J. Nucl. Med. 2017, 58, 1913–1918. [Google Scholar] [CrossRef] [Green Version]
- Triumbari, E.K.A.; de Koster, E.J.; Rufini, V.; Fragomeni, S.M.; Garganese, G.; Collarino, A. 18F-FDG PET and 18F-FDG PET/CT in Vulvar Cancer: A Systematic Review and Meta-Analysis. Clin. Nucl. Med. 2021, 46, 125–132. [Google Scholar] [CrossRef]
- Pounds, R.; O’Neill, D.; Subba, K.; Garg, A.; Scerif, M.; Leong, E.; Nevin, J.; Kehoe, S.; Yap, J. The Role of Preoperative Computerized Tomography (CT) Scan of the Pelvis and Groin in the Management of Clinically Early Staged Vulva Squamous Cell Carcinoma. Gynecol. Oncol. 2020, 157, 444–449. [Google Scholar] [CrossRef]
- Land, R.; Herod, J.; Moskovic, E.; King, M.; Sohaib, S.A.; Trott, P.; Nasiri, N.; Shepherd, J.H.; Bridges, J.E.; Ind, T.E.J.; et al. Routine Computerized Tomography Scanning, Groin Ultrasound with or without Fine Needle Aspiration Cytology in the Surgical Management of Primary Squamous Cell Carcinoma of the Vulva. Int. J. Gynecol. Cancer 2006, 16, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Zobbe, V.; Thranov, I.R.; Pedersen, K.D. Relevance of Computerized Tomography in the Preoperative Evaluation of Patients with Vulvar Cancer: A Prospective Study. Cancer Imaging 2015, 15, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedi, D.G.; Krishnamurthy, R.; Krishnamurthy, S.; Edeiken, B.S.; Le-Petross, H.; Fornage, B.D.; Bassett, R.L.; Hunt, K.K. Cortical Morphologic Features of Axillary Lymph Nodes as a Predictor of Metastasis in Breast Cancer: In Vitro Sonographic Study. Am. J. Roentgenol. 2008, 191, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Añorbe, E.; Alcorta, P.; López, F.; Alonso, I.; Cortés, J. Role of Sonography in the Diagnosis of Axillary Lymph Node Metastases in Breast Cancer: A Systematic Review. American J. Roentgenol. 2006, 186, 1342–1348. [Google Scholar] [CrossRef] [Green Version]
- Nieciecki, M.; Dobruch-Sobczak, K.; Wareluk, P.; Gumińska, A.; Białek, E.; Cacko, M.; Królicki, L. The Role of Ultrasound and Lymphoscintigraphy in the Assessment of Axillary Lymph Nodes in Patients with Breast Cancer. J. Ultrason. 2016, 16, 5–15. [Google Scholar] [CrossRef]
- Querleu, D.; Rychlik, A.; Guyon, F.; Floquet, A.; Planchamp, F. Management of the nodal disease in vulvar cancers. The ESGO guidelines. Bull. Cancer 2020, 107, 715–720. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Vulvar Cancer Guidelines (Version 1.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed on 12 July 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Galbraith, R.F. Graphical Display of Estimates Having Differing Standard Errors. Technometrics 1988, 30, 271–281. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Moskovic, E.C.; Shepherd, J.H.; Barton, D.P.; Trott, P.A.; Nasiri, N.; Thomas, J.M. The Role of High Resolution Ultrasound with Guided Cytology of Groin Lymph Nodes in the Management of Squamous Cell Carcinoma of the Vulva: A Pilot Study. Br. J. Obs. Gynaecol. 1999, 106, 863–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Gregorio, N.; Ebner, F.; Schwentner, L.; Friedl, T.W.P.; Deniz, M.; Látó, K.; Kreienberg, R.; Janni, W.; Varga, D. The Role of Preoperative Ultrasound Evaluation of Inguinal Lymph Nodes in Patients with Vulvar Malignancy. Gynecol. Oncol. 2013, 131, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.B.; Barton, D.P.J.; Trott, P.A.; Nasiri, N.; Shepherd, J.H.; Thomas, J.M.; Moskovic, E.C. The Role of Ultrasound-Guided Cytology of Groin Lymph Nodes in the Management of Squamous Cell Carcinoma of the Vulva: 5-Year Experience in 44 Patients. Clin. Radiol. 2003, 58, 367–371. [Google Scholar] [CrossRef]
- Garganese, G.; Fragomeni, S.M.; Pasciuto, T.; Leombroni, M.; Moro, F.; Evangelista, M.T.; Bove, S.; Gentileschi, S.; Tagliaferri, L.; Paris, I.; et al. Ultrasound Morphometric and Cytologic Preoperative Assessment of Inguinal Lymph-Node Status in Women with Vulvar Cancer: MorphoNode Study. Ultrasound Obs. Gynecol. 2020, 55, 401–410. [Google Scholar] [CrossRef]
- Mäkelä, P.J.; Leminen, A.; Kääriäinen, M.; Lehtovirta, P. Pretreatment Sonographic Evaluation of Inguinal Lymph Nodes in Patients with Vulvar Malignancy. J. Ultrasound Med. 1993, 12, 255–258. [Google Scholar] [CrossRef]
- Pouwer, A.W.; Mus, R.; IntHout, J.; van der Zee, A.; Bulten, J.; Massuger, L.; de Hullu, J.A. The Efficacy of Ultrasound in the Follow up after a Negative Sentinel Lymph Node in Women with Vulvar Cancer: A Prospective Single-Centre Study. BJOG 2018, 125, 1461–1468. [Google Scholar] [CrossRef]
- Abang Mohammed, D.K.; Uberoi, R.; de B Lopes, A.; Monaghan, J.M. Inguinal Node Status by Ultrasound in Vulva Cancer. Gynecol. Oncol. 2000, 77, 93–96. [Google Scholar] [CrossRef]
- Serrado, M.A.; Horta, M.; Cunha, T.M. State of the Art in Vulvar Cancer Imaging. Radiol. Bras. 2019, 52, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Shetty, A.S.; Menias, C.O. MR Imaging of Vulvar and Vaginal Cancer. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 481–502. [Google Scholar] [CrossRef]
- Sohaib, S.A.A.; Richards, P.S.; Ind, T.; Jeyarajah, A.R.; Shepherd, J.H.; Jacobs, I.J.; Reznek, R.H. MR Imaging of Carcinoma of the Vulva. Am. J. Roentgenol. 2002, 178, 373–377. [Google Scholar] [CrossRef]
- Bipat, S.; Fransen, G.A.; Spijkerboer, A.M.; van der Velden, J.; Bossuyt, P.M.M.; Zwinderman, A.H.; Stoker, J. Is There a Role for Magnetic Resonance Imaging in the Evaluation of Inguinal Lymph Node Metastases in Patients with Vulva Carcinoma? Gynecol. Oncol. 2006, 103, 1001–1006. [Google Scholar] [CrossRef]
- Lakhman, Y.; Vargas, H.A.; Reinhold, C.; Akin, E.A.; Bhosale, P.R.; Huang, C.; Kang, S.K.; Khanna, N.; Kilcoyne, A.; Nicola, R.; et al. ACR Appropriateness Criteria® Staging and Follow-up of Vulvar Cancer. J. Am. Coll. Radiol. 2021, 18, S212–S228. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, O.; e Sousa, F.A.; Cunha, T.M.; Nikolić, M.B.; Otero-García, M.M.; Gui, B.; Nougaret, S.; Leonhardt, H.; On behalf of the ESUR Female Pelvic Imaging Working Group. Vulvar Cancer Staging: Guidelines of the European Society of Urogenital Radiology (ESUR). Insights Imaging 2021, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Angelico, G.; Santoro, A.; Inzani, F.; Spadola, S.; Fiorentino, V.; Cianfrini, F.; Carbone, C.; Garganese, G.; Rossi, E.D.; Scambia, G.; et al. Ultrasound-Guided FNA Cytology of Groin Lymph Nodes Improves the Management of Squamous Cell Carcinoma of the Vulva: Results from a Comparative Cytohistological Study. Cancer Cytopathol. 2019, 127, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Masroor, I.; Munir, A.; Idress, R.; Khan, P.; Khan, S. Preoperative Ultrasound-Guided Core Biopsy of Axillary Nodes for Staging of Clinically Negative Axilla in Breast Cancer Patients—A Pilot Study. Cureus 2020, 12, e6718. [Google Scholar] [CrossRef] [Green Version]
- Y L, J.T.; McGowan, K.; Cooley, G.; McLaughlin, R.; Sugrue, M. The Role of Ultrasound Guided Core Biopsy of Axillary Nodes in Predicting Macrometastases and Avoiding Overtreatment Outside ACOSOG Z0011 Parameters. Breast 2015, 24, 57–61. [Google Scholar] [CrossRef]
- Balasubramanian, I.; Fleming, C.A.; Corrigan, M.A.; Redmond, H.P.; Kerin, M.J.; Lowery, A.J. Meta-Analysis of the Diagnostic Accuracy of Ultrasound-Guided Fine-Needle Aspiration and Core Needle Biopsy in Diagnosing Axillary Lymph Node Metastasis. Br. J. Surg. 2018, 105, 1244–1253. [Google Scholar] [CrossRef]
- Fischerova, D.; Garganese, G.; Reina, H.; Fragomeni, S.M.; Cibula, D.; Nanka, O.; Rettenbacher, T.; Testa, A.C.; Epstein, E.; Guiggi, I.; et al. Terms, Definitions and Measurements to Describe Sonographic Features of Lymph Nodes: Consensus Opinion from the Vulvar International Tumor Analysis (VITA) Group. Ultrasound Obs. Gynecol. 2021, 57, 861–879. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verri, D.; Moro, F.; Fragomeni, S.M.; Zaçe, D.; Bove, S.; Pozzati, F.; Gui, B.; Scambia, G.; Testa, A.C.; Garganese, G. The Role of Ultrasound in the Evaluation of Inguinal Lymph Nodes in Patients with Vulvar Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3082. https://doi.org/10.3390/cancers14133082
Verri D, Moro F, Fragomeni SM, Zaçe D, Bove S, Pozzati F, Gui B, Scambia G, Testa AC, Garganese G. The Role of Ultrasound in the Evaluation of Inguinal Lymph Nodes in Patients with Vulvar Cancer: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(13):3082. https://doi.org/10.3390/cancers14133082
Chicago/Turabian StyleVerri, Debora, Francesca Moro, Simona Maria Fragomeni, Drieda Zaçe, Sonia Bove, Federica Pozzati, Benedetta Gui, Giovanni Scambia, Antonia Carla Testa, and Giorgia Garganese. 2022. "The Role of Ultrasound in the Evaluation of Inguinal Lymph Nodes in Patients with Vulvar Cancer: A Systematic Review and Meta-Analysis" Cancers 14, no. 13: 3082. https://doi.org/10.3390/cancers14133082
APA StyleVerri, D., Moro, F., Fragomeni, S. M., Zaçe, D., Bove, S., Pozzati, F., Gui, B., Scambia, G., Testa, A. C., & Garganese, G. (2022). The Role of Ultrasound in the Evaluation of Inguinal Lymph Nodes in Patients with Vulvar Cancer: A Systematic Review and Meta-Analysis. Cancers, 14(13), 3082. https://doi.org/10.3390/cancers14133082






