Management of Early-Stage Vulvar Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
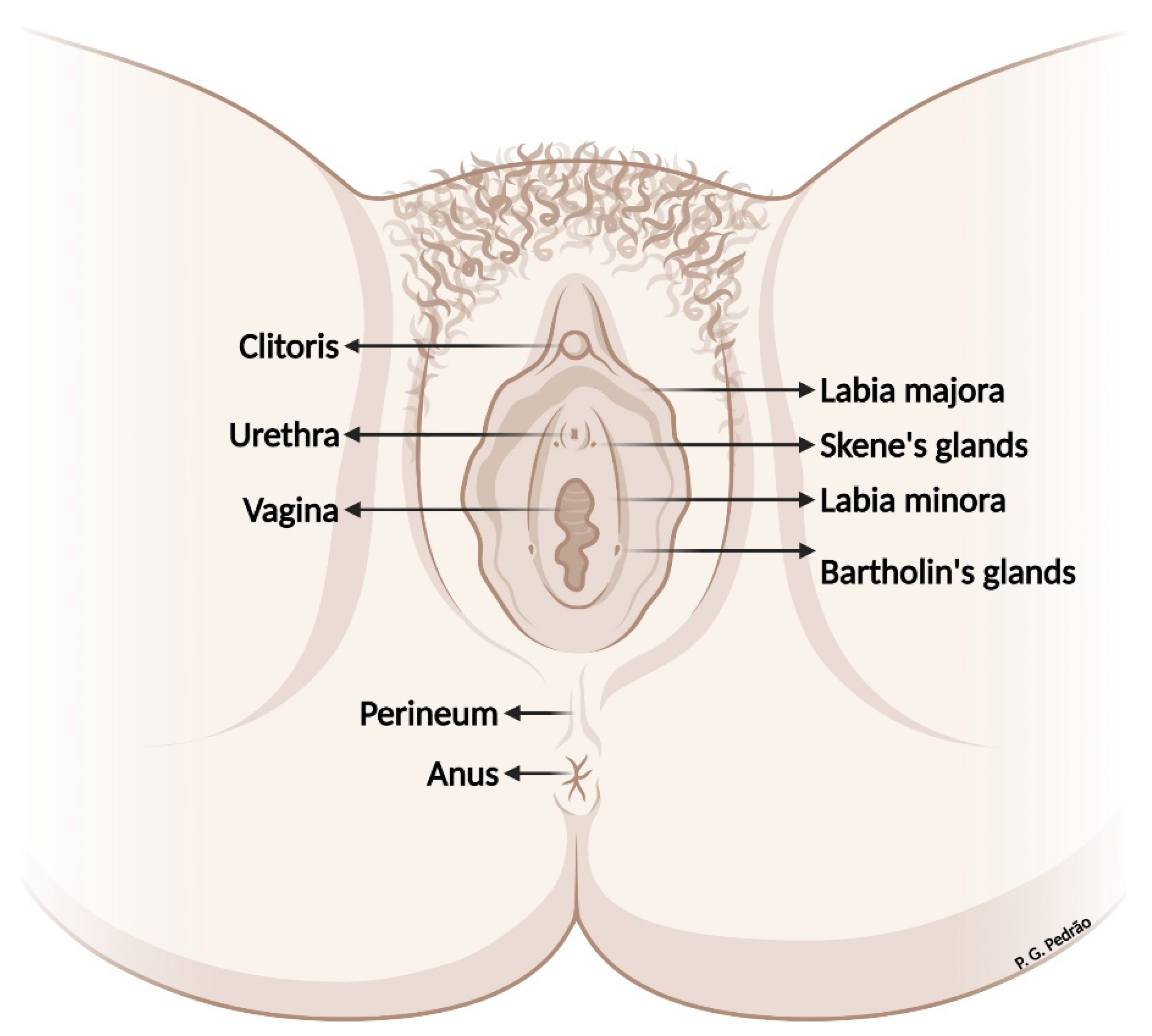
2. Vulvar Anatomy
3. Prevention
3.1. Vaccination (Primary Prevention)
3.2. Screening (Secondary Prevention)
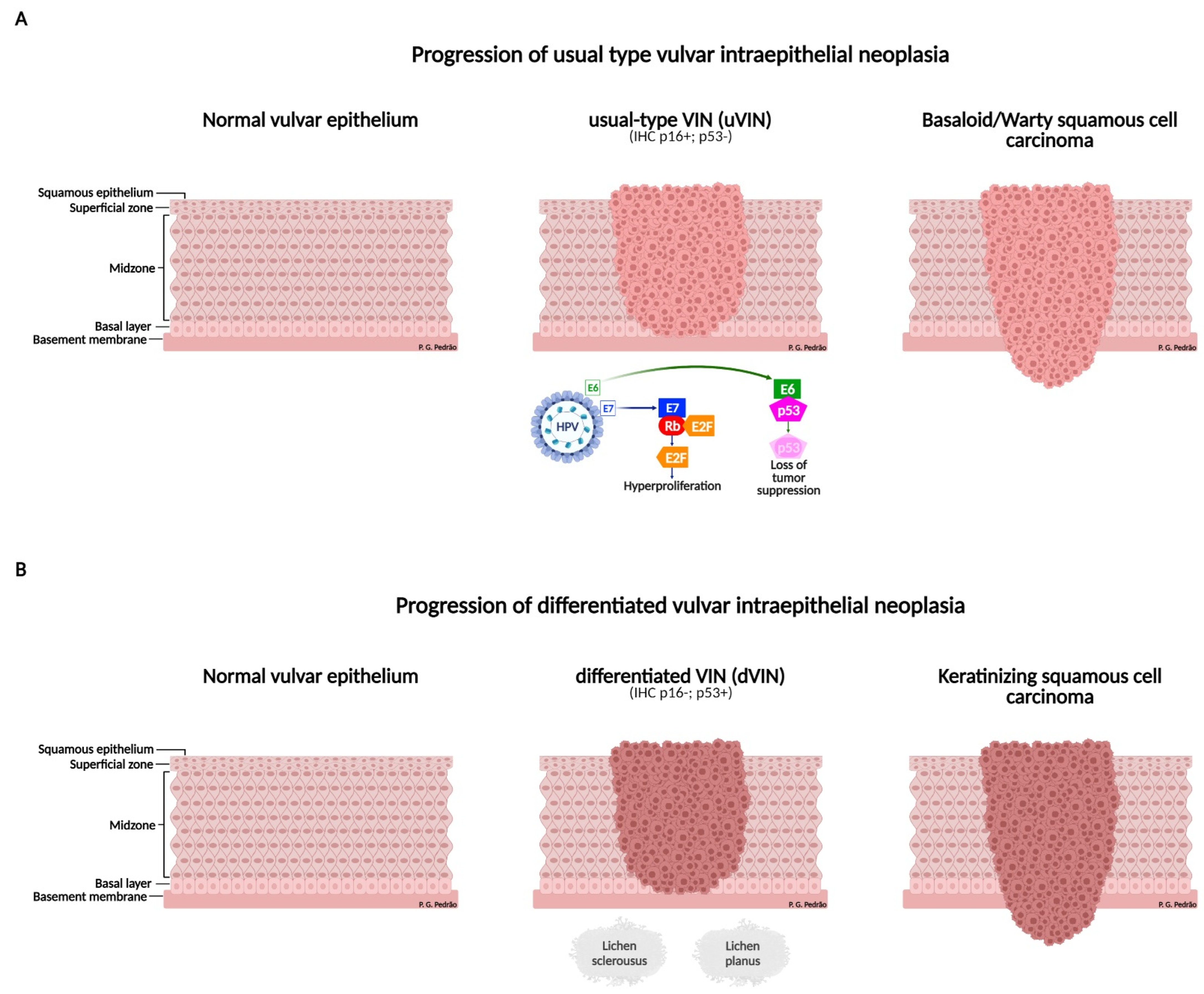
4. Precursor Lesions
| ISSVD 1986 | ISSVD 2004 | LAST 2012/WHO2014 |
|---|---|---|
| VIN I | Flat condylomata or HPV effect | LSIL |
| VIN II and III | VIN, usual type: (i) VIN, warty type (ii) VIN, basaloid type (iii) VIN, mixed | HSIL |
| Differentiated VIN | VIN, differentiated type | Differentiated VIN |
5. Diagnosis
6. Staging
7. Treatment
7.1. Surgical Management
7.1.1. Microinvasive (Stage IA)
7.1.2. Early-Stage
Role of Surgical Margins
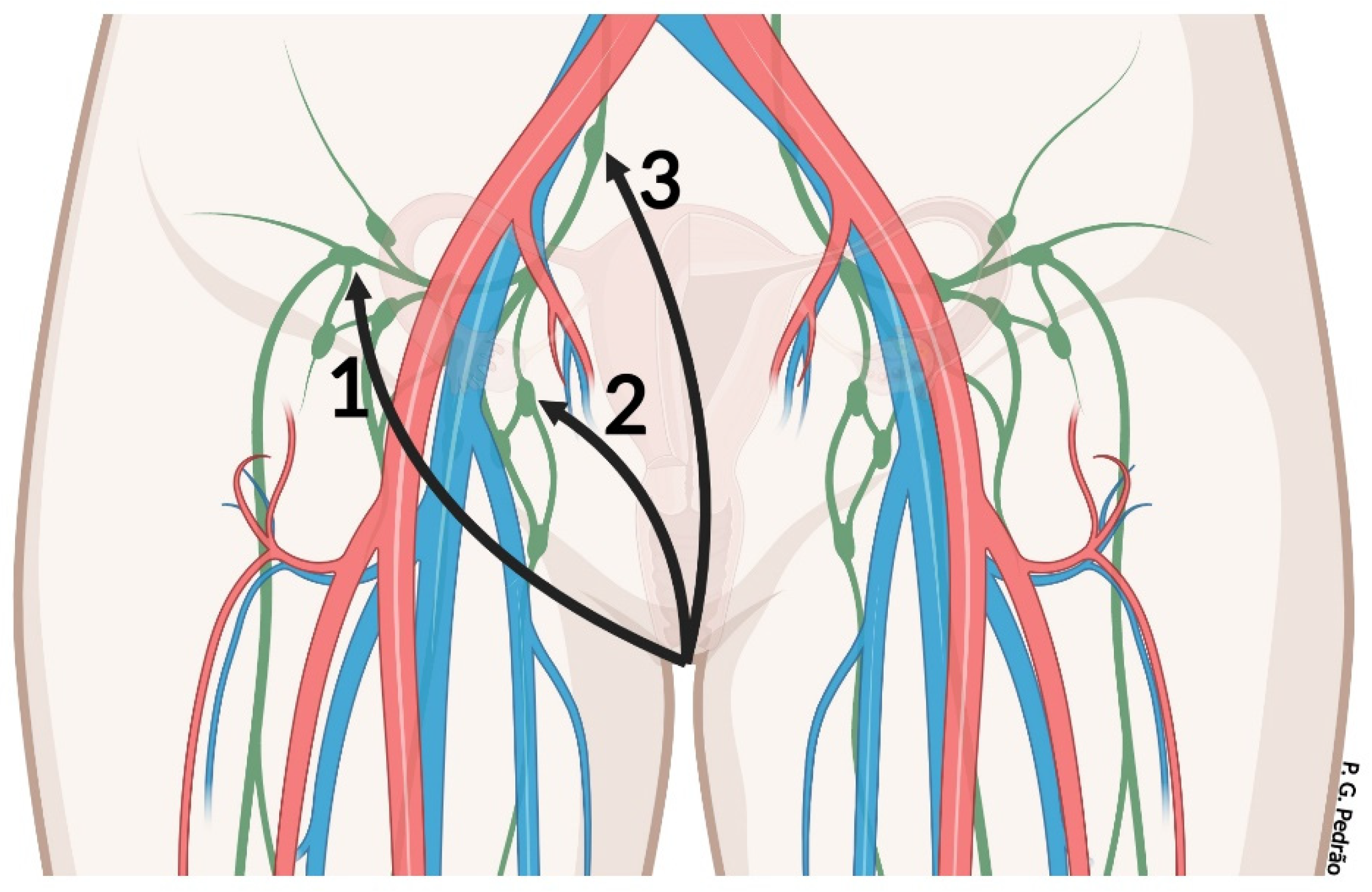
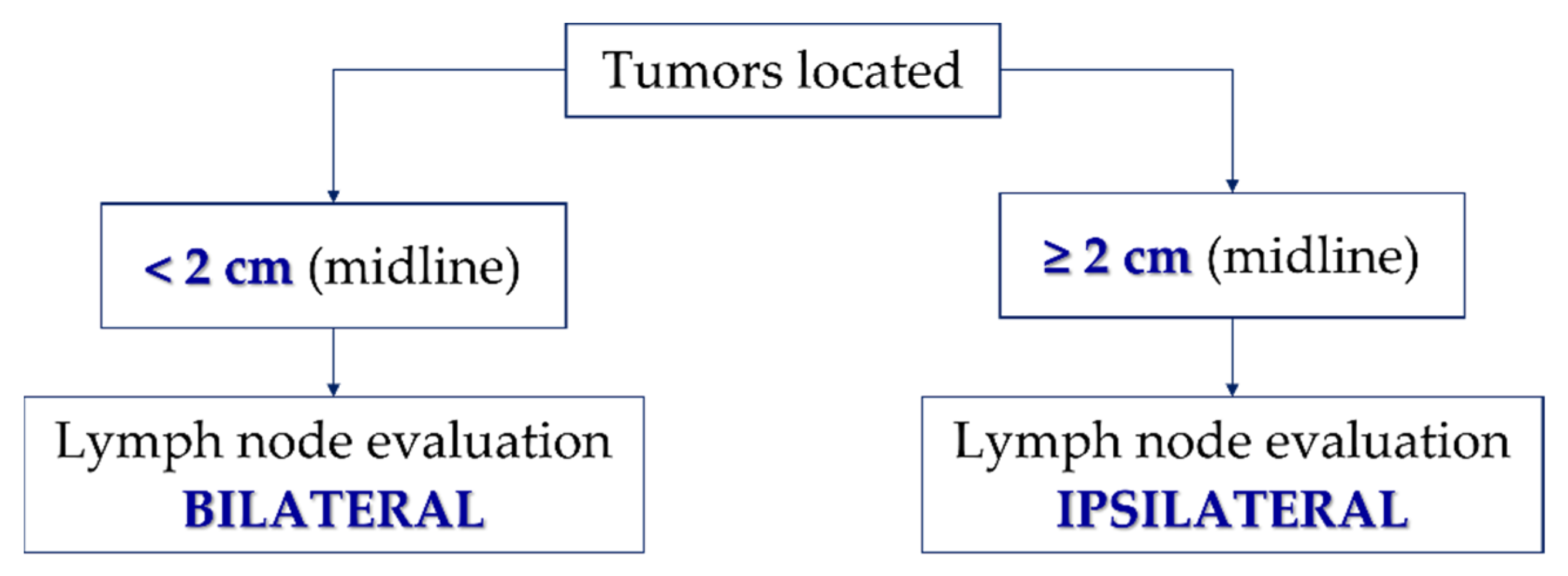
7.2. Management of Inguinal Lymph Nodes
7.3. Role of Sentinel Lymph Node
| Authors | Year | Aim | N | Outcomes |
|---|---|---|---|---|
| Van der Zee, et al. [101] | 2008 | To analyze the clinical utility and safety of SLN biopsy in early-stage vulvar cancer. | 403 | SLN biopsy in patients with early-stage vulvar cancer detects SLN metastases in SLN-negative patients, has a low groin recurrence rate, excellent survival, and decreases treatment-related morbidity. |
| Oonk, et al. [114] | 2010 | To evaluate the association of SLN metastasis size and disease survival risk in patients with early-stage vulvar cancer | 260 | Disease survival is related to the size of the SLN metastasis |
| Levenback, et al. [103] | 2012 | To evaluate whether SLN biopsy replaces inguinofemoral lymphadenectomy in patients with vulvar SCC. | 452 | SLN biopsy can replace inguinofemoral lymphadenectomy in patients with vulvar SCC. |
| Te Grootenhuis, et al. [88] | 2016 | To evaluate the long-term follow-up of patients undergoing SLN biopsy regarding recurrences and survival. | 377 | Patients with negative SLN have a good survival rate, but 36% of these patients and 46% of patients with positive SLN may have local recurrence. However, the surgical morbidity of these patients is significantly reduced. |
| Te Grootenhuis, et al. [72] | 2019 | To evaluate the incidence of local recurrence of vulvar SCC in relation to pathologic margins free of tumor and/or precursor lesion. | 287 | Local recurrences occur frequently in patients with primary vulvar carcinoma and are associated with dVIN at the pathologic margin, rather than any distance from the tumor-free margin. |
| Bedell, et al. [73] | 2019 | To analyze whether re-excision or adjuvant radiation in patients with early-stage vulvar cancer with a close or positive surgical margin improves recurrence-free survival. | 150 | Any additional treatment after primary surgical resection in patients with early-stage vulvar cancer did not show an improvement in local recurrence-free survival and overall survival rates, however, an improvement in the recurrence-free survival of these patients was observed. |
| Barlow, et al. [74] | 2020 | To analyze survival rates after conservative vulvar resection and determine clinicopathological predictors regarding vulvar recurrence, with a focus on surgical margin. | 345 | Treatment by re-excision or radiation therapy in positive or close margins (<5 mm) significantly decreases the risk of recurrence. |
7.4. Adjuvant Treatment
Role of Low-Volume Metastasis
8. Recurrence
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer Stat Facts: Vulvar Cancer. Available online: https://seer.cancer.gov/statfacts/html/vulva.html (accessed on 21 March 2022).
- Global Cancer Observatory—Estimated Number of New Cases in 2020, Vulva, Females, All Ages. Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=population&mode_population=income&population=900&populations=900&key=total&sex=2&cancer=21&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=0&include_nmsc_other=1&half_pie=0&donut=0 (accessed on 21 March 2022).
- Global Cancer Observatory—Estimated Number of Deaths in 2020, Vulva, Female, All Ages. Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=population&mode_population=income&population=900&populations=900&key=total&sex=2&cancer=21&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=0&include_nmsc_other=1&half_pie=0&donut=0 (accessed on 21 March 2022).
- Vulvar Cancer Treatment (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/types/vulvar/hp/vulvar-treatment-pdq#section/_1 (accessed on 29 March 2022).
- Figge, D.C. Rare vulvar malignancies. J. Curr. Top. Obstet. Gynecol. 1991, 239, 257. [Google Scholar]
- Butt, J.L.; Botha, M.H. Vulvar cancer is not a disease of the elderly: Treatment and outcome at a tertiary referral centre in South Africa. S. Afr. Med. J. 2017, 107, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Smith, M.; Barlow, E.; Coffey, K.; Hacker, N.; Canfell, K. Vulvar cancer in high-income countries: Increasing burden of disease. Int. J. Cancer 2017, 141, 2174–2186. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Allbritton, J.I. Vulvar Neoplasms, Benign and Malignant. Obstet. Gynecol. Clin. N. Am. 2017, 44, 339–352. [Google Scholar] [CrossRef]
- Xiao, X.; Meng, Y.B.; Bai, P.; Zou, J.; Zhang, Y.; Nguyen, T.M.B.; Xiao, J.G.; Gao, X.M.; Wen, B.F. Vulvar Cancer in China: Epidemiological Features and Risk Analysis. J. Cancer 2017, 8, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Cobos, G.A.; Pomeranz, M.K. A General Approach to the Evaluation and the Management of Vulvar Disorders. Obstet. Gynecol. Clin. N. Am. 2017, 44, 321–327. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy E-Book: The Anatomical Basis of Clinical Practice, 42th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Coleman, R.L.; Ali, S.; Levenback, C.F.; Gold, M.A.; Fowler, J.M.; Judson, P.L.; Bell, M.C.; De Geest, K.; Spirtos, N.M.; Potkul, R.K.; et al. Is bilateral lymphadenectomy for midline squamous carcinoma of the vulva always necessary? An analysis from Gynecologic Oncology Group (GOG) 173. Gynecol. Oncol. 2013, 128, 155–159. [Google Scholar] [CrossRef]
- Hampl, M.; Sarajuuri, H.; Wentzensen, N.; Bender, H.G.; Kueppers, V. Effect of human papillomavirus vaccines on vulvar, vaginal, and anal intraepithelial lesions and vulvar cancer. Obstet. Gynecol. 2006, 108, 1361–1368. [Google Scholar] [CrossRef]
- Serrano, B.; de Sanjose, S.; Tous, S.; Quiros, B.; Munoz, N.; Bosch, X.; Alemany, L. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur. J. Cancer 2015, 51, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Leodolter, S.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Koutsky, L.A.; Garland, S.M.; Harper, D.M.; Tang, G.W.; Ferris, D.G.; et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: A combined analysis of three randomised clinical trials. Lancet 2007, 369, 1693–1702. [Google Scholar] [CrossRef]
- Pils, S.; Gensthaler, L.; Alemany, L.; Horvat, R.; de Sanjose, S.; Joura, E.A. HPV prevalence in vulvar cancer in Austria. Wien. Klin. Wochenschr. 2017, 129, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.T.; Campbell, S.; Nygard, M. Long-term incidence trends of HPV-related cancers, and cases preventable by HPV vaccination: A registry-based study in Norway. BMJ Open 2018, 8, e019005. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Selk, A.; Garland, S.M.; Bogliatto, F.; Kyrgiou, M.; Weyers, S.; Arbyn, M. Prophylactic vaccination against human papillomaviruses to prevent vulval and vaginal cancer and their precursors. Expert Rev. Vaccines 2019, 18, 1157–1166. [Google Scholar] [CrossRef]
- Dehlendorff, C.; Baandrup, L.; Kjaer, S.K. Real-World Effectiveness of Human Papillomavirus Vaccination Against Vulvovaginal High-Grade Precancerous Lesions and Cancers. J. Natl. Cancer Inst. 2021, 113, 869–874. [Google Scholar] [CrossRef]
- Lehtinen, M.; Lagheden, C.; Luostarinen, T.; Eriksson, T.; Apter, D.; Bly, A.; Gray, P.; Harjula, K.; Heikkila, K.; Hokkanen, M.; et al. Human papillomavirus vaccine efficacy against invasive, HPV-positive cancers: Population-based follow-up of a cluster-randomised trial. BMJ Open 2021, 11, e050669. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.R.; Fasolino, C.; Santoro, G.; Gargano, V.; Rinaldi, M.; Arduino, B.; Belli, M.; Guida, M. Evaluation of Symptoms and Prevention of Cancer in Menopause: The Value of Vulvar Exam. Transl. Med. UniSa 2016, 15, 74–79. [Google Scholar] [PubMed]
- Halonen, P.; Jakobsson, M.; Heikinheimo, O.; Riska, A.; Gissler, M.; Pukkala, E. Lichen sclerosus and risk of cancer. Int. J. Cancer 2017, 140, 1998–2002. [Google Scholar] [CrossRef] [PubMed]
- Hacker, N.F. Revised FIGO staging for carcinoma of the vulva. Int. J. Gynaecol. Obstet. 2009, 105, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, J.; Bogliatto, F.; Haefner, H.K.; Stockdale, C.K.; Preti, M.; Bohl, T.G.; Reutter, J.; Committee, I.T. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) Terminology of Vulvar Squamous Intraepithelial Lesions. J. Low Genit. Tract. Dis. 2016, 20, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Crum, C.P. Vulvar intraepithelial neoplasia: The concept and its application. Hum. Pathol. 1982, 13, 187–189. [Google Scholar] [CrossRef]
- Wilkinson, E.J.; Kneale, B.; Lynch, P.J. Report of the ISSVD terminology committee. J. Reprod. Med. Obstet. Gynecol. 1986, 31, 973–974. [Google Scholar]
- Sideri, M.; Jones, R.W.; Wilkinson, E.J.; Preti, M.; Heller, D.S.; Scurry, J.; Haefner, H.; Neill, S. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J. Reprod. Med. 2005, 50, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Darragh, T.M.; Colgan, T.J.; Thomas Cox, J.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int. J. Gynecol. Pathol. 2013, 32, 76–115. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; World Health Organization: Geneva, Switzerland, 2014; Volume 6.
- Abell, M.R. Intraepithelial carcinomas of epidermis and squamous mucosa of vulva and perineum. Surg. Clin. N. Am. 1965, 45, 1179–1198. [Google Scholar] [CrossRef]
- Faber, M.T.; Sand, F.L.; Albieri, V.; Norrild, B.; Kjaer, S.K.; Verdoodt, F. Prevalence and type distribution of human papillomavirus in squamous cell carcinoma and intraepithelial neoplasia of the vulva. Int. J. Cancer 2017, 141, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Rakislova, N.; Clavero, O.; Alemany, L.; Saco, A.; Quiros, B.; Lloveras, B.; Alejo, M.; Pawlita, M.; Quint, W.; Del Pino, M.; et al. Histological characteristics of HPV-associated and -independent squamous cell carcinomas of the vulva: A study of 1,594 cases. Int. J. Cancer 2017, 141, 2517–2527. [Google Scholar] [CrossRef]
- Barlow, E.L.; Lambie, N.; Donoghoe, M.W.; Naing, Z.; Hacker, N.F. The Clinical Relevance of p16 and p53 Status in Patients with Squamous Cell Carcinoma of the Vulva. J. Oncol. 2020, 2020, 3739075. [Google Scholar] [CrossRef] [PubMed]
- Woelber, L.; Prieske, K.; Eulenburg, C.; Oliveira-Ferrer, L.; de Gregorio, N.; Klapdor, R.; Kalder, M.; Braicu, I.; Fuerst, S.; Klar, M.; et al. p53 and p16 expression profiles in vulvar cancer: A translational analysis by the Arbeitsgemeinschaft Gynakologische Onkologie Chemo and Radiotherapy in Epithelial Vulvar Cancer study group. Am. J. Obstet. Gynecol. 2021, 224, 595-e1–595-e11. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.N.; Park, K.J.; Soslow, R.A.; Murali, R. Squamous precursor lesions of the vulva: Current classification and diagnostic challenges. Pathology 2016, 48, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Sand, F.L.; Thomsen, S.F. Clinician’s Update on the Benign, Premalignant, and Malignant Skin Tumours of the Vulva: The Dermatologist’s View. Int. Sch. Res. Notices 2017, 2017, 2414569. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Bieber, A.K.; Stein, J.A.; Pomeranz, M.K. Diagnosis and management of vulvar cancer: A review. J. Am. Acad. Dermatol. 2019, 81, 1387–1396. [Google Scholar] [CrossRef]
- Rogers, L.J.; Cuello, M.A. Cancer of the vulva. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Zweizig, S.; Korets, S.; Cain, J.M. Key concepts in management of vulvar cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 959–966. [Google Scholar] [CrossRef]
- Frumovitz, M.; Bodurka, D. Neoplastic diseases of the vulva: Lichen sclerosus, intraepithelial neoplasia, paget disease, and carcinoma. In Comprehensive Gynecology, 7th ed.; Elsevier: Philadelphia, PA, USA, 2017. [Google Scholar]
- Ayala, M.; Fatehi, M. Vulvar Intraepithelial Neoplasia; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bhalwal, A.B.; Nick, A.M.; Dos Reis, R.; Chen, C.L.; Munsell, M.F.; Ramalingam, P.; Salcedo, M.P.; Ramirez, P.T.; Sood, A.K.; Schmeler, K.M. Carcinoma of the Bartholin Gland: A Review of 33 Cases. Int. J. Gynecol. Cancer 2016, 26, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L. Pigmented vulvar lesions. Dermatol. Ther. 2010, 23, 449–457. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Wohlmuth-Wieser, I. Vulvar malignancies: An interdisciplinary perspective. JDDG J. Dtsch. Dermatol. Ges. 2019, 17, 1257–1276. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Greer, B.E.; Abu-Rustum, N.R.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Dizon, D.S.; et al. Vulvar Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 92–120. [Google Scholar] [CrossRef]
- Rigel, D.S.; Carucci, J.A. Malignant melanoma: Prevention, early detection, and treatment in the 21st century. CA Cancer J. Clin. 2000, 50, 215–236. [Google Scholar] [CrossRef]
- Hacker, N.F.; Eifel, P.J.; van der Velden, J. Cancer of the vulva. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S2), S76–S83. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Cuello, M.A.; Rogers, L.J. Cancer of the vulva: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 7–18. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.R.; Long, B.J.; Weaver, A.L.; McGree, M.E.; Bakkum-Gamez, J.N.; Brewer, J.D.; Cliby, W.A. Evidence-Based Screening Recommendations for Occult Cancers in the Setting of Newly Diagnosed Extramammary Paget Disease. Mayo Clin. Proc. 2018, 93, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Chen, C.Y.; Liu, F.Y.; Yang, L.Y.; Huang, H.J.; Huang, Y.T.; Jung, S.M.; Chou, H.H.; Lai, C.H.; Ng, K.K. Computed tomography, magnetic resonance imaging and FDG positron emission tomography in the management of vulvar malignancies. Eur. Radiol. 2015, 25, 1267–1278. [Google Scholar] [CrossRef]
- Shetty, A.S.; Menias, C.O. MR Imaging of Vulvar and Vaginal Cancer. Magn. Reson. Imaging Clin. 2017, 25, 481–502. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.J.; Chin, R.I.; Hui, C.; Mutch, D.G.; Powell, M.A.; Schwarz, J.K.; Grigsby, P.W.; Markovina, S. Improved survival with definitive chemoradiation compared to definitive radiation alone in squamous cell carcinoma of the vulva: A review of the National Cancer Database. Gynecol. Oncol. 2017, 146, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Bidzinski, M.; Brannstrom, M.; Landoni, F.; Mahner, S.; Mahantshetty, U.; Mirza, M.; Petersen, C.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients With Vulvar Cancer. Int. J. Gynecol. Cancer 2017, 27, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, T.H.; Hakim, A.A.; Lee, S.J.; Wakabayashi, M.T.; Morgan, R.J.; Han, E.S. Surgical Management of Vulvar Cancer. J. Natl. Compr. Cancer Netw. 2017, 15, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, L.; Preti, M. Surgery of the vulva in vulvar cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 1074–1087. [Google Scholar] [CrossRef]
- Saito, T.; Tabata, T.; Ikushima, H.; Yanai, H.; Tashiro, H.; Niikura, H.; Minaguchi, T.; Muramatsu, T.; Baba, T.; Yamagami, W.; et al. Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of vulvar cancer and vaginal cancer. Int. J. Clin. Oncol. 2018, 23, 201–234. [Google Scholar] [CrossRef]
- van den Einden, L.C.; Massuger, L.F.; Jonkman, J.K.; Bult, P.; de Hullu, J.A.; Bulten, J. An alternative way to measure the depth of invasion of vulvar squamous cell carcinoma in relation to prognosis. Mod. Pathol. 2015, 28, 295–302. [Google Scholar] [CrossRef]
- Skala, S.L.; Ebott, J.A.; Zhao, L.; Lieberman, R.W. Predictive Value of an Alternative Strategy for Measuring Depth and Size of Stage 1 Vulvar Squamous Cell Carcinoma. J. Low Genit. Tract Dis. 2020, 24, 265–271. [Google Scholar] [CrossRef]
- Hacker, N.F.; Barlow, E.L. Staging for vulvar cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 802–811. [Google Scholar] [CrossRef]
- Andersen, B.L.; Hacker, N.F. Psychosexual adjustment after vulvar surgery. Obstet. Gynecol. 1983, 62, 457–462. [Google Scholar]
- de Hullu, J.A.; van der Zee, A.G. Surgery and radiotherapy in vulvar cancer. Crit. Rev. Oncol. Hematol. 2006, 60, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Podratz, K.C.; Symmonds, R.E.; Taylor, W.F.; Williams, T.J. Carcinoma of the vulva: Analysis of treatment and survival. Obstet. Gynecol. 1983, 61, 63–74. [Google Scholar] [PubMed]
- Carlson, J.W.; Kauderer, J.; Walker, J.L.; Gold, M.A.; O’Malley, D.; Tuller, E.; Clarke-Pearson, D.L.; Gynecologic Oncology, G. A randomized phase III trial of VH fibrin sealant to reduce lymphedema after inguinal lymph node dissection: A Gynecologic Oncology Group study. Gynecol. Oncol. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- DiSaia, P.J.; Creasman, W.T.; Rich, W.M. An alternate approach to early cancer of the vulva. Am. J. Obstet. Gynecol. 1979, 133, 825–832. [Google Scholar] [CrossRef]
- Hacker, N.F.; Berek, J.S.; Lagasse, L.D.; Nieberg, R.K.; Leuchter, R.S. Individualization of treatment for stage I squamous cell vulvar carcinoma. Obstet. Gynecol. 1984, 63, 155–162. [Google Scholar]
- Braakhuis, B.J.; Tabor, M.P.; Kummer, J.A.; Leemans, C.R.; Brakenhoff, R.H. A genetic explanation of Slaughter’s concept of field cancerization: Evidence and clinical implications. Cancer Res. 2003, 63, 1727–1730. [Google Scholar] [PubMed]
- Ansink, A.; van der Velden, J. Surgical interventions for early squamous cell carcinoma of the vulva. Cochrane Database Syst. Rev. 2000, 2, CD002036. [Google Scholar] [CrossRef]
- De Hullu, J.A.; Hollema, H.; Lolkema, S.; Boezen, M.; Boonstra, H.; Burger, M.P.; Aalders, J.G.; Mourits, M.J.; Van Der Zee, A.G. Vulvar carcinoma. The price of less radical surgery. Cancer 2002, 95, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Heaps, J.M.; Fu, Y.S.; Montz, F.J.; Hacker, N.F.; Berek, J.S. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol. Oncol. 1990, 38, 309–314. [Google Scholar] [CrossRef]
- Rouzier, R.; Haddad, B.; Plantier, F.; Dubois, P.; Pelisse, M.; Paniel, B.J. Local relapse in patients treated for squamous cell vulvar carcinoma: Incidence and prognostic value. Obstet. Gynecol. 2002, 100, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Te Grootenhuis, N.C.; Pouwer, A.W.; de Bock, G.H.; Hollema, H.; Bulten, J.; van der Zee, A.G.J.; de Hullu, J.A.; Oonk, M.H.M. Margin status revisited in vulvar squamous cell carcinoma. Gynecol. Oncol. 2019, 154, 266–275. [Google Scholar] [CrossRef]
- Bedell, S.M.; Hedberg, C.; Griffin, A.; Pearson, H.; Wilhite, A.; Rubin, N.; Erickson, B.K. Role of adjuvant radiation or re-excision for early stage vulvar squamous cell carcinoma with positive or close surgical margins. Gynecol. Oncol. 2019, 154, 276–279. [Google Scholar] [CrossRef]
- Barlow, E.L.; Jackson, M.; Hacker, N.F. The Prognostic Role of the Surgical Margins in Squamous Vulvar Cancer: A Retrospective Australian Study. Cancers 2020, 12, 3375. [Google Scholar] [CrossRef] [PubMed]
- Groenen, S.M.; Timmers, P.J.; Burger, C.W. Recurrence rate in vulvar carcinoma in relation to pathological margin distance. Int. J. Gynecol. Cancer 2010, 20, 869–873. [Google Scholar] [CrossRef]
- Baiocchi, G.; Mantoan, H.; de Brot, L.; Badiglian-Filho, L.; Kumagai, L.Y.; Faloppa, C.C.; da Costa, A.A. How important is the pathological margin distance in vulvar cancer? Eur. J. Surg. Oncol. 2015, 41, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Woelber, L.; Griebel, L.F.; Eulenburg, C.; Sehouli, J.; Jueckstock, J.; Hilpert, F.; de Gregorio, N.; Hasenburg, A.; Ignatov, A.; Hillemanns, P.; et al. Role of tumour-free margin distance for loco-regional control in vulvar cancer-a subset analysis of the Arbeitsgemeinschaft Gynakologische Onkologie CaRE-1 multicenter study. Eur. J. Cancer 2016, 69, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Woelber, L.; Choschzick, M.; Eulenburg, C.; Hager, M.; Jaenicke, F.; Gieseking, F.; Kock, L.; Ihnen, M.; Petersen, C.; Schwarz, J.; et al. Prognostic value of pathological resection margin distance in squamous cell cancer of the vulva. Ann. Surg. Oncol. 2011, 18, 3811–3818. [Google Scholar] [CrossRef] [PubMed]
- Arvas, M.; Kahramanoglu, I.; Bese, T.; Turan, H.; Sozen, I.; Ilvan, S.; Demirkiran, F. The Role of Pathological Margin Distance and Prognostic Factors After Primary Surgery in Squamous Cell Carcinoma of the Vulva. Int. J. Gynecol. Cancer 2018, 28, 623–631. [Google Scholar] [CrossRef]
- Pleunis, N.; Leermakers, M.E.J.; van der Wurff, A.A.; Klinkhamer, P.; Ezendam, N.P.M.; Boll, D.; de Hullu, J.A.; Pijnenborg, J.M.A. Surgical margins in squamous cell carcinoma, different for the vulva? Eur. J. Surg. Oncol. 2018, 44, 1555–1561. [Google Scholar] [CrossRef]
- Yang, J.; Delara, R.; Ghaith, S.; Newman, H.; Magrina, J.; Butler, K.; Kumar, A.; Dinh, T.; Chen, L.; Magtibay, P. Tumor-free margins and local recurrence in squamous cell carcinoma of the vulva. Gynecol. Oncol. 2020, 158, 555–561. [Google Scholar] [CrossRef]
- Preti, M.; Ronco, G.; Ghiringhello, B.; Micheletti, L. Recurrent squamous cell carcinoma of the vulva: Clinicopathologic determinants identifying low risk patients. Cancer 2000, 88, 1869–1876. [Google Scholar] [CrossRef]
- Nooij, L.S.; van der Slot, M.A.; Dekkers, O.M.; Stijnen, T.; Gaarenstroom, K.N.; Creutzberg, C.L.; Smit, V.T.; Bosse, T.; van Poelgeest, M.I. Tumour-free margins in vulvar squamous cell carcinoma: Does distance really matter? Eur. J. Cancer 2016, 65, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Pinto, A.P.; Schultz, D.; Berkowitz, R.; Crum, C.P. Relationship of margin status and radiation dose to recurrence in post-operative vulvar carcinoma. Gynecol. Oncol. 2013, 130, 545–549. [Google Scholar] [CrossRef]
- Micheletti, L.; Preti, M.; Cintolesi, V.; Corvetto, E.; Privitera, S.; Palmese, E.; Benedetto, C. Prognostic impact of reduced tumor-free margin distance on long-term survival in FIGO stage IB/II vulvar squamous cell carcinoma. J. Gynecol. Oncol. 2018, 29, e61. [Google Scholar] [CrossRef]
- Chan, J.K.; Sugiyama, V.; Pham, H.; Gu, M.; Rutgers, J.; Osann, K.; Cheung, M.K.; Berman, M.L.; Disaia, P.J. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: A multivariate analysis. Gynecol. Oncol. 2007, 104, 636–641. [Google Scholar] [CrossRef]
- Nooij, L.S.; Brand, F.A.; Gaarenstroom, K.N.; Creutzberg, C.L.; de Hullu, J.A.; van Poelgeest, M.I. Risk factors and treatment for recurrent vulvar squamous cell carcinoma. Crit. Rev. Oncol. Hematol. 2016, 106, 1–13. [Google Scholar] [CrossRef]
- Te Grootenhuis, N.C.; van der Zee, A.G.; van Doorn, H.C.; van der Velden, J.; Vergote, I.; Zanagnolo, V.; Baldwin, P.J.; Gaarenstroom, K.N.; van Dorst, E.B.; Trum, J.W.; et al. Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol. Oncol. 2016, 140, 8–14. [Google Scholar] [CrossRef]
- Pouwer, A.; te Grootenhuis, N.; de Bock, G.; Hollema, H.; Bulten, J.; van der Zee, A.; de Hullu, J.; Oonk, M. Local Recurrence in Vulvar Carcinoma; Incidence and Prognostic Impact of Pathological Margin Distance and Lichen Sclerosus. In Proceedings of the International Journal of Gynecological Cancer; 2017; p. 2001. [Google Scholar]
- Vulvar Cancer, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1476 (accessed on 29 March 2022).
- Tock, S.; Wallet, J.; Belhadia, M.; Hudry, D.; Ghesquiere, L.; Narducci, F.; Leblanc, E. Outcomes of the use of different vulvar flaps for reconstruction during surgery for vulvar cancer. Eur. J. Surg. Oncol. 2019, 45, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.; Bryant, A.; Barton, D.P.; Pomel, C.; Naik, R. Exenterative surgery for recurrent gynaecological malignancies. Cochrane Database Syst. Rev. 2014, 2, CD010449. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Schmitt, A.R.; Weaver, A.L.; McGree, M.; Bakkum-Gamez, J.N.; Brewer, J.; Cliby, W.A. A matter of margins: Surgical and pathologic risk factors for recurrence in extramammary Paget’s disease. Gynecol. Oncol. 2017, 147, 358–363. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Wohlmuth-Wieser, I.; May, T.; Vicus, D.; Gien, L.T.; Laframboise, S. Malignant Melanoma of the Vulva and Vagina: A US Population-Based Study of 1863 Patients. Am. J. Clin. Dermatol. 2020, 21, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Berek, J.S.; Hacker, N.F. Berek & Hacker’s Gynecologic Oncology, 7th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2015. [Google Scholar]
- Frey, J.N.; Hampl, M.; Mueller, M.D.; Gunthert, A.R. Should Groin Recurrence Still Be Considered as a Palliative Situation in Vulvar Cancer Patients?: A Brief Report. Int. J. Gynecol. Cancer 2016, 26, 575–579. [Google Scholar] [CrossRef]
- Groningen International Study on Sentinel Nods in Vulvar Cancer-III (GROINSS-VIII). Available online: https://clinicaltrials.gov/ct2/show/NCT05076942 (accessed on 24 June 2022).
- Diehl, A.; Volland, R.; Kirn, V.; Thangarajah, F.; Eichler, C.; Einzmann, T.; Wirtz, M.; Ratiu, D.; Morgenstern, B.; Fridrich, C.; et al. The number of removed lymph nodes by inguinofemoral lymphadenectomy: Impact on recurrence rates in patients with vulva carcinoma. Arch. Gynecol. Obstet. 2016, 294, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Siller, B.S.; Alvarez, R.D.; Conner, W.D.; McCullough, C.H.; Kilgore, L.C.; Partridge, E.E.; Austin, J.M. T2/3 vulva cancer: A case-control study of triple incision versus en bloc radical vulvectomy and inguinal lymphadenectomy. Gynecol. Oncol. 1995, 57, 335–339. [Google Scholar] [CrossRef]
- Levenback, C.; Burke, T.W.; Gershenson, D.M.; Morris, M.; Malpica, A.; Ross, M.I. Intraoperative lymphatic mapping for vulvar cancer. Obstet. Gynecol. 1994, 84, 163–167. [Google Scholar]
- Van der Zee, A.G.; Oonk, M.H.; De Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; Van Dorst, E.B.; et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J. Clin. Oncol. 2008, 26, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Gitas, G.; Proppe, L.; Baum, S.; Kruggel, M.; Rody, A.; Tsolakidis, D.; Zouzoulas, D.; Lagana, A.S.; Guenther, V.; Freytag, D.; et al. A risk factor analysis of complications after surgery for vulvar cancer. Arch. Gynecol. Obstet. 2021, 304, 511–519. [Google Scholar] [CrossRef]
- Levenback, C.F.; Ali, S.; Coleman, R.L.; Gold, M.A.; Fowler, J.M.; Judson, P.L.; Bell, M.C.; De Geest, K.; Spirtos, N.M.; Potkul, R.K.; et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: A gynecologic oncology group study. J. Clin. Oncol. 2012, 30, 3786–3791. [Google Scholar] [CrossRef] [PubMed]
- Brincat, M.R.; Muscat Baron, Y. Sentinel Lymph Node Biopsy in the Management of Vulvar Carcinoma: An Evidence-Based Insight. Int. J. Gynecol. Cancer 2017, 27, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, A.; Bidzinski, M.; Rzepka, J.; Piatek, S. Sentinel lymph node in vulvar cancer. Chin. Clin. Oncol. 2021, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, O.; Farouk, O.; El-Badrawy, A.; Denewer, A.; Setit, A. Sentinel lymph node biopsy in breast cancer guided by CT lymphography; History, evolution and current applications. Breast Dis. 2021, 40, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Vidal-Sicart, S.; Paez, D.; Pellet, O.; Enrique, E.L.; Mikhail-Lette, M.; Morozova, O.; Maria Camila, N.M.; Diana Ivonne, R.S.; Delgado Bolton, R.C.; et al. Sentinel Lymph Node Methods in Breast Cancer. Semin. Nucl. Med. 2022, 52, 551–560. [Google Scholar] [CrossRef]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; Puleo, C.A.; Coventry, B.J.; et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 2014, 370, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Faries, M.B.; Kennedy, E.B.; Agarwala, S.S.; Akhurst, T.J.; Ariyan, C.; Balch, C.M.; Berman, B.S.; Cochran, A.; Delman, K.A.; et al. Sentinel Lymph Node Biopsy and Management of Regional Lymph Nodes in Melanoma: American Society of Clinical Oncology and Society of Surgical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Coit, D.G.; Thompson, J.A.; Albertini, M.R.; Barker, C.; Carson, W.E.; Contreras, C.; Daniels, G.A.; DiMaio, D.; Fields, R.C.; Fleming, M.D. Cutaneous melanoma, version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 367–402. [Google Scholar] [CrossRef]
- Lawrie, T.A.; Patel, A.; Martin-Hirsch, P.P.; Bryant, A.; Ratnavelu, N.D.; Naik, R.; Ralte, A. Sentinel node assessment for diagnosis of groin lymph node involvement in vulval cancer. Cochrane Database Syst. Rev. 2014, 6, CD010409. [Google Scholar] [CrossRef]
- Stehman, F.B.; Ali, S.; DiSaia, P.J. Node count and groin recurrence in early vulvar cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2009, 113, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.; van Hemel, B.M.; Hollema, H.; de Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: Results from GROINSS-V, a multicentre observational study. Lancet Oncol. 2010, 11, 646–652. [Google Scholar] [CrossRef]
- Beneder, C.; Fuechsel, F.G.; Krause, T.; Kuhn, A.; Mueller, M.D. The role of 3D fusion imaging in sentinel lymphadenectomy for vulvar cancer. Gynecol. Oncol. 2008, 109, 76–80. [Google Scholar] [CrossRef]
- Siegenthaler, F.; Imboden, S.; Knabben, L.; Mohr, S.; Papadia, A.; Mueller, M.D. Exploratory Study of the Clinical Value of Near-Infrared Sentinel Lymph Node Mapping With Indocyanine Green in Vulvar Cancer Patients. Front. Oncol. 2021, 11, 652458. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Oonk, M.H.; Abu-Rustum, N.R. Sentinel lymph node biopsy in the management of gynecologic cancer. Curr. Opin. Obstet. Gynecol. 2015, 27, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, F.P.; Tummers, Q.R.; Rietbergen, D.D.; Peters, A.A.; Schaafsma, B.E.; van de Velde, C.J.; Frangioni, J.V.; van Leeuwen, F.W.; Gaarenstroom, K.N.; Vahrmeijer, A.L. Sentinel Lymph Node Biopsy in Vulvar Cancer Using Combined Radioactive and Fluorescence Guidance. Int. J. Gynecol. Cancer 2015, 25, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Darin, M.C.; Gomez-Hidalgo, N.R.; Westin, S.N.; Soliman, P.T.; Escobar, P.F.; Frumovitz, M.; Ramirez, P.T. Role of Indocyanine Green in Sentinel Node Mapping in Gynecologic Cancer: Is Fluorescence Imaging the New Standard? J. Minim Invasive Gynecol. 2016, 23, 186–193. [Google Scholar] [CrossRef]
- Papadia, A.; Imboden, S.; Siegenthaler, F.; Gasparri, M.L.; Mohr, S.; Lanz, S.; Mueller, M.D. Laparoscopic Indocyanine Green Sentinel Lymph Node Mapping in Endometrial Cancer. Ann. Surg. Oncol. 2016, 23, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Ruscito, I.; Gasparri, M.L.; Braicu, E.I.; Bellati, F.; Raio, L.; Sehouli, J.; Mueller, M.D.; Panici, P.B.; Papadia, A. Sentinel Node Mapping in Cervical and Endometrial Cancer: Indocyanine Green Versus Other Conventional Dyes-A Meta-Analysis. Ann. Surg. Oncol. 2016, 23, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Plante, M.; Lee, P.S.; Sandadi, S.; Lilja, J.F.; Escobar, P.F.; Gien, L.T.; Urbauer, D.L.; Abu-Rustum, N.R. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018, 19, 1394–1403. [Google Scholar] [CrossRef]
- Buda, A.; Crivellaro, C.; Elisei, F.; Di Martino, G.; Guerra, L.; De Ponti, E.; Cuzzocrea, M.; Giuliani, D.; Sina, F.; Magni, S.; et al. Impact of Indocyanine Green for Sentinel Lymph Node Mapping in Early Stage Endometrial and Cervical Cancer: Comparison with Conventional Radiotracer (99m)Tc and/or Blue Dye. Ann. Surg. Oncol. 2016, 23, 2183–2191. [Google Scholar] [CrossRef]
- Crane, L.M.; Themelis, G.; Arts, H.J.; Buddingh, K.T.; Brouwers, A.H.; Ntziachristos, V.; van Dam, G.M.; van der Zee, A.G. Intraoperative near-infrared fluorescence imaging for sentinel lymph node detection in vulvar cancer: First clinical results. Gynecol. Oncol. 2011, 120, 291–295. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Nakamura, Y.; Kawachi, Y.; Otsuka, F. Indocyanine green fluorescence-navigated sentinel node biopsy showed higher sensitivity than the radioisotope or blue dye method, which may help to reduce false-negative cases in skin cancer. J. Surg. Oncol. 2012, 106, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Hutteman, M.; van der Vorst, J.R.; Gaarenstroom, K.N.; Peters, A.A.; Mieog, J.S.; Schaafsma, B.E.; Lowik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Optimization of near-infrared fluorescent sentinel lymph node mapping for vulvar cancer. Am. J. Obstet. Gynecol. 2012, 206, 89-e1–89-e5. [Google Scholar] [CrossRef]
- Matheron, H.M.; van den Berg, N.S.; Brouwer, O.R.; Kleinjan, G.H.; van Driel, W.J.; Trum, J.W.; Vegt, E.; Kenter, G.; van Leeuwen, F.W.; Valdes Olmos, R.A. Multimodal surgical guidance towards the sentinel node in vulvar cancer. Gynecol. Oncol. 2013, 131, 720–725. [Google Scholar] [CrossRef]
- Schaafsma, B.E.; Verbeek, F.P.; Peters, A.A.; van der Vorst, J.R.; de Kroon, C.D.; van Poelgeest, M.I.; Trimbos, J.B.; van de Velde, C.J.; Frangioni, J.V.; Vahrmeijer, A.L.; et al. Near-infrared fluorescence sentinel lymph node biopsy in vulvar cancer: A randomised comparison of lymphatic tracers. BJOG 2013, 120, 758–764. [Google Scholar] [CrossRef]
- Soergel, P.; Hertel, H.; Nacke, A.K.; Klapdor, R.; Derlin, T.; Hillemanns, P. Sentinel Lymphadenectomy in Vulvar Cancer Using Near-Infrared Fluorescence From Indocyanine Green Compared With Technetium 99m Nanocolloid. Int. J. Gynecol. Cancer 2017, 27, 805–812. [Google Scholar] [CrossRef] [PubMed]
- KleinJan, G.H.; van Werkhoven, E.; van den Berg, N.S.; Karakullukcu, M.B.; Zijlmans, H.; van der Hage, J.A.; van de Wiel, B.A.; Buckle, T.; Klop, W.M.C.; Horenblas, S.; et al. The best of both worlds: A hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1915–1925. [Google Scholar] [CrossRef]
- Prader, S.; du Bois, A.; Harter, P.; Breit, E.; Schneider, S.; Baert, T.; Heitz, F.; Traut, A.; Ehmann, S.; Pauly, N.; et al. Sentinel lymph node mapping with fluorescent and radioactive tracers in vulvar cancer patients. Arch. Gynecol. Obstet. 2020, 301, 729–736. [Google Scholar] [CrossRef]
- Deken, M.M.; van Doorn, H.C.; Verver, D.; Boogerd, L.S.F.; de Valk, K.S.; Rietbergen, D.D.D.; van Poelgeest, M.I.E.; de Kroon, C.D.; Beltman, J.J.; van Leeuwen, F.W.B.; et al. Near-infrared fluorescence imaging compared to standard sentinel lymph node detection with blue dye in patients with vulvar cancer—A randomized controlled trial. Gynecol. Oncol. 2020, 159, 672–680. [Google Scholar] [CrossRef]
- Laios, A.; Volpi, D.; Tullis, I.D.; Woodward, M.; Kennedy, S.; Pathiraja, P.N.; Haldar, K.; Vojnovic, B.; Ahmed, A.A. A prospective pilot study of detection of sentinel lymph nodes in gynaecological cancers using a novel near infrared fluorescence imaging system. BMC Res. Notes 2015, 8, 608. [Google Scholar] [CrossRef]
- Broach, V.; Abu-Rustum, N.R.; Sonoda, Y.; Brown, C.L.; Jewell, E.; Gardner, G.; Chi, D.S.; Zivanovic, O.; Leitao, M.M., Jr. Evolution and outcomes of sentinel lymph node mapping in vulvar cancer. Int. J. Gynecol. Cancer 2020, 30, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Homesley, H.D.; Bundy, B.N.; Sedlis, A.; Adcock, L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet. Gynecol. 1986, 68, 733–740. [Google Scholar] [PubMed]
- Stehman, F.B.; Bundy, B.N.; Dvoretsky, P.M.; Creasman, W.T. Early stage I carcinoma of the vulva treated with ipsilateral superficial inguinal lymphadenectomy and modified radical hemivulvectomy: A prospective study of the Gynecologic Oncology Group. Obstet. Gynecol. 1992, 79, 490–497. [Google Scholar] [PubMed]
- Stehman, F.B.; Bundy, B.N.; Thomas, G.; Varia, M.; Okagaki, T.; Roberts, J.; Bell, J.; Heller, P.B. Groin dissection versus groin radiation in carcinoma of the vulva: A Gynecologic Oncology Group study. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 389–396. [Google Scholar] [CrossRef]
- Kunos, C.; Simpkins, F.; Gibbons, H.; Tian, C.; Homesley, H. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: A randomized controlled trial. Obstet. Gynecol. 2009, 114, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Benedet, J.L.; Miller, D.M.; Ehlen, T.G.; Bertrand, M.A. Basal cell carcinoma of the vulva: Clinical features and treatment results in 28 patients. Obstet. Gynecol. 1997, 90, 765–768. [Google Scholar] [CrossRef]
- Dalton, A.K.; Wan, K.M.; Gomes, D.; Wyatt, J.M.; Oehler, M.K. Inguinal Metastasis from Basal Cell Carcinoma of the Vulva. Case Rep. Oncol. 2019, 12, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M., Jr. Management of vulvar and vaginal melanomas: Current and future strategies. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e277–e281. [Google Scholar] [CrossRef]
- Bhatla, N.; Tomar, S.; Meena, J.; Sharma, D.N.; Kumar, L. Adjuvant treatment in cervical, vaginal and vulvar cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 78, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.M.; Slomovitz, B.; Baldwin, P.J.W.; van Doorn, H.C.; van der Velden, J.; de Hullu, J.A.; Gaarenstroom, K.N.; Slangen, B.F.M.; Vergote, I.; Brannstrom, M.; et al. Radiotherapy Versus Inguinofemoral Lymphadenectomy as Treatment for Vulvar Cancer Patients With Micrometastases in the Sentinel Node: Results of GROINSS-V II. J. Clin. Oncol. 2021, 39, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Bernard, M.E.; Lin, J.F.; Balasubramani, G.K.; Rajagopalan, M.S.; Sukumvanich, P.; Krivak, T.C.; Olawaiye, A.B.; Kelley, J.L.; Beriwal, S. Impact of adjuvant chemotherapy with radiation for node-positive vulvar cancer: A National Cancer Data Base (NCDB) analysis. Gynecol. Oncol. 2015, 137, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Mahner, S.; Jueckstock, J.; Hilpert, F.; Neuser, P.; Harter, P.; de Gregorio, N.; Hasenburg, A.; Sehouli, J.; Habermann, A.; Hillemanns, P.; et al. Adjuvant therapy in lymph node-positive vulvar cancer: The AGO-CaRE-1 study. J. Natl. Cancer. Inst. 2015, 107, dju426. [Google Scholar] [CrossRef] [PubMed]
- Klapdor, R.; Hillemanns, P.; Wolber, L.; Juckstock, J.; Hilpert, F.; de Gregorio, N.; Iborra, S.; Sehouli, J.; Habermann, A.; Furst, S.T.; et al. Outcome After Sentinel Lymph Node Dissection in Vulvar Cancer: A Subgroup Analysis of the AGO-CaRE-1 Study. Ann. Surg. Oncol. 2017, 24, 1314–1321. [Google Scholar] [CrossRef]
- Woelber, L.; Prieske, K.; Eulenburg, C.Z.; Corradini, S.; Petersen, C.; Bommert, M.; Blankenstein, T.; Hilpert, F.; de Gregorio, N.; Iborra, S.; et al. Adjuvant radiotherapy and local recurrence in vulvar cancer—A subset analysis of the AGO-CaRE-1 study. Gynecol. Oncol. 2022, 164, 68–75. [Google Scholar] [CrossRef]
- Serre, E.; Raimond, E.; Diguisto, C.; Bendifallah, S.; Body, G.; Touboul, C.; Graesslin, O.; Carcopino, X.; Darai, E.; Ouldamer, L.; et al. Inguino-femoral radiotherapy in vulvar squamous cell carcinoma: Clues to revised indications in patients with only one intracapsular lymph node metastasis. Acta Oncol. 2020, 59, 518–524. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, J.; Pleunis, N.; Barlow, E.; Zijlmans, H.; de Hullu, J.; Hacker, N.F.; Fons, G. Radiotherapy is not indicated in patients with vulvar squamous cell carcinoma and only one occult intracapsular groin node metastasis. Gynecol. Oncol. 2021, 160, 128–133. [Google Scholar] [CrossRef]
- Homesley, H.D.; Bundy, B.N.; Sedlis, A.; Yordan, E.; Berek, J.S.; Jahshan, A.; Mortel, R. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study). Am. J. Obstet. Gynecol. 1991, 164, 997–1003; discussion 1003–1004. [Google Scholar] [CrossRef]
- Oonk, M.; Slomovitz, B.; Baldwin, P.; Van Doorn, H.; Van Der Velden, J.; De Hullu, J.; Slangen, B.; Gaarenstroom, K.; Vergote, I.; Brannstrom, M. Radiotherapy instead of inguinofemoral lymphadenectomy in vulvar cancer patients with a metastatic sentinel node: Results of GROINSS-V II. Int. J. Gynecol. Cancer 2019, 29, 3623–3632. [Google Scholar]
- Observation in Patients with Early-Stage Vulvar Cancer Undergoing Sentinel Lymph Node Dissection (GROINSS-V). Available online: https://clinicaltrials.gov/ct2/show/NCT01500512 (accessed on 29 March 2022).
- Chow, S.; Karam, A. Role of sentinel lymph node biopsy for gynecologic cancers. Curr. Opin. Obstet. Gynecol. 2022, 34, 15–19. [Google Scholar] [CrossRef]
- Nica, A.; Covens, A.; Vicus, D.; Kupets, R.; Osborne, R.; Cesari, M.; Gien, L.T. Sentinel lymph nodes in vulvar cancer: Management dilemmas in patients with positive nodes and larger tumors. Gynecol. Oncol. 2019, 152, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.; van Os, M.A.; de Bock, G.H.; de Hullu, J.A.; Ansink, A.C.; van der Zee, A.G. A comparison of quality of life between vulvar cancer patients after sentinel lymph node procedure only and inguinofemoral lymphadenectomy. Gynecol. Oncol. 2009, 113, 301–305. [Google Scholar] [CrossRef]
- Maggino, T.; Landoni, F.; Sartori, E.; Zola, P.; Gadducci, A.; Alessi, C.; Solda, M.; Coscio, S.; Spinetti, G.; Maneo, A.; et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF Study. Cancer 2000, 89, 116–122. [Google Scholar] [CrossRef]
- Gonzalez Bosquet, J.; Magrina, J.F.; Gaffey, T.A.; Hernandez, J.L.; Webb, M.J.; Cliby, W.A.; Podratz, K.C. Long-term survival and disease recurrence in patients with primary squamous cell carcinoma of the vulva. Gynecol. Oncol. 2005, 97, 828–833. [Google Scholar] [CrossRef]
- Elit, L.; Reade, C.J. Recommendations for Follow-up Care for Gynecologic Cancer Survivors. Obstet. Gynecol. 2015, 126, 1207–1214. [Google Scholar] [CrossRef]
- Salani, R.; Backes, F.J.; Fung, M.F.; Holschneider, C.H.; Parker, L.P.; Bristow, R.E.; Goff, B.A. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am. J. Obstet. Gynecol. 2011, 204, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Salani, R.; Khanna, N.; Frimer, M.; Bristow, R.E.; Chen, L.M. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol. Oncol. 2017, 146, 3–10. [Google Scholar] [CrossRef]
- Pouwer, A.W.; Mus, R.; IntHout, J.; van der Zee, A.; Bulten, J.; Massuger, L.; de Hullu, J.A. The efficacy of ultrasound in the follow up after a negative sentinel lymph node in women with vulvar cancer: A prospective single-centre study. BJOG 2018, 125, 1461–1468. [Google Scholar] [CrossRef]
- Gadducci, A.; Tana, R.; Barsotti, C.; Guerrieri, M.E.; Genazzani, A.R. Clinico-pathological and biological prognostic variables in squamous cell carcinoma of the vulva. Crit. Rev. Oncol. Hematol. 2012, 83, 71–83. [Google Scholar] [CrossRef]
- Woelber, L.; Mahner, S.; Voelker, K.; Eulenburg, C.Z.; Gieseking, F.; Choschzick, M.; Jaenicke, F.; Schwarz, J. Clinicopathological prognostic factors and patterns of recurrence in vulvar cancer. Anticancer Res. 2009, 29, 545–552. [Google Scholar]
- Fonseca-Moutinho, J.A. Recurrent vulvar cancer. Clin. Obstet. Gynecol. 2005, 48, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Grimes, W.R.; Stratton, M. Pelvic Exenteration; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]

| Stage | Description |
|---|---|
| I | Tumor confined to the vulva |
| IA | Tumor size ≤ 2 cm and stromal invasion ≤ 1 mm a |
| IB | Tumor size > 2 cm or stromal invasion > 1 mm a |
| II | Tumor of any size with extension to lower one-third of the urethra, lower one-third of the vagina, lower one-third of the anus with negative nodes |
| III | Tumor of any size with extension to the upper part of adjacent perineal structures, or with any number of non-fixed, non-ulcerated lymph nodes |
| IIIA | Tumor of any size with disease extension to the upper two-thirds of the urethra, upper two-thirds of the vagina, bladder mucosa, rectal mucosa, or regional lymph node metastases ≤ 5 mm |
| IIIB | Regional b lymph node metastases > 5 mm |
| IIIC | Regional b lymph node metastases with extracapsular spread |
| IV | Tumors of any size fixed to bone, or fixed, ulcerated lymph node metastases, or distant metastases |
| IVA | Disease fixed to pelvic bone or fixed or ulcerated regional b lymph node metastases |
| IVB | Distant metastases |
| Variable | Months | Years | ||
|---|---|---|---|---|
| 0–12 | 12–24 | 3–5 | >5 | |
| Physical examination | Every 3–6 months | Every 3–6 months | Every 6–12 months | Yearly |
| Papanicolaou test/cytologic evidence | Yearly a | |||
| Radiographic imaging b | Insufficient data to support routine use | |||
| Recurrence suspected | CT scans or PET/CT scans | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrão, P.G.; Guimarães, Y.M.; Godoy, L.R.; Possati-Resende, J.C.; Bovo, A.C.; Andrade, C.E.M.C.; Longatto-Filho, A.; dos Reis, R. Management of Early-Stage Vulvar Cancer. Cancers 2022, 14, 4184. https://doi.org/10.3390/cancers14174184
Pedrão PG, Guimarães YM, Godoy LR, Possati-Resende JC, Bovo AC, Andrade CEMC, Longatto-Filho A, dos Reis R. Management of Early-Stage Vulvar Cancer. Cancers. 2022; 14(17):4184. https://doi.org/10.3390/cancers14174184
Chicago/Turabian StylePedrão, Priscila Grecca, Yasmin Medeiros Guimarães, Luani Rezende Godoy, Júlio César Possati-Resende, Adriane Cristina Bovo, Carlos Eduardo Mattos Cunha Andrade, Adhemar Longatto-Filho, and Ricardo dos Reis. 2022. "Management of Early-Stage Vulvar Cancer" Cancers 14, no. 17: 4184. https://doi.org/10.3390/cancers14174184
APA StylePedrão, P. G., Guimarães, Y. M., Godoy, L. R., Possati-Resende, J. C., Bovo, A. C., Andrade, C. E. M. C., Longatto-Filho, A., & dos Reis, R. (2022). Management of Early-Stage Vulvar Cancer. Cancers, 14(17), 4184. https://doi.org/10.3390/cancers14174184









