Clinical Management of Neuroendocrine Neoplasms in Clinical Practice: A Formal Consensus Exercise
Abstract
:Simple Summary
Abstract
1. Introduction
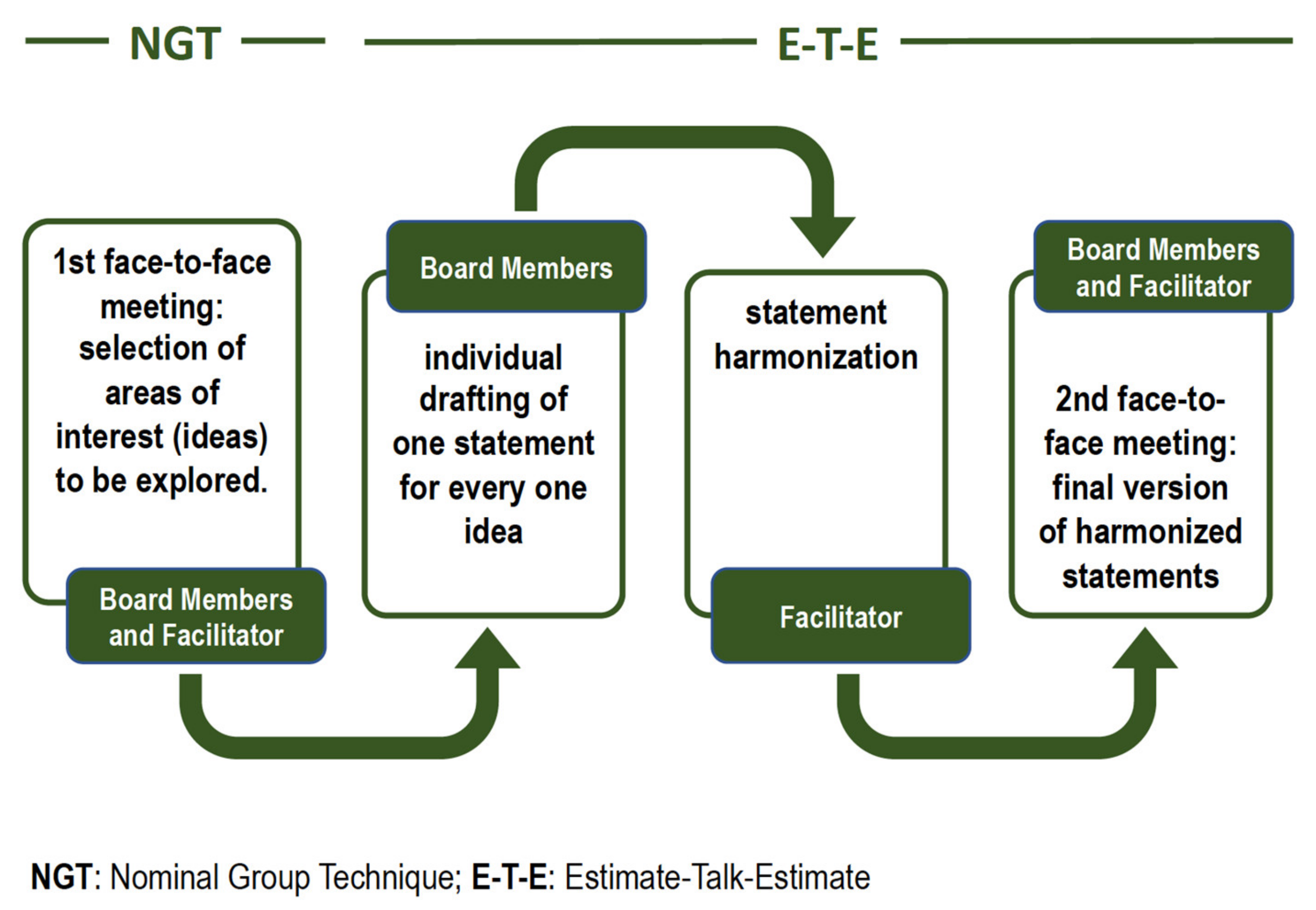
2. Materials and Methods
3. Results
3.1. Multidisciplinary Management
3.2. Baseline Prognostic Characterization
3.3. Watchful Waiting
3.4. Follow-Up of Radically Resected NENs
3.5. Therapeutic Strategies
3.6. Informed Consent for RLT
3.7. Dosimetry of RLT
3.8. Management of Patients with Comorbidities
3.9. Management of Therapy with SSA during RLT
3.10. Evaluation of Tumor Response (Morphological vs. Functional and Clinical) after RLT
3.11. Follow-Up after RLT
3.12. Off-Label Use of RLT
3.13. Approach to Patients with Bone Metastases
3.14. Role of Patient-Reported Outcomes in Management
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, L.; Dai, S.; Chen, M.; Li, F.; Sun, J.; Luo, F. Epidemiologic Trends of and Factors Associated With Overall Survival for Patients With Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw. Open 2021, 4, e2124750. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; De Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Kaderli, R.M.; Spanjol, M.; Kollar, A.; Butikofer, L.; Gloy, V.; Dumont, R.A.; Seiler, C.A.; Christ, E.R.; Radojewski, P.; Briel, M.; et al. Therapeutic Options for Neuroendocrine Tumors: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2019, 5, 480–489. [Google Scholar] [CrossRef]
- NCCN. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf (accessed on 15 February 2022).
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Pavel, M.; Oberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Delbecq, A.L.; Van de Ven, A.H. A Group Process Model for Problem Identification and Program Planning. J. Appl. Behav. Sci. 1971, 7, 466–492. [Google Scholar] [CrossRef]
- Rohrbaugh, J. Improving the quality of group judgment: Social judgment analysis and the nominal group technique. Organ. Behav. Hum. Perform. 1981, 28, 272–288. [Google Scholar] [CrossRef]
- Rowe Wright, G. Expert opinions in forecasting: Role of the Delphi technique. In Principles of Forecasting; Armstrong, J.S., Ed.; Kluwer Academic Press: Norwell, MA, USA, 2001. [Google Scholar]
- Gustafson, D.H.; Shukla, R.K.; Delbecq, A.; Walster, G.W. A comparative study of differences in subjective likelihood estimates made by individuals, interacting groups, Delphi groups, and nominal groups. Organ. Behav. Hum. Perform. 1973, 9, 280–291. [Google Scholar] [CrossRef]
- Gallego, D.; Bueno, S. Exploring the application of the Delphi method as a forecasting tool in Information Systems and Technologies research. Technol. Anal. Strateg. Manag. 2014, 26, 987–999. [Google Scholar] [CrossRef]
- Singh, S.; Law, C. Multidisciplinary reference centers: The care of neuroendocrine tumors. J. Oncol. Pract. 2010, 6, e11–e16. [Google Scholar] [CrossRef] [Green Version]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Kloppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Ramage, J.K.; De Herder, W.W.; Delle Fave, G.; Ferolla, P.; Ferone, D.; Ito, T.; Ruszniewski, P.; Sundin, A.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 139–143. [Google Scholar] [CrossRef]
- Ramage, J.K.; Punia, P.; Faluyi, O.; Frilling, A.; Meyer, T.; Saharan, R.; Valle, J.W. Observational Study to Assess Quality of Life in Patients with Pancreatic Neuroendocrine Tumors Receiving Treatment with Everolimus: The OBLIQUE Study (UK Phase IV Trial). Neuroendocrinology 2019, 108, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Nockel, P.; Babic, B.; Millo, C.; Herscovitch, P.; Patel, D.; Nilubol, N.; Sadowski, S.M.; Cochran, C.; Gorden, P.; Kebebew, E. Localization of Insulinoma Using 68Ga-DOTATATE PET/CT Scan. J. Clin. Endocrinol. Metab. 2017, 102, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Crown, A.; Rocha, F.G.; Raghu, P.; Lin, B.; Funk, G.; Alseidi, A.; Hubka, M.; Rosales, J.; Lee, M.; Kennecke, H. Impact of initial imaging with gallium-68 dotatate PET/CT on diagnosis and management of patients with neuroendocrine tumors. J. Surg. Oncol. 2020, 121, 480–485. [Google Scholar] [CrossRef]
- Ezziddin, S.; Adler, L.; Sabet, A.; Poppel, T.D.; Grabellus, F.; Yuce, A.; Fischer, H.P.; Simon, B.; Holler, T.; Biersack, H.J.; et al. Prognostic stratification of metastatic gastroenteropancreatic neuroendocrine neoplasms by 18F-FDG PET: Feasibility of a metabolic grading system. J. Nucl. Med. 2014, 55, 1260–1266. [Google Scholar] [CrossRef] [Green Version]
- Rinzivillo, M.; Partelli, S.; Prosperi, D.; Capurso, G.; Pizzichini, P.; Iannicelli, E.; Merola, E.; Muffatti, F.; Scopinaro, F.; Schillaci, O.; et al. Clinical Usefulness of (18)F-Fluorodeoxyglucose Positron Emission Tomography in the Diagnostic Algorithm of Advanced Entero-Pancreatic Neuroendocrine Neoplasms. Oncologist 2018, 23, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Pulvirenti, A.; Rao, D.; McIntyre, C.A.; Gonen, M.; Tang, L.H.; Klimstra, D.S.; Fleisher, M.; Ramanathan, L.V.; Reidy-Lagunes, D.; Allen, P.J. Limited role of Chromogranin A as clinical biomarker for pancreatic neuroendocrine tumors. HPB 2019, 21, 612–618. [Google Scholar] [CrossRef]
- Genc, C.G.; Jilesen, A.P.; Partelli, S.; Falconi, M.; Muffatti, F.; van Kemenade, F.J.; van Eeden, S.; Verheij, J.; Van Dieren, S.; Van Eijck, C.H.J.; et al. A New Scoring System to Predict Recurrent Disease in Grade 1 and 2 Nonfunctional Pancreatic Neuroendocrine Tumors. Ann. Surg. 2018, 267, 1148–1154. [Google Scholar] [CrossRef]
- Pulvirenti, A.; Javed, A.A.; Landoni, L.; Jamieson, N.B.; Chou, J.F.; Miotto, M.; He, J.; Gonen, M.; Pea, A.; Tang, L.H.; et al. Multi-institutional Development and External Validation of a Nomogram to Predict Recurrence After Curative Resection of Pancreatic Neuroendocrine Tumors. Ann. Surg. 2019, 274, 1051–1057. [Google Scholar] [CrossRef]
- Zaidi, M.Y.; Lopez-Aguiar, A.G.; Switchenko, J.M.; Lipscomb, J.; Andreasi, V.; Partelli, S.; Gamboa, A.C.; Lee, R.M.; Poultsides, G.A.; Dillhoff, M.; et al. A Novel Validated Recurrence Risk Score to Guide a Pragmatic Surveillance Strategy After Resection of Pancreatic Neuroendocrine Tumors: An International Study of 1006 Patients. Ann. Surg. 2019, 270, 422–433. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. Neoplasms of the neuroendocrine pancreas. In WHO Classification of Tumours of Endocrine Organs, 4th ed.; WHO/IARC Classification of Tumours; WHO: Geneva, Switzerland, 2017; Volume 10, pp. 209–239. [Google Scholar]
- Crino, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Conti Bellocchi, M.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology 2021, 21, 443–450. [Google Scholar] [CrossRef]
- Genc, C.G.; Falconi, M.; Partelli, S.; Muffatti, F.; van Eeden, S.; Doglioni, C.; Klumpen, H.J.; Van Eijck, C.H.J.; Nieveen van Dijkum, E.J.M. Recurrence of Pancreatic Neuroendocrine Tumors and Survival Predicted by Ki67. Ann. Surg. Oncol. 2018, 25, 2467–2474. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.; Zheng, Z.; Bi, J.; Wang, X.; Feng, Q. Risk Factors for Lymph Node Metastasis and Survival Outcomes in Colorectal Neuroendocrine Tumors. Cancer Manag. Res. 2020, 12, 7151–7164. [Google Scholar] [CrossRef]
- Fazio, N. Watch and wait policy in advanced neuroendocrine tumors: What does it mean? World J. Clin. Oncol. 2017, 8, 96–99. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Cwikla, J.B.; Phan, A.T.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Muller, H.H.; Arnold, R.; Group, P.S. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef]
- Howe, J.R.; Merchant, N.B.; Conrad, C.; Keutgen, X.M.; Hallet, J.; Drebin, J.A.; Minter, R.M.; Lairmore, T.C.; Tseng, J.F.; Zeh, H.J.; et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 1–33. [Google Scholar] [CrossRef]
- Partelli, S.; Bartsch, D.K.; Capdevila, J.; Chen, J.; Knigge, U.; Niederle, B.; Nieveen van Dijkum, E.J.M.; Pape, U.F.; Pascher, A.; Ramage, J.; et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017, 105, 255–265. [Google Scholar] [CrossRef] [PubMed]
- De Mestier, L.; Lepage, C.; Baudin, E.; Coriat, R.; Courbon, F.; Couvelard, A.; Do Cao, C.; Frampas, E.; Gaujoux, S.; Gincul, R.; et al. Digestive Neuroendocrine Neoplasms (NEN): French Intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig. Liver Dis. 2020, 52, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Knigge, U.; Capdevila, J.; Bartsch, D.K.; Baudin, E.; Falkerby, J.; Kianmanesh, R.; Kos-Kudla, B.; Niederle, B.; Nieveen van Dijkum, E.; O’Toole, D.; et al. ENETS Consensus Recommendations for the Standards of Care in Neuroendocrine Neoplasms: Follow-Up and Documentation. Neuroendocrinology 2017, 105, 310–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Moody, L.; Chan, D.L.; Metz, D.C.; Strosberg, J.; Asmis, T.; Bailey, D.L.; Bergsland, E.; Brendtro, K.; Carroll, R.; et al. Follow-up Recommendations for Completely Resected Gastroenteropancreatic Neuroendocrine Tumors. JAMA Oncol. 2018, 4, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.L.; Frilling, A.; de Herder, W.W.; Horsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. (177)Lu-Dotatate plus long-acting octreotide versus highdose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Pozzari, M.; Maisonneuve, P.; Spada, F.; Berruti, A.; Amoroso, V.; Cella, C.A.; Laffi, A.; Pellicori, S.; Bertani, E.; Fazio, N. Systemic therapies in patients with advanced well-differentiated pancreatic neuroendocrine tumors (PanNETs): When cytoreduction is the aim. A critical review with meta-analysis. Cancer Treat. Rev. 2018, 71, 39–46. [Google Scholar] [CrossRef]
- Citterio, D.; Pusceddu, S.; Facciorusso, A.; Coppa, J.; Milione, M.; Buzzoni, R.; Bongini, M.; deBraud, F.; Mazzaferro, V. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur. J. Surg. Oncol. 2017, 43, 380–387. [Google Scholar] [CrossRef]
- Partelli, S.; Cirocchi, R.; Rancoita, P.M.V.; Muffatti, F.; Andreasi, V.; Crippa, S.; Tamburrino, D.; Falconi, M. A Systematic review and meta-analysis on the role of palliative primary resection for pancreatic neuroendocrine neoplasm with liver metastases. HPB 2018, 20, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Tovazzi, V.; Ferrari, V.D.; Dalla Volta, A.; Consoli, F.; Amoroso, V.; Berruti, A. Should everolimus be stopped after radiological progression in metastatic insulinoma? A “cons” point of view. Endocrine 2020, 69, 481–484. [Google Scholar] [CrossRef]
- Berruti, A.; Pia, A.; Terzolo, M. Advances in pancreatic neuroendocrine tumor treatment. N. Engl. J. Med. 2011, 364, 1871–1872. [Google Scholar]
- Kennedy, A.; Bester, L.; Salem, R.; Sharma, R.A.; Parks, R.W.; Ruszniewski, P. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): Guidelines from the NET-Liver-Metastases Consensus Conference. HPB 2015, 17, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Hicks, R.J.; Kwekkeboom, D.J.; Krenning, E.; Bodei, L.; Grozinsky-Glasberg, S.; Arnold, R.; Borbath, I.; Cwikla, J.; Toumpanakis, C.; Kaltsas, G.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology 2017, 105, 295–309. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Bison, S.M.; Konijnenberg, M.W.; Melis, M.; Pool, S.E.; Bernsen, M.R.; Teunissen, J.J.; Kwekkeboom, D.J.; De Jong, M. Peptide receptor radionuclide therapy using radiolabeled somatostatin analogs: Focus on future developments. Clin. Transl. Imaging 2014, 2, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Sandstrom, M.; Garske-Roman, U.; Granberg, D.; Johansson, S.; Widstrom, C.; Eriksson, B.; Sundin, A.; Lundqvist, H.; Lubberink, M. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J. Nucl. Med. 2013, 54, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Garske-Roman, U.; Sandstrom, M.; Fross Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of (177)Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 970–988. [Google Scholar] [CrossRef] [Green Version]
- Bodei, L.; Cremonesi, M.; Ferrari, M.; Pacifici, M.; Grana, C.M.; Bartolomei, M.; Baio, S.M.; Sansovini, M.; Paganelli, G. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: The role of associated risk factors. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1847–1856. [Google Scholar] [CrossRef]
- Yordanova, A.; Wicharz, M.M.; Mayer, K.; Brossart, P.; Gonzalez-Carmona, M.A.; Strassburg, C.P.; Fimmers, R.; Essler, M.; Ahmadzadehfar, H. The Role of Adding Somatostatin Analogues to Peptide Receptor Radionuclide Therapy as a Combination and Maintenance Therapy. Clin. Cancer Res. 2018, 24, 4672–4679. [Google Scholar] [CrossRef] [Green Version]
- Prasad, V.; Srirajaskanthan, R.; Toumpanakis, C.; Grana, C.M.; Baldari, S.; Shah, T.; Lamarca, A.; Courbon, F.; Scheidhauer, K.; Baudin, E.; et al. Lessons from a multicentre retrospective study of peptide receptor radionuclide therapy combined with lanreotide for neuroendocrine tumours: A need for standardised practice. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2358–2371. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Carbonero, R.; Garcia-Figueiras, R.; Carmona-Bayonas, A.; Sevilla, I.; Teule, A.; Quindos, M.; Grande, E.; Capdevila, J.; Aller, J.; Arbizu, J.; et al. Imaging approaches to assess the therapeutic response of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Current perspectives and future trends of an exciting field in development. Cancer Metastasis Rev. 2015, 34, 823–842. [Google Scholar] [CrossRef] [Green Version]
- Huizing, D.M.V.; Aalbersberg, E.A.; Versleijen, M.W.J.; Tesselaar, M.E.T.; Walraven, I.; Lahaye, M.J.; De Wit-van der Veen, B.J.; Stokkel, M.P.M. Early response assessment and prediction of overall survival after peptide receptor radionuclide therapy. Cancer Imaging 2020, 20, 57. [Google Scholar] [CrossRef]
- Haug, A.R.; Auernhammer, C.J.; Wangler, B.; Schmidt, G.P.; Uebleis, C.; Goke, B.; Cumming, P.; Bartenstein, P.; Tiling, R.; Hacker, M. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J. Nucl. Med. 2010, 51, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Ramage, J.; Naraev, B.G.; Halfdanarson, T.R. Peptide receptor radionuclide therapy for patients with advanced pancreatic neuroendocrine tumors. Semin. Oncol. 2018, 45, 236–248. [Google Scholar] [CrossRef]
- Sansovini, M.; Severi, S.; Ianniello, A.; Nicolini, S.; Fantini, L.; Mezzenga, E.; Ferroni, F.; Scarpi, E.; Monti, M.; Bongiovanni, A.; et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with (177)Lu-D OTATATE. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 490–499. [Google Scholar] [CrossRef]
- Swiha, M.M.; Sutherland, D.E.K.; Sistani, G.; Khatami, A.; Abazid, R.M.; Mujoomdar, A.; Wiseman, D.P.; Romsa, J.G.; Reid, R.H.; Laidley, D.T. Survival predictors of (177)Lu-Dotatate peptide receptor radionuclide therapy (PRRT) in patients with progressive well-differentiated neuroendocrine tumors (NETS). J. Cancer Res. Clin. Oncol. 2021, 148, 225–236. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Carreras, C. Peptides and receptors in image-guided therapy: Theranostics for neuroendocrine neoplasms. Semin. Nucl. Med. 2012, 42, 190–207. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J. Radionuclide Therapy for Neuroendocrine Tumors. Curr. Oncol. Rep. 2017, 19, 9. [Google Scholar] [CrossRef]
- Kunikowska, J.; Krolicki, L.; Hubalewska-Dydejczyk, A.; Mikolajczak, R.; Sowa-Staszczak, A.; Pawlak, D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: Which is a better therapy option? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1788–1797. [Google Scholar] [CrossRef] [Green Version]
- Villard, L.; Romer, A.; Marincek, N.; Brunner, P.; Koller, M.T.; Schindler, C.; Ng, Q.K.; Macke, H.R.; Muller-Brand, J.; Rochlitz, C.; et al. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J. Clin. Oncol. 2012, 30, 1100–1106. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, M.; Krenning, E.P.; Kam, B.L.; De Herder, W.W.; Feelders, R.A.; Kwekkeboom, D.J. Salvage therapy with (177)Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 2010, 51, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strosberg, J.; Leeuwenkamp, O.; Siddiqui, M.K. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 93, 102141. [Google Scholar] [CrossRef] [PubMed]
- Rudisile, S.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Boning, G.; Gildehaus, F.J.; Fendler, W.P.; Auernhammer, C.J.; Spitzweg, C.; Bartenstein, P.; et al. Salvage PRRT with (177)Lu-DOTA-octreotate in extensively pretreated patients with metastatic neuroendocrine tumor (NET): Dosimetry, toxicity, efficacy, and survival. BMC Cancer 2019, 19, 788. [Google Scholar] [CrossRef] [Green Version]
- Van der Zwan, W.A.; Brabander, T.; Kam, B.L.R.; Teunissen, J.J.M.; Feelders, R.A.; Hofland, J.; Krenning, E.P.; De Herder, W.W. Salvage peptide receptor radionuclide therapy with [(177)Lu-DOTA,Tyr(3)]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 704–717. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, E.; Machta, J.; Walker, M.; Toumpanakis, C.; Caplin, M.; Navalkissoor, S. Retreatment with peptide receptor radionuclide therapy in patients with progressing neuroendocrine tumours: Efficacy and prognostic factors for response. Br. J. Radiol. 2018, 91, 20180041. [Google Scholar] [CrossRef]
- Severi, S.; Sansovini, M.; Ianniello, A.; Bodei, L.; Nicolini, S.; Ibrahim, T.; Di Iorio, V.; D’Errico, V.; Caroli, P.; Monti, M.; et al. Feasibility and utility of re-treatment with (177)Lu-DOTATATE in GEP-NENs relapsed after treatment with (90)Y-DOTATOC. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1955–1963. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Singh, A.; Kaemmerer, D.; Mueller, D.; Prasad, V.; Hommann, M.; Robiller, F.C.; Niepsch, K.; Franz, H.; et al. Results and adverse events of personalized peptide receptor radionuclide therapy with (90)Yttrium and (177)Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 2018, 9, 16932–16950. [Google Scholar] [CrossRef] [Green Version]
- Sabet, A.; Haslerud, T.; Pape, U.F.; Sabet, A.; Ahmadzadehfar, H.; Grunwald, F.; Guhlke, S.; Biersack, H.J.; Ezziddin, S. Outcome and toxicity of salvage therapy with 177Lu-octreotate in patients with metastatic gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 205–210. [Google Scholar] [CrossRef]
- Sorbye, H.; Kong, G.; Grozinsky-Glasberg, S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr. Relat. Cancer 2020, 27, R67–R77. [Google Scholar] [CrossRef]
- Thang, S.P.; Lung, M.S.; Kong, G.; Hofman, M.S.; Callahan, J.; Michael, M.; Hicks, R.J. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN)—A single-institution retrospective analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 262–277. [Google Scholar] [CrossRef]
- Kashyap, R.; Hofman, M.S.; Michael, M.; Kong, G.; Akhurst, T.; Eu, P.; Zannino, D.; Hicks, R.J. Favourable outcomes of (177)Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 176–185. [Google Scholar] [CrossRef]
- Claringbold, P.G.; Price, R.A.; Turner, J.H. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother. Radiopharm. 2012, 27, 561–569. [Google Scholar] [CrossRef]
- Claringbold, P.G.; Turner, J.H. Pancreatic Neuroendocrine Tumor Control: Durable Objective Response to Combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy. Neuroendocrinology 2016, 103, 432–439. [Google Scholar] [CrossRef]
- Ostwal, V.; Basu, S.; Bhargava, P.; Shah, M.; Parghane, R.V.; Srinivas, S.; Chaudhari, V.; Bhandare, M.S.; Shrikhande, S.V.; Ramaswamy, A. Capecitabine-Temozolomide in Advanced Grade 2 and Grade 3 Neuroendocrine Neoplasms: Benefits of Chemotherapy in Neuroendocrine Neoplasms with Significant 18FDG Uptake. Neuroendocrinology 2021, 111, 998–1004. [Google Scholar] [CrossRef]
- Claringbold, P.G.; Turner, J.H. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): A Phase I Study. Cancer Biother. Radiopharm. 2015, 30, 261–269. [Google Scholar] [CrossRef]
- Kim, C.; Liu, S.V.; Subramaniam, D.S.; Torres, T.; Loda, M.; Esposito, G.; Giaccone, G. Phase I study of the (177)Lu-DOTA(0)-Tyr(3)-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J. Immunother. Cancer 2020, 8, e000980. [Google Scholar] [CrossRef]
- Cullinane, C.; Waldeck, K.; Kirby, L.; Rogers, B.E.; Eu, P.; Tothill, R.W.; Hicks, R.J. Enhancing the anti-tumour activity of (177)Lu-DOTA-octreotate radionuclide therapy in somatostatin receptor-2 expressing tumour models by targeting PARP. Sci. Rep. 2020, 10, 10196. [Google Scholar] [CrossRef]
- Strosberg, J.; Kunz, P.L.; Hendifar, A.; Yao, J.; Bushnell, D.; Kulke, M.H.; Baum, R.P.; Caplin, M.; Ruszniewski, P.; Delpassand, E.; et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with (177)Lu-Dotatate: An analysis of the NETTER-1 study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2372–2382. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, E.; Engin, M.N.; Ozkan, Z.G.; Kovan, B.; Buyukkaya, F.; Poyanli, A.; Saglam, S.; Basaran, M.; Turkmen, C. Y90 selective internal radiation therapy and peptide receptor radionuclide therapy for the treatment of metastatic neuroendocrine tumors: Combination or not? Nucl. Med. Commun. 2020, 41, 1242–1249. [Google Scholar] [CrossRef]
- Braat, A.; Bruijnen, R.C.G.; van Rooij, R.; Braat, M.; Wessels, F.J.; van Leeuwaarde, R.S.; van Treijen, M.J.C.; de Herder, W.W.; Hofland, J.; Tesselaar, M.E.T.; et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (HEPAR PLuS): A single-centre, single-arm, open-label, phase 2 study. Lancet Oncol. 2020, 21, 561–570. [Google Scholar] [CrossRef]
- Kunikowska, J.; Pawlak, D.; Bak, M.I.; Kos-Kudla, B.; Mikolajczak, R.; Krolicki, L. Long-term results and tolerability of tandem peptide receptor radionuclide therapy with (90)Y/(177)Lu-DOTATATE in neuroendocrine tumors with respect to the primary location: A 10-year study. Ann. Nucl. Med. 2017, 31, 347–356. [Google Scholar] [CrossRef]
- Seregni, E.; Maccauro, M.; Chiesa, C.; Mariani, L.; Pascali, C.; Mazzaferro, V.; De Braud, F.; Buzzoni, R.; Milione, M.; Lorenzoni, A.; et al. Treatment with tandem [90Y]DOTA-TATE and [177Lu]DOTA-TATE of neuroendocrine tumours refractory to conventional therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 223–230. [Google Scholar] [CrossRef]
- Claringbold, P.G.; Brayshaw, P.A.; Price, R.A.; Turner, J.H. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 302–311. [Google Scholar] [CrossRef]
- Nicolini, S.; Bodei, L.; Bongiovanni, A.; Sansovini, M.; Grassi, I.; Ibrahim, T.; Monti, M.; Caroli, P.; Sarnelli, A.; Diano, D.; et al. Combined use of 177Lu-DOTATATE and metronomic capecitabine (Lu-X) in FDG-positive gastro-entero-pancreatic neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3260–3267. [Google Scholar] [CrossRef]
- Kong, G.; Thompson, M.; Collins, M.; Herschtal, A.; Hofman, M.S.; Johnston, V.; Eu, P.; Michael, M.; Hicks, R.J. Assessment of predictors of response and long-term survival of patients with neuroendocrine tumour treated with peptide receptor chemoradionuclide therapy (PRCRT). Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1831–1844. [Google Scholar] [CrossRef] [Green Version]
- Hartrampf, P.E.; Hanscheid, H.; Kertels, O.; Schirbel, A.; Kreissl, M.C.; Flentje, M.; Sweeney, R.A.; Buck, A.K.; Polat, B.; Lapa, C. Long-term results of multimodal peptide receptor radionuclide therapy and fractionated external beam radiotherapy for treatment of advanced symptomatic meningioma. Clin. Transl. Radiat. Oncol. 2020, 22, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Braat, A.; Ahmadzadehfar, H.; Kappadath, S.C.; Stothers, C.L.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with (90)Y Resin Microspheres of Neuroendocrine Liver Metastases After Initial Peptide Receptor Radionuclide Therapy. Cardiovasc. Interv. Radiol. 2020, 43, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Bushnell, D.L.; Bodeker, K.L.; O’Dorisio, T.M.; Madsen, M.T.; Menda, Y.; Graves, S.; Zamba, G.K.D.; O’Dorisio, M.S. Addition of (131)I-MIBG to PRRT ((90)Y-DOTATOC) for Personalized Treatment of Selected Patients with Neuroendocrine Tumors. J. Nucl. Med. 2021, 62, 1274–1277. [Google Scholar] [CrossRef]
- Kos-Kudla, B.; O’Toole, D.; Falconi, M.; Gross, D.; Kloppel, G.; Sundin, A.; Ramage, J.; Oberg, K.; Wiedenmann, B.; Komminoth, P.; et al. ENETS consensus guidelines for the management of bone and lung metastases from neuroendocrine tumors. Neuroendocrinology 2010, 91, 341–350. [Google Scholar] [CrossRef]
- Putzer, D.; Gabriel, M.; Henninger, B.; Kendler, D.; Uprimny, C.; Dobrozemsky, G.; Decristoforo, C.; Bale, R.J.; Jaschke, W.; Virgolini, I.J. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J. Nucl. Med. 2009, 50, 1214–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadowski, S.M.; Neychev, V.; Millo, C.; Shih, J.; Nilubol, N.; Herscovitch, P.; Pacak, K.; Marx, S.J.; Kebebew, E. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J. Clin. Oncol. 2016, 34, 588–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, M.; He, I.; Luu, M.; David, J.; Gong, J.; Placencio-Hickok, V.R.; Reznik, R.S.; Tuli, R.; Hendifar, A.E. Palliative Radiation Therapy for Bone Metastases in Neuroendocrine Neoplasms. Adv. Radiat. Oncol. 2019, 4, 513–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cives, M.; Pelle, E.; Rinzivillo, M.; Prosperi, D.; Tucci, M.; Silvestris, F.; Panzuto, F. Bone Metastases in Neuroendocrine Tumors: Molecular Pathogenesis and Implications in Clinical Practice. Neuroendocrinology 2021, 111, 207–216. [Google Scholar] [CrossRef]
- Costa, L.; Major, P.P. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat. Clin. Pract. Oncol. 2009, 6, 163–174. [Google Scholar] [CrossRef]
- Ezziddin, S.; Sabet, A.; Heinemann, F.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Guhlke, S.; Holler, T.; Willinek, W.; Boy, C.; Biersack, H.J. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with (177)Lu-octreotate. J. Nucl. Med. 2011, 52, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Sabet, A.; Khalaf, F.; Haslerud, T.; Al-Zreiqat, A.; Sabet, A.; Simon, B.; Poppel, T.D.; Biersack, H.J.; Ezziddin, S. Bone metastases in GEP-NET: Response and long-term outcome after PRRT from a follow-up analysis. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 437–445. [Google Scholar]
- Newman, C.B.; Melmed, S.; Snyder, P.J.; Young, W.F.; Boyajy, L.D.; Levy, R.; Stewart, W.N.; Klibanski, A.; Molitch, M.E.; Gagel, R.F. Safety and efficacy of long-term octreotide therapy of acromegaly: Results of a multicenter trial in 103 patients—A clinical research center study. J. Clin. Endocrinol. Metab. 1995, 80, 2768–2775. [Google Scholar] [CrossRef]
- Ruszniewski, P.; Valle, J.W.; Lombard-Bohas, C.; Cuthbertson, D.J.; Perros, P.; Holubec, L.; Delle Fave, G.; Smith, D.; Niccoli, P.; Maisonobe, P.; et al. Patient-reported outcomes with lanreotide Autogel/Depot for carcinoid syndrome: An international observational study. Dig. Liver Dis. 2016, 48, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Granberg, D.; Wolin, E.; Warner, R.; Sissons, M.; Kolarova, T.; Goldstein, G.; Pavel, M.; Oberg, K.; Leyden, J. Patient-Reported Burden of a Neuroendocrine Tumor (NET) Diagnosis: Results From the First Global Survey of Patients With NETs. J. Glob. Oncol. 2017, 3, 43–53. [Google Scholar] [CrossRef]
- Wolin, E.M.; Leyden, J.; Goldstein, G.; Kolarova, T.; Hollander, R.; Warner, R.R.P. Patient-Reported Experience of Diagnosis, Management, and Burden of Neuroendocrine Tumors: Results From a Large Patient Survey in the United States. Pancreas 2017, 46, 639–647. [Google Scholar] [CrossRef]
- Snyder, C.F.; Aaronson, N.K. Use of patient-reported outcomes in clinical practice. Lancet 2009, 374, 369–370. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.S.; Singh, A.; van der Zwan, W.A.; Severi, S.; Drozdov, I.A.; Malczewska, A.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G.; et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: The NETest. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 895–906. [Google Scholar] [CrossRef]
- Roll, W.; Weckesser, M.; Seifert, R.; Bodei, L.; Rahbar, K. Imaging and liquid biopsy in the prediction and evaluation of response to PRRT in neuroendocrine tumors: Implications for patient management. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4016–4027. [Google Scholar] [CrossRef]
- Woodhouse, B.; Pattison, S.; Segelov, E.; Singh, S.; Parker, K.; Kong, G.; Macdonald, W.; Wyld, D.; Meyer-Rochow, G.; Pavlakis, N.; et al. Consensus-Derived Quality Performance Indicators for Neuroendocrine Tumour Care. J. Clin. Med. 2019, 8, 1455. [Google Scholar] [CrossRef] [Green Version]

| Statement | |
|---|---|
| 1. Multidisciplinary discussion | A network among “tumor boards” working on NEN patients is advisable NEN-dedicated multidisciplinary teams should adopt the same main criteria independently of local experience. |
| 2. Initial prognostic characterization | Initial prognostic characterization should be based on clinical information (functioning/non-functioning, performance status, comorbidity), histopathology (differentiation and grading), and morphological and functional imaging. There is no recommended definition of disease at high risk after radical surgery across NEN primary diseases. |
| 3. Watchful waiting | A watchful waiting strategy is generally not recommended in locally advanced/metastatic patients. |
| 4. Follow-up of radically resected NENs | Follow-up should be patient-tailored in patients with NEN after radical surgery and should include a panel of conventional tests, including circulating markers, plus a list of optional instrumental tests, chosen based on the characteristics of the tumor and patient. A patient-tailored long term follow-up strategy is still lacking and needs to be defined. The timing should be modulated on the basis of prognostic parameters, while strongly taking into account safety issues related to potentially invasive exams. |
| 5. Therapeutic strategies | There is poor evidence regarding a specific sequence or integration of various treatments in NENs. The therapeutic strategy with sequence and type of treatments should be decided in a tumor board considering the characteristics of the patient, literature data, and regulatory aspects. |
| 6. Informed consent for RLT | A standard informed consent form for RLT should be used. Informed consent should include specific information about the purpose, mode of execution, risk-benefit balance, and potential for early and late side effects, allowing optimization of communication about the risks, benefits, and possible alternative options, to provide the same level of information within all institutions. |
| 7. Dosimetry of RLT (for therapy) | Dosimetry evaluation should be recommended to prevent potential risks to bone marrow and kidney function to provide data to clinicians, especially in patients with long survival expectancy. |
| 8. Management of patients with comorbidities | Comorbidities not representing an absolute contraindication to RLT (i.e., severe hypertension, brittle diabetes, functioning tumors, concomitant meningioma, etc.) should require specific protocols. |
| 9. Management of therapy with SSA during RLT | SSA therapy should be continued during the entire course of RLT. Dosage may be adjusted in case of functioning tumors. |
| 10. Evaluation of response (morphological vs. functional and clinical) after RLT | Assessment of tumor response after RLT should carefully consider both morphological and functional imaging. However, the timing of imaging should be correlated with characteristics of the individual tumor. |
| 11. Follow-up after RLT | Follow-up should be patient-tailored and include morphological (CT and/or MRI) and/or functional (PET/CT with radiolabeled somatostatin analogs and/or FDG) imaging and biomarkers, chosen based on the characteristics of the tumor. The timing should be modulated based on prognostic parameters, while strongly considering safety issues. It is suggested to intercalate morphological and functional imaging to reduce the patient’s irradiation dose given the very long follow-up. |
| 12. Off-label use of RLT | Alternative schedules, means of administration, indications other than approved, and rechallenge should be limited to specific clinical studies. |
| 13. Approach to patients with bone metastases | Bone involvement with appropriate imaging techniques must be carefully assessed in patients with a metastatic NEN to identify those at risk of skeletal-related events. |
| 14. Role of PROs in management | Patient-reported outcomes (PROs) should be considered as a critical endpoint of benefit. Thus, guidelines should consider PROs, pointing out that their lack may have a bearing on the ultimate recommendation. |
| Study | Number of Patients | Initial RLT | Re-Treatment RLT | PFS (Months) | 95% CI |
|---|---|---|---|---|---|
| Sabet et al., 2014 [72] | 33 | 177Lu-DOTATATE | 177Lu-DOTATATE | 13.0 | 9.0–18.0 |
| Severi et al., 2015 [70] | 26 | 90Y-DOTATOC | 177Lu-DOTATATE | 9.0 | 5.0–17.0 |
| Vaughan et al., 2018 [69] | 47 | 177Lu-DOTATATE or 90Y-DOTATOC | 177Lu-DOTATATE or 90Y-DOTATOC | 17.5 | 11.0–23.8 |
| Baum et al. [71] | 470 | 177Lu-DOTATATE or 90Y-DOTATOC | 177Lu-DOTATATE or 90Y-DOTATOC | 11.0 | 9.4–12.5 |
| Van der Zwal et al., 2019 [68] | 168 | 177Lu-DOTATATE | 177Lu-DOTATATE | 14.6 | 12.4–19.6 |
| Rudisile S et al., 2019 [67] | 32 | 177Lu-DOTATATE | 177Lu-DOTATATE | 6.0 | 0.0–16.00 |
| Combination Partner | ORR (%) | OS (Months) | PFS (Months) | SAE (%) | Reference |
|---|---|---|---|---|---|
| SSA | 37 | NR | 48 | 3% hepatoxicity | [52,53,65] |
| Capecitabine | 24–30 | NR | 31 | 15% hematotoxicity | [87,88] |
| CAPTEM | 53–70 | NR | 22–48 | 6% hematotoxicity | [76,77,78] |
| 5-FU | 25 | NR | - | - | [89] |
| Everolimus | 44 | NR | 63 at 2 years | 100% hematotoxicity | [79] |
| EBRT | 0 | NR | 108 | - | [90] |
| Liver Embolization | |||||
| (90Y) | 16 | 42–68 | - | 50% liver enzyme elevation | [83,84,91] |
| (166Ho) | 43 | - | 10% abdominal pain | ||
| Dual RLT (177Lu/90Y) | 42 | 66–127 | - | 2% MDS | [63,85,86] |
| (177Lu/225Ac) | - | 7% hematotoxicity | |||
| MIBG (131I) | 0 | - | 33% thrombocytopenia | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomei, M.; Berruti, A.; Falconi, M.; Fazio, N.; Ferone, D.; Lastoria, S.; Pappagallo, G.; Seregni, E.; Versari, A. Clinical Management of Neuroendocrine Neoplasms in Clinical Practice: A Formal Consensus Exercise. Cancers 2022, 14, 2501. https://doi.org/10.3390/cancers14102501
Bartolomei M, Berruti A, Falconi M, Fazio N, Ferone D, Lastoria S, Pappagallo G, Seregni E, Versari A. Clinical Management of Neuroendocrine Neoplasms in Clinical Practice: A Formal Consensus Exercise. Cancers. 2022; 14(10):2501. https://doi.org/10.3390/cancers14102501
Chicago/Turabian StyleBartolomei, Mirco, Alfredo Berruti, Massimo Falconi, Nicola Fazio, Diego Ferone, Secondo Lastoria, Giovanni Pappagallo, Ettore Seregni, and Annibale Versari. 2022. "Clinical Management of Neuroendocrine Neoplasms in Clinical Practice: A Formal Consensus Exercise" Cancers 14, no. 10: 2501. https://doi.org/10.3390/cancers14102501
APA StyleBartolomei, M., Berruti, A., Falconi, M., Fazio, N., Ferone, D., Lastoria, S., Pappagallo, G., Seregni, E., & Versari, A. (2022). Clinical Management of Neuroendocrine Neoplasms in Clinical Practice: A Formal Consensus Exercise. Cancers, 14(10), 2501. https://doi.org/10.3390/cancers14102501







