Characterization of Poorly Cohesive and Signet Ring Cell Carcinomas and Identification of PTPRM as a Diagnostic Marker
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. GC Cases
2.2. Analysis of the mRNA Expression of Genes Related to Cancer Progression Using the NanoString Assay
2.3. Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.4. Immunohistochemical Staining
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of SRC, PC, and Combined PC/SRC Carcinomas
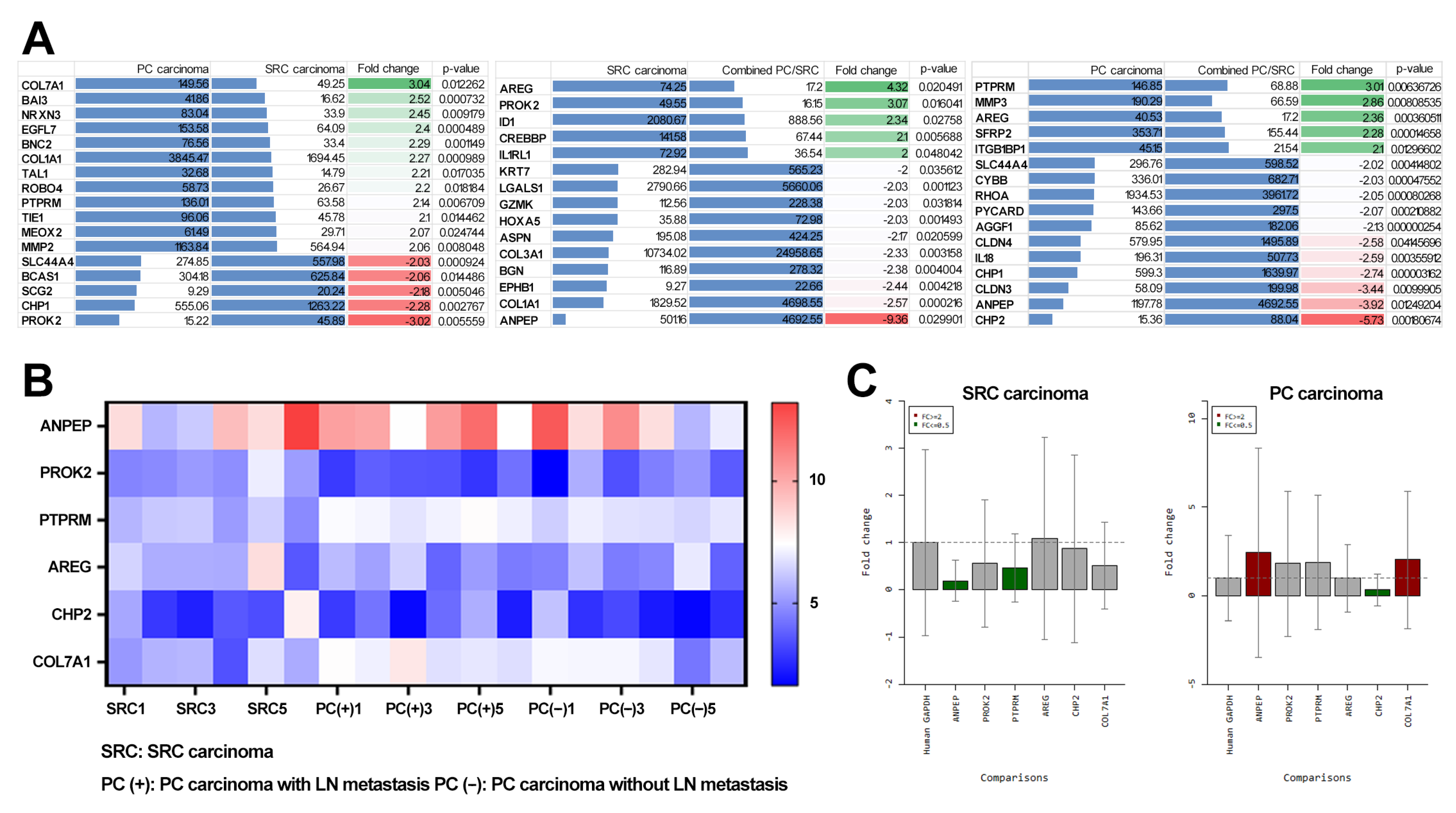
3.2. Transcriptome Analysis of SRC and PC Carcinomas
3.3. Identification of Diagnostic Markers for SRC and PC Carcinoma
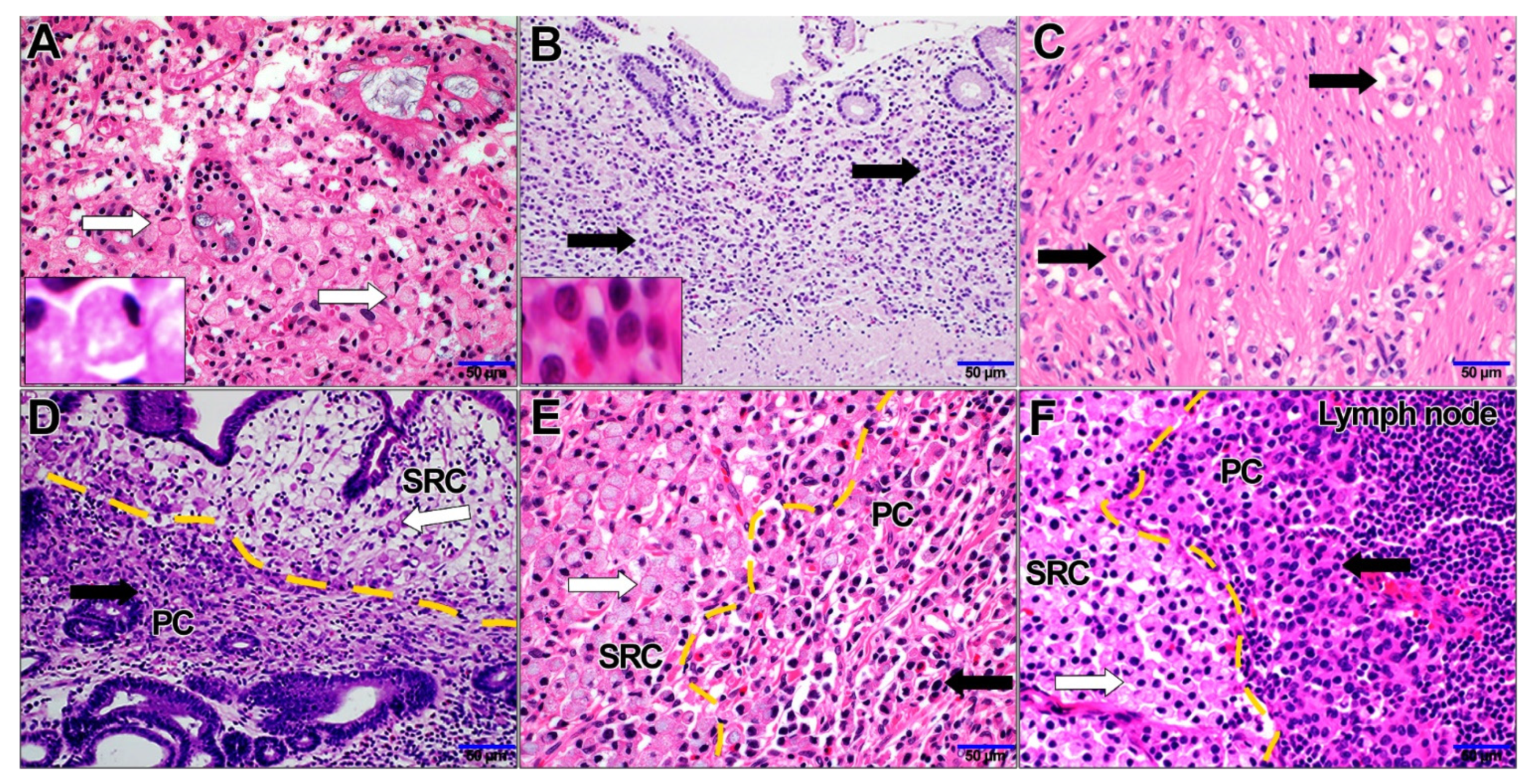
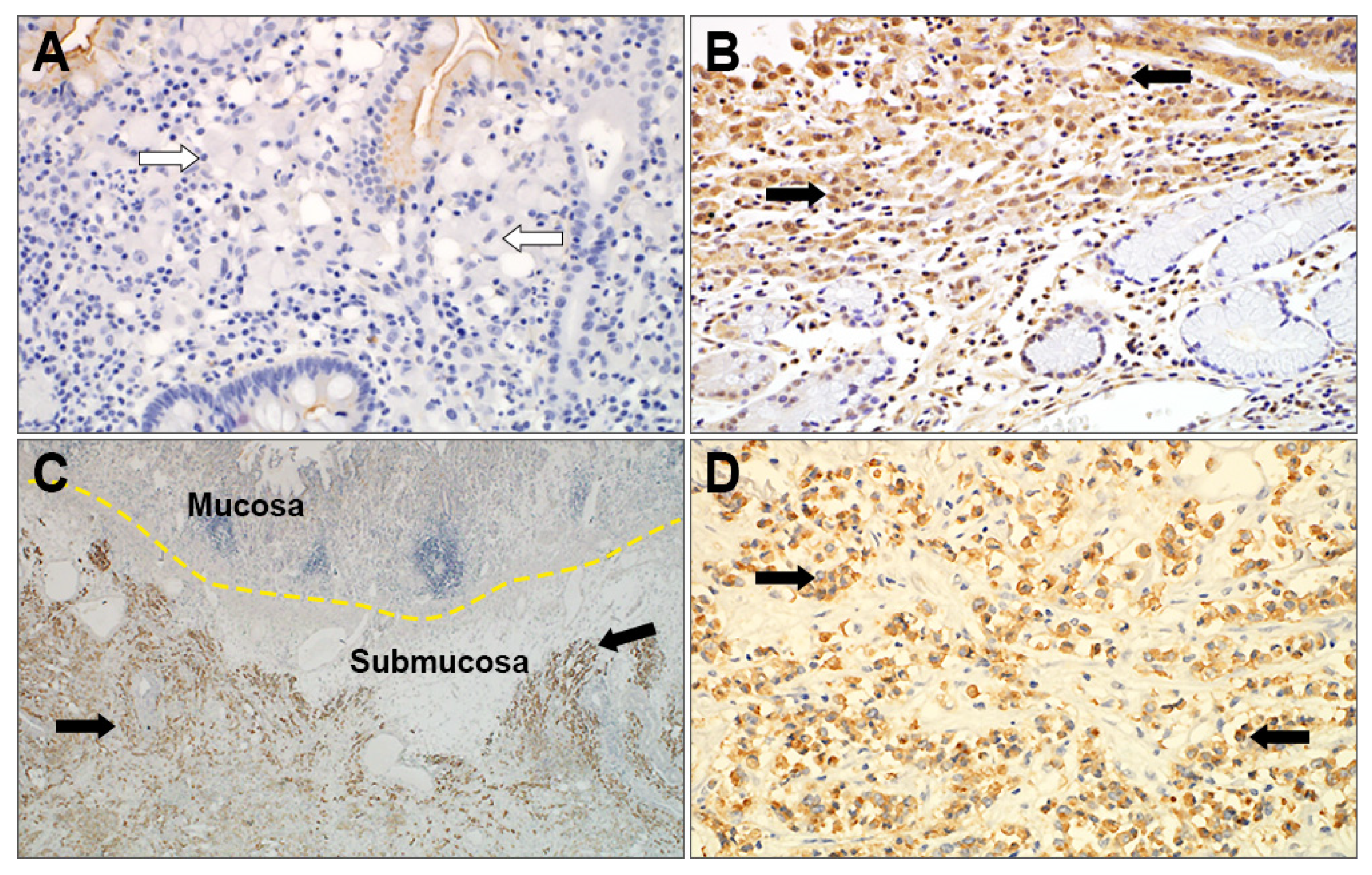
3.4. Immunohistochemical Analysis of SRC and PC Carcinomas and its Clinical Significance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Henson, D.E.; Dittus, C.; Younes, M.; Nguyen, H.; Albores-Saavedra, J.A. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: Increase in the signet ring cell type. Arch. Pathol. Lab. Med. 2004, 128, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma: An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System; World Health Organization: Geneva, Switzerland, 2010.
- Hamilton, S.R.; Aalttonen, L.A. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System; IARC Press: Lyon, France, 2000. [Google Scholar]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumors: Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019.
- Theuer, C.P.; Nastanski, F.; Brewster, W.R.; Butler, J.A.; Anton-Culver, H. Signet ring cell histology is associated with unique clinical features but does not affect gastric cancer survival. Am. Surg. 1999, 65, 915. [Google Scholar] [PubMed]
- Bamboat, Z.M.; Tang, L.H.; Vinuela, E.; Kuk, D.; Gonen, M.; Shah, M.A.; Brennan, M.F.; Coit, D.G.; Strong, V.E. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Annal. Surg. Oncol. 2014, 21, 1678–1685. [Google Scholar] [CrossRef]
- Liu, K.; Wan, J.; Bei, Y.; Chen, X.; Lu, M. Prognostic impact of different histological types on gastric adenocarcinoma: A surveillance, epidemiology, and end results database analysis. Pathol. Oncol. Res. 2017, 23, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Mariette, C.; Carneiro, F.; Grabsch, H.; Van der Post, R.; Allum, W.; de Manzoni, G. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer 2019, 22, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Park, J.H.; Park, C.K.; Kim, J.-H.; Lee, S.K.; Lee, Y.C.; Noh, S.H.; Kim, H. Histologic purity of signet ring cell carcinoma is a favorable risk factor for lymph node metastasis in poorly cohesive, submucosa-invasive early gastric carcinoma. Gastric Cancer 2017, 20, 583–590. [Google Scholar] [CrossRef]
- Kwon, C.H.; Kim, Y.K.; Lee, S.; Kim, A.; Park, H.J.; Choi, Y.; Won, Y.J.; Park, D.Y.; Lauwers, G.Y. Gastric poorly cohesive carcinoma: A correlative study of mutational signatures and prognostic significance based on histopathological subtypes. Histopathlogy 2018, 72, 556–568. [Google Scholar] [CrossRef]
- Allred, D.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- McCarty Jr, K.; Miller, L.; Cox, E.; Konrath, J.; McCarty Sr, K. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch. Pathol. Lab. Med. 1985, 109, 716–721. [Google Scholar]
- Nakamura, K.; Sugano, H.; Takagi, K. Carcinoma of the stomach in incipient phase Its histogenesis and histological appearances. GANN Jpn. J. Cancer Res. 1968, 59, 251–258. [Google Scholar]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric cancer: Classification, histology and application of molecular pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Bencivenga, M.; Treppiedi, E.; Dal Cero, M.; Torroni, L.; Verlato, G.; Iglesias, M.; Renaud, F.; Tomezzoli, A.; Castelli, C.; Piessen, G. The amount of signet ring cells is significantly associated with tumour stage and survival in gastric poorly cohesive tumours. J. Surg. Oncol. 2020, 121, 1084–1089. [Google Scholar] [CrossRef]
- Shiotsuki, K.; Takizawa, K.; Ono, H. Indications of endoscopic submucosal dissection for undifferentiated early gastric cancer: Current status and future perspectives for further expansion. Digestion 2022, 103, 76–82. [Google Scholar] [CrossRef]
- Taghavi, S.; Jayarajan, S.N.; Davey, A.; Willis, A.I. Prognostic significance of signet ring gastric cancer. J. Clin. Oncol. 2012, 30, 3493. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.G.; Wang, Z.N.; Sun, Z.; Liu, F.N.; Yu, M.; Xu, H.M. Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: Results from a Chinese mono-institutional study. J. Surg. Oncol. 2011, 103, 700–703. [Google Scholar] [CrossRef]
- Ohyama, H.; Yoshimura, D.; Hirotsu, Y.; Amemiya, K.; Amano, H.; Miura, Y.; Ashizawa, H.; Nakagomi, K.; Takaoka, S.; Hosoda, K. Rapidly declining trend of signet ring cell cancer of the stomach may parallel the infection rate of Helicobacter pylori. BMC Gastroenterol. 2019, 19, 178. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, J.S.; Moon, H.S.; Lee, E.S.; Kim, S.H.; Sung, J.K.; Lee, B.S.; Jeong, H.Y. Signet ring cell carcinoma of early gastric cancer, is endoscopic treatment really risky? Medicine 2017, 96, e7532. [Google Scholar] [CrossRef]
- Hunter, T. Tyrosine phosphorylation: Thirty years and counting. Curr. Opin. Cell Biol. 2009, 21, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Ostman, A.; Hellberg, C.; Böhmer, F.D. Protein-tyrosine phosphatases and cancer. Nat. Rev. Cancer 2006, 6, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Barazeghi, E.; Hellman, P.; Westin, G.; Stålberg, P. PTPRM, a candidate tumor suppressor gene in small intestinal neuroendocrine tumors. Endocr. Connect. 2019, 8, 1126–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhir, P.R.; Lin, S.T.; Chia-Wen, C.; Yang, S.H.; Li, A.F.-Y.; Lai, R.H.; Wang, M.J.; Chen, Y.T.; Chen, C.F.; Jou, Y.S. Loss of PTPRM associates with the pathogenic development of colorectal adenoma-carcinoma sequence. Sci. Rep. 2015, 5, 9633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, P.H.; Ye, L.; Mason, M.D.; Jiang, W.G. Protein tyrosine phosphatase µ (PTP µ or PTPRM), a negative regulator of proliferation and invasion of breast cancer cells, is associated with disease prognosis. PLoS ONE 2012, 7, e50183. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, C.; Liao, Y.; Liu, J.; Huang, J.; Xia, M.; Chen, M.; Tan, H.; He, W.; Xu, M. High expression of PTPRM predicts poor prognosis and promotes tumor growth and lymph node metastasis in cervical cancer. Cell Death Dis. 2020, 11, 687. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]

| Characteristics | Gastric Cancer Patients | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TAC | SRC Carcinoma | PC Carcinoma | Combined PC/SRC Carcinoma | Mixed Carcinoma | EBV-Associated Carcinoma | Others | Total | p | |
| Sex | 0.000 | ||||||||
| Male | 626 (77.0) | 8 (50.0) | 18 (46.2) | 38 (53.5) | 25 (59.5) | 31 (88.6) | 12 (92.3) | 758 (73.7) | |
| Female | 187 (23.0) | 8 (50.0) | 21 (53.8) | 33 (46.5) | 17 (40.5) | 4 (11.4) | 1 (7.7) | 271 (26.3) | |
| Age | 0.000 | ||||||||
| ≤61 | 355 (43.7) | 13 (81.3) | 23 (59.0) | 49 (69.0) | 30 (71.4) | 26 (74.3) | 6 (76.2) | 502 (48.8) | |
| >61 | 458 (56.3) | 3 (18.8) | 16 (41.0) | 22 (31.0) | 12 (28.6) | 9 (25.7) | 7 (53.8) | 527 (51.2) | |
| EGV vs. AGC | 0.000 | ||||||||
| EGC | 723 (88.9) | 16 (100) | 17 (43.6) | 51 (71.8) | 32 (76.2) | 21 (60.0) | 4 (30.8) | 864 (84.0) | |
| AGC | 90 (11.1) | 0 (0) | 22 (56.4) | 20 (28.2) | 10 (23.8) | 14 (40.0) | 9 (69.2) | 165 (16.0) | |
| T stage | 0.000 | ||||||||
| T1 | 724 (89.1) | 16 (100) | 17 (43.6) | 51 (71.8) | 32 (76.2) | 21 (60.0) | 4 (30.8) | 865 (84.1) | |
| T2 | 45 (5.5) | 0 (0) | 1 (2.6) | 4 (5.6) | 7 (16.7) | 4 (11.4) | 0 (0) | 61 (5.9) | |
| T3 | 19 (2.3) | 0 (0) | 8 (20.5) | 8 (11.3) | 1 (2.4) | 8 (22.9) | 5 (38.5) | 49 (4.8) | |
| T4 | 25 (3.1) | 0 (0) | 13 (33.3) | 8 (11.3) | 2 (4.8) | 2 (5.7) | 4 (30.8) | 54 (5.2) | |
| LN metastasis | 0.000 | ||||||||
| Absent | 742 (91.3) | 16 (100) | 20 (51.3) | 54 (76.1) | 30 (71.4) | 27 (77.1) | 4 (30.8) | 893 (86.8) | |
| Present | 71 (8.7) | 0 (0) | 19 (48.7) | 17 (23.9) | 12 (28.6) | 8 (22.9) | 9 (69.2) | 136 (13.2) | |
| Pathologic stage | 0.000 | ||||||||
| I–II | 774 (95.2) | 16 (100) | 25 (64.1) | 60 (84.5) | 38 (90.5) | 32 (91.4) | 7 (53.8) | 952 (92.5) | |
| III–IV | 39 (4.8) | 0 (0) | 14 (35.9) | 11 (15.5) | 4 (9.5) | 3 (8.6) | 6 (46.2) | 77 (7.5) | |
| Characteristics | Gastric Cancer Patients | ||||
|---|---|---|---|---|---|
| SRC Carcinoma | PC Carcinoma | Combined PC/SRC Carcinoma | Total | p | |
| Sex | 0.759 | ||||
| Male | 8 (50.0) | 18 (46.2) | 38 (53.5) | 64 (50.8) | |
| Female | 8 (50.0) | 21 (53.8) | 33 (46.5) | 62 (49.2) | |
| Age | 0.254 | ||||
| <61 | 13 (81.3) | 23 (59.0) | 46 (69.0) | 85 (67.5) | |
| ≥61 | 3 (18.8) | 16 (41.0) | 22 (31.0) | 41 (32.5) | |
| Surgical treatment | 0.012 | ||||
| Endoscopic resection | 8 (50) | 7 (17.9) | 12 (16.9) | 27 (21.4) | |
| Gastrectomy | 8 (50) | 32 (82.1) | 59 (83.1) | 99 (78.6) | |
| EGV vs. AGC | 0.000 | ||||
| EGC | 16 (100) | 17 (43.6) | 51 (71.8) | 84 (66.7) | |
| AGC | 0 (0) | 22 (56.4) | 20 (28.2) | 42 (33.3) | |
| T stage | 0.001 | ||||
| T1 | 16 (100) | 17 (43.6) | 51 (71.8) | 84 (66.7) | |
| T2 | 0 (0) | 1 (2.6) | 4 (5.6) | 5 (4.0) | |
| T3 | 0 (0) | 8 (20.5) | 8 (11.3) | 16 (12.7) | |
| T4 | 0 (0) | 13 (33.3) | 8 (11.3) | 21 (16.7) | |
| LN metastasis | 0.001 | ||||
| Absent | 16 (100) | 20 (51.3) | 54 (76.1) | 90 (71.4) | |
| Present | 0 (0) | 19 (48.7) | 17 (23.9) | 36 (28.6) | |
| Pathologic stage | 0.004 | ||||
| I–II | 16 (100) | 25 (64.1) | 60 (84.5) | 101 (80.2) | |
| III–IV | 0 (0) | 14 (35.9) | 11 (15.5) | 25 (19.8) | |
| Characteristics | Patients | PTPRM | Patients | PROK2 | ||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Low | High | P | No. (%) | Low | High | P | |
| GC subtype | 0.001 | 0.015 | ||||||
| PC carcinoma | 35 (74.5) | 13 (54.2) | 22 (95.7) | 35 (74.5) | 15 (60.0) | 20 (90.9) | ||
| SRC carcinoma | 12 (25.5) | 11 (45.8) | 1 (4.3) | 12 (25.5) | 10 (40.0) | 2 (9.1) | ||
| EGV vs. AGC | 0.059 | 0.319 | ||||||
| EGC | 25 (53.2) | 16 (66.7) | 9 (39.1) | 25 (53.2) | 15 (60.0) | 10 (45.5) | ||
| AGC | 22 (46.8) | 8 (33.3) | 14 (60.9) | 22 (46.8) | 10 (40.0) | 12 (54.5) | ||
| T stage | 0.192 | 0.706 | ||||||
| T1–T2 | 27 (57.4) | 16 (66.7) | 11 (47.8) | 27 (57.4) | 15 (60.0) | 12 (54.5) | ||
| T3–T4 | 20 (42.6) | 8 (33.3) | 12 (52.2) | 20 (42.6) | 10 (40.0) | 10 (45.5) | ||
| LN metastasis | 0.005 | 0.210 | ||||||
| Absent | 28 (59.6) | 19 (79.2) | 9 (39.1) | 28 (59.6) | 17 (68.0) | 11 (50.0) | ||
| Present | 19 (40.4) | 5 (20.8) | 14 (60.9) | 19 (40.4) | 8 (32.0) | 11 (50.0) | ||
| Distant metastasis | 0.276 | 0.237 | ||||||
| Absent | 43 (91.5) | 23 (95.8) | 20 (87.0) | 43 (91.5) | 24 (96.0) | 19 (86.4) | ||
| Present | 4 (8.5) | 1 (4.2) | 3 (13.0) | 4 (8.5) | 1 (4.0) | 3 (13.6) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, G.E.; Kang, S.H.; Kim, J.S.; Kim, S.-H.; Kim, K.-H.; Kim, J.-M.; Suh, K.-S.; Park, H.K.; Kang, D.-W.; Lee, H.; et al. Characterization of Poorly Cohesive and Signet Ring Cell Carcinomas and Identification of PTPRM as a Diagnostic Marker. Cancers 2022, 14, 2502. https://doi.org/10.3390/cancers14102502
Bae GE, Kang SH, Kim JS, Kim S-H, Kim K-H, Kim J-M, Suh K-S, Park HK, Kang D-W, Lee H, et al. Characterization of Poorly Cohesive and Signet Ring Cell Carcinomas and Identification of PTPRM as a Diagnostic Marker. Cancers. 2022; 14(10):2502. https://doi.org/10.3390/cancers14102502
Chicago/Turabian StyleBae, Go Eun, Sun Hyung Kang, Ju Seok Kim, Seok-Hwan Kim, Kyung-Hee Kim, Jin-Man Kim, Kwang-Sun Suh, Hyung Kyu Park, Dong-Wook Kang, Hyunjung Lee, and et al. 2022. "Characterization of Poorly Cohesive and Signet Ring Cell Carcinomas and Identification of PTPRM as a Diagnostic Marker" Cancers 14, no. 10: 2502. https://doi.org/10.3390/cancers14102502
APA StyleBae, G. E., Kang, S. H., Kim, J. S., Kim, S.-H., Kim, K.-H., Kim, J.-M., Suh, K.-S., Park, H. K., Kang, D.-W., Lee, H., & Yeo, M.-K. (2022). Characterization of Poorly Cohesive and Signet Ring Cell Carcinomas and Identification of PTPRM as a Diagnostic Marker. Cancers, 14(10), 2502. https://doi.org/10.3390/cancers14102502







