Novel T Follicular Helper-like T-Cell Lymphoma Therapies: From Preclinical Evaluation to Clinical Reality
Abstract
Simple Summary
Abstract
1. Introduction
2. Tfh PTCL Oncogenesis and Mutational Landscape
3. Recently Developed Mouse Models Recapitulating AITL Malignancy
4. Current Treatment Strategies for AITL and Tfh PTCL Patients
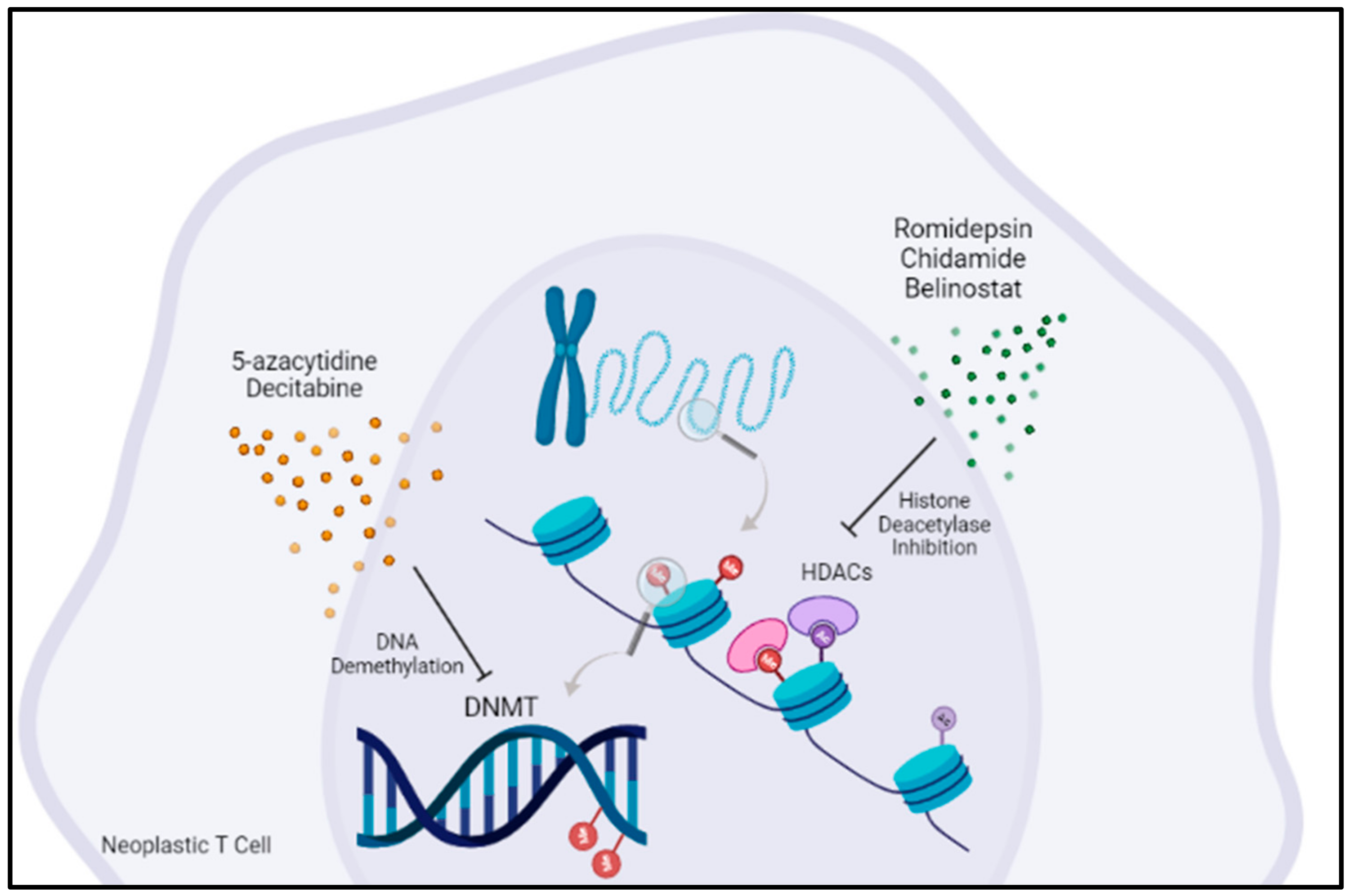
5. Targeting Epigenetic Regulators Is an Emerging Concept in AITL Treatment
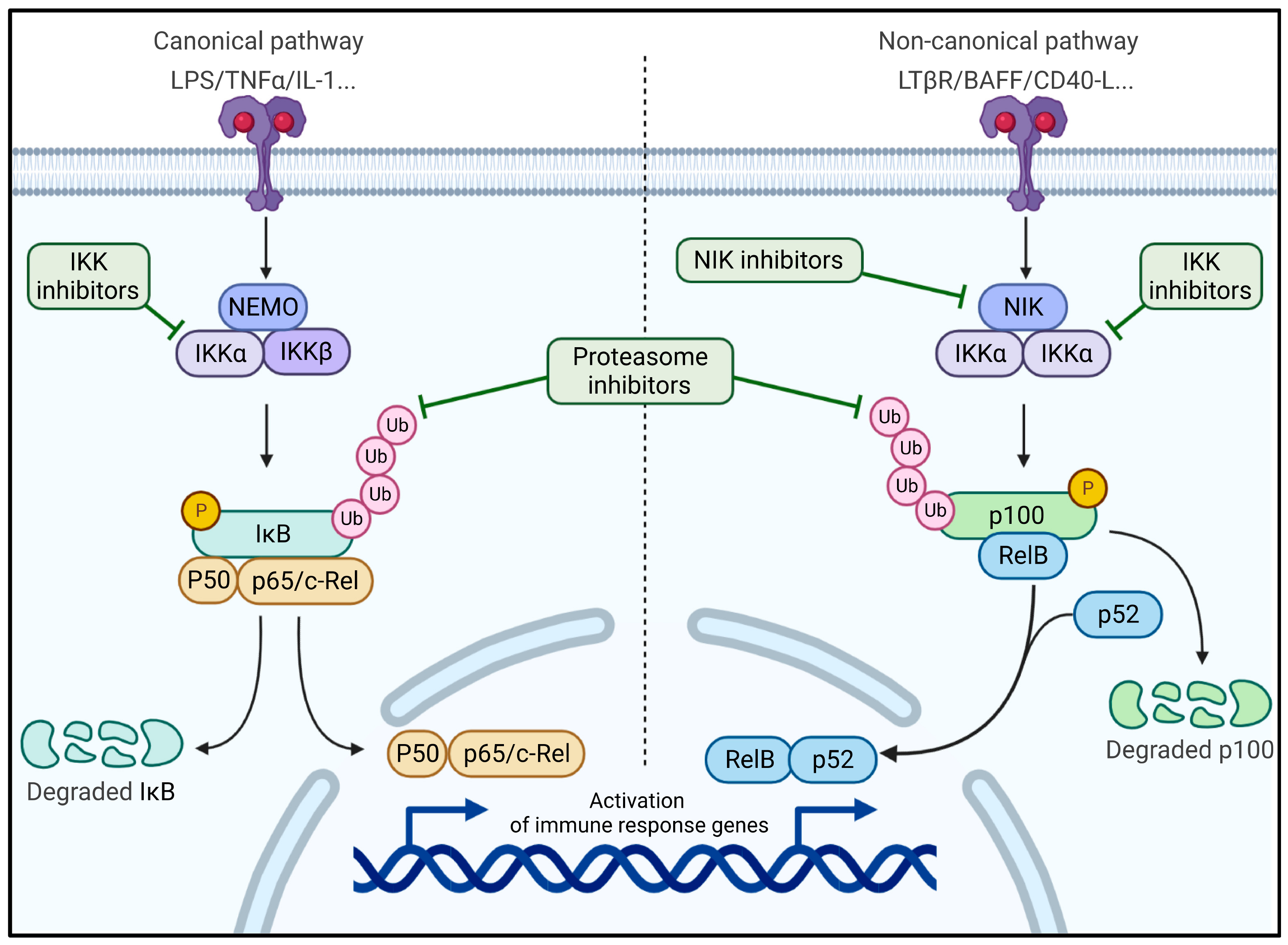
6. The NF-κB Pathway Revealed as Therapeutic Target in a New AITL Preclinical Mouse Model
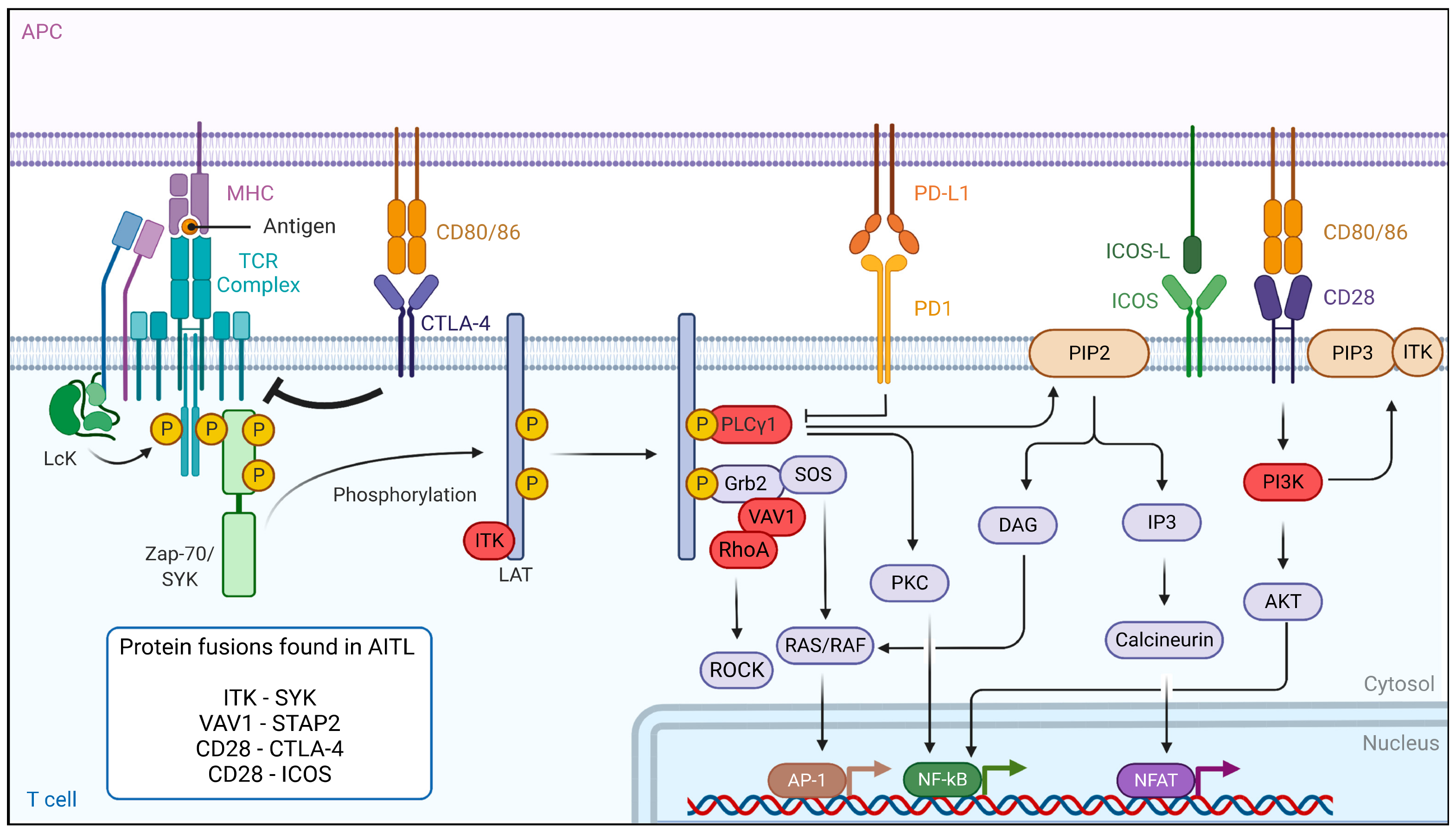
7. Dysregulation of the TCR Signaling Pathway in AITL Reveals New Treatment Options
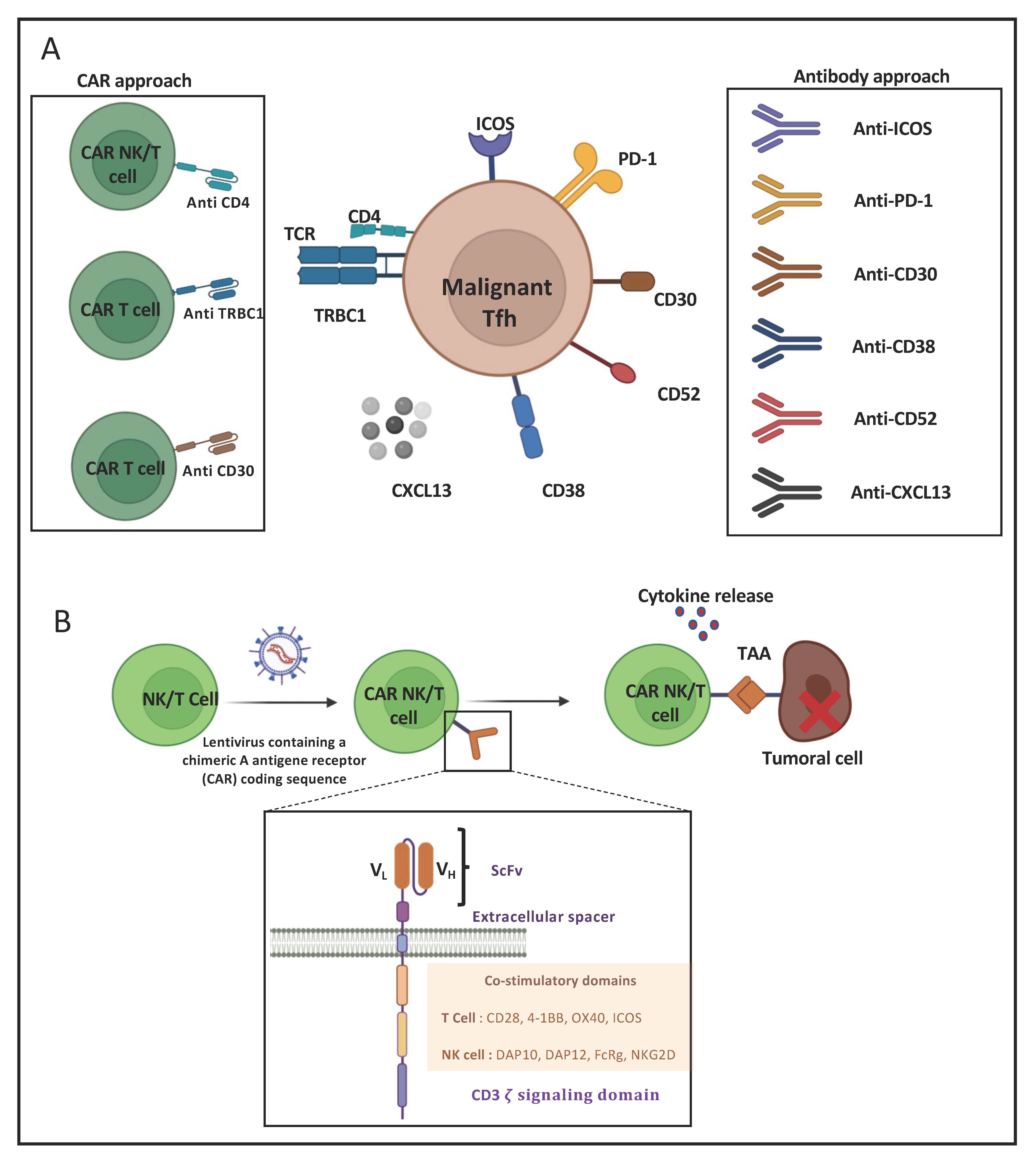
8. Immunotherapeutic Approaches for AITL
8.1. Monoclonal-Antibody-Based Immunotherapies for AITL Treatment
8.2. CAR-T-Cell-Based Immunotherapies for AITL Treatment
9. Metabolic Interference, a Future New Therapeutic Option for AITL and Tfh-like Lymphoma?
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- de Leval, L.; Gisselbrecht, C.; Gaulard, P. Advances in the Understanding and Management of Angioimmunoblastic T-Cell Lymphoma. Br. J. Haematol. 2010, 148, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Baron, M.; Amara, N.; Haioun, C.; Dandoit, M.; Maynadié, M.; Parrens, M.; Vergier, B.; Copie-Bergman, C.; Fabiani, B.; et al. Impact of Expert Pathologic Review of Lymphoma Diagnosis: Study of Patients From the French Lymphopath Network. J. Clin. Oncol. 2017, 35, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.; Arber, D.; Campo, E.; Harris, N. Quintanilla-Fend L. In Hematopathology e-Book; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- de Leval, L.; Parrens, M.; Le Bras, F.; Jais, J.-P.; Fataccioli, V.; Martin, A.; Lamant, L.; Delarue, R.; Berger, F.; Arbion, F.; et al. Angioimmunoblastic T-Cell Lymphoma Is the Most Common T-Cell Lymphoma in Two Distinct French Information Data Sets. Haematologica 2015, 100, e361–e364. [Google Scholar] [CrossRef] [PubMed]
- Federico, M.; Rudiger, T.; Bellei, M.; Nathwani, B.N.; Luminari, S.; Coiffier, B.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Savage, K.J. Clinicopathologic Characteristics of Angioimmunoblastic T-Cell Lymphoma: Analysis of the International Peripheral T-Cell Lymphoma Project. J. Clin. Oncol. 2013, 31, 240. [Google Scholar] [CrossRef]
- de Leval, L.; Rickman, D.S.; Thielen, C.; de Reynies, A.; Huang, Y.-L.; Delsol, G.; Lamant, L.; Leroy, K.; Brière, J.; Molina, T.; et al. The Gene Expression Profile of Nodal Peripheral T-Cell Lymphoma Demonstrates a Molecular Link between Angioimmunoblastic T-Cell Lymphoma (AITL) and Follicular Helper T (TFH) Cells. Blood 2007, 109, 4952–4963. [Google Scholar] [CrossRef]
- Dobay, M.P.; Lemonnier, F.; Missiaglia, E.; Bastard, C.; Vallois, D.; Jais, J.-P.; Scourzic, L.; Dupuy, A.; Fataccioli, V.; Pujals, A.; et al. Integrative Clinicopathological and Molecular Analyses of Angioimmunoblastic T-Cell Lymphoma and Other Nodal Lymphomas of Follicular Helper T-Cell Origin. Haematologica 2017, 102, e148–e151. [Google Scholar] [CrossRef]
- Rodríguez, M.; Alonso-Alonso, R.; Tomás-Roca, L.; Rodríguez-Pinilla, S.M.; Manso-Alonso, R.; Cereceda, L.; Borregón, J.; Villaescusa, T.; Córdoba, R.; Sánchez-Beato, M.; et al. Peripheral T-Cell Lymphoma: Molecular Profiling Recognizes Subclasses and Identifies Prognostic Markers. Blood Adv. 2021, 5, 5588–5598. [Google Scholar] [CrossRef]
- Vallois, D.; Dobay, M.P.D.; Morin, R.D.; Lemonnier, F.; Missiaglia, E.; Juilland, M.; Iwaszkiewicz, J.; Fataccioli, V.; Bisig, B.; Roberti, A.; et al. Activating Mutations in Genes Related to TCR Signaling in Angioimmunoblastic and Other Follicular Helper T-Cell–Derived Lymphomas. Blood 2016, 128, 1490–1502. [Google Scholar] [CrossRef]
- Kohli, R.M.; Zhang, Y. TET Enzymes, TDG and the Dynamics of DNA Demethylation. Nature 2013, 502, 472–479. [Google Scholar] [CrossRef]
- Yang, L.; Rau, R.; Goodell, M.A. DNMT3A in Haematological Malignancies. Nat. Rev. Cancer 2015, 15, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, F.; Cairns, R.A.; Inoue, S.; Li, W.Y.; Dupuy, A.; Broutin, S.; Martin, N.; Fataccioli, V.; Pelletier, R.; Wakeham, A.; et al. The IDH2 R172K Mutation Associated with Angioimmunoblastic T-Cell Lymphoma Produces 2HG in T Cells and Impacts Lymphoid Development. Proc. Natl. Acad. Sci. USA 2016, 113, 15084–15089. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, F.; Poullot, E.; Dupuy, A.; Couronné, L.; Martin, N.; Scourzic, L.; Fataccioli, V.; Bruneau, J.; Cairns, R.A.; Mak, T.W.; et al. Loss of 5-Hydroxymethylcytosine Is a Frequent Event in Peripheral T Cell Lymphomas. Haematologica 2018, 103, e115–e118. [Google Scholar] [CrossRef] [PubMed]
- Bick, A.G.; Weinstock, J.S.; Nandakumar, S.K.; Fulco, C.P.; Bao, E.L.; Zekavat, S.M.; Szeto, M.D.; Liao, X.; Leventhal, M.J.; Nasser, J. Inherited Causes of Clonal Haematopoiesis in 97,691 Whole Genomes. Nature 2020, 586, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.E.; Petrova-Drus, K.; Huet, S.; Epstein-Peterson, Z.D.; Gao, Q.; Sigler, A.E.; Baik, J.; Ozkaya, N.; Moskowitz, A.J.; Kumar, A. Clonal Hematopoiesis in Angioimmunoblastic T-Cell Lymphoma with Divergent Evolution to Myeloid Neoplasms. Blood Adv. 2020, 4, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Mhaidly, R.; Krug, A.; Gaulard, P.; Lemonnier, F.; Ricci, J.-E.; Verhoeyen, E. New Preclinical Models for Angioimmunoblastic T-Cell Lymphoma: Filling the GAP. Oncogenesis 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.R.; Ambesi-Impiombato, A.; Couronné, L.; Quinn, S.A.; Kim, C.S.; da Silva Almeida, A.C.; West, Z.; Belver, L.; Martin, M.S.; Scourzic, L.; et al. RHOA G17V Induces T Follicular Helper Cell Specification and Promotes Lymphomagenesis. Cancer Cell 2018, 33, 259–273.e7. [Google Scholar] [CrossRef]
- Sakata-Yanagimoto, M.; Enami, T.; Yoshida, K.; Shiraishi, Y.; Ishii, R.; Miyake, Y.; Muto, H.; Tsuyama, N.; Sato-Otsubo, A.; Okuno, Y.; et al. Somatic RHOA Mutation in Angioimmunoblastic T Cell Lymphoma. Nat. Genet. 2014, 46, 171–175. [Google Scholar] [CrossRef]
- Palomero, T.; Couronné, L.; Khiabanian, H.; Kim, M.-Y.; Ambesi-Impiombato, A.; Perez-Garcia, A.; Carpenter, Z.; Abate, F.; Allegretta, M.; Haydu, J.E.; et al. Recurrent Mutations in Epigenetic Regulators, RHOA and FYN Kinase in Peripheral T Cell Lymphomas. Nat. Genet. 2014, 46, 166. [Google Scholar] [CrossRef]
- Steinhilber, J.; Mederake, M.; Bonzheim, I.; Serinsöz-Linke, E.; Müller, I.; Fallier-Becker, P.; Lemonnier, F.; Gaulard, P.; Fend, F.; Quintanilla-Martinez, L. The Pathological Features of Angioimmunoblastic T-Cell Lymphomas with IDH2R172 Mutations. Mod. Pathol. 2019, 32, 1123–1134. [Google Scholar] [CrossRef]
- Streubel, B.; Vinatzer, U.; Willheim, M.; Raderer, M.; Chott, A. Novel t(5;9)(Q33;Q22) Fuses ITK to SYK in Unspecified Peripheral T-Cell Lymphoma. Leukemia 2006, 20, 313–318. [Google Scholar] [CrossRef]
- Vallois, D.; Dupuy, A.; Lemonnier, F.; Allen, G.; Missiaglia, E.; Fataccioli, V.; Ortonne, N.; Clavert, A.; Delarue, R.; Rousselet, M.-C.; et al. RNA Fusions Involving CD28 Are Rare in Peripheral T-Cell Lymphomas and Concentrate Mainly in Those Derived from Follicular Helper T Cells. Haematologica 2018, 103, e360–e363. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Li, J.; Yang, H.; Zeng, H.; Han, W.; Zhang, J.; Lee, M.; Moczygemba, M.; Isgandarova, S.; Yang, Y.; et al. Mutations in 5-Methylcytosine Oxidase TET2 and RhoA Cooperatively Disrupt T Cell Homeostasis. J. Clin. Investig. 2017, 127, 2998–3012. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Brown, L.; Stevenson, K.; deSouza, T.; Aster, J.C.; Louissaint, A.; Weinstock, D.M. RhoA G17V Is Sufficient to Induce Autoimmunity and Promotes T-Cell Lymphomagenesis in Mice. Blood 2018, 132, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Delfau-Larue, M.-H.; de Leval, L.; Joly, B.; Plonquet, A.; Challine, D.; Parrens, M.; Delmer, A.; Salles, G.; Morschhauser, F.; Delarue, R. Targeting Intratumoral B Cells with Rituximab in Addition to CHOP in Angioimmunoblastic T-Cell Lymphoma. A Clinicobiological Study of the GELA. Haematologica 2012, 97, 1594. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, F.; Safar, V.; Beldi-Ferchiou, A.; Cottereau, A.-S.; Bachy, E.; Cartron, G.; Fataccioli, V.; Pelletier, L.; Robe, C.; Letourneau, A. Integrative Analysis of a Phase 2 Trial Combining Lenalidomide with CHOP in Angioimmunoblastic T-Cell Lymphoma. Blood Adv. 2021, 5, 539–548. [Google Scholar] [CrossRef]
- Wulf, G.G.; Altmann, B.; Ziepert, M.; D’amore, F.; Held, G.; Greil, R.; Tournilhac, O.; Relander, T.; Viardot, A.; Wilhelm, M. Alemtuzumab plus CHOP versus CHOP in Elderly Patients with Peripheral T-Cell Lymphoma: The DSHNHL2006-1B/ACT-2 Trial. Leukemia 2021, 35, 143–155. [Google Scholar] [CrossRef]
- Bachy, E.; Camus, V.; Thieblemont, C.; Sibon, D.; Casasnovas, R.-O.; Ysebaert, L.; Damaj, G.; Guidez, S.; Pica, G.M.; Kim, W.S.; et al. Romidepsin Plus CHOP Versus CHOP in Patients With Previously Untreated Peripheral T-Cell Lymphoma: Results of the Ro-CHOP Phase III Study (Conducted by LYSA). J. Clin. Oncol. 2022, 40, 242–251. [Google Scholar] [CrossRef]
- Pro, B.; Horwitz, S.M.; Prince, H.M.; Foss, F.M.; Sokol, L.; Greenwood, M.; Caballero, D.; Morschhauser, F.; Wilhelm, M.; Iyer, S.P.; et al. Romidepsin Induces Durable Responses in Patients with Relapsed or Refractory Angioimmunoblastic T-Cell Lymphoma. Hematol. Oncol. 2017, 35, 914–917. [Google Scholar] [CrossRef]
- Mohammed Saleh, M.F.; Kotb, A.; Abdallah, G.E.M.; Muhsen, I.N.; El Fakih, R.; Aljurf, M. Recent Advances in Diagnosis and Therapy of Angioimmunoblastic T Cell Lymphoma. Curr. Oncol. 2021, 28, 5480–5498. [Google Scholar] [CrossRef]
- Coiffier, B.; Pro, B.; Prince, H.M.; Foss, F.; Sokol, L.; Greenwood, M.; Caballero, D.; Borchmann, P.; Morschhauser, F.; Wilhelm, M.; et al. Results from a Pivotal, Open-Label, Phase II Study of Romidepsin in Relapsed or Refractory Peripheral T-Cell Lymphoma after Prior Systemic Therapy. J. Clin. Oncol. 2012, 30, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Su, L.; Liu, L.; Gao, Y.; Wang, Q.; Su, H.; Song, Y.; Zhang, H.; Shen, J.; Jing, H.; et al. The Combination of Chidamide with the CHOEP Regimen in Previously Untreated Patients with Peripheral T-Cell Lymphoma: A Prospective, Multicenter, Single Arm, Phase 1b/2 Study. Cancer Biol. Med. 2021, 18, 841–848. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, O.A.; Horwitz, S.; Masszi, T.; Van Hoof, A.; Brown, P.; Doorduijn, J.; Hess, G.; Jurczak, W.; Knoblauch, P.; Chawla, S.; et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J. Clin. Oncol. 2015, 33, 2492–2499. [Google Scholar] [CrossRef]
- Ghione, P.; Faruque, P.; Mehta-Shah, N.; Seshan, V.; Ozkaya, N.; Bhaskar, S.; Yeung, J.; Spinner, M.A.; Lunning, M.; Inghirami, G.; et al. T Follicular Helper Phenotype Predicts Response to Histone Deacetylase Inhibitors in Relapsed/Refractory Peripheral T-Cell Lymphoma. Blood Adv. 2020, 4, 4640–4647. [Google Scholar] [CrossRef]
- Lemonnier, F.; Dupuis, J.; Sujobert, P.; Tournillhac, O.; Cheminant, M.; Sarkozy, C.; Pelletier, L.; Marçais, A.; Robe, C.; Fataccioli, V. Treatment with 5-Azacytidine Induces a Sustained Response in Patients with Angioimmunoblastic T-Cell Lymphoma. Blood J. Am. Soc. Hematol. 2018, 132, 2305–2309. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Moskowitz, A.J.; Mehta-Shah, N.; Sokol, L.; Chen, Z.; Rahim, R.; Song, W.; Van Besien, K.; Horwitz, S.M.; Rutherford, S.C.; et al. Multi-Center Phase II Study of Oral Azacitidine (CC-486) Plus CHOP As Initial Treatment for Peripheral T-Cell Lymphoma (PTCL). Blood 2020, 136, 33–34. [Google Scholar] [CrossRef]
- Falchi, L.; Ma, H.; Klein, S.; Lue, J.K.; Montanari, F.; Marchi, E.; Deng, C.; Kim, H.A.; Rada, A.; Jacob, A.T.; et al. Combined Oral 5-Azacytidine and Romidepsin Are Highly Effective in Patients with PTCL: A Multicenter Phase 2 Study. Blood 2021, 137, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Koch, R.; Porcu, P.; Oki, Y.; Moskowitz, A.; Perez, M.; Myskowski, P.; Officer, A.; Jaffe, J.D.; Morrow, S.N.; et al. Activity of the PI3K-δ,γ Inhibitor Duvelisib in a Phase 1 Trial and Preclinical Models of T-Cell Lymphoma. Blood 2018, 131, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Brammer, J. Duvelisib in Patients with Relapsed/Refractory Peripheral T-Cell Lymphoma from the Phase 2 Primo Trial: Results of an Interim Analysis; ASH: Washington, DC, USA, 12 December 2021. [Google Scholar]
- Mondragón, L.; Mhaidly, R.; De Donatis, G.M.; Tosolini, M.; Dao, P.; Martin, A.R.; Pons, C.; Chiche, J.; Jacquin, M.; Imbert, V.; et al. GAPDH Overexpression in the T Cell Lineage Promotes Angioimmunoblastic T Cell Lymphoma through an NF-ΚB-Dependent Mechanism. Cancer Cell 2019, 36, 268–287.e10. [Google Scholar] [CrossRef]
- Colell, A.; Ricci, J.-E.; Tait, S.; Milasta, S.; Maurer, U.; Bouchier-Hayes, L.; Fitzgerald, P.; Guio-Carrion, A.; Waterhouse, N.J.; Li, C.W.; et al. GAPDH and Autophagy Preserve Survival after Apoptotic Cytochrome c Release in the Absence of Caspase Activation. Cell 2007, 129, 983–997. [Google Scholar] [CrossRef]
- Colell, A.; Green, D.R.; Ricci, J.-E. Novel Roles for GAPDH in Cell Death and Carcinogenesis. Cell Death Differ. 2009, 16, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Cildir, G.; Low, K.C.; Tergaonkar, V. Noncanonical NF-ΚB Signaling in Health and Disease. Trends Mol. Med. 2016, 22, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Myles, A.; Cancro, M.P. The NIK of Time for B Cells. Eur. J. Immunol. 2016, 46, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, S.; Nakamura, Y.; Suzuka, H.; Koba, M.; Yasumizu, R.; Ikehara, S.; Shibata, Y. A New Mutation, Aly, That Induces a Generalized Lack of Lymph Nodes Accompanied by Immunodeficiency in Mice. Eur. J. Immunol. 1994, 24, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Zhou, X.; Xie, X.; Chen, X.; Jie, Z.; Zou, Q.; Hu, H.; Zhu, L.; Cheng, X.; et al. Cell Intrinsic Role of NF-ΚB-Inducing Kinase in Regulating T Cell-Mediated Immune and Autoimmune Responses. Sci. Rep. 2016, 6, 22115. [Google Scholar] [CrossRef]
- Xiao, G.; Harhaj, E.W.; Sun, S.C. NF-KappaB-Inducing Kinase Regulates the Processing of NF-KappaB2 P100. Mol. Cell 2001, 7, 401–409. [Google Scholar] [CrossRef]
- Cheng, J.; Feng, X.; Li, Z.; Zhou, F.; Yang, J.-M.; Zhao, Y. Pharmacological Inhibition of NF-ΚB-Inducing Kinase (NIK) with Small Molecules for the Treatment of Human Diseases. RSC Med. Chem. 2022, 12, 552–565. [Google Scholar] [CrossRef]
- Gray, C.M.; Remouchamps, C.; McCorkell, K.A.; Solt, L.A.; Dejardin, E.; Orange, J.S.; May, M.J. Noncanonical NF-ΚB Signaling Is Limited by Classical NF-ΚB Activity. Sci. Signal. 2014, 7, ra13. [Google Scholar] [CrossRef][Green Version]
- Bram, R.J. TBK1 Suppression of IgA in the NIK of Time. Nat. Immunol. 2012, 13, 1027–1029. [Google Scholar] [CrossRef]
- Jin, J.; Xiao, Y.; Chang, J.-H.; Yu, J.; Hu, H.; Starr, R.; Brittain, G.C.; Chang, M.; Cheng, X.; Sun, S.-C. The Kinase TBK1 Controls IgA Class Switching by Negatively Regulating Noncanonical NF-ΚB Signaling. Nat. Immunol. 2012, 13, 1101–1109. [Google Scholar] [CrossRef]
- Iqbal, J.; Weisenburger, D.D.; Greiner, T.C.; Vose, J.M.; McKeithan, T.; Kucuk, C.; Geng, H.; Deffenbacher, K.; Smith, L.; Dybkaer, K.; et al. Molecular Signatures to Improve Diagnosis in Peripheral T-Cell Lymphoma and Prognostication in Angioimmunoblastic T-Cell Lymphoma. Blood 2010, 115, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-I.; Chang, S.-T.; Lin, M.-Y.; Hsieh, Y.-C.; Chu, P.-Y.; Chen, C.-J.; Lin, K.-J.; Jung, Y.-C.; Hwang, W.-S.; Huang, W.-T.; et al. Angioimmunoblastic T-Cell Lymphoma in Taiwan Shows a Frequent Gain of ITK Gene. Int. J. Clin. Exp. Pathol. 2014, 7, 6097–6107. [Google Scholar] [PubMed]
- Feldman, A.L.; Sun, D.X.; Law, M.E.; Novak, A.J.; Attygalle, A.D.; Thorland, E.C.; Fink, S.R.; Vrana, J.A.; Caron, B.L.; Morice, W.G.; et al. Overexpression of Syk Tyrosine Kinase in Peripheral T-Cell Lymphomas. Leukemia 2008, 22, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T Cell Activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Fujisawa, M.; Sakata-Yanagimoto, M.; Nishizawa, S.; Komori, D.; Gershon, P.; Kiryu, M.; Tanzima, S.; Fukumoto, K.; Enami, T.; Muratani, M.; et al. Activation of RHOA-VAV1 Signaling in Angioimmunoblastic T-Cell Lymphoma. Leukemia 2018, 32, 694–702. [Google Scholar] [CrossRef]
- Rohr, J.; Guo, S.; Huo, J.; Bouska, A.; Lachel, C.; Li, Y.; Simone, P.D.; Zhang, W.; Gong, Q.; Wang, C.; et al. Recurrent Activating Mutations of CD28 in Peripheral T-Cell Lymphomas. Leukemia 2016, 30, 1062–1070. [Google Scholar] [CrossRef]
- Ohmoto, A.; Fuji, S. Cyclosporine for Angioimmunoblastic T-Cell Lymphoma: A Literature Review. Expert Rev. Hematol. 2019, 12, 975–981. [Google Scholar] [CrossRef]
- Boddicker, R.L.; Razidlo, G.L.; Dasari, S.; Zeng, Y.; Hu, G.; Knudson, R.A.; Greipp, P.T.; Davila, J.I.; Johnson, S.H.; Porcher, J.C.; et al. Integrated Mate-Pair and RNA Sequencing Identifies Novel, Targetable Gene Fusions in Peripheral T-Cell Lymphoma. Blood 2016, 128, 1234–1245. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Deng, L.; Ping, L.; Shi, Y.; Zheng, W.; Lin, N.; Wang, X.; Tu, M.; Xie, Y.; et al. ITK Inhibition Induced in Vitro and in Vivo Anti-Tumor Activity through Downregulating TCR Signaling Pathway in Malignant T Cell Lymphoma. Cancer Cell Int. 2019, 19, 32. [Google Scholar] [CrossRef]
- Pechloff, K.; Holch, J.; Ferch, U.; Schweneker, M.; Brunner, K.; Kremer, M.; Sparwasser, T.; Quintanilla-Martinez, L.; Zimber-Strobl, U.; Streubel, B.; et al. The Fusion Kinase ITK-SYK Mimics a T Cell Receptor Signal and Drives Oncogenesis in Conditional Mouse Models of Peripheral T Cell Lymphoma. J. Exp. Med. 2010, 207, 1031–1044. [Google Scholar] [CrossRef]
- Dierks, C.; Adrian, F.; Fisch, P.; Ma, H.; Maurer, H.; Herchenbach, D.; Forster, C.U.; Sprissler, C.; Liu, G.; Rottmann, S.; et al. The ITK-SYK Fusion Oncogene Induces a T-Cell Lymphoproliferative Disease in Mice Mimicking Human Disease. Cancer Res. 2010, 70, 6193–6204. [Google Scholar] [CrossRef] [PubMed]
- Lechner, K.S.; Neurath, M.F.; Weigmann, B. Role of the IL-2 Inducible Tyrosine Kinase ITK and Its Inhibitors in Disease Pathogenesis. J. Mol. Med. 2020, 98, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Dong, S.; Strattan, E.; Ren, L.; Butchar, J.P.; Thornton, K.; Mishra, A.; Porcu, P.; Bradshaw, J.M.; Bisconte, A.; et al. Targeting Interleukin-2-Inducible T-Cell Kinase (ITK) and Resting Lymphocyte Kinase (RLK) Using a Novel Covalent Inhibitor PRN694. J. Biol. Chem. 2015, 290, 5960–5978. [Google Scholar] [CrossRef] [PubMed]
- Dubovsky, J.A.; Beckwith, K.A.; Natarajan, G.; Woyach, J.A.; Jaglowski, S.; Zhong, Y.; Hessler, J.D.; Liu, T.-M.; Chang, B.Y.; Larkin, K.M.; et al. Ibrutinib Is an Irreversible Molecular Inhibitor of ITK Driving a Th1-Selective Pressure in T Lymphocytes. Blood 2013, 122, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vardhana, S.; Moskowitz, A.J.; Porcu, P.; Dogan, A.; Dubovsky, J.A.; Matasar, M.J.; Zhang, Z.; Younes, A.; Horwitz, S.M. Pilot Trial of Ibrutinib in Patients with Relapsed or Refractory T-Cell Lymphoma. Blood Adv. 2018, 2, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.Y.; Kim, P.; Kim, W.S.; Lee, S.H.; Kim, S.; Kang, S.Y.; Jang, H.Y.; Lee, J.-E.; Kim, J.; Kim, S.J.; et al. Frequent CTLA4-CD28 Gene Fusion in Diverse Types of T-Cell Lymphoma. Haematologica 2016, 101, 757–763. [Google Scholar] [CrossRef]
- Lee, G.J.; Jun, Y.; Jeon, Y.K.; Lee, D.; Lee, S.; Kim, J. Mice Transgenic for Human CTLA4-CD28 Fusion Gene Show Proliferation and Transformation of ATLL-like and AITL-like T Cells. Oncoimmunology 2022, 11, 2015170. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Sakata-Yanagimoto, M.; Fujisawa, M.; Nuhat, S.T.; Miyoshi, H.; Nannya, Y.; Hashimoto, K.; Fukumoto, K.; Bernard, O.A.; Kiyoki, Y. Dasatinib Is an Effective Treatment for Angioimmunoblastic T-Cell Lymphoma. Cancer Res. 2020, 80, 1875–1884. [Google Scholar] [CrossRef]
- Kridin, K.; Ahmed, A.R. Post-Rituximab Immunoglobulin M (IgM) Hypogammaglobulinemia. Autoimmun. Rev. 2020, 19, 102466. [Google Scholar] [CrossRef]
- Stone, E.L.; Pepper, M.; Katayama, C.D.; Kerdiles, Y.M.; Lai, C.-Y.; Emslie, E.; Lin, Y.C.; Yang, E.; Goldrath, A.W.; Li, M.O.; et al. ICOS Coreceptor Signaling Inactivates the Transcription Factor FOXO1 to Promote Tfh Cell Differentiation. Immunity 2015, 42, 239–251. [Google Scholar] [CrossRef]
- Weber, J.P.; Fuhrmann, F.; Feist, R.K.; Lahmann, A.; Al Baz, M.S.; Gentz, L.-J.; Vu Van, D.; Mages, H.W.; Haftmann, C.; Riedel, R.; et al. ICOS Maintains the T Follicular Helper Cell Phenotype by Down-Regulating Krüppel-like Factor 2. J. Exp. Med. 2015, 212, 217–233. [Google Scholar] [CrossRef]
- Warnatz, K.; Bossaller, L.; Salzer, U.; Skrabl-Baumgartner, A.; Schwinger, W.; van der Burg, M.; van Dongen, J.J.M.; Orlowska-Volk, M.; Knoth, R.; Durandy, A.; et al. Human ICOS Deficiency Abrogates the Germinal Center Reaction and Provides a Monogenic Model for Common Variable Immunodeficiency. Blood 2006, 107, 3045–3052. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Hou, S.; Fang, Q.; Liu, X.; Liu, X.; Qi, H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018, 49, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Good-Jacobson, K.L.; Szumilas, C.G.; Chen, L.; Sharpe, A.H.; Tomayko, M.M.; Shlomchik, M.J. PD-1 Regulates Germinal Center B Cell Survival and the Formation and Affinity of Long-Lived Plasma Cells. Nat. Immunol. 2010, 11, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, F.; Li, R.; Li, Z.; Chen, X.; Zhou, Z.; Zhang, X.; Hu, T.; Zhang, Y.; Young, K.; et al. Role of Programmed Death Ligands in Effective T-Cell Interactions in Extranodal Natural Killer/T-Cell Lymphoma. Oncol. Lett. 2014, 8, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Fiore, D.; Cappelli, L.V.; Broccoli, A.; Zinzani, P.L.; Chan, W.C.; Inghirami, G. Peripheral T Cell Lymphomas: From the Bench to the Clinic. Nat. Rev. Cancer 2020, 20, 323–342. [Google Scholar] [CrossRef]
- Wartewig, T.; Kurgyis, Z.; Keppler, S.; Pechloff, K.; Hameister, E.; Öllinger, R.; Maresch, R.; Buch, T.; Steiger, K.; Winter, C.; et al. PD-1 Is a Haploinsufficient Suppressor of T Cell Lymphomagenesis. Nature 2017, 552, 121–125. [Google Scholar] [CrossRef]
- Rauch, D.A.; Conlon, K.C.; Janakiram, M.; Brammer, J.E.; Harding, J.C.; Ye, B.H.; Zang, X.; Ren, X.; Olson, S.; Cheng, X.; et al. Rapid Progression of Adult T-Cell Leukemia/Lymphoma as Tumor-Infiltrating Tregs after PD-1 Blockade. Blood 2019, 134, 1406–1414. [Google Scholar] [CrossRef]
- Barta, S.K.; Zain, J.; MacFarlane, A.W.; Smith, S.M.; Ruan, J.; Fung, H.C.; Tan, C.R.; Yang, Y.; Alpaugh, R.K.; Dulaimi, E.; et al. Phase II Study of the PD-1 Inhibitor Pembrolizumab for the Treatment of Relapsed or Refractory Mature T-Cell Lymphoma. Clin. Lymphoma Myeloma Leuk 2019, 19, 356–364.e3. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef]
- Neuwelt, A.; Al-Juhaishi, T.; Davila, E.; Haverkos, B. Enhancing Antitumor Immunity through Checkpoint Blockade as a Therapeutic Strategy in T-Cell Lymphomas. Blood Adv. 2020, 4, 4256–4266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mai, W.; Jiang, W.; Geng, Q. Sintilimab: A Promising Anti-Tumor PD-1 Antibody. Front. Oncol. 2020, 10, 594558. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Liu, X.F.; Jiao, L.J.; Yin, S.Y.; Wang, Z.; Li, X.X.; Ma, Z.P.; Yang, J.M.; He, M.X. Angioimmunoblastic T-cell lymphoma: Histopathological grading and prognosis. Zhonghua Bing Li Xue Za Zhi 2019, 48, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Guo, W.; Wang, Y.; Li, J.; Zhao, Y.; Qu, L.; Yan, X.; Li, J.; Guo, Q.; Young, K.H.; et al. The Short-Term Efficacy and Safety of Brentuximab Vedotin Plus Cyclophosphamide, Epirubicin and Prednisone in Untreated PTCL: A Real-World, Retrospective Study. Adv. Ther. 2022, 39, 532–543. [Google Scholar] [CrossRef]
- Sabattini, E.; Pizzi, M.; Tabanelli, V.; Baldin, P.; Sacchetti, C.S.; Agostinelli, C.; Zinzani, P.L.; Pileri, S.A. CD30 Expression in Peripheral T-Cell Lymphomas. Haematologica 2013, 98, e81–e82. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef]
- Brodfuehrer, J.; Rankin, A.; Edmonds, J.; Keegan, S.; Andreyeva, T.; Lawrence-Henderson, R.; Ozer, J.; Gao, H.; Bloom, L.; Boisvert, A.; et al. Quantitative Analysis of Target Coverage and Germinal Center Response by a CXCL13 Neutralizing Antibody in a T-Dependent Mouse Immunization Model. Pharm. Res. 2014, 31, 635–648. [Google Scholar] [CrossRef]
- Bhamidipati, K.; Silberstein, J.L.; Chaichian, Y.; Baker, M.C.; Lanz, T.V.; Zia, A.; Rasheed, Y.S.; Cochran, J.R.; Robinson, W.H. CD52 Is Elevated on B Cells of SLE Patients and Regulates B Cell Function. Front. Immunol. 2020, 11, 626820. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, C.M.; Hubacheck, J.; Janik, J.E.; Wilson, W.; Morris, J.C.; Jasper, G.A.; Stetler-Stevenson, M. Variable CD52 Expression in Mature T Cell and NK Cell Malignancies: Implications for Alemtuzumab Therapy. Br. J. Haematol. 2009, 145, 173–179. [Google Scholar] [CrossRef]
- Buckstein, R.; Fraser, G.; Cheung, M.; Kukreti, V.; Kuruvilla, J.; Imrie, K.; Piliotis, E.; Pond, G.; Windsor, J.; Ghorab, Z.; et al. Alemtuzumab and CHOP Chemotherapy for the Treatment of Aggressive Histology Peripheral T Cell Lymphomas: A Multi-Center Phase I Study. Clin. Lymphoma Myeloma Leuk 2016, 16, 18–28.e4. [Google Scholar] [CrossRef]
- Krug, A.; Martinez-Turtos, A.; Verhoeyen, E. Importance of T, NK, CAR T and CAR NK Cell Metabolic Fitness for Effective Anti-Cancer Therapy: A Continuous Learning Process Allowing the Optimization of T, NK and CAR-Based Anti-Cancer Therapies. Cancers 2021, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Breman, E.; Demoulin, B.; Agaugué, S.; Mauën, S.; Michaux, A.; Springuel, L.; Houssa, J.; Huberty, F.; Jacques-Hespel, C.; Marchand, C.; et al. Overcoming Target Driven Fratricide for T Cell Therapy. Front. Immunol. 2018, 9, 2940. [Google Scholar] [CrossRef] [PubMed]
- Scarfò, I.; Ormhøj, M.; Frigault, M.J.; Castano, A.P.; Lorrey, S.; Bouffard, A.A.; van Scoyk, A.; Rodig, S.J.; Shay, A.J.; Aster, J.C.; et al. Anti-CD37 Chimeric Antigen Receptor T Cells Are Active against B- and T-Cell Lymphomas. Blood 2018, 132, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Pinz, K.G.; Yakaboski, E.; Jares, A.; Liu, H.; Firor, A.E.; Chen, K.H.; Wada, M.; Salman, H.; Tse, W.; Hagag, N.; et al. Targeting T-Cell Malignancies Using Anti-CD4 CAR NK-92 Cells. Oncotarget 2017, 8, 112783–112796. [Google Scholar] [CrossRef] [PubMed]
- Maciocia, P.M.; Wawrzyniecka, P.A.; Philip, B.; Ricciardelli, I.; Akarca, A.U.; Onuoha, S.C.; Legut, M.; Cole, D.K.; Sewell, A.K.; Gritti, G.; et al. Targeting the T Cell Receptor β-Chain Constant Region for Immunotherapy of T Cell Malignancies. Nat. Med. 2017, 23, 1416–1423. [Google Scholar] [CrossRef]
- Pearce, E.J.; Pearce, E.L. Immunometabolism in 2017: Driving Immunity: All Roads Lead to Metabolism. Nat. Rev. Immunol. 2018, 18, 81–82. [Google Scholar] [CrossRef]
- Chang, C.-H.; Curtis, J.D.; Maggi, L.B.; Faubert, B.; Villarino, A.V.; O’Sullivan, D.; Huang, S.C.-C.; van der Windt, G.J.W.; Blagih, J.; Qiu, J.; et al. Posttranscriptional Control of T Cell Effector Function by Aerobic Glycolysis. Cell 2013, 153, 1239–1251. [Google Scholar] [CrossRef]
- Xu, Y.; Chaudhury, A.; Zhang, M.; Savoldo, B.; Metelitsa, L.S.; Rodgers, J.; Yustein, J.T.; Neilson, J.R.; Dotti, G. Glycolysis Determines Dichotomous Regulation of T Cell Subsets in Hypoxia. J. Clin. Investig. 2016, 126, 2678–2688. [Google Scholar] [CrossRef]
- Stauss, D.; Brunner, C.; Berberich-Siebelt, F.; Höpken, U.E.; Lipp, M.; Müller, G. The Transcriptional Coactivator Bob1 Promotes the Development of Follicular T Helper Cells via Bcl6. EMBO J. 2016, 35, 881–898. [Google Scholar] [CrossRef]
- Basso, K.; Dalla-Favera, R. Roles of BCL6 in Normal and Transformed Germinal Center B Cells. Immunol. Rev. 2012, 247, 172–183. [Google Scholar] [CrossRef]
- Manso, R.; Martínez-Magunacelaya, N.; Chamizo, C.; Rojo, F.; Piris, M.Á.; Rodriguez-Pinilla, S.M. Mutual Regulation between BCL6 and a Specific Set of MiRNAs Controls TFH Phenotype in Peripheral T-Cell Lymphoma. Br. J. Haematol. 2018, 182, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Bunting, K.L.; Melnick, A.M. New Effector Functions and Regulatory Mechanisms of BCL6 in Normal and Malignant Lymphocytes. Curr. Opin. Immunol. 2013, 25, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, S.; Sakata-Yanagimoto, M.; Hattori, K.; Muto, H.; Nguyen, T.; Izutsu, K.; Yoshida, K.; Ogawa, S.; Nakamura, N.; Chiba, S. BCL6 Locus Is Hypermethylated in Angioimmunoblastic T-Cell Lymphoma. Int. J. Hematol. 2017, 105, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Leeman-Neill, R.J.; Bhagat, G. BCL6 as a Therapeutic Target for Lymphoma. Expert Opin. Ther. Targets 2018, 22, 143–152. [Google Scholar] [CrossRef]
- Cardenas, M.G.; Oswald, E.; Yu, W.; Xue, F.; MacKerell, A.D.; Melnick, A.M. The Expanding Role of the BCL6 Oncoprotein as a Cancer Therapeutic Target. Clin. Cancer Res. 2017, 23, 885–893. [Google Scholar] [CrossRef]
- Kerres, N.; Steurer, S.; Schlager, S.; Bader, G.; Berger, H.; Caligiuri, M.; Dank, C.; Engen, J.R.; Ettmayer, P.; Fischerauer, B.; et al. Chemically Induced Degradation of the Oncogenic Transcription Factor BCL6. Cell Rep. 2017, 20, 2860–2875. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef]
- Klein Geltink, R.I.; O’Sullivan, D.; Corrado, M.; Bremser, A.; Buck, M.D.; Buescher, J.M.; Firat, E.; Zhu, X.; Niedermann, G.; Caputa, G.; et al. Mitochondrial Priming by CD28. Cell 2017, 171, 385–397.e11. [Google Scholar] [CrossRef]
- Waickman, A.T.; Powell, J.D. MTOR, Metabolism, and the Regulation of T-Cell Differentiation and Function. Immunol. Rev. 2012, 249, 43–58. [Google Scholar] [CrossRef]
- Patsoukis, N.; Bardhan, K.; Chatterjee, P.; Sari, D.; Liu, B.; Bell, L.N.; Karoly, E.D.; Freeman, G.J.; Petkova, V.; Seth, P.; et al. PD-1 Alters T-Cell Metabolic Reprogramming by Inhibiting Glycolysis and Promoting Lipolysis and Fatty Acid Oxidation. Nat. Commun. 2015, 6, 6692. [Google Scholar] [CrossRef] [PubMed]
- Scharping, N.E.; Menk, A.V.; Moreci, R.S.; Whetstone, R.D.; Dadey, R.E.; Watkins, S.C.; Ferris, R.L.; Delgoffe, G.M. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity 2016, 45, 374–388. [Google Scholar] [CrossRef] [PubMed]
- MacIver, N.J.; Michalek, R.D.; Rathmell, J.C. Metabolic Regulation of T Lymphocytes. Annu. Rev. Immunol. 2013, 31, 259–283. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, D.; Spier, C.; Della Croce, K.; Miller, S.; George, B.; Riley, C.; Warner, S.; Grogan, T.M.; Miller, T.P. Transcript Profiling in Peripheral T-Cell Lymphoma, Not Otherwise Specified, and Diffuse Large B-Cell Lymphoma Identifies Distinct Tumor Profile Signatures. Mol. Cancer Ther. 2005, 4, 1867–1879. [Google Scholar] [CrossRef]
- Xiong, J.; Bian, J.; Wang, L.; Zhou, J.-Y.; Wang, Y.; Zhao, Y.; Wu, L.-L.; Hu, J.-J.; Li, B.; Chen, S.-J.; et al. Dysregulated Choline Metabolism in T-Cell Lymphoma: Role of Choline Kinase-α and Therapeutic Targeting. Blood Cancer J. 2015, 5, 287. [Google Scholar] [CrossRef]
- Bachow, S.H.; O’Connor, O.A. Emerging Therapies in Relapsed and Refractory Peripheral T-Cell Lymphoma. Clin. Adv. Hematol. Oncol. 2015, 13, 837–846. [Google Scholar]
- Poirier, F.; Joubert-Caron, R.; Labas, V.; Caron, M. Proteomic Analysis of a Lymphoma-Derived Cell Line (DG75) Following Treatment with a Demethylating Drug: Modification of Membrane-Associated Proteins. Proteomics 2003, 3, 1028–1036. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krug, A.; Tari, G.; Saidane, A.; Gaulard, P.; Ricci, J.-E.; Lemonnier, F.; Verhoeyen, E. Novel T Follicular Helper-like T-Cell Lymphoma Therapies: From Preclinical Evaluation to Clinical Reality. Cancers 2022, 14, 2392. https://doi.org/10.3390/cancers14102392
Krug A, Tari G, Saidane A, Gaulard P, Ricci J-E, Lemonnier F, Verhoeyen E. Novel T Follicular Helper-like T-Cell Lymphoma Therapies: From Preclinical Evaluation to Clinical Reality. Cancers. 2022; 14(10):2392. https://doi.org/10.3390/cancers14102392
Chicago/Turabian StyleKrug, Adrien, Gamze Tari, Aymen Saidane, Philippe Gaulard, Jean-Ehrland Ricci, François Lemonnier, and Els Verhoeyen. 2022. "Novel T Follicular Helper-like T-Cell Lymphoma Therapies: From Preclinical Evaluation to Clinical Reality" Cancers 14, no. 10: 2392. https://doi.org/10.3390/cancers14102392
APA StyleKrug, A., Tari, G., Saidane, A., Gaulard, P., Ricci, J.-E., Lemonnier, F., & Verhoeyen, E. (2022). Novel T Follicular Helper-like T-Cell Lymphoma Therapies: From Preclinical Evaluation to Clinical Reality. Cancers, 14(10), 2392. https://doi.org/10.3390/cancers14102392





