Histopathologic and MR Imaging Appearance of Spontaneous and Radiation-Induced Necrosis in Uveal Melanomas: Initial Results
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Radiotherapy Protocol
2.3. MRI Protocol
2.4. Histopathology
2.5. Image Analysis
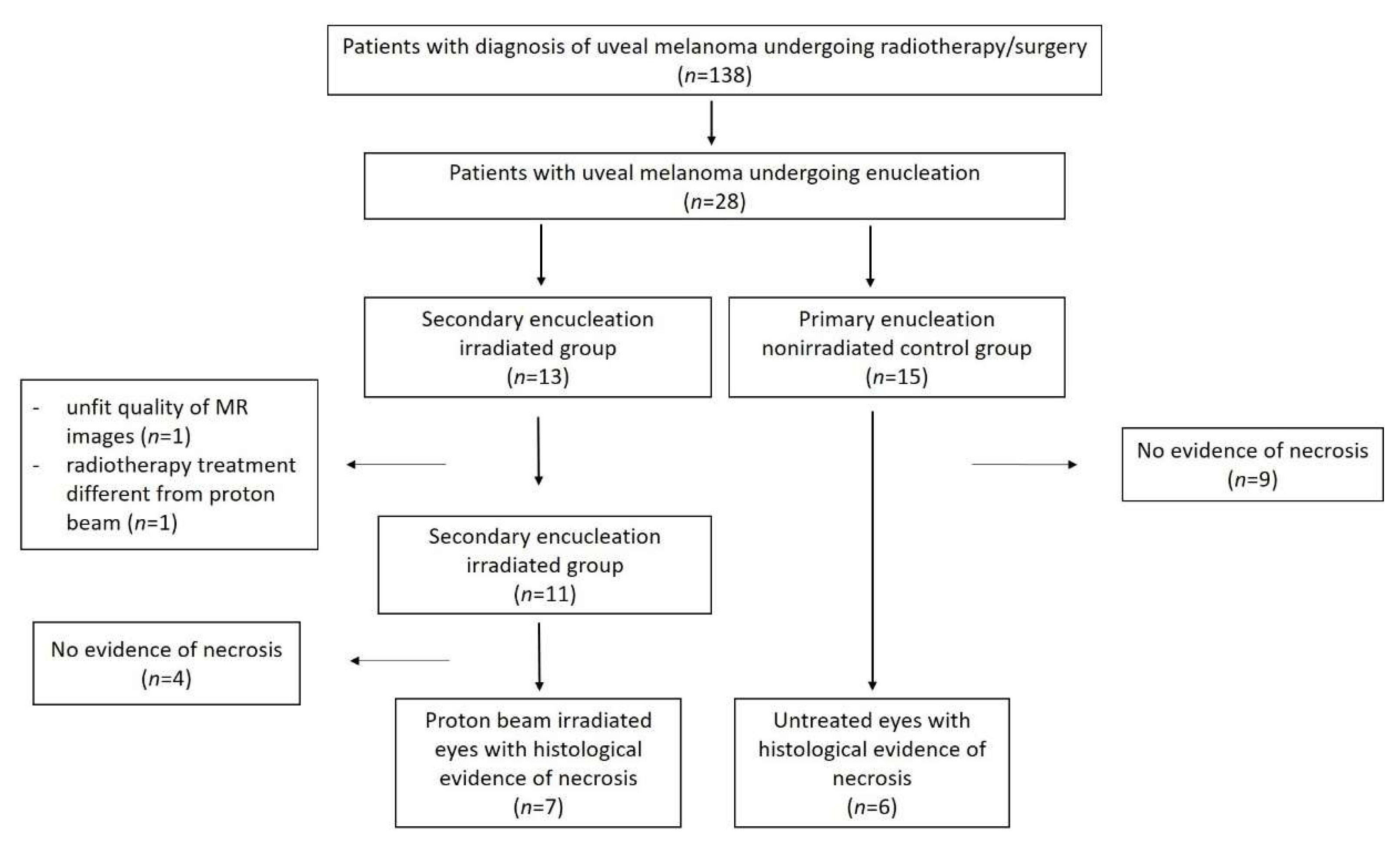
3. Results
3.1. Study Populaton
3.2. Histopathologic Findings
3.2.1. Histopathologic Findings in the Irradiated Group
3.2.2. Histopathologic Findings in the Control Group
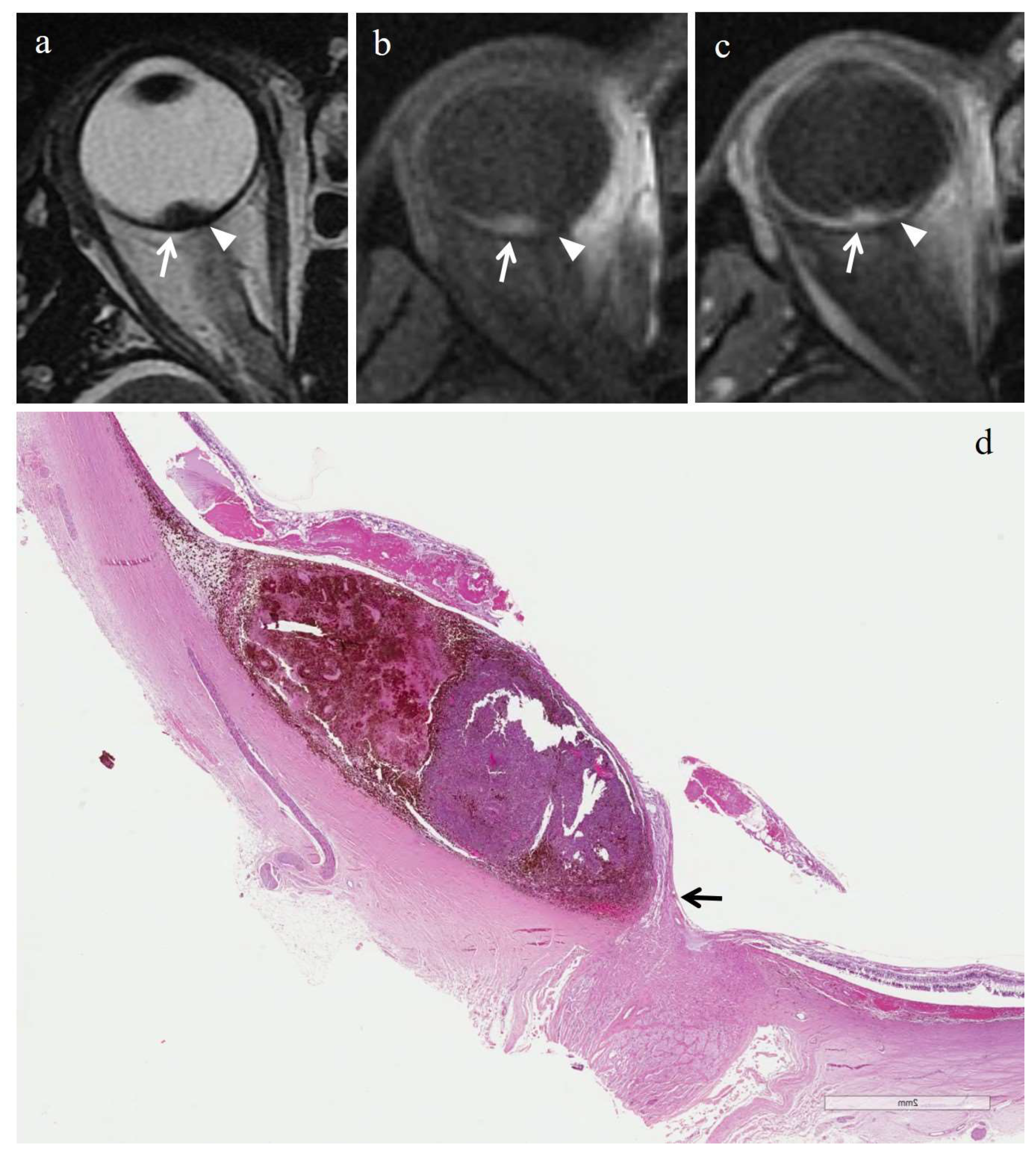
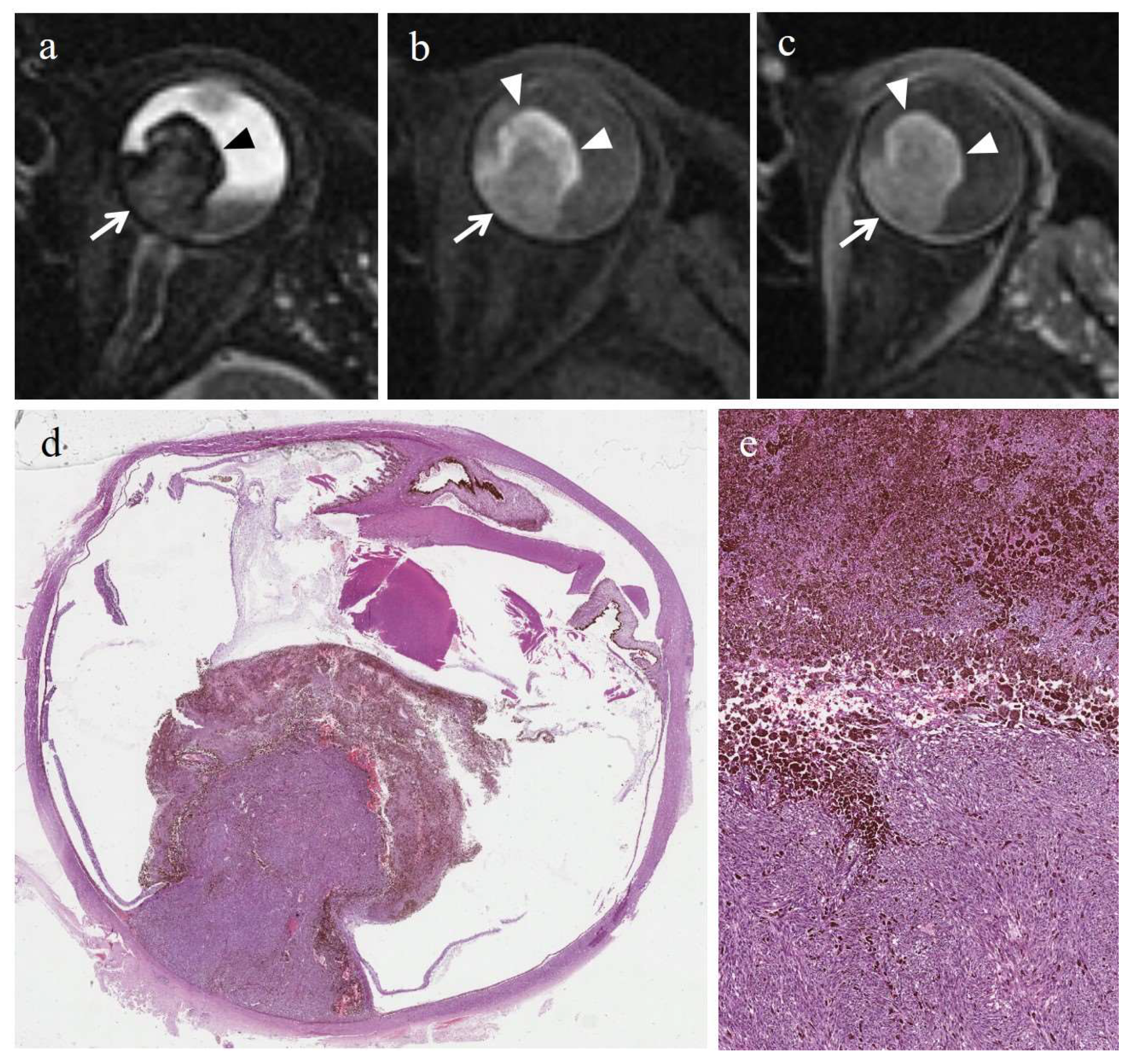
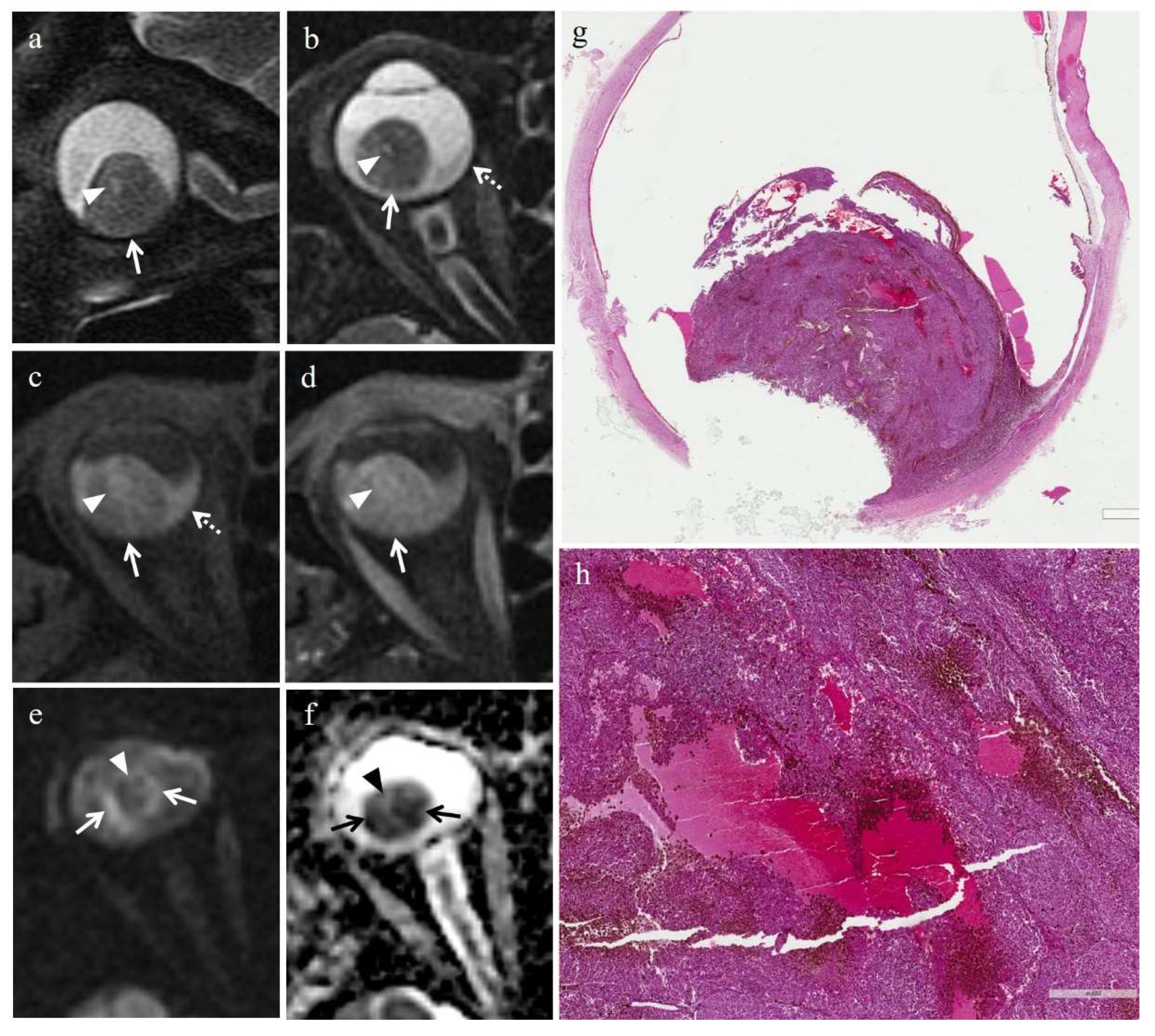
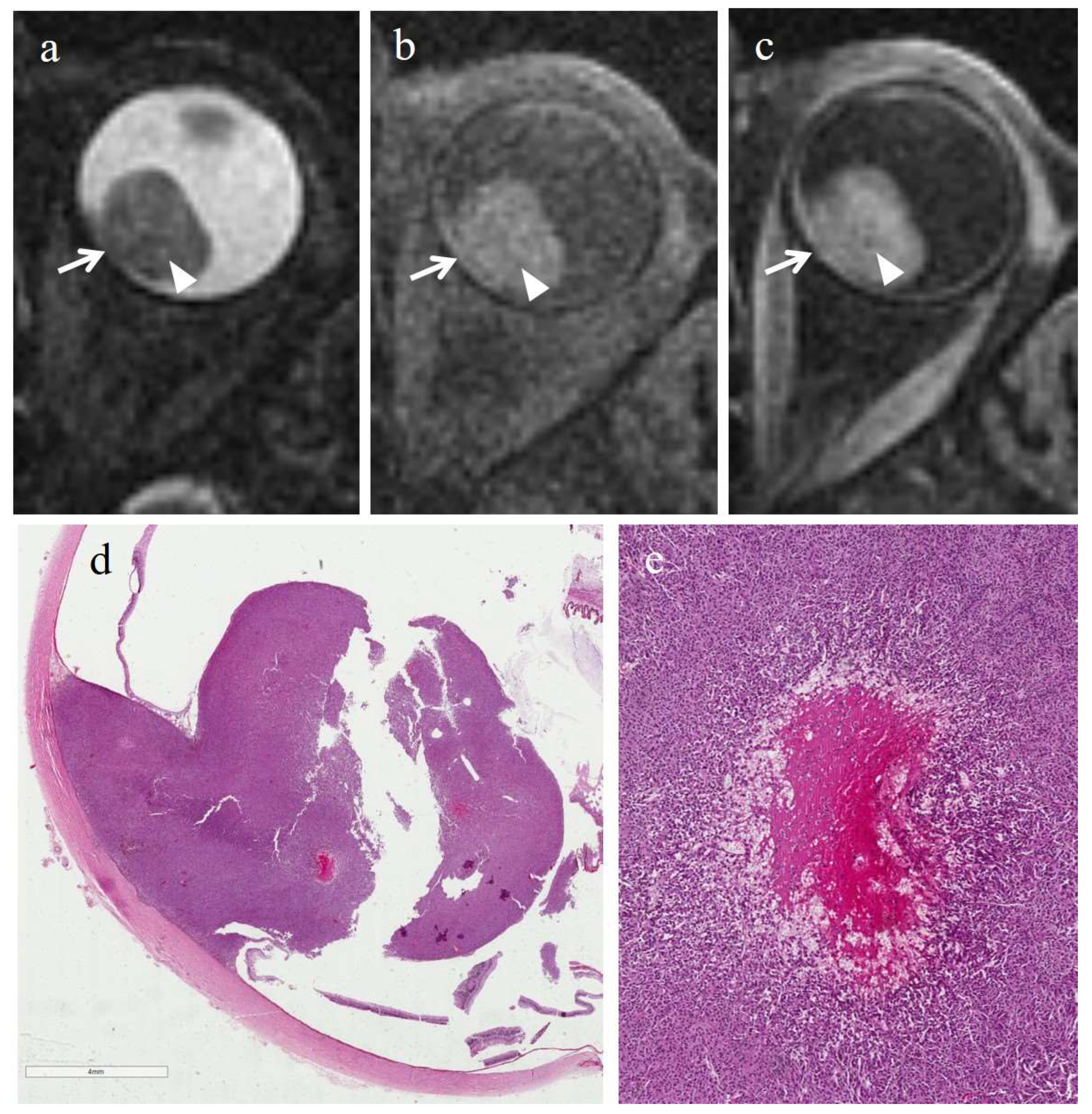
3.3. MRI Findings
3.3.1. MRI Findings in the Irradiated Group
3.3.2. MRI Findings in the Control Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keraliya, A.R.; Krajewski, K.M.; Braschi-Amirfarzan, M.; Tirumani, S.H.; Shinagare, A.B.; Jagannathan, J.P.; Ramaiya, N.H. Extracutaneous melanomas: A primer for the radiologist. Insights Imaging 2015, 6, 707–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purohit, B.S.; Vargas, M.I.; Ailianou, A.; Merlini, L.; Poletti, P.A.; Platon, A.; Delattre, B.M.; Rager, O.; Burkhardt, K.; Becker, M. Orbital tumours and tumour-like lesions: Exploring the armamentarium of multiparametric imaging. Insights Imaging 2016, 7, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Mallone, S.; De Vries, E.; Guzzo, M.; Midena, E.; Verne, J.; Coebergh, J.W.; Marcos-Gragera, R.; Ardanaz, E.; Martinez, R.; Chirlaque, M.D.; et al. Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur. J. Cancer 2012, 48, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, C.; Caltabiano, R.; Broggi, G.; Russo, A.; Puzzo, L.; Avitabile, T.; Longo, A.; Reibaldi, M.; Barbagallo, D.; Di Pietro, C.; et al. LncRNA LINC00518 Acts as an Oncogene in Uveal Melanoma by Regulating an RNA-Based Network. Cancers 2020, 12, 3867. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Raffaele, L.; Salamone, V.; Caltabiano, R.; Broggi, G.; Puzzo, L.; et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging-Part I: MR imaging with pathologic correlation and technical considerations. Insights Imaging 2021, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Zielinska, A.; Luis, M.; Carbone, C.; Martins-Gomes, C.; Souto, S.B.; Silva, A.M. Uveal melanoma: Physiopathology and new in situ-specific therapies. Cancer Chemother Pharmacol. 2019, 84, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Chalada, M.; Ramlogan-Steel, C.A.; Dhungel, B.P.; Layton, C.J.; Steel, J.C. The Impact of Ultraviolet Radiation on the Aetiology and Development of Uveal Melanoma. Cancers 2021, 13, 1700. [Google Scholar] [CrossRef]
- Broggi, G.; Russo, A.; Reibaldi, M.; Russo, D.; Varricchio, S.; Bonfiglio, V.; Spatola, C.; Barbagallo, C.; Foti, P.V.; Avitabile, T.; et al. Histopathology and Genetic Biomarkers of Choroidal Melanoma. Appl. Sci. 2020, 10, 8081. [Google Scholar] [CrossRef]
- Russo, D.; Di Crescenzo, R.M.; Broggi, G.; Merolla, F.; Martino, F.; Varricchio, S.; Ilardi, G.; Borzillo, A.; Carandente, R.; Pignatiello, S.; et al. Expression of P16INK4a in Uveal Melanoma: New Perspectives. Front Oncol. 2020, 13, 562074. [Google Scholar] [CrossRef]
- Esposito, E.; Zoroquiain, P.; Mastromonaco, C.; Lasiste, J.M.; Aldrees, S.; Moreira Neto, C.A.; Coblentz, J.; Alshareef, R.; Burnier, M., Jr.; Chhablani, J.; et al. Choroidal Histopathology. In Choroidal Disorders, 1st ed.; Chapter 3; Academic Press: New York, NY, USA; Elsevier: New York, NY, USA, 2017; pp. 21–48. [Google Scholar]
- Putri, C.A.; Salvi, S.M. Necrotic choroidal melanoma masquerading as scleritis. Indian J. Ophthalmol. 2020, 68, 1979–1981. [Google Scholar] [CrossRef]
- Beenakker, J.W.; Ferreira, T.A.; Soemarwoto, K.P.; Genders, S.W.; Teeuwisse, W.M.; Webb, A.G.; Luyten, G.P. Clinical evaluation of ultra-high-field MRI for three-dimensional visualisation of tumour size in uveal melanoma patients, with direct relevance to treatment planning. Magn. Reson. Mater. Phys. Biol. Med. 2016, 29, 571–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, T.A.; Grech Fonk, L.; Jaarsma-Coes, M.G.; van Haren, G.G.R.; Marinkovic, M.; Beenakker, J.M. MRI of Uveal Melanoma. Cancers 2019, 11, 377. [Google Scholar] [CrossRef] [Green Version]
- Grech Fonk, L.; Ferreira, T.A.; Webb, A.G.; Luyten, G.P.M.; Beenakker, J.M. The Economic Value of MR-Imaging for Uveal Melanoma. Clin. Ophthalmol. 2020, 14, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma-Coes, M.G.; Marinkovic, M.; Astreinidou, E.; Schuurmans, M.S.; Peters, F.P.; Luyten, G.P.M.; Rasch, C.R.N.; Beenakker, J.M. Measuring eye deformation between planning and proton beam therapy position using magnetic resonance imaging. Phys. Imaging Radiat. Oncol. 2020, 16, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Niendorf, T.; Beenakker, J.M.; Langner, S.; Erb-Eigner, K.; Bach Cuadra, M.; Beller, E.; Millward, J.M.; Niendorf, T.M.; Stachs, O. Ophthalmic Magnetic Resonance Imaging: Where Are We (Heading To)? Curr. Eye Res. 2021, 46, 1251–1270. [Google Scholar] [CrossRef]
- Tang, M.C.Y.; Jaarsma-Coes, M.G.; Ferreira, T.A.; Zwirs-Grech Fonk, L.; Marinkovic, M.; Luyten, G.P.M.; Beenakker, J.M. A Comparison of 3 T and 7 T MRI for the Clinical Evaluation of Uveal Melanoma. J. Magn. Reson. Imaging 2021. [Google Scholar] [CrossRef]
- Foti, P.V.; Farina, R.; Coronella, M.; Palmucci, S.; Montana, A.; Sigona, A.; Reibaldi, M.; Longo, A.; Russo, A.; Avitabile, T.; et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting the response of ocular melanoma to proton beam therapy: Initial results. Radiol. Med. 2015, 120, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Longo, A.; Reibaldi, M.; Russo, A.; Privitera, G.; Spatola, C.; Raffaele, L.; Salamone, V.; Farina, R.; Palmucci, S.; et al. Uveal melanoma: Quantitative evaluation of difusion-weighted MR imaging in the response assessment after proton-beam therapy, long-term follow-up. Radiol. Med. 2017, 122, 131–139. [Google Scholar] [CrossRef]
- Midena, E.; Segato, T.; Piermarocchi, S.; Boccato, P. Fine needle aspiration biopsy in ophthalmology. Surv. Ophthalmol. 1985, 29, 410–422. [Google Scholar] [CrossRef]
- Midena, E.; Parrozzani, R. Biopsies in uveal melanoma. Dev. Ophthalmol. 2012, 49, 81–95. [Google Scholar] [CrossRef]
- Frizziero, L.; Midena, E.; Trainiti, S.; Londei, D.; Bonaldi, L.; Bini, S.; Parrozzani, R. Uveal Melanoma Biopsy: A Review. Cancers 2019, 11, 1075. [Google Scholar] [CrossRef] [Green Version]
- Midena, E.; Parrozzani, R.; Midena, G.; Trainiti, S.; Marchione, G.; Cosmo, E.; Londei, D.; Frizziero, L. In vivo intraocular biomarkers: Changes of aqueous humor cytokines and chemokines in patients affected by uveal melanoma. Medicine 2020, 99, e22091. [Google Scholar] [CrossRef] [PubMed]
- Valpione, S.; Moser, J.C.; Parrozzani, R.; Bazzi, M.; Mansfield, A.S.; Mocellin, S.; Pigozzo, J.; Midena, E.; Markovic, S.N.; Aliberti, C.; et al. Development and external validation of a prognostic nomogram for metastatic uveal melanoma. PLoS ONE 2015, 10, e0120181. [Google Scholar] [CrossRef] [PubMed]
- Szeligo, B.M.; Ivey, A.D.; Boone, B.A. Poor Response to Checkpoint Immunotherapy in Uveal Melanoma Highlights the Persistent Need for Innovative Regional Therapy Approaches to Manage Liver Metastases. Cancers 2021, 13, 3426. [Google Scholar] [CrossRef]
- Rusňák, Š.; Hecová, L.; Kasl, Z.; Sobotová, M.; Hauer, L. Therapy of uveal melanoma A Review. Cesk. Slov. Oftalmol. 2020, 77, 1–13. [Google Scholar] [CrossRef]
- Zimmerman, L.E.; McLean, I.W.; Foster, W.D. Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells. Br. J. Ophthalmol. 1978, 62, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; Gragoudas, E.S.; Egan, K.M.; Glynn, R.J.; Howard, S.; Fante, S.G.; Albert, D.M. Relative survival rates after alternative therapies for uveal melanoma. Ophthalmology 1990, 97, 769–777. [Google Scholar] [CrossRef]
- Diener-West, M.; Earle, J.D.; Fine, S.L.; Hawkins, B.S.; Moy, C.S.; Reynolds, S.M.; Schachat, A.P.; Straatsma, B.R.; Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS Report No. 18. Arch. Ophthalmol. 2001, 119, 969–982. [Google Scholar] [CrossRef]
- Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine125 brachytherapy for choroidal melanoma, IV: Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar] [CrossRef]
- Midena, G.; Parrozzani, R.; Frizziero, L.; Midena, E. Chorioretinal Side Effects of Therapeutic Ocular Irradiation: A Multimodal Imaging Approach. J. Clin. Med. 2020, 9, 3496. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Boersma, L.J.; Rasch, C.R.N.; van Vulpen, M.; Reitsma, J.B.; van der Schaaf, A.; Schuit, E. Clinical Trial Strategies to Compare Protons With Photons. Semin. Radiat. Oncol. 2018, 28, 79–87. [Google Scholar] [CrossRef]
- Yonekawa, Y.; Kim, I.K. Epidemiology and Management of Uveal Melanoma. Hematol. Clin. N. Am. 2012, 26, 1169–1184. [Google Scholar] [CrossRef]
- Journée-de Korver, H.G.; Midena, E.; Singh, A.D. Infrared thermotherapy: From laboratory to clinic. Ophthalmol. Clin. N. Am. 2005, 18, 99–110, viii–ix. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; Caltabiano, R.; et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging-Part II: Treatment indications and complications. Insights Imaging 2021, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.B.; Char, D.H. Histopathology of uveal melanomas treated with charged particle radiation. Ophthalmology 1987, 94, 639–643. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; Karlsson, U.; Menduke, H.; Brady, L.W. Enucleation after plaque radiotherapy for posterior uveal melanoma. Histopathol. Find. Ophthalmol. 1990, 97, 1665–1670. [Google Scholar] [CrossRef]
- Saornil, M.A.; Egan, K.M.; Gragoudas, E.S.; Seddon, J.M.; Walsh, S.M.; Albert, D.M. Histopathology of proton beam-irradiated vs enucleated uveal melanomas. Arch. Ophthalmol. 1992, 110, 1112–1118. [Google Scholar] [CrossRef]
- Avery, R.B.; Diener-West, M.; Reynolds, S.M.; Grossniklaus, H.E.; Green, W.R.; Albert, D.M. Histopathologic characteristics of choroidal melanoma in eyes enucleated after iodine 125 brachytherapy in the collaborative ocular melanoma study. Arch. Ophthalmol. 2008, 126, 207–212. [Google Scholar] [CrossRef] [Green Version]
- De Jong, M.C.; de Graaf, P.; Pouwels, P.J.W.; Beenakker, J.W.; Jansen, R.W.; Geurts, J.J.G.; Moll, A.C.; Castelijns, J.A.; van der Valk, P.; van der Weerd, L. 9.4T and 17.6T MRI of Retinoblastoma: Ex Vivo evaluation of microstructural anatomy and disease extent compared with histopathology. J. Magn. Reson. Imaging 2018, 47, 1487–1497. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Jaarsma-Coes, M.G.; Marinkovic, M.; Verbist, B.; Verdijk, R.M.; Jager, M.J.; Luyten, G.P.M.; Beenakker, J.M. MR imaging characteristics of uveal melanoma with histopathological validation. Neuroradiology 2021. [Google Scholar] [CrossRef] [PubMed]
- Spatola, C.; Privitera, G. Clinical aspects and potential clinical applications of laser accelerated proton beams. AIP Conf. Proc. 2013, 1546, 108–111. [Google Scholar] [CrossRef]
- Foti, P.V.; Inì, C.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; et al. MR Imaging–Pathologic Correlation of Uveal Melanomas Undergoing Secondary Enucleation after Proton Beam Radiotherapy. Appl. Sci. 2021, 11, 4310. [Google Scholar] [CrossRef]
- McLean, I.W.; Foster, W.D.; Zimmerman, L.E.; Gamel, J.W. Modifications of Callender’s Classification of Uveal Melanoma at the Armed Forces Institute of Pathology. Am. J. Ophthalmol. 2018, 96, 502–509. [Google Scholar] [CrossRef]
- Ferry, A.P.; Blair, C.J.; Gragoudas, E.S.; Volk, S.C. Pathologic examination of ciliary body melanoma treated with proton beam irradiation. Arch. Ophthalmol. 1985, 103, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Manschot, W.A.; van Strik, R. Uveal melanoma: Therapeutic consequences of doubling times and irradiation results; a review. Int. Ophthalmol. 1992, 16, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Albert, D.M.; Diener-West, M.; Robinson, N.L.; Grossniklaus, H.E.; Green, W.R.; Vine, A.K.; Willis, J.; Frueh, B.; Kurtz, R.M.; Elner, S.; et al. Histopathologic characteristics of uveal melanomas in eyes enucleated from the Collaborative Ocular Melanoma Study. COMS report no. 6. Am. J. Ophthalmol. 1998, 125, 745–766. [Google Scholar] [CrossRef]
- Seddon, J.M.; Gragoudas, E.S.; Albert, D.M. Ciliary body and choroidal melanomas treated by proton beam irradiation. Histopathologic study of eyes. Arch. Ophthalmol. 1983, 101, 1402–1408. [Google Scholar] [CrossRef]
- Singh, M.; Durairaj, P.; Yeung, J. Uveal Melanoma: A Review of the Literature. Oncol Ther. 2018, 6, 87–104, Erratum in Oncol. Ther. 2019, 7, 93. [Google Scholar] [CrossRef] [Green Version]
- Brannan, S.; Browne, B.; Clark, B.J. Massive infarction of ocular tissues complicating a necrotic uveal melanoma. Eye 1998, 12 Pt 2, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Bujara, K. Necrotic malignant melanomas of the choroid and ciliary body. A clinicopathological and statistical study. Graefes. Arch. Clin. Exp. Ophthalmol. 1982, 219, 40–43. [Google Scholar] [CrossRef]
- Baumann, C.; Iannetta, D.; Coupland, S.E.; Groenewald, C.; Vishwanath, M.; Heimann, H. Spontaneous Necrosis of a Large Choroidal Melanoma: Unusual Presentation in a 49-Year-Old Male. Ocul. Oncol. Pathol. 2020, 6, 174–179. [Google Scholar] [CrossRef]
- Thareja, S.; Rashid, A.; Grossniklaus, H.E. Spontaneous Necrosis of Choroidal Melanoma. Ocul. Oncol. Pathol. 2014, 1, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palamar, M.; Thangappan, A.; Shields, C.L.; Ehya, H.; Shields, J.A. Necrotic choroidal melanoma with scleritis and choroidal effusion. Cornea 2009, 28, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Moshari, A.; Cheeseman, E.W.; McLean, I.W. Totally necrotic choroidal and ciliary body melanomas: Associations with prognosis, episcleritis, and scleritis. Am. J. Ophthalmol. 2001, 131, 232–236. [Google Scholar] [CrossRef]
- Bhagat, S.; Ramaesh, K.; Wharton, S.B.; Dhillon, B. Spontaneous acute scleritis and scleral necrosis in choroidal malignant melanoma. Eye 1999, 13 Pt 6, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Tabassian, A.; Zuravieff, J.J. Necrotic choroidal melanoma with orbital inflammation. Arch. Ophthalmol. 1995, 113, 1576–1577. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Santos, M.C.; Gündüz, K.; Singh, A.D.; Othmane, I. Incomplete spontaneous regression of choroidal melanoma associated with inflammation. Arch. Ophthalmol. 1999, 117, 1245–1247. [Google Scholar] [PubMed]
- Reese, A.B.; Archila, E.A.; Jones, I.S.; Cooper, W.C. Necrosis of malignant melanoma of the choroid. Am. J. Ophthalmol. 1970, 69, 91–104. [Google Scholar] [CrossRef]
- Fraser, D.J., Jr.; Font, R.L. Ocular inflammation and hemorrhage as initial manifestations of uveal malignant melanoma. Incidence and prognosis. Arch. Ophthalmol. 1979, 97, 1311–1314. [Google Scholar] [CrossRef]
- Erb-Eigner, K.; Willerding, G.; Taupitz, M.; Hamm, B.; Asbach, P. Diffusion-weighted imaging of ocular melanoma. Investig. Radiol. 2013, 48, 702–707. [Google Scholar] [CrossRef]
- Beenakker, J.W.; van Rijn, G.A.; Luyten, G.P.; Webb, A.G. High-resolution MRI of uveal melanoma using a microcoil phased array at 7 T. NMR Biomed. 2013, 26, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma-Coes, M.G.; Ferreira, T.A.; van Houdt, P.J.; van der Heide, U.A.; Luyten, G.P.M.; Beenakker, J.M. Eye-specific quantitative dynamic contrast-enhanced MRI analysis for patients with intraocular masses. Magn. Reson. Mater. Phys. Biol. Med. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Kamrava, M.; Sepahdari, A.R.; Leu, K.; Wang, P.-C.; Roberts, K.; Demanes, D.J.; McCannel, T.; Ellingson, B.M. Quantitative multiparametric MRI in uveal melanoma: Increased tumor permeability may predict monosomy 3. Neuroradiology 2015, 57, 833–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| MRI Protocol | T2W FSE | T2W FSE STIR | T1W FSE | T1W FSE Fat Sat | DWI SE EPI |
|---|---|---|---|---|---|
| Acquisition plane | axial, coronal | axial, coronal | axial, coronal | axial, coronal | axial |
| Repetition time/echo time (msec) | 3220/120 | 3700/50 | 550/14.9 | 450/15.1 | 4800/89.9 |
| Flip angle | 90° | 90° | 90° | 90° | 90° |
| Echo train length | 19 | 12 | 2 | 2 | - |
| N. of averages | 4 | 3 | 3 | 2 | 8 |
| Section thickness (mm) | 3 | 3 | 3 | 3 | 4 |
| Interslice gap (mm) | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 |
| Field of view (mm) | 160 × 160 | 160 × 160 | 160 × 160 | 160 × 160 | 200 × 200 |
| Matrix | 352 × 256 | 256 × 256 | 256 × 224 | 256 × 256 | 192 × 192 |
| Frequency direction | Superior to inferior | Anterior to posterior | Right to left | Right to left | Right to left |
| b-value (s/mm2) | - | - | - | - | 0–1000 |
| Patient | Gender | Age | Eye | Tumor Location | Interval between Irradiation and Enucleation | Reasons for Enucleation | Pretreatment Tumor Size * (TP, LBD) |
|---|---|---|---|---|---|---|---|
| 1 | Female | 72 | Left | Choroid | 46 months | Radiotherapy-related complications | 7.8 mm, 18 mm |
| 2 | Male | 60 | Right | Choroid | 35 months | Tumor regrowth | 6 mm, 13.4 mm |
| 3 | Male | 29 | Right | Choroid | 34 months | Tumor regrowth | 8 mm, 16.2 mm |
| 4 | Male | 44 | Right | Choroid | 32 months | Tumor regrowth | 2.7 mm, 8 mm |
| 5 | Female | 78 | Right | Choroid | 18 months | Tumor regrowth | 5.4 mm, 12.6 mm |
| 6 | Male | 54 | Right | Choroid | 36 months | Tumor regrowth | 4.2 mm, 9.1 mm |
| 7 | Female | 60 | Left | Choroid | 29 months | Tumor regrowth | 6.3 mm, 11.2 mm |
| Patient | Gender | Age | Eye | Tumor Location | Reasons for Enucleation | Pretreatment Tumor Size * (TP, LBD) |
|---|---|---|---|---|---|---|
| 1 | Male | 55 | Left | Choroid | Optic nerve invasion | 8 mm, 16 mm |
| 2 | Female | 80 | Right | Choroid | Large tumor | 8 mm, 13 mm |
| 3 | Male | 77 | Right | Choroid | Optic nerve invasion | 15 mm, 20 mm |
| 4 | Male | 70 | Right | Choroid | Large tumor | 12.2 mm, 10.7 mm |
| 5 | Male | 40 | Left | Choroid | Large tumor | 11 mm, 14 mm |
| 6 | Female | 79 | Left | Choroid | Large tumor | 12.1 mm, 12.3 mm |
| Patient | Histologic Type | Degree of Pigmentation | Degree of Necrosis | Necrotic Pattern |
|---|---|---|---|---|
| 1 | Necrosis without viable tumor tissue | - | Grade III | Sharply demarcated tumor necrosis |
| 2 | Spindle cell | Pigmented | Grade I | Sharply demarcated tumor necrosis |
| 3 | Epithelioid cell | Pigmented | Grade II | Sharply demarcated tumor necrosis |
| 4 | Spindle cell | Pigmented | Grade III | Sharply demarcated tumor necrosis |
| 5 | Mixed cell type | Poorly pigmented | Grade I | Multiple foci- hemorrhagic/coagulative-type |
| 6 | Mixed cell type | Poorly pigmented | Grade III | Sharply demarcated tumor necrosis |
| 7 | Spindle cell | Poorly pigmented | Grade III | Sharply demarcated tumor necrosis |
| Patient | Histologic Type | Degree of Pigmentation | Degree of Necrosis | Necrotic Pattern |
|---|---|---|---|---|
| 1 | Epithelioid cell | Pigmented | Grade I | Multiple foci- hemorrhagic/coagulative-type, tumor necrosis |
| 2 | Epithelioid cell | Poorly pigmented | Grade I | Multiple foci- hemorrhagic/coagulative-type, tumor necrosis |
| 3 | Spindle cell | Pigmented | Grade II | Multiple foci tumor necrosis |
| 4 | Mixed cell type | Pigmented | Grade I | Multiple foci tumor necrosis |
| 5 | Spindle cell | Pigmented | Grade I | Multiple foci- hemorrhagic/coagulative-type, tumor necrosis |
| 6 | Mixed cell type | Poorly pigmented | Grade I | Multiple foci- hemorrhagic/coagulative-type |
| MR Finding | T2 | T1 | Gd-T1 | DWI |
|---|---|---|---|---|
| Radiation-induced necrosis |  Low signal |  High signal |  No enhancement |  Low signal |
| Radiation-induced necrosis with viable tumor tissue |  M RIN |  M RIN |  M RIN |  M RIN |
| Hemorrhagic necrosis in untreated melanoma |  High signal |  Low signal |  No enhancement |  Low signal |
 low signal;
low signal;  high signal;
high signal;  no enhancement; M: melanoma; RIN: radiation induced necrosis.
no enhancement; M: melanoma; RIN: radiation induced necrosis.| Patient | T2 | T1 | Gd-T1 | DWI | ADC × 10−3 mm2/s ** |
|---|---|---|---|---|---|
| 1 | Hypointense | Hyperintense | No enhancement | No restriction | - |
| 2 | - | - | - | - | 0.76 |
| 3 | Hypointense | Hyperintense | No enhancement | No restriction | 0.84 |
| 4 | Hypointense | Hyperintense | No enhancement | No restriction | - |
| 5 | Hyperintense | Hypointense | No enhancement | No restriction | 0.67 |
| 6 | Hypointense | Hyperintense | No enhancement | No restriction | - |
| 7 | Hypointense | Hyperintense | No enhancement | No restriction | 0.86 |
| Patient | T2 | T1 | Gd-T1 | DWI | ADC × 10−3 mm2/s ** |
|---|---|---|---|---|---|
| 1 | - | - | - | - | 1.04 |
| 2 | - | - | - | - | 1.22 |
| 3 | - | - | - | - | 0.63 |
| 4 | - | - | - | - | 0.78 |
| 5 | - | - | - | - | 1.05 |
| 6 | Hyperintense | Hypointense | No enhancement | No restriction | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, P.V.; Inì, C.; Broggi, G.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; et al. Histopathologic and MR Imaging Appearance of Spontaneous and Radiation-Induced Necrosis in Uveal Melanomas: Initial Results. Cancers 2022, 14, 215. https://doi.org/10.3390/cancers14010215
Foti PV, Inì C, Broggi G, Farina R, Palmucci S, Spatola C, Liardo RLE, Milazzotto R, Raffaele L, Salamone V, et al. Histopathologic and MR Imaging Appearance of Spontaneous and Radiation-Induced Necrosis in Uveal Melanomas: Initial Results. Cancers. 2022; 14(1):215. https://doi.org/10.3390/cancers14010215
Chicago/Turabian StyleFoti, Pietro Valerio, Corrado Inì, Giuseppe Broggi, Renato Farina, Stefano Palmucci, Corrado Spatola, Rocco Luca Emanuele Liardo, Roberto Milazzotto, Luigi Raffaele, Vincenzo Salamone, and et al. 2022. "Histopathologic and MR Imaging Appearance of Spontaneous and Radiation-Induced Necrosis in Uveal Melanomas: Initial Results" Cancers 14, no. 1: 215. https://doi.org/10.3390/cancers14010215
APA StyleFoti, P. V., Inì, C., Broggi, G., Farina, R., Palmucci, S., Spatola, C., Liardo, R. L. E., Milazzotto, R., Raffaele, L., Salamone, V., Caltabiano, R., Puzzo, L., Russo, A., Reibaldi, M., Longo, A., Vigneri, P., Venturini, M., Giurazza, F., Avitabile, T., & Basile, A. (2022). Histopathologic and MR Imaging Appearance of Spontaneous and Radiation-Induced Necrosis in Uveal Melanomas: Initial Results. Cancers, 14(1), 215. https://doi.org/10.3390/cancers14010215










