Retinal Glial Cells in Von Hippel–Lindau Disease: A Novel Approach in the Pathophysiology of Retinal Hemangioblastoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. OCT
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Population Characteristics
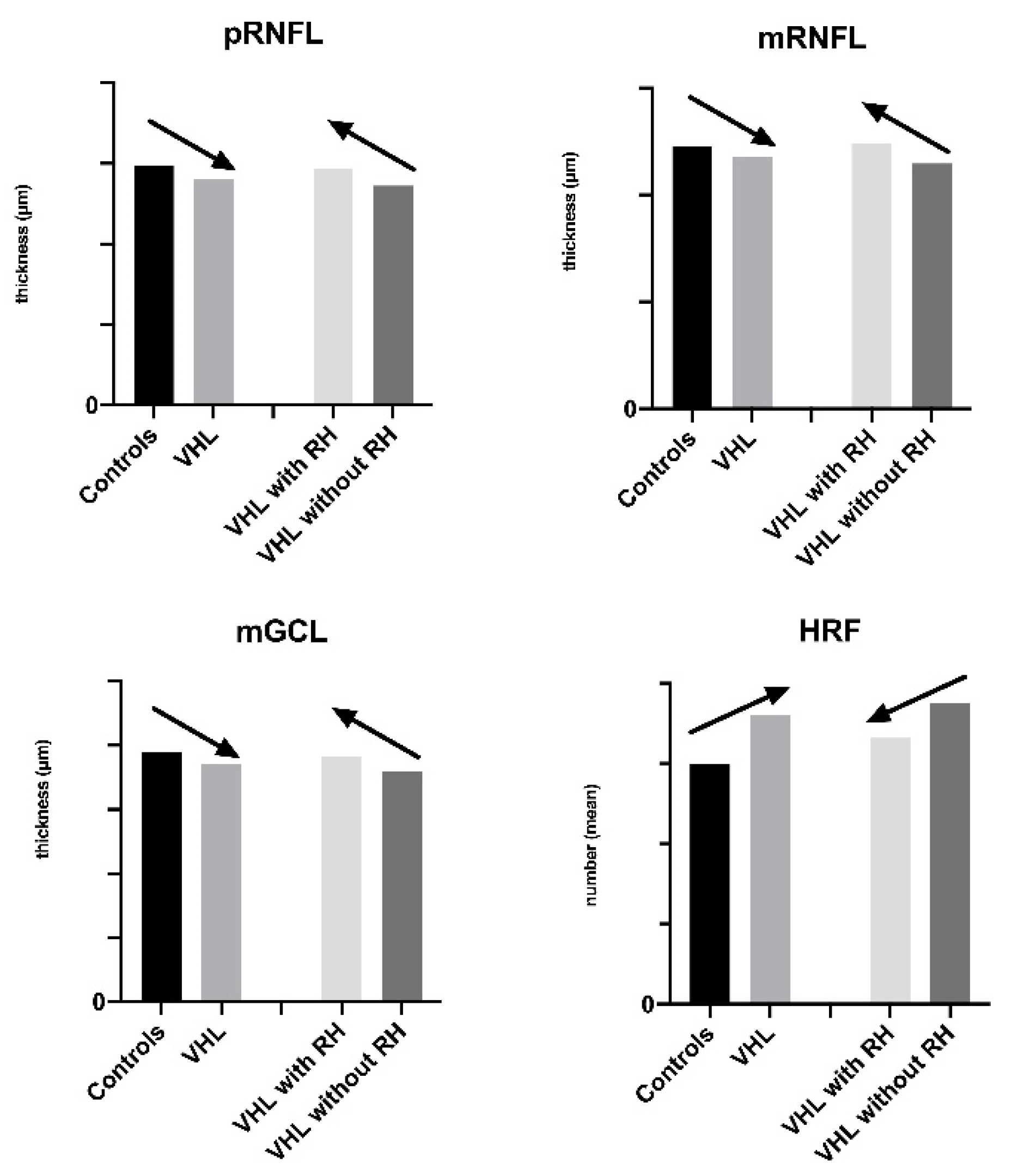
3.2. Peripapillary Retinal Nerve Fiber Layer Thickness
3.3. Macular Layers Thickness and Volume Analysis
3.3.1. Retinal Nerve Fiber Layer Analysis
3.3.2. Ganglion Cells Layer Analysis
3.4. Best Corrected Visual Acuity and Central Retinal Thickness
3.5. Quantification and Distribution of HRF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aronow, M.; Wiley, H.; Gaudric, A.; Krivosic, V.; Gorin, M.B.; Shields, C.; Shields, J.A.; Jonasch, E.W.; Singh, A.D.; Chew, E.Y. Von Hippel–Lindau Disease: Update on Pathogenesis and Systemic Aspects. Retina 2019, 39, 2243–2253. [Google Scholar] [CrossRef]
- Gossage, L.; Eisen, T.; Maher, E.R. VHL, the story of a tumour suppressor gene. Nat. Rev. Cancer 2015, 15, 55–64. [Google Scholar] [CrossRef]
- Gläsker, S.; Vergauwen, E.; Koch, C.A.; Kutikov, A.; Vortmeyer, A.O. Von Hippel–Lindau Disease: Current Challenges and Future Prospects. OncoTargets Ther. 2020, 13, 5669–5690. [Google Scholar] [CrossRef]
- Pierscianek, D.; Wolf, S.; Keyvani, K.; El Hindy, N.; Stein, K.P.; Sandalcioglu, I.E.; Sure, U.; Mueller, O.; Zhu, Y. Study of angiogenic signaling pathways in hemangioblastoma. Neuropathology 2017, 37, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.V.; Zheng, X.; Pereira, T.; Gradin, K.; Jin, S.; Lundkvist, J.; Ruas, J.L.; Poellinger, L.; Lendahl, U.; Bondesson, M. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev. Cell 2005, 9, 617–628. [Google Scholar] [CrossRef] [Green Version]
- Pear, W.S.; Simon, M.C. Lasting longer without oxygen: The influence of hypoxia on Notch signaling. Cancer Cell 2005, 8, 435–437. [Google Scholar] [CrossRef] [Green Version]
- Karimi, S.; Arabi, A.; Shahraki, T.; Safi, S.V. Hyppel-Lindau disease and the eye. J. Ophthalmic. Vis. Res. 2020, 15, 78–94. [Google Scholar]
- Chew, E.Y. Ocular manifestations of Von Hippel–Lindau disease: Clinical and genetic investigations. Trans. Am. Ophthalmol. Soc. 2005, 103, 495–511. [Google Scholar] [PubMed]
- Ishizawa, K.; Komori, T.; Hirose, T. Stromal cells in hemangioblastoma: Neuroectodermal differentiation and morphological similarities to ependymoma. Pathol. Int. 2005, 55, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Thomas, J.W.; Vignesvaran, N.; Jarrett, W.H. Retinal hemangioblastoma. A histologic, immunohistochemical, and ultrastructural evaluation. Ophthalmology 1992, 99, 140–145. [Google Scholar] [CrossRef]
- Vortmeyer, A.O.; Alomari, A.K. Morphologic and genetic analysis of retinal angioma associated with massive gliosis in a patient with Von Hippel–Lindau disease. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Park, D.M.; Zhuang, Z.; Chen, L.; Szerlip, N.; Maric, I.; Li, J.; Sohn, T.; Kim, S.H.; Lubensky, I.A.; Vortmeyer, A.O.; et al. Von Hippel–Lindau disease-associated hemangioblastoma sare derived from embryologic multipotent cells. PLoS Med. 2007, 4, e60. [Google Scholar] [CrossRef]
- Takada, S.; Hojo, M.; Takebe, N.; Tanigaki, K.; Miyamoto, S. Stromal cells of hemangioblastomas exhibit mesenchymal stem cell-derived vascular progenitor cell properties. Brain Tumor. Pathol. 2018, 35, 193–201. [Google Scholar] [CrossRef]
- Nicholson, D.H.; Green, W.R.; Kenyon, K.R. Light and electron microscopic study of early lesions in angiomatosis retinae. Am. J. Ophthalmol. 1976, 82, 193–204. [Google Scholar] [CrossRef]
- Pilotto, E.; Nacci, E.B.; De Mojà, G.; Ferrara, A.M.; Parrozzani, R.; Londei, D.; Zovato, S.; Midena, E. Structural and microvascular changes of the peripapillary retinal nerve fiber layer in Von Hippel–Lindau disease: An OCT and OCT angiography study. Sci. Rep. 2021, 11, 25. [Google Scholar] [CrossRef]
- Frizziero, L.; Parrozzani, R.; Midena, G.; Miglionico, G.; Vujosevic, S.; Pilotto, E.; Midena, E. Hyperreflective intraretinal spots in radiation macular edema on spectral domain optical coherence tomography. Retina 2016, 36, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, W.; Zhao, L.; Fariss, R.N.; Wong, W.T. Adaptative Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J. Neuroinflamm. 2011, 8, 173–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vujosevic, S.; Bini, S.; Midena, G.; Berton, M.; Pilotto, E.; Midena, E. Hyperreflective intraretinal spots in diabetics without and with nonproliferative diabetic retinopathy: An in vivo study using spectral domain OCT. J. Diabetes Res. 2013, 491835. [Google Scholar] [CrossRef] [Green Version]
- Framme, C.; Schweizer, P.; Imesch, M.; Wolf, S.; Wolf-Schnurrbusch, U. Behavior of SD-OCT-detected Hyperreflective Foci in the retina of anti-VEGF-treated patients with diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5814–5818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilotto, E.; Leonardi, F.; Stefanon, G.; Longhin, E.; Torresin, T.; Deganello, D.; Cavarzeran, F.; Miglionico, G.; Parrozzani, R.; Midena, E. Early retinal and choroidal OCT and OCT angiography signs of inflammation after uncomplicated cataract surgery. Br. J. Ophthalmol. 2019, 103, 1001–1007. [Google Scholar] [CrossRef]
- Pilotto, E.; Miante, S.; Torresin, T.; Puthenparampil, M.; Frizziero, L.; Federle, L.; Gallo, P.; Midena, E. Hyperreflective Foci in the Retina of Active Relapse-Onset Multiple Sclerosis. Ophthalmology 2020, 127, 1774–1776. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Bini, S.; Torresin, T.; Berton, M.; Midena, G.; Parrozzani, R.; Martini, F.; Pucci, P.; Daniele, A.R.; Cavarzeran, F.; et al. Hyperreflective retinal spots in normal and diabetic eyes: B-Scan and En Face Spectral Domain Optical Coherence Tomography Evaluation. Retina 2017, 37, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Jakobiec, F.A.; Font, R.L.; Johnson, F.B. Angiomatosis retinae. An ultrastrtuctural study and lipid analysis. Cancer 1976, 38, 2042–2056. [Google Scholar] [CrossRef]
- Hasselblatt, M.; Jeibmann, A.; Gerss, J.; Behrens, C.; Rama, B.; Wassmann, H.; Paulus, W. Cellular and reticular variants of haemangioblastoma revisited: A clinicopathologic study of 88 cases. Neuropathol. Appl. Neurobiol. 2005, 31, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Dollfus, H.; Massin, P.; Taupin, P.; Nemeth, C.; Amara, S.; Giraud, S.; Béroud, C.; Dureau, P.; Gaudric, A.; Landais, P.; et al. Retinal hemangioblastomas in Von Hippel–Lindau disease: A clinical and molecular study. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3067–3074. [Google Scholar]
- Chan, C.C.; Vormeyer, A.O.; Chew, E.Y.; Green, W.R.; Matteson, D.M.; Shen, D.F.; Linehan, W.M.; Lubensky, I.A.; Zhuang, Z. VHL gene deletion and enhanced VEGF gene expression detected in the stromal cells of retinal angioma. Arch. Ophthalmol. 1999, 117, 625–630. [Google Scholar] [CrossRef]
- De Hoz, R.; Rojas, B.; Ramírez, A.I.; Salazar, J.J.; Gallego, B.I.; Triviño, A.; Ramírez, J.M. Retinal macroglial responses in health and disease. Biomed. Res. Int. 2016, 954721. [Google Scholar] [CrossRef] [Green Version]
- Acaz-Fonseca, E.; Ortiz-Rodriguez, A.; Azcoitia, I.; Garcia-Segura, L.M.; Arevalo, M.A. Notch signalingin astrocytes mediates their morphological response to an inflammatory challenge. Cell Death Discov. 2019, 5, 85–103. [Google Scholar] [CrossRef]
- Weidemann, A.; Krohne, T.U.; Aguilar, E.; Kurihara, T.; Takeda, N.; Dorrell, M.I.; Simon, M.C.; Haase, V.H.; Friedlander, M.; Johnson, R.S. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 2020, 58, 1177–1185. [Google Scholar] [CrossRef] [Green Version]
- Puthenparampil, M.; Federle, L.; Poggiali, D.; Miante, S.; Signori, A.; Pilotto, E.; Rinaldi, F.; Perini, P.; Sormani, M.P.; Midena, E.; et al. Trans-synaptic degeneration in the optic pathway. A study in clinically isolated syndrome and early relapsing-remitting multiple sclerosis with or without optic neuritis. PLoS ONE 2017, 12, e0183957. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef]
- Pilotto, E.; Nacci, E.B.; Ferrara, A.M.; De Mojà, G.; Zovato, S.; Midena, E. Macular Perfusion impairment in Von Hippel–Lindau disease suggests a generalized retinal vessel alteration. J. Clin. Med. 2020, 9, 2677. [Google Scholar] [CrossRef]
- Büssow, H. The astrocytes in the retina and optic nerve head of mammals: A special glia for the ganglion cell axons. Cell Tissue Res. 1980, 206, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Ogden, T.E. Nerve fiber layer of the primate retina: Thickness and glial content. Vis. Res. 1983, 23, 581–587. [Google Scholar] [CrossRef]
- Forstreuter, F.; Lucius, R.; Mentlein, R. Vascular endothelial growth factor induces chemotaxis and proliferation of microglial cells. J. Neuroimmunol. 2002, 132, 93–98. [Google Scholar] [CrossRef]
- Couturier, A.; Bousquet, E.; Zhao, M.; Naud, M.C.; Klein, C.; Jonet, L.; Tadayoni, R.; De Kozak, Y.; Behar-Cohen, F. Anti-vascular endothelial growth factor acts on retinal microglia/macrophage activation in a rat model of ocular inflammation. Mol. Vis. 2014, 20, 908–920. [Google Scholar]
- Uemura, A.; Fruttiger, M.; D’Amore, P.; De Falco, S.; Joussen, A.M.; Sennlaub, F.; Brunck, L.R.; Johnson, K.T.; Lambrou, G.N.; Rittenhouse, K.D.; et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 2021, 25, 100954. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Linke, S.; Dias, J.M.; Zheng, X.; Gradin, K.; Wallis, T.P.; Hamilton, B.R.; Gustafsson, M.; Ruas, J.L.; Wilkins, S.; et al. Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 3368–3373. [Google Scholar] [CrossRef] [Green Version]
- Grandbarbe, L.; Michelucci, A.; Heurtaux, T.; Hemmer, K.; Morga, E.; Heuschling, P. Notch signaling modulates the activation of microglial cells. Glia 2007, 55, 1519–1530. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Bao, J.; Bai, Q.Q.; Wang, G.Q. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Knudson, A.G.J. Genetics of human cancer. Ann. Rev. Genet. 1986, 20, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Spraul, C.W.; Lynn, M.L.; Drack, A.; Grossniklaus, H.E. The two-stage mutation model in retinal hemangioblastoma. Ophthalmic Genet. 1998, 19, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Shields, C.L.; Shields, J.A. Von Hippel–Lindau disease. Surv. Ophthalmol. 2001, 46, 117–142. [Google Scholar] [CrossRef]
- Singh, A.D.; Nouri, M.; Shields, C.L.; Shields, J.A.; Perez, N. Treatment of retinal capillary hemangioma. Ophthalmology 2002, 109, 1799–1806. [Google Scholar] [CrossRef]

| Mutation | Patients (Number, %) |
|---|---|
| c.485 G > T p.Cys162Phe | 1, 1.6 |
| c.202C > T p.Ser68Pro | 1, 1.6 |
| c.257C > T p.Pro86Leu | 7, 11.5 |
| c.277G > A p.Gly93Ser | 2, 3.3 |
| c.277G > C p.Gly93Arg | 5, 8.2 |
| c.314_315insC | 2, 3.3 |
| c.407T > C p.Phe136Ser | 1, 1.6 |
| c.456_457del AC p.Thr152fs | 1, 1.6 |
| c.462A > Cp.Pro154 = r.341_463del | 1, 1.6 |
| c.463 + 2T > C | 1, 1.6 |
| c.464-1G > A | 2, 3.3 |
| c.481C > T p.Arg161 * | 2, 3.3 |
| c.482G > A p. Arg161Gly | 1, 1.6 |
| c.486C > G p.Cys162Trp | 4, 6.6 |
| c.499C > T p.Arg167Trp | 2, 3.3 |
| c.548C > A p.Ser183 * | 2, 3.3 |
| c.551T > C p.Leu184Arg | 1, 1.6 |
| c.552 del A p. R113RfsX45 | 2, 3.3 |
| c.563T > G p.Leu188Arg | 1, 1.6 |
| c.640T > G p. * 213Gly | 1, 1.6 |
| Complete deletion | 7, 11.5 |
| Exon 1 deletion | 4, 6.6 |
| Exon 2 deletion | 1, 1.6 |
| Exons 1 and 2 deletion | 2, 3.3 |
| Exon 3 deletion | 4, 6.6 |
| Exons 2 and 3 deletion | 3, 5.0 |
| pRNFL | VHL | VHL with RH | VHL without RH | Controls |
|---|---|---|---|---|
| pRNFL (μm: mean ± SD) | 101.1 ± 10.6 | 102.8 ± 8.2 | 100.1 ± 11.8 | 103.9 ± 7.0 |
| T-pRNFL (μm: mean ± SD) | 68.2 ± 10.1 | 71.4 ± 7.5 | 66.4 ± 10.9 | 69.9 ± 9.9 |
| TS-pRNFL (μm: mean ± SD) | 140.1 ± 17.4 | 146.9 ± 15.3 | 136.3 ± 17.6 | 148.9 ± 14.2 |
| NS-pRNFL (μm: mean ± SD) | 111.6 ± 25.5 | 113.2 ± 22.8 | 110.7 ± 27.2 | 108.0 ± 19.0 |
| N-pRNFL (μm: mean ± SD) | 78.5 ± 16.6 | 77.5 ± 11.6 | 79.0 ± 18.9 | 82.7 ± 13.3 |
| NI-pRNFL (μm: mean ± SD) | 118.7 ± 23.0 | 113.6 ± 15.5 | 121.5 ± 26.0 | 122.4 ± 18.8 |
| TI-pRNFL (μm: mean ± SD) | 145.3 ± 18.8 | 151.4 ± 17.2 | 141.8 ± 19.0 | 147.1 ± 16.8 |
| PMB (μm: mean ± SD) | 52.6 ± 8.1 | 55.0 ± 6.5 | 51.2 ± 8.6 | 52.8 ± 6.9 |
| pRNFL | VHL vs. Controls | VHL without RH vs. Control | VHL with RH vs. Control | VHL without RH vs. VHL with RH |
|---|---|---|---|---|
| RNFL | 0.2131 | 0.1086 | 0.6360 | 0.3422 |
| T-pRNFL | 0.4615 | 0.1835 | 0.6359 | 0.0587 |
| TS-pRNFL | 0.0227 | 0.0029 | 0.6355 | 0.0181 |
| NS-pRNFL | 0.5074 | 0.6494 | 0.3813 | 0.7177 |
| N-pRNFL | 0.2443 | 0.3833 | 0.1832 | 0.7065 |
| NI-pRNFL | 0.4595 | 0.8827 | 0.0929 | 0.1405 |
| TI-pRNFL | 0.6603 | 0.2434 | 0.3695 | 0.0647 |
| PMB | 0.8931 | 0.4223 | 0.3238 | 0.0793 |
| mRNFL | VHL | VHL without RH | VHL with RH | Controls |
|---|---|---|---|---|
| mRNFL-Vol (mm3: mean ± SD) | 0.889 ± 0.077 | 0.876 ± 0.075 | 0.912 ± 0.077 | 0.929 ± 0.114 |
| mRNFL-C (μm: mean ± SD) | 12.6 ± 2.0 | 13.0 ± 1.5 | 12.0 ± 2.6 | 12.4 ± 2.2 |
| mRNFL-SI (μm: mean ± SD) | 23.6 ± 2.7 | 23.2 ± 2.6 | 24.3 ± 2.9 | 25.2 ± 2.9 |
| mRNFL-NI (μm: mean ± SD) | 20.7 ± 1.8 | 20.5 ± 1.8 | 21.1 ± 1.6 | 20.5 ± 1.7 |
| mRNFL-II (μm: mean ± SD) | 25.0 ± 2.6 | 24.8 ± 2.7 | 25.2 ± 2.4 | 24.8 ± 2.4 |
| mRNFL-TI (μm: mean ± SD) | 16.7 ± 1.0 | 16.8 ± 1.1 | 16.7 ± 0.8 | 16.9 ± 1.2 |
| mRNFL-SO (μm: mean ± SD) | 35.8 ± 4.0 | 35.4 ± 3.7 | 36.6 ± 4.5 | 39.6 ± 5.8 |
| mRNFL-NO (μm: mean ± SD) | 47.2 ± 5.9 | 45.9 ± 5.8 | 49.6 ± 5.5 | 49.1 ± 8.2 |
| mRNFL-IO (μm: mean ± SD) | 39.2 ± 5.1 | 38.7 ± 5.0 | 40.0 ± 5.4 | 40.4 ± 6.6 |
| mRNFL-TO (μm: mean ± SD) | 18.1 ± 0.9 | 18.1 ± 1.0 | 18.2 ± 0.9 | 18.5 ± 1.3 |
| mRNFL | VHL vs. Controls | VHL without RH vs. Controls | VHL with RH vs. Controls | VHL without RH vs. VHL with RH |
|---|---|---|---|---|
| mRNFL-Vol | 0.0554 | 0.0270 | 0.5964 | 0.0809 |
| mRNFL-C | 0.7662 | 0.5036 | 0.7206 | 0.2448 |
| mRNFL-SI | 0.0585 | 0.0335 | 0.4261 | 0.2150 |
| mRNFL-NI | 0.7919 | 0.9889 | 0.5996 | 0.5095 |
| mRNFL-II | 0.8440 | 0.9699 | 0.7249 | 0.6801 |
| mRNFL-TI | 0.8533 | 0.8934 | 0.8513 | 0.9205 |
| mRNFL-SO | <0.0001 | <0.0001 | 0.0111 | 0.1454 |
| mRNFL-NO | 0.0301 | 0.0007 | 0.6157 | <0.0001 |
| mRNFL-IO | 0.1542 | 0.0699 | 0.7576 | 0.1302 |
| mRNFL-TO | 0.6321 | 0.6268 | 0.7774 | 0.8816 |
| GCL | VHL | VHL without RH | VHL with RH | Controls |
|---|---|---|---|---|
| GCL-Vol (mm3: mean ± SD) | 1.111 ± 0.078 | 1.095 ± 0.078 | 1.138 ± 0.072 | 1.135 ± 0.072 |
| GCL-C (μm: mean ± SD) | 15.6 ± 3.7 | 16.1 ± 3.6 | 14.9 ± 4.0 | 14.6 ± 3.1 |
| GCL-SI (μm: mean ± SD) | 53.7 ± 4.4 | 53.0 ± 4.8 | 55.0 ± 3.5 | 54.5 ± 3.1 |
| GCL-NI (μm: mean ± SD) | 52.6 ± 4.9 | 52.4 ± 5.3 | 53.0 ± 4.0 | 52.4 ± 4.2 |
| GCL-II (μm: mean ± SD) | 53.3 ± 4.7 | 52.9 ± 4.8 | 53.9 ± 4.6 | 54.3 ± 3.4 |
| GCL-TI (μm: mean ± SD) | 49.3 ± 5.0 | 48.8 ± 5.4 | 50.4 ± 4.0 | 50.8 ± 3.3 |
| GCL-SO (μm: mean ± SD) | 35.1 ± 2.5 | 34.6 ± 2.6 | 36.0 ± 2.3 | 35.6 ± 2.8 |
| GCL-NO (μm: mean ± SD) | 38.9 ± 3.0 | 38.1 ± 2.9 | 40.3 ± 2.7 | 39.4 ± 3.3 |
| GCL-IO (μm: mean ± SD) | 34.2 ± 2.7 | 33.7 ± 2.6 | 35.0 ± 2.9 | 34.9 ± 2.7 |
| GCL-TO (μm: mean ± SD) | 37.1 ± 3.8 | 36.5 ± 3.7 | 38.2 ± 3.8 | 39.0 ± 3.7 |
| GCL | VHL vs. Controls | VHL without RH vs. Controls | VHL with RH vs. Controls | VHL without RH vs. VHL with RH |
|---|---|---|---|---|
| GCL-Vol | 0.1533 | 0.0412 | 0.9601 | 0.0561 |
| GCL-C | 0.2316 | 0.1190 | 0.7938 | 0.2478 |
| GCL-SI | 0.3452 | 0.1092 | 0.6695 | 0.0665 |
| GCL-NI | 0.7896 | 0.9930 | 0.5061 | 0.5289 |
| GCL-II | 0.2684 | 0.1674 | 0.7283 | 0.3602 |
| GCL-TI | 0.1035 | 0.0381 | 0.6938 | 0.1291 |
| GCL-SO | 0.5576 | 0.2850 | 0.6962 | 0.1855 |
| GCL-NO | 0.5413 | 0.1677 | 0.3898 | 0.0580 |
| GCL-IO | 0.4439 | 0.2373 | 0.8842 | 0.2315 |
| GCL-TO | 0.0212 | 0.0073 | 0.3844 | 0.1068 |
| BCVA and CRT | VHL | VHL without RH | VHL with RH | Controls |
|---|---|---|---|---|
| BCVA (letters: mean± SD) | 85.5 ± 2.4 | 85.2 ± 2.3 | 85.9 ± 2.5 | 87.6 ± 3.4 |
| CRT (μm: mean ± SD) | 273.0 ± 18.2 | 274.6 ± 16.4 | 270.2 ± 21.1 | 272.6 ± 18.4 |
| BCVA and CRT | VHL vs. Controls | VHL without RH vs. Controls | VHL with RH vs. Controls | VHL without RH vs. VHL with RH |
|---|---|---|---|---|
| BCVA | 0.0007 | 0.0010 | 0.0461 | 0.2732 |
| CRT | 0.9359 | 0.6553 | 0.6030 | 0.3763 |
| Groups | HRF IR | HRF OR | HRF Total Retina |
|---|---|---|---|
| VHL (mean ± SD) | 28.4 ± 8.1 | 7.5 ± 2.6 | 36.0 ± 9.3 |
| VHL without RH (mean ± SD) | 29.7 ± 7.7 | 7.8 ± 2.5 | 37.5 ± 8.8 |
| VHL with RH (mean ± SD) | 26.2 ± 8.5 | 7.0 ± 2.8 | 33.2 ± 9.8 |
| Controls (mean ± SD) | 24.9 ± 7.7 | 8.0 ± 2.5 | 32.9 ± 8.9 |
| Comparison between Groups | HRF IR | HRF OR | HRF Total Retina |
|---|---|---|---|
| VHL vs. Controls | 0.0554 | 0.3985 | 0.1505 |
| VHL without RH vs. Controls | 0.0121 | 0.7616 | 0.0302 |
| VHL with RH vs. Controls | 0.5735 | 0.1798 | 0.9143 |
| VHL without RH vs. VHL with RH | 0.1086 | 0.2295 | 0.0677 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilotto, E.; Midena, G.; Torresin, T.; De Mojà, G.; Bacelle, M.L.; Ferrara, A.M.; Zovato, S.; Midena, E. Retinal Glial Cells in Von Hippel–Lindau Disease: A Novel Approach in the Pathophysiology of Retinal Hemangioblastoma. Cancers 2022, 14, 170. https://doi.org/10.3390/cancers14010170
Pilotto E, Midena G, Torresin T, De Mojà G, Bacelle ML, Ferrara AM, Zovato S, Midena E. Retinal Glial Cells in Von Hippel–Lindau Disease: A Novel Approach in the Pathophysiology of Retinal Hemangioblastoma. Cancers. 2022; 14(1):170. https://doi.org/10.3390/cancers14010170
Chicago/Turabian StylePilotto, Elisabetta, Giulia Midena, Tommaso Torresin, Gilda De Mojà, Maria Laura Bacelle, Alfonso Massimiliano Ferrara, Stefania Zovato, and Edoardo Midena. 2022. "Retinal Glial Cells in Von Hippel–Lindau Disease: A Novel Approach in the Pathophysiology of Retinal Hemangioblastoma" Cancers 14, no. 1: 170. https://doi.org/10.3390/cancers14010170
APA StylePilotto, E., Midena, G., Torresin, T., De Mojà, G., Bacelle, M. L., Ferrara, A. M., Zovato, S., & Midena, E. (2022). Retinal Glial Cells in Von Hippel–Lindau Disease: A Novel Approach in the Pathophysiology of Retinal Hemangioblastoma. Cancers, 14(1), 170. https://doi.org/10.3390/cancers14010170









