Molecular Crosstalk between the Hepatitis C Virus and the Extracellular Matrix in Liver Fibrogenesis and Early Carcinogenesis
Abstract
Simple Summary
Abstract
1. Introduction
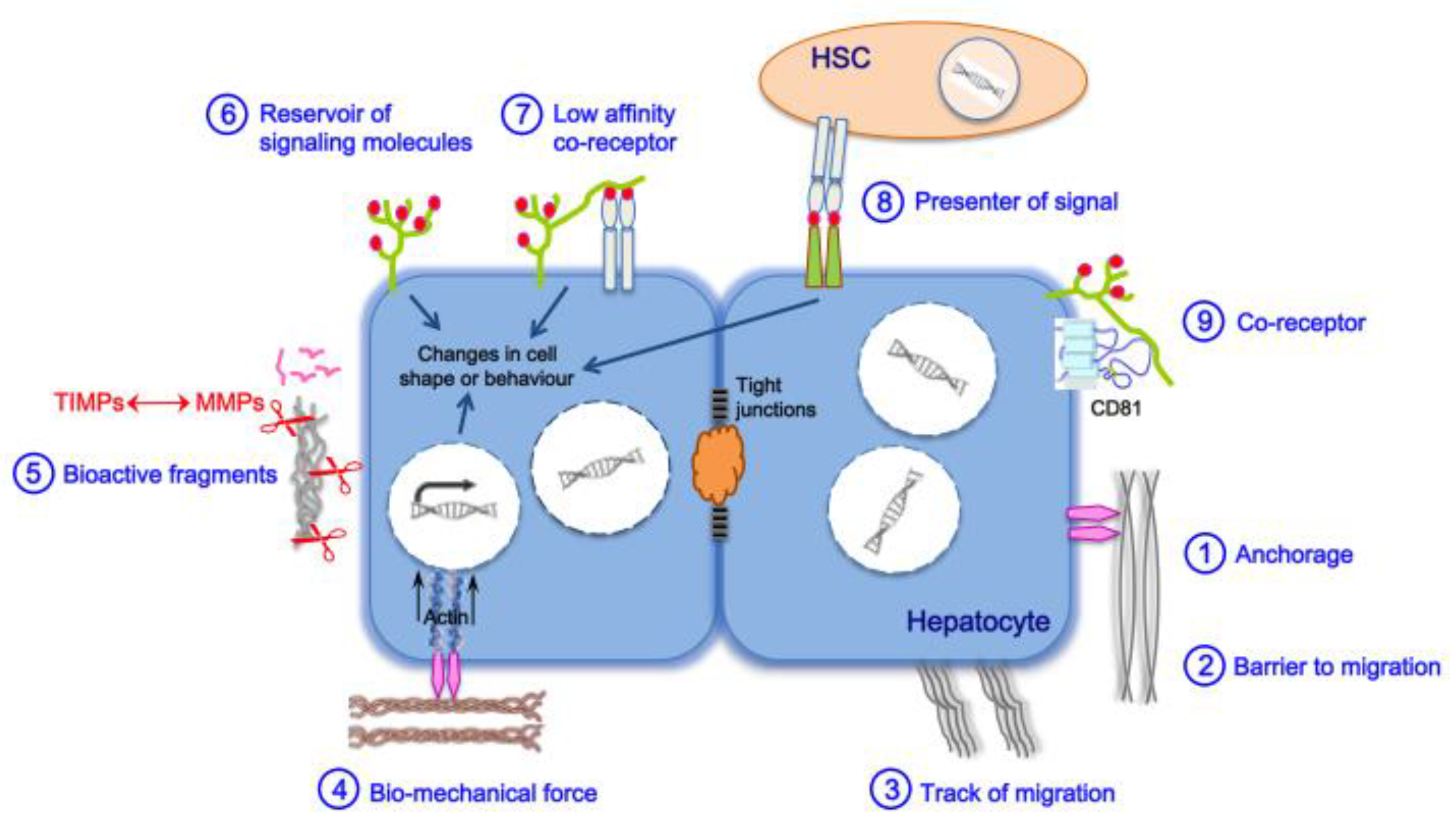
2. Main Actors of Liver Fibrosis
3. Liver Fibrosis and Cirrhosis
3.1. General Pan-Etiology Features
3.2. Features Linked to HCV Pathogenesis
3.2.1. Collagens and Derived Fragments
3.2.2. Enzymes of the ECM
3.2.2.1. Lysyl Oxidases
3.2.2.2. Matrix Metalloproteases (MMPs) and Their Inhibitors
3.2.2.3. A Disintegrin and Metalloprotease with Thrombospondin Motifs (ADAM and ADAM-TS)
3.2.3. Proteoglycans
3.2.3.1. Membrane-Associated PGs
3.2.3.2. Soluble Extracellular PGs
3.2.4. Matricellular Proteins
3.2.4.1. Tenascins
3.2.4.2. Osteopontin
3.2.4.3. CCN2 or CTGF
3.2.5. Adhesive Glycoproteins
3.2.5.1. Fibronectin
3.2.5.2. Laminin and Nidogen
3.2.6. Elastin
3.2.7. TGF-β and HCV Pathogenesis
4. Are HSCs Direct Targets of HCV Infection?
5. Fibrosis Reversal in the Era of DAAs in HCV-Induced Liver Fibrosis
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM | a disintegrin and metalloprotease |
| ADAM-TS | ADAM with thrombospondin motifs |
| ALT | alanine amino-transferase |
| COX-2 | cyclo-oxygenase-2 |
| CREBH | cAMP-responsive element binding protein H |
| CS | chondroitin sulfate |
| CTGF | connective tissue growth factor |
| DAAs | direct-acting antivirals |
| ECM | extracellular matrix |
| ER | endoplasmic reticulum |
| GAG(s) | glycosaminoglycan(s) |
| GPC3 | glypican-3 |
| HA | Hyaluronan or hyaluronic acid |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HGF | hepatocyte growth factor |
| HNF4α | hepatocyte nuclear factor 4α |
| HPC | hepatic progenitor cells |
| HSC(s) | hepatic stellate cell(s) |
| HSPG(s) | heparan sulfate proteoglycan(s) |
| IP-10 | interferon gamma-inducible protein 10 |
| LOX | lysyl oxidase |
| LOXL | LOX-like |
| LTBP | large latent TGF-β-binding protein |
| MMP(s) | matrix metalloprotease(s) |
| NAFLD | nonalcoholic fatty liver disease |
| NF-κB | nuclear factor-κB |
| NK | natural killer cell |
| PDGF | platelet-derived growth factor |
| PG(s) | proteoglycan(s) |
| SMA | smooth muscle actin |
| SNP | single-nucleotide polymorphism |
| SVR | sustained virological response |
| TGF-β1 and -β2 | transforming growth factor-β1 and -β2 |
| THBS1 | thrombospondin-1 gene |
| TIMP | tissue inhibitor of MMP |
| TLR | toll-like receptor |
References
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Heal. 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Lemon, S.M.; McGivern, D.R. How do persistent infections with hepatitis C virus cause liver cancer? Curr. Opin. Virol. 2015, 14, 101–108. [Google Scholar] [CrossRef]
- Lemon, S.M.; McGivern, D.R. Is Hepatitis C Virus Carcinogenic? Gastroenterology 2012, 142, 1274–1278. [Google Scholar] [CrossRef]
- World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Blach, S.; Zeuzem, S.; Manns, M.; Altraif, I.; Duberg, A.-S.; Muljono, D.H.; Waked, I.; Alavian, S.M.; Lee, M.-H.; Negro, F.; et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis C. Available online: http://www.who.int.gate2.inist.fr/mediacentre/factsheets/fs164/en/ (accessed on 29 January 2018).
- Hajarizadeh, B.; Grebely, J.; Dore, G.J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.-C.; Colombo, M. Hepatocellular carcinoma and direct acting antiviral treatments: Controversy after the revolution. J. Hepatol. 2016, 65, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Baumert, T.F.; Jühling, F.; Ono, A.; Hoshida, Y. Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Med. 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hamdane, N.; Jühling, F.; Crouchet, E.; Saghire, H.E.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Suarez, A.A.R.; et al. HCV-Induced Epigenetic Changes Associated with Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329. [Google Scholar] [CrossRef] [PubMed]
- Hengst, J.; Falk, C.S.; Schlaphoff, V.; Deterding, K.; Manns, M.P.; Cornberg, M.; Wedemeyer, H. Direct-Acting Antiviral-Induced Hepatitis C Virus Clearance Does Not Completely Restore the Altered Cytokine and Chemokine Milieu in Patients with Chronic Hepatitis C. J. Infect. Dis. 2016, 214, 1965–1974. [Google Scholar] [CrossRef]
- Akuta, N.; Suzuki, F.; Hirakawa, M.; Kawamura, Y.; Sezaki, H.; Suzuki, Y.; Hosaka, T.; Kobayashi, M.; Kobayashi, M.; Saitoh, S.; et al. Amino acid substitutions in hepatitis C virus core region predict hepatocarcinogenesis following eradication of HCV RNA by antiviral therapy. J. Med. Virol. 2011, 83, 1016–1022. [Google Scholar] [CrossRef]
- Takeda, H.; Takai, A.; Iguchi, E.; Mishima, M.; Arasawa, S.; Kumagai, K.; Eso, Y.; Shimizu, T.; Takahashi, K.; Ueda, Y.; et al. Oncogenic transcriptomic profile is sustained in the liver after the eradication of the hepatitis C virus. Carcinogenesis 2021. [Google Scholar] [CrossRef]
- Paul, D.; Madan, V.; Bartenschlager, R. Hepatitis C Virus RNA Replication and Assembly: Living on the Fat of the Land. Cell Host Microbe 2014, 16, 569–579. [Google Scholar] [CrossRef]
- Sebae, G.K.E.; Malatos, J.M.; Cone, M.-K.E.; Rhee, S.; Angelo, J.R.; Mager, J.; Tremblay, K.D. Single-cell murine genetic fate mapping reveals bipotential hepatoblasts and novel multi-organ endoderm progenitors. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Wallace, M.C.; Friedman, S.L. Pathobiology of liver fibrosis: A translational success story. Gut 2015, 64, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Rauterberg, J.; Voss, B.; Pott, G.; Gerlach, U. Connective tissue components of the normal and fibrotic liver. Klin. Wochenschr. 1981, 59, 767–779. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- De Minicis, S.; Seki, E.; Uchinami, H.; Kluwe, J.; Zhang, Y.; Brenner, D.A.; Schwabe, R.F. Gene Expression Profiles during Hepatic Stellate Cell Activation in Culture and in Vivo. Gastroenterology 2007, 132, 1937–1946. [Google Scholar] [CrossRef]
- Lin, X.Z.; Horng, M.H.; Sun, Y.N.; Shiesh, S.C.; Chow, N.H.; Guo, X.Z. Computer morphometry for quantitative measurement of liver fibrosis: Comparison with knodell’s score, colorimetry and conventional description reports. J. Gastroenterol. Hepatol. 1998, 13, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rojkind, M.; Giambrone, M.A.; Biempica, L. Collagen Types in Normal and Cirrhotic Liver. Gastroenterology 1979, 76, 710–719. [Google Scholar] [CrossRef]
- Kagan, H.M. Lysyl Oxidase: Mechanism, Regulation and Relationship to Liver Fibrosis. Pathol. Res. Pr. 1994, 190, 910–919. [Google Scholar] [CrossRef]
- Andez, A.M.; Amenta, P.S. The extracellular matrix in hepatic regeneration. FASEB J. 1995, 9, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Kanta, J. Elastin in the Liver. Front. Physiol. 2016, 7, 491. [Google Scholar] [CrossRef] [PubMed]
- Musso, O.; Rehn, M.; Saarela, J.; Théret, N.; Liétard, J.; Hintikka, E.; Lotrian, D.; Campion, J.-P.; Pihlajaniemi, T.; Clément, B. Collagen XVIII is localized in sinusoids and basement membrane zones and expressed by hepatocytes and activated stellate cells in fibrotic human liver. Hepatology 1998, 28, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Vallet, S.D. Fragments generated upon extracellular matrix remodeling: Biological regulators and potential drugs. Matrix Biol. 2019, 75–76, 170–189. [Google Scholar] [CrossRef]
- Sun, S.; Song, Z.; Cotler, S.J.; Cho, M. Biomechanics and functionality of hepatocytes in liver cirrhosis. J. Biomech. 2014, 47, 2205–2210. [Google Scholar] [CrossRef]
- Grigorov, B.; Reungoat, E.; Maurin, A.G.D.; Varbanov, M.; Blaising, J.; Michelet, M.; Manuel, R.; Parent, R.; Bartosch, B.; Zoulim, F.; et al. Hepatitis C virus infection propagates through interactions between Syndecan-1 and CD81 and impacts the hepatocyte glycocalyx. Cell. Microbiol. 2017, 19. [Google Scholar] [CrossRef]
- Wells, J.M.; Gaggar, A.; Blalock, J.E. MMP generated matrikines. Matrix Biol. 2015, 44–46, 122–129. [Google Scholar] [CrossRef]
- Schuppan, D.; Ashfaq-Khan, M.; Yang, A.T.; Kim, Y.O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018, 68–69, 435–451. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Baffet, G.; Théret, N. Molecular and tissue alterations of collagens in fibrosis. Matrix Biol. 2018. [Google Scholar] [CrossRef]
- Jung, Y.; Witek, R.P.; Syn, W.-K.; Choi, S.S.; Omenetti, A.; Premont, R.; Guy, C.D.; Diehl, A.M. Signals from dying hepatocytes trigger growth of liver progenitors. Gut 2010, 59, 655–665. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Zhao, S.-X.; Li, W.-C.; Fu, N.; Kong, L.-B.; Zhang, Q.-S.; Han, F.; Ren, W.-G.; Cui, P.; Du, J.-H.; Wang, B.-Y.; et al. CD14+ monocytes and CD163+ macrophages correlate with the severity of liver fibrosis in patients with chronic hepatitis C. Exp. Ther. Med. 2020, 20, 228. [Google Scholar] [CrossRef]
- Douam, F.; Lavillette, D.; Cosset, F.-L. The Mechanism of HCV Entry into Host Cells. Prog. Mol. Biol. Transl. Sci. 2015, 129, 63–107. [Google Scholar] [CrossRef] [PubMed]
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Berditchevski, F. Complexes of tetraspanins with integrins: More than meets the eye. J. Cell Sci. 2001, 114, 4143–4151. [Google Scholar] [CrossRef]
- Alisi, A.; Arciello, M.; Petrini, S.; Conti, B.; Missale, G.; Balsano, C. Focal Adhesion Kinase (FAK) Mediates the Induction of Pro-Oncogenic and Fibrogenic Phenotypes in Hepatitis C Virus (HCV)-Infected Cells. PLoS ONE 2012, 7, e44147. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Webb, D.J.; Horwitz, A.R. Cell migration at a glance. J. Cell Sci. 2005, 118, 4917–4919. [Google Scholar] [CrossRef]
- Martínez, S.M.; Crespo, G.; Navasa, M.; Forns, X. Noninvasive assessment of liver fibrosis. Hepatology 2011, 53, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Manon-Jensen, T.; Genovese, F.; Kristensen, J.H.; Nielsen, M.J.; Sand, J.M.B.; Hansen, N.-U.B.; Bay-Jensen, A.-C.; Bager, C.L.; Krag, A.; et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. Liver Physiol. 2015, 308, G807–G830. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, L. Liver Pathology: Cirrhosis, Hepatitis, and Primary Liver Tumors—Update and Diagnostic Problems. Mod. Pathol. 2000, 13, 679–704. [Google Scholar] [CrossRef] [PubMed]
- Govaere, O.; Cockell, S.; Van Haele, M.; Wouters, J.; Van Delm, W.; Van den Eynde, K.; Bianchi, A.; Van Eijsden, R.; Van Steenbergen, W.; Monbaliu, D.; et al. High-throughput sequencing identifies aetiology-dependent differences in ductular reaction in human chronic liver disease. J. Pathol. 2019, 248, 66–76. [Google Scholar] [CrossRef]
- Trivedi, S.; Murthy, S.; Sharma, H.; Hartlage, A.S.; Kumar, A.; Gadi, S.; Simmonds, P.; Chauhan, L.V.; Scheel, T.K.H.; Billerbeck, E.; et al. Viral persistence, liver disease and host response in hepatitis c-like virus rat model. Hepatology 2017. [Google Scholar] [CrossRef]
- Khatun, M.; Ray, R.B. Mechanisms Underlying Hepatitis C Virus-Associated Hepatic Fibrosis. Cells 2019, 8, 1249. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Karsdal, M.A.; Kazankov, K.; Grønbaek, H.; Krag, A.; Leeming, D.J.; Schuppan, D.; George, J. Fibrosis is not just fibrosis-basement membrane modelling and collagen metabolism differs between hepatitis B- and C-induced injury. Aliment. Pharmacol. Ther. 2016, 44, 1242–1252. [Google Scholar] [CrossRef]
- Guido, M.; Mangia, A.; Faa, G. Chronic viral hepatitis: The histology report. Dig. Liver Dis. 2011, 43, S331–S343. [Google Scholar] [CrossRef]
- Beltra, J.-C.; Decaluwe, H. Cytokines and persistent viral infections. Cytokine 2016, 82, 4–15. [Google Scholar] [CrossRef]
- Govaere, O.; Petz, M.; Wouters, J.; Vandewynckel, Y.-P.; Scott, E.J.; Topal, B.; Nevens, F.; Verslype, C.; Anstee, Q.M.; Van Vlierberghe, H.; et al. The PDGFRα-laminin B1-keratin 19 cascade drives tumor progression at the invasive front of human hepatocellular carcinoma. Oncogene 2017, 36, 6605–6616. [Google Scholar] [CrossRef]
- Martinez-Quetglas, I.; Pinyol, R.; Dauch, D.; Torrecilla, S.; Tovar, V.; Moeini, A.; Alsinet, C.; Portela, A.; Rodriguez-Carunchio, L.; Solé, M.; et al. IGF2 Is Up-Regulated by Epigenetic Mechanisms in Hepatocellular Carcinomas and is an Actionable Oncogene Product in Experimental Models. Gastroenterology 2016, 151, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Preisser, L.; Miot, C.; Le Guillou-Guillemette, H.; Beaumont, E.; Foucher, E.D.; Garo, E.; Blanchard, S.; Frémaux, I.; Croué, A.; Fouchard-Hubert, I.; et al. IL-34 and macrophage colony-stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology 2014, 60, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Tummala, K.S.; Brandt, M.; Teijeiro, A.; Graña, O.; Schwabe, R.F.; Perna, C.; Djouder, N. Hepatocellular Carcinomas Originate Predominantly from Hepatocytes and Benign Lesions from Hepatic Progenitor Cells. Cell Rep. 2017, 19, 584–600. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Krebs, A.; Preimel, D.; Popov, Y.; Bartenschlager, R.; Lohmann, V.; Pinzani, M.; Schuppan, D. Hepatitis C Virus-Replicating Hepatocytes Induce Fibrogenic Activation of Hepatic Stellate Cells. Gastroenterology 2005, 129, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Hur, W.; Wang, J.S.; Jang, J.W.; Kim, C.W.; Bae, S.H.; Jang, S.K.; Yang, S.-H.; Sung, Y.C.; Kwon, O.-J.; et al. HCV core protein promotes liver fibrogenesis via up-regulation of CTGF with TGF-β1. Exp. Mol. Med. 2005, 37, 138–145. [Google Scholar] [CrossRef]
- Baiocchini, A.; Montaldo, C.; Conigliaro, A.; Grimaldi, A.; Correani, V.; Mura, F.; Ciccosanti, F.; Rotiroti, N.; Brenna, A.; Montalbano, M.; et al. Extracellular Matrix Molecular Remodeling in Human Liver Fibrosis Evolution. PLoS ONE 2016, 11, e0151736. [Google Scholar] [CrossRef]
- Mormone, E.; Lu, Y.; Ge, X.; Fiel, M.I.; Nieto, N. Fibromodulin, an Oxidative Stress-Sensitive Proteoglycan, Regulates the Fibrogenic Response to Liver Injury in Mice. Gastroenterology 2012, 142, 612–621. [Google Scholar] [CrossRef]
- Benzoubir, N.; Lejamtel, C.; Battaglia, S.; Testoni, B.; Benassi, B.; Gondeau, C.; Perrin-Cocon, L.; Desterke, C.; Thiers, V.; Samuel, D.; et al. HCV core-mediated activation of latent TGF-β via thrombospondin drives the crosstalk between hepatocytes and stromal environment. J. Hepatol. 2013, 59, 1160–1168. [Google Scholar] [CrossRef]
- Bataller, R.; Paik, Y.-H.; Lindquist, J.N.; Lemasters, J.J.; Brenner, D.A. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology 2004, 126, 529–540. [Google Scholar] [CrossRef]
- Nunez, O.; Fernández-Martínez, A.; Majano, P.L.; Apolinario, A.; Gómez-Gonzalo, M.; Benedicto, I.; López-Cabrera, M.; Boscá, L.; Clemente, G.; García-Monzón, C.; et al. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: Role of viral core and NS5A proteins. Gut 2004, 53, 1665–1672. [Google Scholar] [CrossRef]
- Iqbal, J.; Sarkar-Dutta, M.; McRae, S.; Ramachandran, A.; Kumar, B.; Waris, G. Osteopontin Regulates Hepatitis C Virus (HCV) Replication and Assembly by Interacting with HCV Proteins and Lipid Droplets and by Binding to Receptors AVβ3 and CD44. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, T.; Honda, M.; Yamashita, T.; Nio, K.; Shimakami, T.; Shimizu, R.; Nakasyo, S.; Murai, K.; Shirasaki, N.; Okada, H.; et al. The osteopontin-CD44 axis in hepatic cancer stem cells regulates IFN signaling and HCV replication. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Jee, M.H.; Hong, K.Y.; Park, J.H.; Lee, J.S.; Kim, H.S.; Lee, S.H.; Jang, S.K. New Mechanism of Hepatic Fibrogenesis: Hepatitis C Virus Infection Induces Transforming Growth Factor Β1 Production through Glucose-Regulated Protein 94. J. Virol. 2015, 90, 3044–3055. [Google Scholar] [CrossRef]
- Chida, T.; Ito, M.; Nakashima, K.; Kanegae, Y.; Aoshima, T.; Takabayashi, S.; Kawata, K.; Nakagawa, Y.; Yamamoto, M.; Shimano, H.; et al. Critical role of CREBH-mediated induction of transforming growth factor β2 by hepatitis C virus infection in fibrogenic responses in hepatic stellate cells. Hepatology 2017, 66, 1430–1443. [Google Scholar] [CrossRef]
- Kwon, Y.-C.; Sasaki, R.; Meyer, K.; Ray, R. Hepatitis C Virus Core Protein Modulates Endoglin (CD105) Signaling Pathway for Liver Pathogenesis. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Xue, Y.; Mars, W.M.; Bowen, W.; Singhi, A.D.; Stoops, J.; Michalopoulos, G.K. Hepatitis C Virus Mimics Effects of Glypican-3 on CD81 and Promotes Development of Hepatocellular Carcinomas via Activation of Hippo Pathway in Hepatocytes. Am. J. Pathol. 2018, 188, 1469–1477. [Google Scholar] [CrossRef]
- Kim, H.; Bose, S.K.; Meyer, K.; Ray, R.; Lyles, D.S. Hepatitis C Virus Impairs Natural Killer Cell-Mediated Augmentation of Complement Synthesis. J. Virol. 2014, 88, 2564–2571. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Q.; Wu, K.; Chen, X.; Zheng, Y.; Zhu, C.; Wu, J. Hepatitis C virus NS3 protein enhances cancer cell invasion by activating matrix metalloproteinase-9 and cyclooxygenase-2 through ERK/p38/NF-κB signal cascade. Cancer Lett. 2015, 356, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Presser, L.D.; Haskett, A.; Waris, G. Hepatitis C virus-induced furin and thrombospondin-1 activate TGF-β1: Role of TGF-β1 in HCV replication. Virology 2011, 412, 284–296. [Google Scholar] [CrossRef]
- Sakata, K.; Hara, M.; Terada, T.; Watanabe, N.; Takaya, D.; Yaguchi, S.-I.; Matsumoto, T.; Matsuura, T.; Shirouzu, M.; Yokoyama, S.; et al. HCV NS3 protease enhances liver fibrosis via binding to and activating TGF-β type I receptor. Sci. Rep. 2013, 3, 3243. [Google Scholar] [CrossRef]
- Wen, C.; He, X.; Ma, H.; Hou, N.; Wei, C.; Song, T.; Zhang, Y.; Sun, L.; Ma, Q.; Zhong, H. Hepatitis C Virus Infection Downregulates the Ligands of the Activating Receptor NKG2D. Cell. Mol. Immunol. 2008, 5, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Verga-Gérard, A.; Porcherot, M.; Meyniel-Schicklin, L.; André, P.; Lotteau, V.; Perrin-Cocon, L. Hepatitis C virus/human interactome identifies SMURF2 and the viral protease as critical elements for the control of TGF-β signaling. FASEB J. 2013, 27, 4027–4040. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Liu, Y.; Luo, Z.; Kang, L.; Qu, J.; Liu, W.; Xia, X.; Wu, K.; Wu, J. Hepatitis C Virus Activates Bcl-2 and MMP-2 Expression through Multiple Cellular Signaling Pathways. J. Virol. 2012, 86, 12531–12543. [Google Scholar] [CrossRef] [PubMed]
- Chusri, P.; Kumthip, K.; Hong, J.; Zhu, C.; Duan, X.; Jilg, N.; Fusco, D.N.; Brisac, C.; Schaefer, E.A.; Cai, D.; et al. HCV induces transforming growth factor β1 through activation of endoplasmic reticulum stress and the unfolded protein response. Sci. Rep. 2016, 6, 22487. [Google Scholar] [CrossRef]
- Choi, S.-H.; Hwang, S.B. Modulation of the Transforming Growth Factor-β Signal Transduction Pathway by Hepatitis C Virus Nonstructural 5A Protein. J. Biol. Chem. 2006, 281, 7468–7478. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The Matrisome: In Silico Definition and In Vivo Characterization by Proteomics of Normal and Tumor Extracellular Matrices. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Whittaker, C.A.; Carr, S.A.; Tanabe, K.K.; Hynes, R.O. Extracellular matrix signatures of human primary metastatic colon cancers and their metastases to liver. BMC Cancer 2014, 14, 518. [Google Scholar] [CrossRef]
- Decaris, M.L.; Emson, C.L.; Li, K.; Gatmaitan, M.; Luo, F.; Cattin, J.; Nakamura, C.; Holmes, W.E.; Angel, T.E.; Peters, M.G.; et al. Turnover Rates of Hepatic Collagen and Circulating Collagen-Associated Proteins in Humans with Chronic Liver Disease. PLoS ONE 2015, 10, e0123311. [Google Scholar] [CrossRef]
- Asselah, T.; Bièche, I.; Laurendeau, I.; Paradis, V.; Vidaud, D.; Degott, C.; Martinot, M.; Bedossa, P.; Valla, D.; Vidaud, M.; et al. Liver Gene Expression Signature of Mild Fibrosis in Patients with Chronic Hepatitis C. Gastroenterology 2005, 129, 2064–2075. [Google Scholar] [CrossRef]
- Yasui, Y.; Abe, T.; Kurosaki, M.; Matsunaga, K.; Higuchi, M.; Tamaki, N.; Watakabe, K.; Okada, M.; Wang, W.; Shimizu, T.; et al. Non-invasive liver fibrosis assessment correlates with collagen and elastic fiber quantity in patients with hepatitis C virus infection. Hepatol. Res. 2019, 49, 33–41. [Google Scholar] [CrossRef]
- Bracht, T.; Schweinsberg, V.; Trippler, M.; Kohl, M.; Ahrens, M.; Padden, J.; Naboulsi, W.; Barkovits, K.; Megger, D.A.; Eisenacher, M.; et al. Analysis of Disease-Associated Protein Expression Using Quantitative Proteomics—Fibulin-5 is Expressed in Association with Hepatic Fibrosis. J. Proteome Res. 2015, 14, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.J.; Veidal, S.S.; Karsdal, M.A.; Ørsnes-Leeming, D.J.; Vainer, B.; Gardner, S.D.; Hamatake, R.; Goodman, Z.D.; Schuppan, D.; Patel, K. Plasma Pro-C3 (N-terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 2015, 35, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Murawaki, Y.; Ikuta, Y.; Koda, M.; Kawasaki, H. Serum Type III Procollagen Peptide, Type IV Collagen 7S Domain, Central Triple-Helix of Type IV Collagen and Tissue Inhibitor of Metalloproteinases in Patients with Chronic Viral Liver Disease: Relationship to Liver Histology. Hepatology 1994, 20, 780–787. [Google Scholar] [CrossRef]
- Valva, P.; Casciato, P.; Carrasco, J.M.D.; Gadano, A.; Galdame, O.; Galoppo, M.C.; Mullen, E.; De Matteo, E.; Preciado, M.V. The Role of Serum Biomarkers in Predicting Fibrosis Progression in Pediatric and Adult Hepatitis C Virus Chronic Infection. PLoS ONE 2011, 6, e23218. [Google Scholar] [CrossRef]
- Lichtinghagen, R.; Michels, D.; Haberkorn, C.; Arndt, B.; Bahr, M.; Flemming, P.; Manns, M.P.; Boeker, K.H. Matrix metalloproteinase (MMP)-2, MMP-7, and tissue inhibitor of metalloproteinase-1 are closely related to the fibroproliferative process in the liver during chronic hepatitis C. J. Hepatol. 2001, 34, 239–247. [Google Scholar] [CrossRef]
- Ljumovic, D.; Diamantis, I.; Alegakis, A.K.; Kouroumalis, E.A. Differential expression of matrix metalloproteinases in viral and non-viral chronic liver diseases. Clin. Chim. Acta 2004, 349, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Castillo, M.; Hernandez-Barragan, A.; Flores-Vasconcelos, I.; Galicia-Moreno, M.; Rosique-Oramas, D.; Perez-Hernandez, J.L.; La Tijera, F.H.-D.; Montalvo-Jave, E.; Torre-Delgadillo, A.; Cordero-Perez, P.; et al. Production and activity of matrix metalloproteinases during liver fibrosis progression of chronic hepatitis C patients. World J. Hepatol. 2021, 13, 218–232. [Google Scholar] [CrossRef]
- Murawaki, Y.; Ikuta, Y.; Kawasaki, H. Clinical usefulness of serum tissue inhibitor of metalloproteinases (TIMP)-2 assay in patients with chronic liver disease in comparison with serum TIMP. Clin. Chim. Acta 1999, 281, 109–120. [Google Scholar] [CrossRef]
- Dudás, M.J.; Kovalszky, I.; Gallai, M.; Nagy, M.J.O.; Schaff, Z.; Knittel, T.; Mehde, M.; Neubauer, K.; Szalay, F.; Ramadori, G. Expression of Decorin, Transforming Growth Factor-beta1, Tissue Inhibitor Metalloproteinase 1 and 2, and Type IV Collagenases in Chronic Hepatitis. Am. J. Clin. Pathol. 2001, 115, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Ramnath, D.; Irvine, K.M.; Lukowski, S.W.; Horsfall, L.U.; Loh, Z.; Clouston, A.D.; Patel, P.J.; Fagan, K.J.; Iyer, A.; Lampe, G.; et al. Hepatic expression profiling identifies steatosis-independent and steatosis-driven advanced fibrosis genes. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Li, H.-J.; Chang, S.; Liao, H.-J.; Zhang, Z.-P.; Huang, P.; Tang, H.-H. A Disintegrin and Metalloprotease with Thrombospondin Motif 2 May Contribute to Cirrhosis in Humans through the Transforming Growth Factor-β/SMAD Pathway. Gut Liver 2013, 7, 213–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kühn, J.; Gressner, O.A.; Götting, C.; Gressner, A.M.; Kleesiek, K. Increased serum xylosyltransferase activity in patients with liver fibrosis. Clin. Chim. Acta 2009, 409, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Gao, C. Monitoring fibrogenic progression in the liver. Clin. Chim. Acta 2014, 433, 111–122. [Google Scholar] [CrossRef]
- Zhu, Z.-W.; Friess, H.; Wang, L.; Abou-Shady, M.; Zimmermann, A.; Lander, A.D.; Korc, M.; Kleeff, J.; Büchler, M.W. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut 2001, 48, 558–564. [Google Scholar] [CrossRef]
- Llovet, J.M.; Chen, Y.; Wurmbach, E.; Roayaie, S.; Fiel, M.I.; Schwartz, M.; Thung, S.N.; Khitrov, G.; Zhang, W.; Villanueva, A.; et al. A Molecular Signature to Discriminate Dysplastic Nodules from Early Hepatocellular Carcinoma in HCV Cirrhosis. Gastroenterology 2006, 131, 1758–1767. [Google Scholar] [CrossRef]
- Wurmbach, E.; Chen, Y.-B.; Khitrov, G.; Zhang, W.; Roayaie, S.; Schwartz, M.; Fiel, I.; Thung, S.; Mazzaferro, V.; Bruix, J.; et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology 2007, 45, 938–947. [Google Scholar] [CrossRef]
- Clemente, M.; Nunez, O.; Lorente, R.; Rincon, D.; Matilla, A.; Salcedo, M.; Catalina, M.V.; Ripoll, C.; Iacono, O.L.; Banares, R.; et al. Increased intrahepatic and circulating levels of endoglin, a TGF-β1 co-receptor, in patients with chronic hepatitis C virus infection: Relationship to histological and serum markers of hepatic fibrosis. J. Viral Hepat. 2006, 13, 625–632. [Google Scholar] [CrossRef]
- Guéchot, J.; Laudat, A.; Loria, A.; Serfaty, L.; Poupon, R.; Giboudeau, J. Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin. Chem. 1996, 42, 558–563. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Daniels, S.J.; Nielsen, S.H.; Bager, C.; Rasmussen, D.G.K.; Loomba, R.; Surabattula, R.; Villesen, I.F.; Luo, Y.; Shevell, D.; et al. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int. 2020, 40, 736–750. [Google Scholar] [CrossRef]
- Taleb, R.S.Z.; Moez, P.; Younan, D.; Eisenacher, M.; Tenbusch, M.; Sitek, B.; Bracht, T. Quantitative proteome analysis of plasma microparticles for the characterization of HCV-induced hepatic cirrhosis and hepatocellular carcinoma. Proteom. Clin. Appl. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- El-Karef, A.; Kaito, M.; Tanaka, H.; Ikeda, K.; Nishioka, T.; Fujita, N.; Inada, H.; Adachi, Y.; Kawada, N.; Nakajima, Y.; et al. Expression of large tenascin-C splice variants by hepatic stellate cells/myofibroblasts in chronic hepatitis C. J. Hepatol. 2007, 46, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Benbow, J.H.; Elam, A.D.; Bossi, K.L.; Massengill, D.L.; Brandon-Warner, E.; Anderson, W.E.; Culberson, C.R.; Russo, M.W.; Delemos, A.S.; Schrum, L.W. Analysis of Plasma Tenascin-C in Post-HCV Cirrhosis: A Prospective Study. Dig. Dis. Sci. 2018, 63, 653–664. [Google Scholar] [CrossRef]
- Choi, S.S.; Claridge, L.C.; Jhaveri, R.; Swiderska-Syn, M.; Clark, P.; Suzuki, A.; Pereira, T.A.; Mi, Z.; Kuo, P.C.; Guy, C.D.; et al. Osteopontin is up-regulated in chronic hepatitis C and is associated with cellular permissiveness for hepatitis C virus replication. Clin. Sci. 2014, 126, 845–855. [Google Scholar] [CrossRef]
- Urtasun, R.; Lopategi, A.; George, J.; Leung, T.-M.; Lu, Y.; Wang, X.; Ge, X.; Fiel, M.I.; Nieto, N. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin αVβ3 engagement and PI3K/pAkt/NFκB signaling. Hepatology 2011, 55, 594–608. [Google Scholar] [CrossRef]
- Hackl, N.J.; Bersch, C.; Feick, P.; Antoni, C.; Franke, A.; Singer, M.V.; Nakchbandi, I.A. Circulating fibronectin isoforms predict the degree of fibrosis in chronic hepatitis C. Scand. J. Gastroenterol. 2009, 45, 349–356. [Google Scholar] [CrossRef]
- Bracht, T.; Mölleken, C.; Ahrens, M.; Poschmann, G.; Schlosser, A.; Eisenacher, M.; Stühler, K.; Meyer, H.E.; Schmiegel, W.H.; Holmskov, U.; et al. Evaluation of the biomarker candidate MFAP4 for non-invasive assessment of hepatic fibrosis in hepatitis C patients. J. Transl. Med. 2016, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Mölleken, C.; Ahrens, M.; Schlosser, A.; Dietz, J.; Eisenacher, M.; Meyer, H.E.; Schmiegel, W.; Holmskov, U.; Sarrazin, C.; Sorensen, G.L.; et al. Direct-acting antivirals-based therapy decreases hepatic fibrosis serum biomarker microfibrillar-associated protein 4 in hepatitis C patients. Clin. Mol. Hepatol. 2019, 25, 42–51. [Google Scholar] [CrossRef]
- Castilla, A.; Prieto, J.; Fausto, N. Transforming Growth Factors β1 and α in Chronic Liver Disease. N. Engl. J. Med. 1991, 324, 933–940. [Google Scholar] [CrossRef]
- Kinnman, U.A.N. In Situ Expression of Transforming Growth Factor-ß1?3, Latent Transforming Growth Factor-ß Binding Protein and Tumor Necrosis Factor-a in Liver Tissue from Patients with Chronic Hepatitis C. Scand. J. Gastroenterol. 2000, 35, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Divella, R.; Daniele, A.; Gadaleta, C.; Tufaro, A.; Venneri, M.T.; Paradiso, A.; Quaranta, M. Circulating Transforming Growth Factor-β and Epidermal Growth Factor Receptor as Related to Virus Infection in Liver Carcinogenesis. Anticancer Res. 2012, 32, 141–145. [Google Scholar] [PubMed]
- Bader, H.L.; Lambert, E.; Guiraud, A.; Malbouyres, M.; Driever, W.; Koch, M.; Ruggiero, F. Zebrafish Collagen XIV Is Transiently Expressed in Epithelia and Is Required for Proper Function of Certain Basement Membranes. J. Biol. Chem. 2013, 288, 6777–6787. [Google Scholar] [CrossRef]
- Grässel, S.; Unsöld, C.; Schäcke, H.; Bruckner-Tuderman, L.; Bruckner, P. Collagen XVI is expressed by human dermal fibroblasts and keratinocytes and is associated with the microfibrillar apparatus in the upper papillary dermis. Matrix Biol. 1999, 18, 309–317. [Google Scholar] [CrossRef]
- Uitto, J.; Pulkkinen, L. Molecular complexity of the cutaneous basement membrane zone. Mol. Biol. Rep. 1996, 23, 35–46. [Google Scholar] [CrossRef]
- Schuppan, D.; Cramer, T.; Bauer, M.; Strefeld, T.; Hahn, E.G.; Herbst, H. Hepatocytes as a source of collagen type XVIII endostatin. Lancet 1998, 352, 879–880. [Google Scholar] [CrossRef]
- Heljasvaara, R.; Aikio, M.; Ruotsalainen, H.; Pihlajaniemi, T. Collagen XVIII in tissue homeostasis and dysregulation—Lessons learned from model organisms and human patients. Matrix Biol. 2017, 57–58, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Vadasz, Z.; Kessler, O.; Akiri, G.; Gengrinovitch, S.; Kagan, H.M.; Baruch, Y.; Ben Izhak, O.; Neufeld, G. Abnormal deposition of collagen around hepatocytes in Wilson’s disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J. Hepatol. 2005, 43, 499–507. [Google Scholar] [CrossRef]
- Barry-Hamilton, V.; Spangler, R.; Marshall, D.; McCauley, S.A.; Rodriguez, H.M.; Oyasu, M.; Mikels, A.; Vaysberg, M.; Ghermazien, H.; Wai, C.; et al. Allosteric inhibition of lysyl oxidase–like-2 impedes the development of a pathologic microenvironment. Nat. Med. 2010, 16, 1009–1017. [Google Scholar] [CrossRef]
- Liu, S.B.; Ikenaga, N.; Peng, Z.; Sverdlov, D.Y.; Greenstein, A.; Smith, V.; Schuppan, D.; Popov, Y. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2015, 30, 1599–1609. [Google Scholar] [CrossRef]
- Ikenaga, N.; Peng, Z.-W.; Vaid, K.A.; Liu, S.B.; Yoshida, S.; Sverdlov, D.Y.; Mikels-Vigdal, A.; Smith, V.; Schuppan, D.; Popov, Y.V. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut 2017, 66, 1697–1708. [Google Scholar] [CrossRef]
- Giovannini, C.; Fornari, F.; Indio, V.; Trerè, D.; Renzulli, M.; Vasuri, F.; Cescon, M.; Ravaioli, M.; Perrucci, A.; Astolfi, A.; et al. Direct Antiviral Treatments for Hepatitis C Virus Have Off-Target Effects of Oncologic Relevance in Hepatocellular Carcinoma. Cancers 2020, 12, 2674. [Google Scholar] [CrossRef]
- Fontana, R.J.; Dienstag, J.L.; Bonkovsky, H.L.; Sterling, R.K.; Naishadham, D.; Goodman, Z.D.; Lok, A.S.F.; Wright, E.C.; Su, G.L. Serum fibrosis markers are associated with liver disease progression in non-responder patients with chronic hepatitis C. Gut 2010, 59, 1401–1409. [Google Scholar] [CrossRef]
- Medeiros, T.; Saraiva, G.N.; Moraes, L.A.; Gomes, A.C.; Lacerda, G.S.; Leite, P.E.; Esberard, E.B.; Andrade, T.G.; Xavier, A.R.; Quírico-Santos, T.; et al. Liver fibrosis improvement in chronic hepatitis C after direct acting-antivirals is accompanied by reduced profibrogenic biomarkers–a role for MMP-9/TIMP-1. Dig. Liver Dis. 2020. [Google Scholar] [CrossRef]
- Schwettmann, L.; Wehmeier, M.; Jokovic, D.; Aleksandrova, K.; Brand, K.; Manns, M.P.; Lichtinghagen, R.; Bahr, M.J. Hepatic expression of A Disintegrin And Metalloproteinase (ADAM) and ADAMs with thrombospondin motives (ADAM-TS) enzymes in patients with chronic liver diseases. J. Hepatol. 2008, 49, 243–250. [Google Scholar] [CrossRef]
- Bourd-Boittin, K.; Basset, L.; Bonnier, M.; L’Helgoualc’H, A.; Samson, M.; Théret, N. CX3CL1/fractalkine shedding by human hepatic stellate cells: Contribution to chronic inflammation in the liver. J. Cell. Mol. Med. 2009, 13, 1526–1535. [Google Scholar] [CrossRef]
- Parkes, J.; Guha, I.N.; Roderick, P.; Harris, S.; Cross, R.; Manos, M.M.; Irving, W.; Zaitoun, A.; Wheatley, M.; Ryder, S.; et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J. Viral Hepat. 2010, 18, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kato, N.; Urabe, Y.; Takahashi, A.; Muroyama, R.; Hosono, N.; Otsuka, M.; Tateishi, R.; Omata, M.; Nakagawa, H.; et al. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat. Genet. 2011, 43, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, N.W.; Dole, K.; Stastny, P. Differential surface expression of MICA by endothelial cells, fibroblasts, keratinocytes, and monocytes. Hum. Immunol. 1999, 60, 323–330. [Google Scholar] [CrossRef]
- Goto, K.; Kato, N. MICA SNPs and the NKG2D system in virus-induced HCC. J. Gastroenterol. 2014, 50, 261–272. [Google Scholar] [CrossRef]
- Waldhauer, I.; Goehlsdorf, D.; Gieseke, F.; Weinschenk, T.; Wittenbrink, M.; Ludwig, A.; Stevanovic, S.; Rammensee, H.-G.; Steinle, A. Tumor-Associated MICA Is Shed by ADAM Proteases. Cancer Res. 2008, 68, 6368–6376. [Google Scholar] [CrossRef] [PubMed]
- Kohga, K.; Takehara, T.; Tatsumi, T.; Ishida, H.; Miyagi, T.; Hosui, A.; Hayashi, N. Sorafenib inhibits the shedding of major histocompatibility complex class I-related chain A on hepatocellular carcinoma cells by down-regulating a disintegrin and metalloproteinase 9. Hepatology 2009, 51, 1264–1273. [Google Scholar] [CrossRef]
- Kohga, K.; Takehara, T.; Tatsumi, T.; Miyagi, T.; Ishida, H.; Ohkawa, K.; Kanto, T.; Hiramatsu, N.; Hayashi, N. Anticancer Chemotherapy Inhibits MHC Class I–Related Chain A Ectodomain Shedding by Downregulating ADAM10 Expression in Hepatocellular Carcinoma. Cancer Res. 2009, 69, 8050–8057. [Google Scholar] [CrossRef]
- Goto, K.; Arai, J.; Stephanou, A.; Kato, N. Novel therapeutic features of disulfiram against hepatocellular carcinoma cells with inhibitory effects on a disintegrin and metalloproteinase 10. Oncotarget 2018, 9, 18821–18831. [Google Scholar] [CrossRef]
- Huang, C.-F.; Huang, C.-Y.; Yeh, M.-L.; Wang, S.-C.; Chen, K.-Y.; Ko, Y.-M.; Lin, C.-C.; Tsai, Y.-S.; Tsai, P.-C.; Lin, Z.-Y.; et al. Genetics Variants and Serum Levels of MHC Class I Chain-related A in Predicting Hepatocellular Carcinoma Development in Chronic Hepatitis C Patients Post Antiviral Treatment. EBioMedicine 2017, 15, 81–89. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Tzanakakis, G.N.; Karamanos, N.K. Proteoglycans in health and disease: Novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010, 277, 3904–3923. [Google Scholar] [CrossRef]
- Shi, Q.; Jiang, J.; Luo, G. Syndecan-1 Serves as the Major Receptor for Attachment of Hepatitis C Virus to the Surfaces of Hepatocytes. J. Virol. 2013, 87, 6866–6875. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, M.; Felmlee, D.J.; Parnot, M.; Baumert, T.F.; Schuster, C. Syndecan 4 Is Involved in Mediating HCV Entry through Interaction with Lipoviral Particle-Associated Apolipoprotein E. PLoS ONE 2014, 9, e95550. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ono, M.; Fujimoto, Y.; Gallo, R.L.; Bernfield, M.; Kohgo, Y. Reduced expression of syndecan-1 in human hepatocellular carcinoma with high metastatic potential. Int. J. Cancer 1997, 74, 482–491. [Google Scholar] [CrossRef]
- Regős, E.; Karászi, K.; Reszegi, A.; Kiss, A.; Schaff, Z.; Baghy, K.; Kovalszky, I. Syndecan-1 in Liver Diseases. Pathol. Oncol. Res. 2020, 26, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Poönighaus, C.; Ambrosius, M.; Casanova, J.C.; Prante, C.; Kuhn, J.; Esko, J.D.; Kleesiek, K.; Goötting, C. Human Xylosyltransferase II Is Involved in the Biosynthesis of the Uniform Tetrasaccharide Linkage Region in Chondroitin Sulfate and Heparan Sulfate Proteoglycans. J. Biol. Chem. 2007, 282, 5201–5206. [Google Scholar] [CrossRef] [PubMed]
- Baghy, K.; Tátrai, P.; Regős, E.; Kovalszky, I. Proteoglycans in liver cancer. World J. Gastroenterol. 2016, 22, 379–393. [Google Scholar] [CrossRef]
- Liu, B.; Paranjpe, S.; Bowen, W.C.; Bell, A.W.; Luo, J.-H.; Yu, Y.-P.; Mars, W.M.; Michalopoulos, G.K. Investigation of the Role of Glypican 3 in Liver Regeneration and Hepatocyte Proliferation. Am. J. Pathol. 2009, 175, 717–724. [Google Scholar] [CrossRef]
- Toretsky, J.A.; Zitomersky, N.L.; Eskenazi, A.E.; Voigt, R.W.; Strauch, E.D.; Sun, C.C.; Huber, R.; Meltzer, S.J.; Schlessinger, D. Glypican-3 Expression in Wilms Tumor and Hepatoblastoma. J. Pediatr. Hematol. 2001, 23, 496–499. [Google Scholar] [CrossRef]
- Bhave, V.S.; Mars, W.; Donthamsetty, S.; Zhang, X.; Tan, L.; Luo, J.; Bowen, W.C.; Michalopoulos, G.K. Regulation of Liver Growth by Glypican 3, CD81, Hedgehog, and Hhex. Am. J. Pathol. 2013, 183, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Zvibel, I.; Halfon, P.; Fishman, S.; Penaranda, G.; Leshno, M.; Bet Or, A.; Halpern, Z.; Oren, R. Syndecan 1 (CD138) serum levels: A novel biomarker in predicting liver fibrosis stage in patients with hepatitis C. Liver Int. 2009, 29, 208–212. [Google Scholar] [CrossRef]
- Shimizu, Y.; Mizuno, S.; Fujinami, N.; Suzuki, T.; Saito, K.; Konishi, M.; Takahashi, S.; Gotohda, N.; Tada, T.; Toyoda, H.; et al. Plasma and tumoral glypican-3 levels are correlated in patients with hepatitis C virus-related hepatocellular carcinoma. Cancer Sci. 2019, 111, 334–342. [Google Scholar] [CrossRef]
- Tsuchiya, N. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 10573–10583. [Google Scholar] [CrossRef]
- Sun, C.K.; Chua, M.-S.; He, J.; Samuel, K.S. Suppression of Glypican 3 Inhibits Growth of Hepatocellular Carcinoma Cells through Up-Regulation of TGF-β2. Neoplasia 2011, 13, 735. [Google Scholar] [CrossRef]
- Regős, E.; Abdelfattah, H.H.; Reszegi, A.; Szilák, L.; Werling, K.; Szabó, G.; Kiss, A.; Schaff, Z.; Kovalszky, I.; Baghy, K. Syndecan-1 inhibits early stages of liver fibrogenesis by interfering with TGFβ1 action and upregulating MMP14. Matrix Biol. 2018, 68–69, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, S.S.; Karalis, T.T.; Chatzopoulos, A.; Karamanos, N.K. Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cell. Signal. 2019, 63, 109377. [Google Scholar] [CrossRef] [PubMed]
- Park, N.R.; Cha, J.H.; Jang, J.W.; Bae, S.H.; Jang, B.; Kim, J.-H.; Hur, W.; Choi, J.Y.; Yoon, S.K. Synergistic effects of CD44 and TGF-β1 through AKT/GSK-3β/β-catenin signaling during epithelial-mesenchymal transition in liver cancer cells. Biochem. Biophys. Res. Commun. 2016, 477, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Castelli, G.; Pelosi, E.; Testa, U. Liver Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Cancers 2017, 9, 127. [Google Scholar] [CrossRef]
- Kakehashi, A.; Ishii, N.; Sugihara, E.; Gi, M.; Saya, H.; Wanibuchi, H. CD 44 variant 9 is a potential biomarker of tumor initiating cells predicting survival outcome in hepatitis C virus-positive patients with resected hepatocellular carcinoma. Cancer Sci. 2016, 107, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Fukuhara, T.; Wen, X.; Ninomiya, A.; Moriishi, K.; Maehara, Y.; Takeuchi, O.; Kawai, T.; Akira, S.; Matsuura, Y. CD44 Participates in IP-10 Induction in Cells in Which Hepatitis C Virus RNA Is Replicating, through an Interaction with Toll-Like Receptor 2 and Hyaluronan. J. Virol. 2012, 86, 6159–6170. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Tang, F.; Rnjak-Kovacina, J.; Smith, J.G.; Melrose, J.; Whitelock, J.M. The multifaceted roles of perlecan in fibrosis. Matrix Biol. 2018, 68–69, 150–166. [Google Scholar] [CrossRef]
- Batmunkh, E.; Tátrai, P.; Szabó, E.; Lódi, C.; Holczbauer, Á.; Páska, C.; Kupcsulik, P.; Kiss, A.; Schaff, Z.; Kovalszky, I. Comparison of the expression of agrin, a basement membrane heparan sulfate proteoglycan, in cholangiocarcinoma and hepatocellular carcinoma. Hum. Pathol. 2007, 38, 1508–1515. [Google Scholar] [CrossRef]
- Binder, M.J.; McCoombe, S.; Williams, E.D.; McCulloch, D.R.; Ward, A.C. The extracellular matrix in cancer progression: Role of hyalectan proteoglycans and ADAMTS enzymes. Cancer Lett. 2017, 385, 55–64. [Google Scholar] [CrossRef]
- Sobhy, A.; Fakhry, M.M.; Azeem, H.A.; Ashmawy, A.M.; Khalifa, H.O. Significance of biglycan and osteopontin as non-invasive markers of liver fibrosis in patients with chronic hepatitis B virus and chronic hepatitis C virus. J. Investig. Med. 2018, 67, 681–685. [Google Scholar] [CrossRef]
- Roedig, H.; Damiescu, R.; Zeng-Brouwers, J.; Kutija, I.; Trebicka, J.; Wygrecka, M.; Schaefer, L. Danger matrix molecules orchestrate CD14/CD44 signaling in cancer development. Semin. Cancer Biol. 2020, 62, 31–47. [Google Scholar] [CrossRef]
- Baghy, K.; Iozzo, R.V.; Kovalszky, I. Decorin–TGFβ Axis in Hepatic Fibrosis and Cirrhosis. J. Histochem. Cytochem. 2012, 60, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Bartosch, B.; Smirnova, O.A.; Isaguliants, M.G.; Kochetkov, S.N. HCV and Oxidative Stress in the Liver. Viruses 2013, 5, 439–469. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Radu, C.M.; Zanetto, A.; Bulato, C.; Shalaby, S.; Spiezia, L.; Franceschet, E.; Burra, P.; Russo, F.P.; Simioni, P. Changes in plasma circulating microvesicles in patients with HCV-related cirrhosis after treatment with direct-acting antivirals. Liver Int. 2019, 40, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Gerarduzzi, C.; Hartmann, U.; Leask, A.; Drobetsky, E. The Matrix Revolution: Matricellular Proteins and Restructuring of the Cancer Microenvironment. Cancer Res. 2020, 80, 2705–2717. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, Y.; Knops, N.; Elmonem, M.A.; Nguyen, T.Q.; Arcolino, F.O.; Heuvel, L.V.D.; Levtchenko, E.; Kuypers, D.; Goldschmeding, R. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 2018, 68–69, 44–66. [Google Scholar] [CrossRef]
- Tanaka, H.; El-Karef, A.; Kaito, M.; Kinoshita, N.; Fujita, N.; Horiike, S.; Watanabe, S.; Yoshida, T.; Adachi, Y. Circulating level of large splice variants of tenascin-C is a marker of piecemeal necrosis activity in patients with chronic hepatitis C. Liver Int. 2006, 26, 311–318. [Google Scholar] [CrossRef]
- Coombes, J.D.; Swiderska-Syn, M.; Dollé, L.; Reid, D.; Eksteen, B.; Claridge, L.; Briones-Orta, M.; Shetty, S.; Oo, Y.H.; Riva, A.; et al. Osteopontin neutralisation abrogates the liver progenitor cell response and fibrogenesis in mice. Gut 2015, 64, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Bruha, R.; Vitek, L.; Smid, V. Osteopontin – A potential biomarker of advanced liver disease. Ann. Hepatol. 2020, 19, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Matsue, Y.; Tsutsumi, M.; Hayashi, N.; Saito, T.; Tsuchishima, M.; Toshikuni, N.; Arisawa, T.; George, J. Serum Osteopontin Predicts Degree of Hepatic Fibrosis and Serves as a Biomarker in Patients with Hepatitis C Virus Infection. PLoS ONE 2015, 10, e0118744. [Google Scholar] [CrossRef]
- Athwal, V.S.; Pritchett, J.; Martin, K.; Llewellyn, J.; Scott, J.; Harvey, E.; Zaitoun, A.M.; Mullan, A.F.; Zeef, L.A.H.; Friedman, S.L.; et al. Publisher Correction: SOX9 regulated matrix proteins are increased in patients serum and correlate with severity of liver fibrosis. Sci. Rep. 2019, 9, 1. [Google Scholar] [CrossRef]
- Wang, X.; Lopategi, A.; Ge, X.; Lu, Y.; Kitamura, N.; Urtasun, R.; Leung, T.-M.; Fiel, M.I.; Nieto, N. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut 2014, 63, 1805–1818. [Google Scholar] [CrossRef]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Iso, Y.; Sawada, T.; Okada, T.; Kubota, K. Loss of E-cadherin mRNA and gain of osteopontin mRNA are useful markers for detecting early recurrence of HCV-related hepatocellular carcinoma. J. Surg. Oncol. 2005, 92, 304–311. [Google Scholar] [CrossRef]
- Mochida, S.; Hashimoto, M.; Matsui, A.; Naito, M.; Inao, M.; Nagoshi, S.; Nagano, M.; Egashira, T.; Mishiro, S.; Fujiwara, K. Genetic polymorphims in promoter region of osteopontin gene may be a marker reflecting hepatitis activity in chronic hepatitis C patients. Biochem. Biophys. Res. Commun. 2004, 313, 1079–1085. [Google Scholar] [CrossRef]
- Gressner, O.A.; Gressner, A.M. Connective tissue growth factor: A fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008, 28, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Paradis, V.; Dargere, D.; Vidaud, M.; De Gouville, A.-C.; Huet, S.; Martinez, V.; Gauthier, J.-M.; Bâ, N.; Sobesky, R.; Ratziu, V.; et al. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology 1999, 30, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Hora, C.; Negro, F.; Leandro, G.; Oneta, C.M.; Rubbia-Brandt, L.; Muellhaupt, B.; Helbling, B.; Malinverni, R.; Gonvers, J.-J.; Dufour, J.-F.; et al. Connective tissue growth factor, steatosis and fibrosis in patients with chronic hepatitis C. Liver Int. 2007, 28, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, T.; Chen, L.; Balasubramanian, A.; Groopman, J.E.; Ghoshal, K.; Jacob, S.T.; Leask, A.; Brigstock, D.R.; Anand, A.R.; Ganju, R.K. Activation of the Connective Tissue Growth Factor (CTGF)-Transforming Growth Factor β 1 (TGF-β 1) Axis in Hepatitis C Virus-Expressing Hepatocytes. PLoS ONE 2012, 7, e46526. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.J.; Clouston, A.D.; Forbes, S.J. Links Between Hepatic Fibrosis, Ductular Reaction, and Progenitor Cell Expansion. Gastroenterology 2014, 146, 349–356. [Google Scholar] [CrossRef]
- Vasel, M.; Rutz, R.; Bersch, C.; Feick, P.; Singer, M.V.; Kirschfink, M.; Nakchbandi, I.A. Complement activation correlates with liver necrosis and fibrosis in chronic hepatitis C. Clin. Immunol. 2014, 150, 149–156. [Google Scholar] [CrossRef]
- Lorenzini, S.; Bird, T.G.; Boulter, L.; Bellamy, C.; Samuel, K.; Aucott, R.; Clayton, E.; Andreone, P.; Bernardi, M.; Golding, M.; et al. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut 2010, 59, 645–654. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yamamoto, K.; Nagano, T.; Okamoto, R.; Ibuki, N.; Tagashira, M.; Tsuji, T. Immunohistochemical study on phenotypical changes of hepatocytes in liver disease with reference to extracellular matrix composition. Liver Int. 1999, 19, 32–38. [Google Scholar] [CrossRef]
- Kanta, J.; Dooley, S.; Delvoux, B.; Breuer, S.; D’Amico, T.; Gressner, A.M. Tropoelastin expression is up-regulated during activation of hepatic stellate cells and in the livers of CCl4-cirrhotic rats. Liver Int. 2002, 22, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ye, L.; Bennett, S.; Xu, H.; He, D.; Xu, J. Molecular structure and function of microfibrillar-associated proteins in skeletal and metabolic disorders and cancers. J. Cell. Physiol. 2021, 236, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Papke, C.L.; Yanagisawa, H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: Insights from mouse and human studies. Matrix Biol. 2014, 37, 142–149. [Google Scholar] [CrossRef]
- Kanzaki, T.; Olofsson, A.; Morén, A.; Wernstedt, C.; Hellman, U.; Miyazono, K.; Claesson-Welsh, L.; Heldin, C.-H. TGF-β1 binding protein: A component of the large latent complex of TGF-β1 with multiple repeat sequences. Cell 1990, 61, 1051–1061. [Google Scholar] [CrossRef]
- Kusakabe, M.; Cheong, P.-L.; Nikfar, R.; McLennan, I.S.; Koishi, K. The structure of the TGF-β latency associated peptide region determines the ability of the proprotein convertase furin to cleave TGF-βs. J. Cell. Biochem. 2007, 103, 311–320. [Google Scholar] [CrossRef]
- Fausto, N.; Mead, J.E.; Gruppuso, P.A.; Castilla, A.; Jakowlew, S.B. Effects of TGF-Beta s in the Liver: Cell Proliferation and Fibrogenesis. Ciba Found. Symp. 1991, 157, 165–174. [Google Scholar]
- Li, G.; Jiang, Q.; Xu, K. CREB family: A significant role in liver fibrosis. Biochimie 2019, 163, 94–100. [Google Scholar] [CrossRef]
- Lua, I.; Li, Y.; Zagory, J.A.; Wang, K.S.; French, S.W.; Sévigny, J.; Asahina, K. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J. Hepatol. 2016, 64, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Kataria, Y.; Deaton, R.J.; Enk, E.; Jin, M.; Petrauskaite, M.; Dong, L.; Goldenberg, J.R.; Cotler, S.J.; Jensen, D.M.; Van Breemen, R.B.; et al. Retinoid and carotenoid status in serum and liver among patients at high-risk for liver cancer. BMC Gastroenterol. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Dijke, P.T. IT-LIVER Consortium TGF-β signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef]
- Sasaki, R.; Devhare, P.; Ray, R.B.; Ray, R. Hepatitis C virus-induced tumor-initiating cancer stem-like cells activate stromal fibroblasts in a xenograft tumor model. Hepatology 2017, 66, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R.; Gonzalezperalta, R.P.; Qian, K.; Xu, Y.; Marousis, C.G.; Davis, G.L.; Lau, J.Y.N. Transforming growth factor-ß 1 in chronic hepatitis C. J. Viral Hepat. 1997, 4, 29–35. [Google Scholar] [CrossRef]
- Taniguchi, H.; Kato, N.; Otsuka, M.; Goto, T.; Yoshida, H.; Shiratori, Y.; Omata, M. Hepatitis C virus core protein upregulates transforming growth factor-?1 transcription. J. Med. Virol. 2003, 72, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Miyanari, Y.; Atsuzawa, K.; Usuda, N.; Watashi, K.; Hishiki, T.; Zayas, M.; Bartenschlager, R.; Wakita, T.; Hijikata, M.; Shimotohno, K. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 2007, 9, 1089–1097. [Google Scholar] [CrossRef]
- Waris, G.; Tardif, K.D.; Siddiqui, A. Endoplasmic reticulum (ER) stress: Hepatitis C virus induces an ER-nucleus signal transduction pathway and activates NF-κB and STAT-3. Biochem. Pharmacol. 2002, 64, 1425–1430. [Google Scholar] [CrossRef]
- Meurer, S.K.; Alsamman, M.; Scholten, D.; Weiskirchen, R. Endoglin in liver fibrogenesis: Bridging basic science and clinical practice. World J. Biol. Chem. 2014, 5, 180–203. [Google Scholar] [CrossRef]
- About, F.; Bibert, S.; Jouanguy, E.; Nalpas, B.; Lorenzo, L.; Rattina, V.; Zarhrate, M.; Hanein, S.; Munteanu, M.; Müllhaupt, B.; et al. Identification of an Endoglin Variant Associated with HCV-Related Liver Fibrosis Progression by Next-Generation Sequencing. Front. Genet. 2019, 10, 1024. [Google Scholar] [CrossRef]
- Xu, L.; Hui, A.Y.; Albanis, E.; Arthur, M.J.; O’Byrne, S.M.; Blaner, W.S.; Mukherjee, P.; Friedman, S.L.; Eng, F.J. Human hepatic stellate cell lines, LX-1 and LX-2: New tools for analysis of hepatic fibrosis. Gut 2005, 54, 142–151. [Google Scholar] [CrossRef]
- Florimond, A.; Chouteau, P.; Bruscella, P.; Le Seyec, J.; Mérour, E.; Ahnou, N.; Mallat, A.; Lotersztajn, S.; Pawlotsky, J.-M. Human hepatic stellate cells are not permissive for hepatitis C virus entry and replication. Gut 2015, 64, 957–965. [Google Scholar] [CrossRef]
- Aoudjehane, L.; Bisch, G.; Scatton, O.; Granier, C.; Gaston, J.; Housset, C.; Roingeard, P.; Cosset, F.-L.; Perdigão, F.; Balladur, P.; et al. Infection of Human Liver Myofibroblasts by Hepatitis C Virus: A Direct Mechanism of Liver Fibrosis in Hepatitis C. PLoS ONE 2015, 10, e0134141. [Google Scholar] [CrossRef]
- Devhare, P.B.; Sasaki, R.; Shrivastava, S.; Di Bisceglie, A.M.; Ray, R.; Ray, R.B. Exosome-Mediated Intercellular Communication between Hepatitis C Virus-Infected Hepatocytes and Hepatic Stellate Cells. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, B. Piecing together the key players of fibrosis in chronic hepatitis C: What roles do non-hepatic liver resident cell types play? Gut 2014, 64, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Foschi, F.G.; Domenicali, M.; Giacomoni, P.; Dall’Aglio, A.C.; Conti, F.; Borghi, A.; Bevilacqua, V.; Napoli, L.; Mirici, F.; Cucchetti, A.; et al. Is there an association between commonly employed biomarkers of liver fibrosis and liver stiffness in the general population? Ann. Hepatol. 2020, 19, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Meissner, E.G.; McLaughlin, M.; Matthews, L.; Gharib, A.M.; Wood, B.J.; Levy, E.; Sinkus, R.; Virtaneva, K.; Sturdevant, D.; Martens, C.; et al. Simtuzumab treatment of advanced liver fibrosis in HIV and HCV-infected adults: Results of a 6-month open-label safety trial. Liver Int. 2016, 36, 1783–1792. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]

| Virus | HBV | HCV |
|---|---|---|
| Viral family | Hepadnaviridae | Flaviviridae |
| Genome | DNA and cccDNA | RNA |
| Life cycle | Genome integration, expression of HBx protein, insertional activation of cellular oncogenes, cccDNA (minichromosome) | Exclusively cytoplasmic |
| Persistence | Nucleus-located cccDNA | Chronic inflammation, oxidative stress, alterations in cellular signaling and metabolism |
| HCV Proteins | ECM Proteins or Cytokines |
|---|---|
| Capsid core | LOX ∞ [61] Procollagen I ∞ [62] Collagen I ∞ [61] MMP-2 ∞ [58] |
| MMP-9 ∞ [63] | |
| COX-2 ∞ [63] | |
| Syndecan-1 * [31] | |
| Thrombospondin-1 ∞ [61] | |
| Osteopontin * [64,65] | |
| CTGF ∞ [58] | |
| TGF-β1 ◊ [58,61,62,66] | |
| TGF-β2 ◊ [67] | |
| Endoglin ∞ [68] | |
| Envelope glycoproteins E1 and/or E2 | Glypican-3 * [69] |
| TGF-β1 ◊ [66] | |
| Cysteine autoprotease NS2 | MICA ∞ [70] TGF-β2 ◊ [67] |
| Serine protease and helicase NS3 | Procollagen I ∞ [62] MMP-9 ∞ [71] |
| COX-2 ∞ [71] | |
| Thrombospondin-1 [72] | |
| Osteopontin * [64] | |
| TGF-β1 ◊ [62,72] | |
| TGF-β type I receptor * [73] | |
| NS3 with its cofactor NS4A | MMP-9 ∞ [71] |
| COX-2 ∞ [71] MICA ∞ [74] | |
| TGF-β ◊ [72,75] | |
| NS4B | MMP-2 ∞ [76] |
| NS5A | MMP-2 ∞ [63] |
| MMP-9 ∞ [63] | |
| COX-2 ∞ [63] | |
| Thrombospondin-1 ∞ [72] | |
| Osteopontin * [64] | |
| TGF-β1 ◊ [72,77,78] | |
| RNA-dependent RNA polymerase NS5B | Osteopontin * [64] |
| MICA ∞ [70] TGF-β ◊ [75] |
| ECM Proteins/Cytokine | F0/F1 | F2 | F3 | F4 | HCC | References |
|---|---|---|---|---|---|---|
| Collagens I, III, V | F1 | [45,59,60,79,80,81,82,83] | ||||
| Collagen XII | [59,84] | |||||
| Collagen XIV | [59,84] | |||||
| Collagen XVI | [59] | |||||
| Collagen XVIII | [59] | |||||
| PIIINP | F1 | [85,86,87] | ||||
| MMP-2, -7, -9 | F1 | [63,82,88,89,90] | ||||
| TIMP-1 | [82,86,88,91,92] | |||||
| ADAM-TS1 | [93] | |||||
| ADAM-TS2 | [94] | |||||
| Xylosyltransferase-2 | F1 | [95,96] | ||||
| Glypican-3 | [97,98,99] | |||||
| Hyaluronic acid | [87,100,101] | |||||
| Decorin | F1 | [92] | ||||
| Biglycan | [59] | |||||
| Fibromodulin | [60] | |||||
| Lumican | [59,81,84,102] | |||||
| Versican | F1 | [93,103] | ||||
| Tenascin-C | [104,105] | |||||
| Osteopontin | F1 | [82,106,107] | ||||
| Fibronectin | [103,108] | |||||
| Fibronectin isoforms | [108] | |||||
| Elastin | [59,83,84,102] | |||||
| MFAP-4 † | F1 | [84,109,110] | ||||
| Fibulin-5 | [84] | |||||
| TGF-β1 (protein, mRNA) | [59,100,103,111,112] | |||||
| TGF-β1 (serum levels) | F1 | [87,113] | ||||
| TGF-β2 | F1 | F0 | [67] | |||
| Endoglin (protein, serum levels) | [100] | |||||
| Endoglin (mRNA) § | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reungoat, E.; Grigorov, B.; Zoulim, F.; Pécheur, E.-I. Molecular Crosstalk between the Hepatitis C Virus and the Extracellular Matrix in Liver Fibrogenesis and Early Carcinogenesis. Cancers 2021, 13, 2270. https://doi.org/10.3390/cancers13092270
Reungoat E, Grigorov B, Zoulim F, Pécheur E-I. Molecular Crosstalk between the Hepatitis C Virus and the Extracellular Matrix in Liver Fibrogenesis and Early Carcinogenesis. Cancers. 2021; 13(9):2270. https://doi.org/10.3390/cancers13092270
Chicago/Turabian StyleReungoat, Emma, Boyan Grigorov, Fabien Zoulim, and Eve-Isabelle Pécheur. 2021. "Molecular Crosstalk between the Hepatitis C Virus and the Extracellular Matrix in Liver Fibrogenesis and Early Carcinogenesis" Cancers 13, no. 9: 2270. https://doi.org/10.3390/cancers13092270
APA StyleReungoat, E., Grigorov, B., Zoulim, F., & Pécheur, E.-I. (2021). Molecular Crosstalk between the Hepatitis C Virus and the Extracellular Matrix in Liver Fibrogenesis and Early Carcinogenesis. Cancers, 13(9), 2270. https://doi.org/10.3390/cancers13092270









