In Vitro and In Vivo Cell Uptake of a Cell-Penetrating Peptide Conjugated with Fluorescent Dyes Having Different Chemical Properties
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and General Information
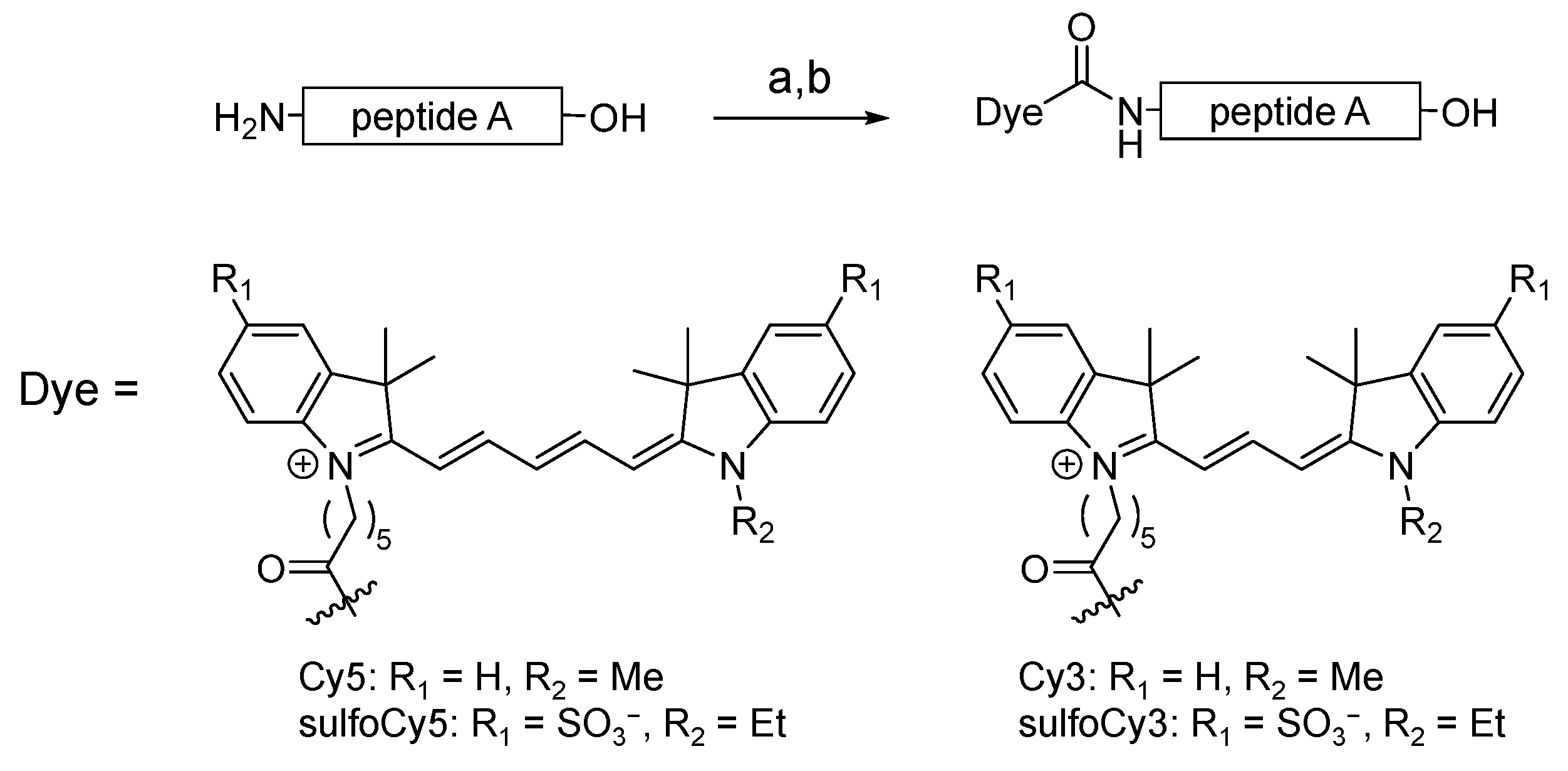
2.2. Synthesis
2.3. Cell Culture
2.4. Fluorescence Microscopy
2.5. Flow Cytometry
2.6. Animal Experiments
2.6.1. Animal and Tumor Model
2.6.2. In Vivo and Ex Vivo Fluorescence Imaging
2.6.3. Measurement of the Concentration of Dye-Peptide A in Blood
2.6.4. Stability of Dye-Peptide A in Mouse Plasma
3. Results
3.1. Synthesis of Fluorescently Labeled Peptide A
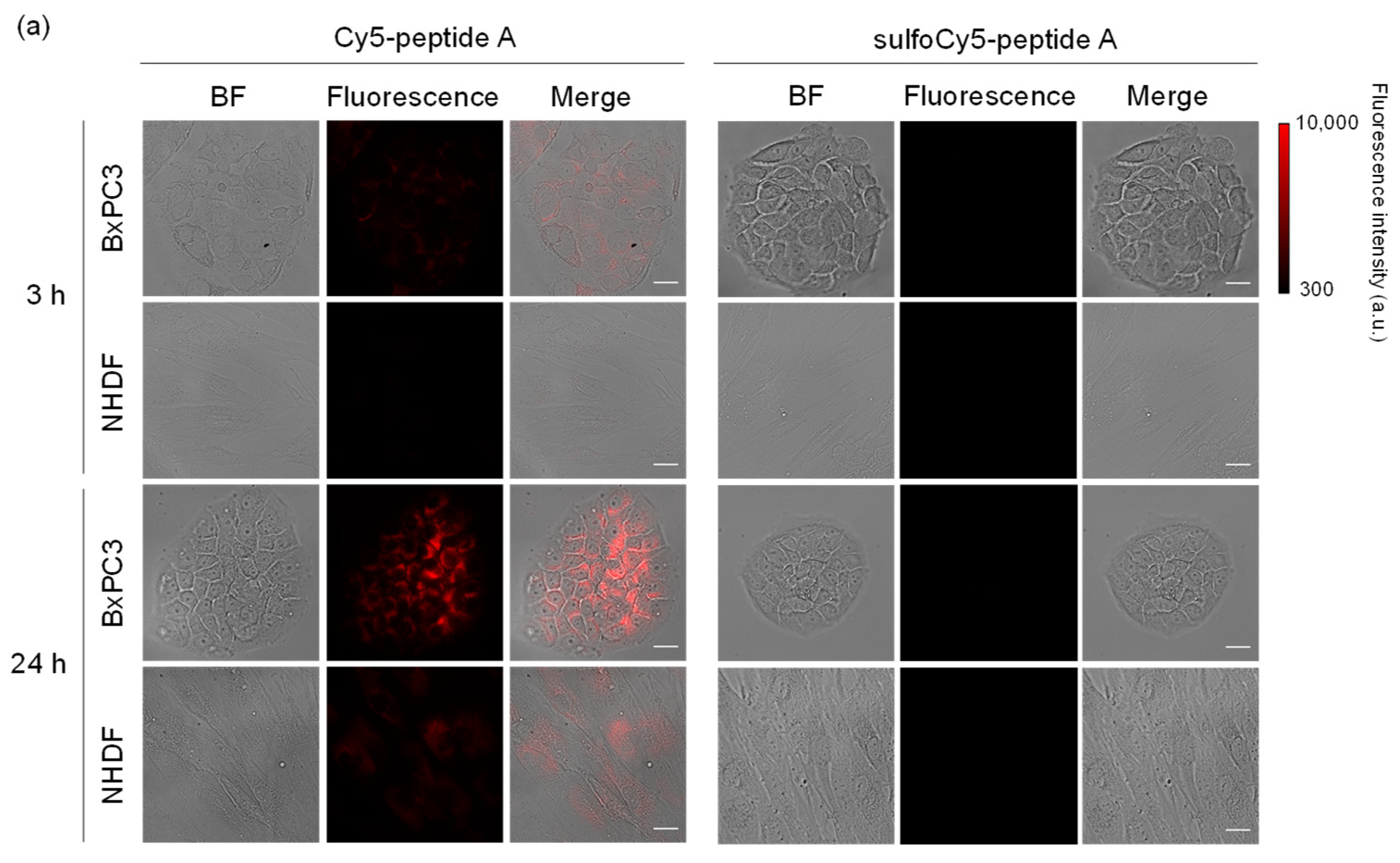
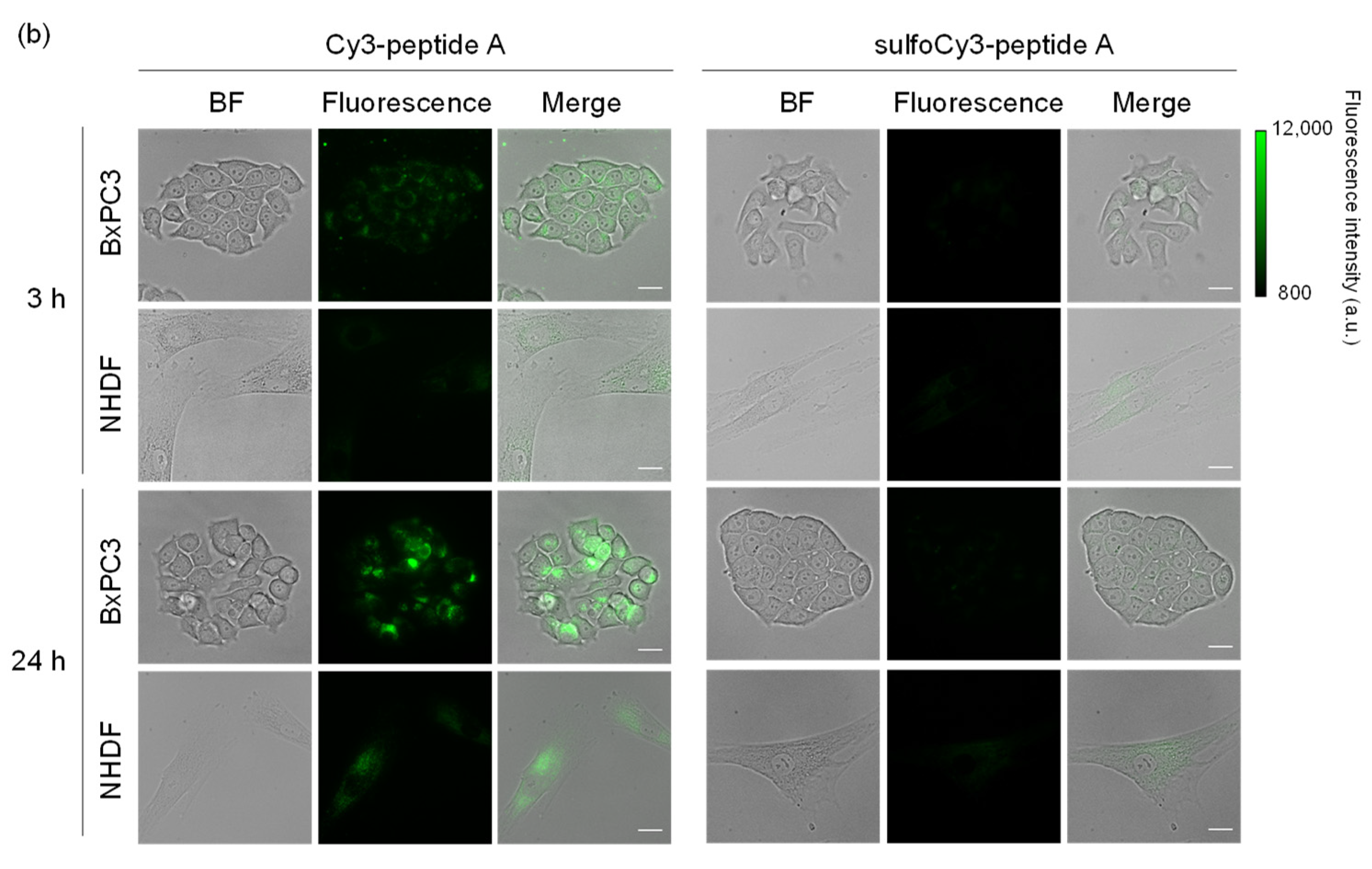
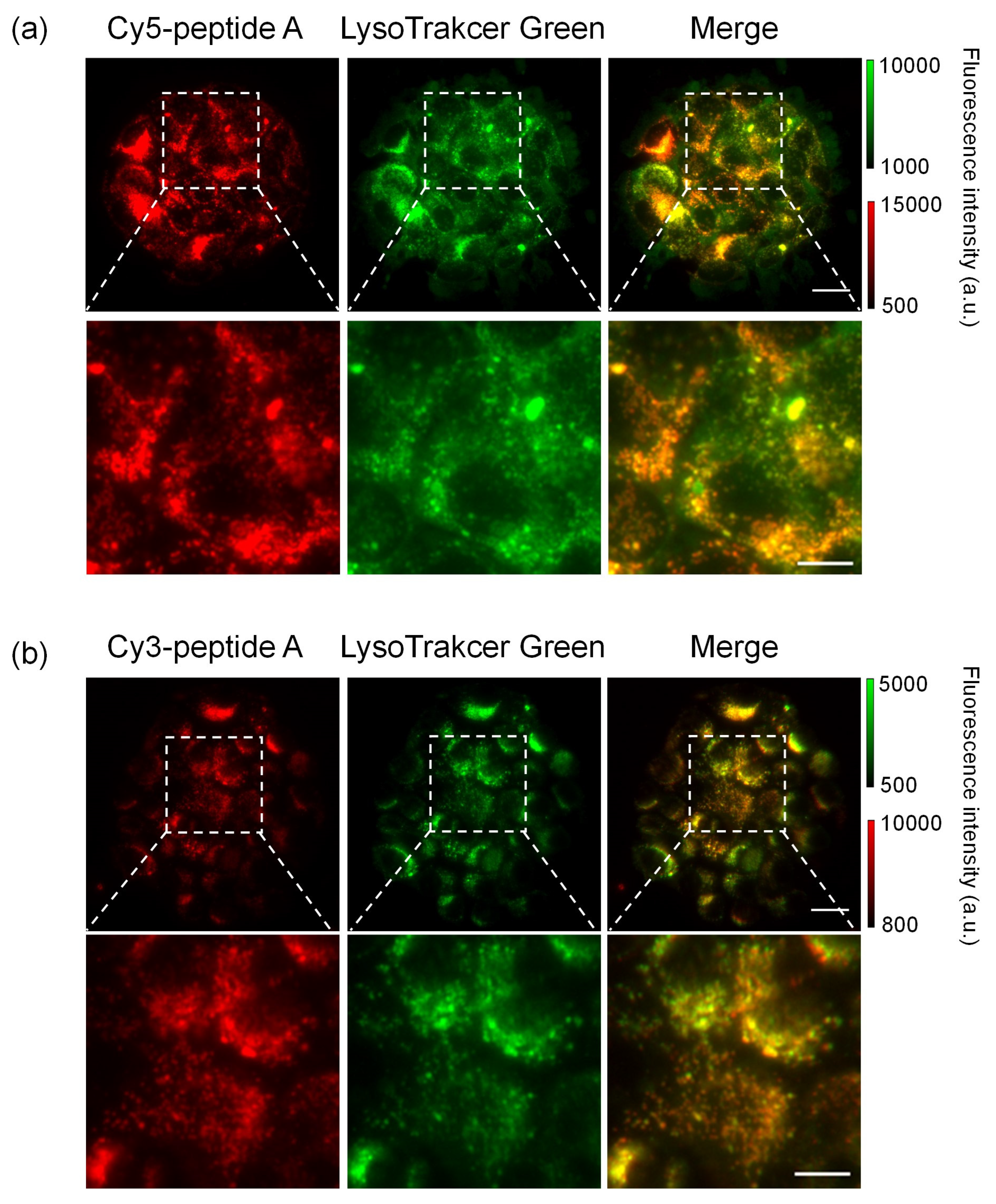
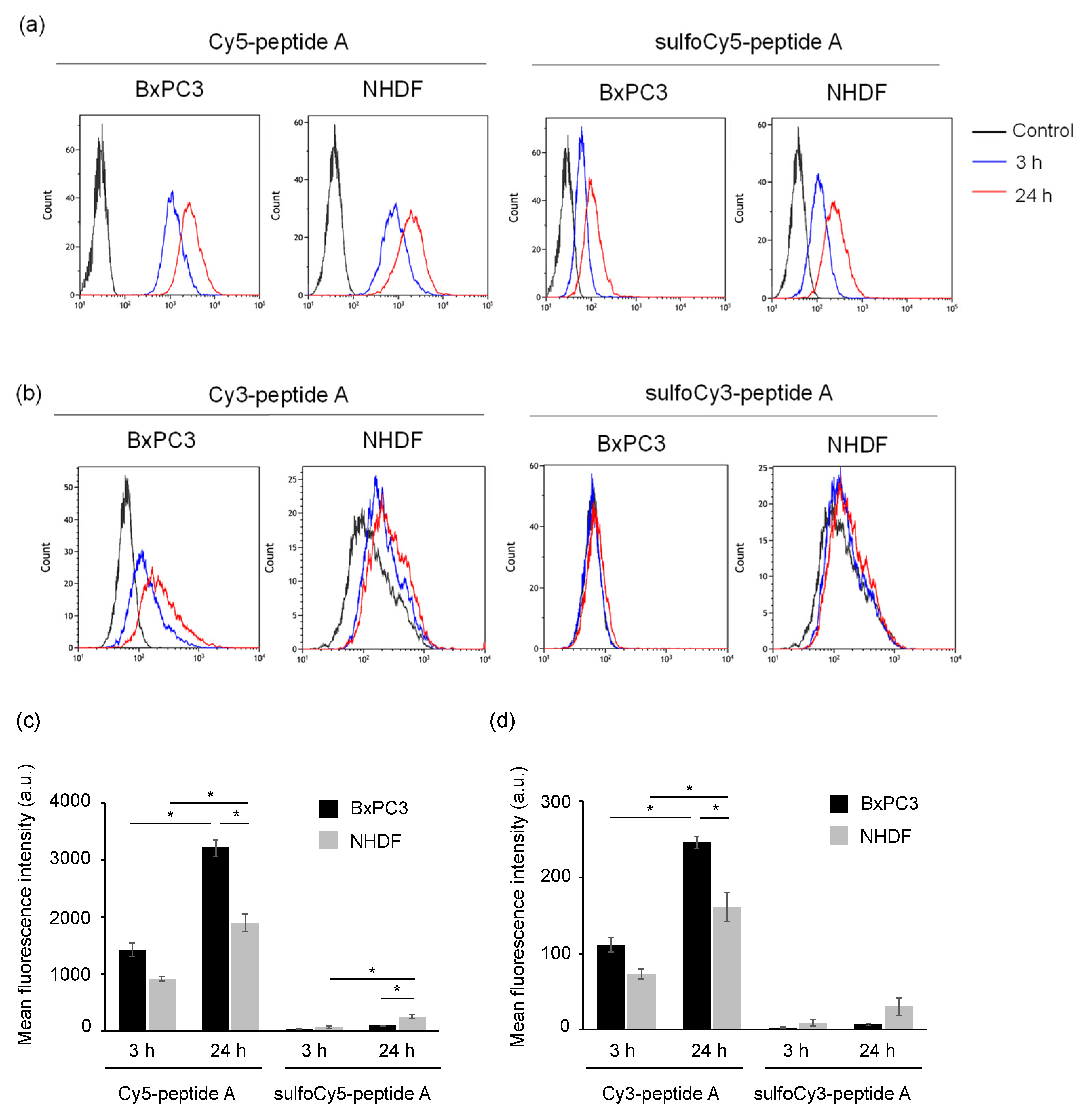
3.2. In Vitro Analysis of Dye-Peptide A by Microscopy and Flow Cytometry
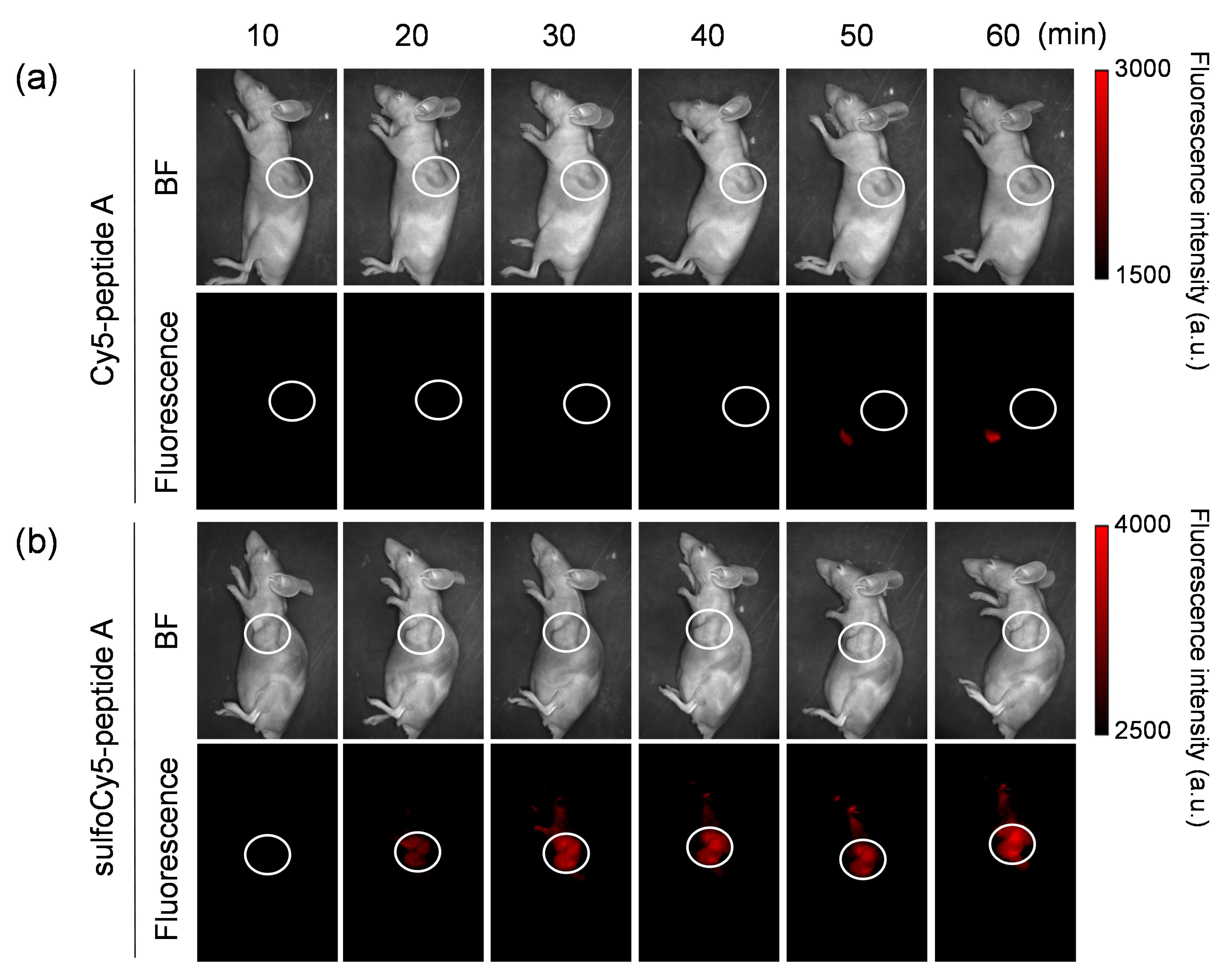
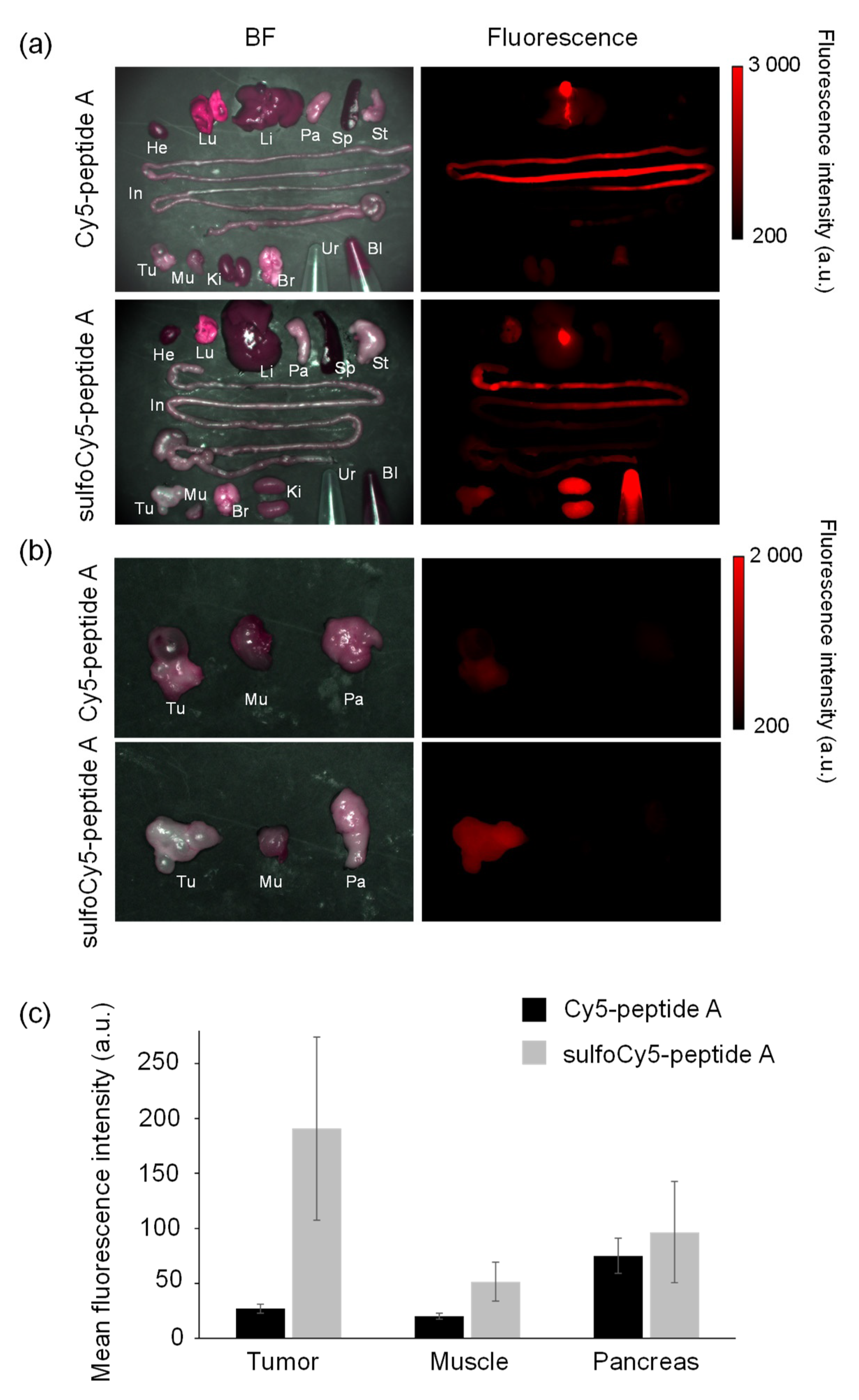
3.3. In Vivo and Ex Vivo Analysis of Dye-Peptide A Conjugates
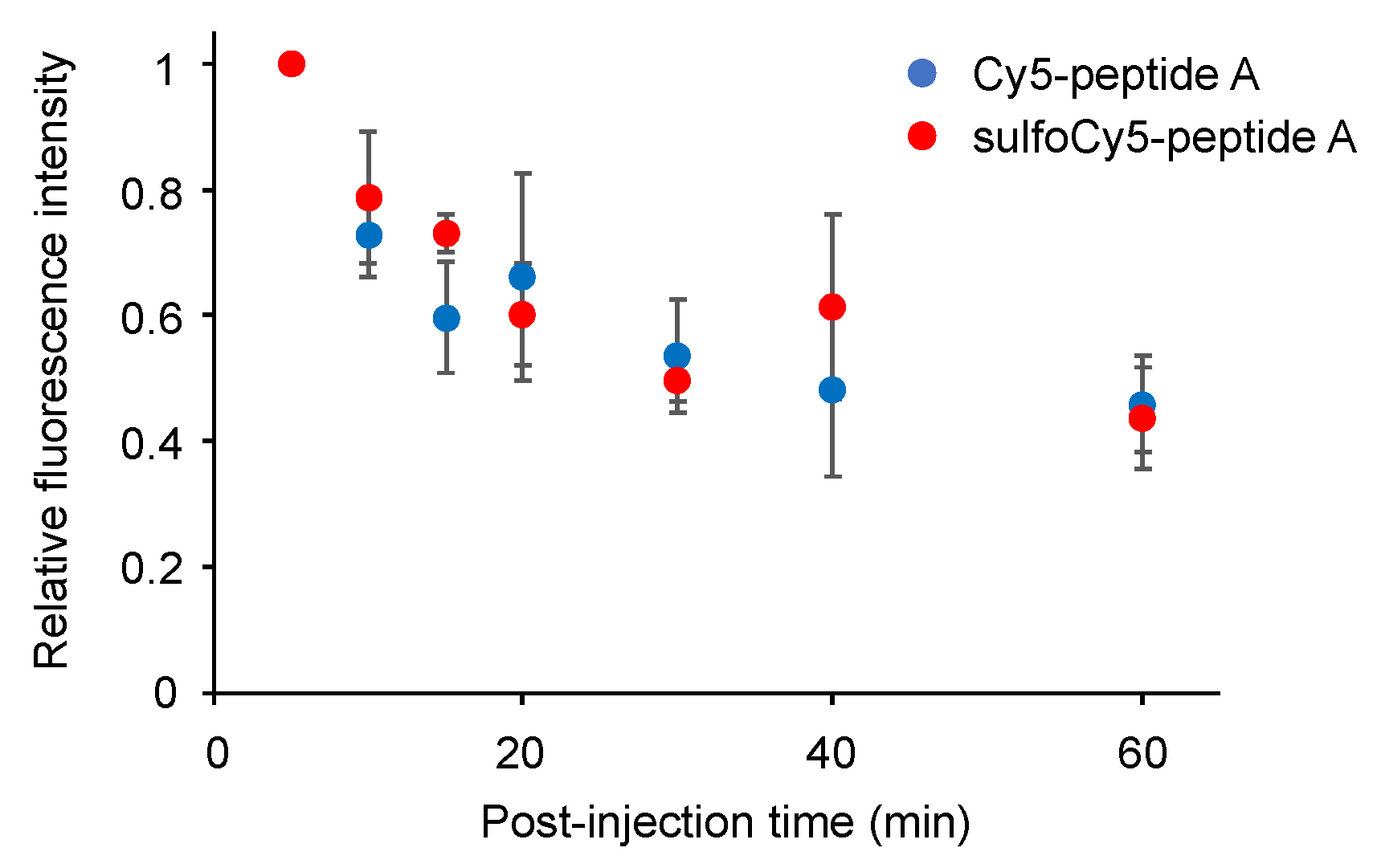
3.4. Stability of Dye-Peptide A Conjugates in Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasarao, M.; Low, P.S. Ligand-Targeted Drug Delivery. Chem. Rev. 2017, 117, 12133–12164. [Google Scholar] [CrossRef]
- Mukai, H.; Watanabe, Y. Review: PET imaging with macro- and middle-sized molecular probes. Nucl. Med. Biol. 2021, 92, 156–170. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Jiang, Z.; Guan, J.; Qian, J.; Zhan, C. Peptide ligand-mediated targeted drug delivery of nanomedicines. Biomater. Sci. 2019, 7, 461–471. [Google Scholar] [CrossRef]
- Wang, W.; Hu, Z. Targeting Peptide-Based Probes for Molecular Imaging and Diagnosis. Adv. Mater. 2019, 31, e1804827. [Google Scholar] [CrossRef]
- Böhmová, E.; Machová, D.; Pechar, M.; Pola, R.; Venclíková, K.; Janoušková, O.; Etrych, T. Cell-Penetrating Peptides: A Useful Tool for the Delivery of Various Cargoes into Cells. Physiol. Res. 2018, 67, S267–S279. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Habault, J.; Poyet, J.-L. Recent Advances in Cell Penetrating Peptide-Based Anticancer Therapies. Molecules 2019, 24, 927. [Google Scholar] [CrossRef]
- Kondo, E.; Saito, K.; Tashiro, Y.; Kamide, K.; Uno, S.; Furuya, T.; Mashita, M.; Nakajima, K.; Tsumuraya, T.; Kobayashi, N.; et al. Tumour lineage-homing cell-penetrating peptides as anticancer molecular delivery systems. Nat. Commun. 2012, 3, 951. [Google Scholar] [CrossRef]
- Maity, S.K.; Stahl, P.; Hensel, A.; Knauer, S.; Hirschhauser, C.; Schmuck, C. Cancer-cell-specific drug delivery by a tu-mor-homing cpp-gossypol conjugate employing a tracelessly cleavable linker. Chem. Eur. J. 2020, 26, 3010–3015. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Gibbs, S.L.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Liu, F.; Hyun, H.; Park, G.; Xie, Y.; Bae, S.; et al. Targeted zwitteri-onic near-infrared fluorophores for improved optical imaging. Nat. Biotechnol. 2013, 31, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Gorka, A.P.; Nagaya, T.; Michie, M.S.; Nani, R.R.; Nakamura, Y.; Coble, V.L.; Vasalatiy, O.V.; Swenson, R.E.; Choyke, P.L.; et al. Role of Fluorophore Charge on the In Vivo Optical Imaging Properties of Near-Infrared Cyanine Dye/Monoclonal Antibody Conjugates. Bioconjug. Chem. 2016, 27, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Bunschoten, A.; Van Willigen, D.M.; Buckle, T.; Berg, N.S.V.D.; Welling, M.M.; Spa, S.J.; Wester, H.-J.; Van Leeuwen, F.W.B. Tailoring Fluorescent Dyes to Optimize a Hybrid RGD-Tracer. Bioconjug. Chem. 2016, 27, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Lee, J.H.; Kang, H.; Park, G.K.; El Fakhri, G.; Choi, H.S. PSMA-targeted contrast agents for intraoperative imaging of prostate cancer. Chem. Commun. 2017, 53, 1611–1614. [Google Scholar] [CrossRef]
- Hübner, R.; Benkert, V.; Cheng, X.; Wängler, B.; Krämer, R.; Wängler, C. Probing two PESIN-indocyanine-dye-conjugates: Significance of the used fluorophore. J. Mater. Chem. B 2020, 8, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Buckle, T.; van Willigen, D.M.; Spa, S.J.; Hensbergen, A.W.; van der Wal, S.; de Korne, C.M.; Welling, M.M.; van der Poel, H.G.; Hardwick, J.C.H.; van Leeuwen, F.W.B. Tracers for fluorescence-guided surgery: How elongation of the polymethine chain in cyanine dyes alters the pharmacokinetics of a dual-modality c[rgdyk] tracer. J. Nucl. Med. 2018, 59, 986–992. [Google Scholar] [CrossRef]
- Temming, K.; Schiffelers, R.M.; Molema, G.; Kok, R.J. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist. Updates 2005, 8, 381–402. [Google Scholar] [CrossRef]
- Gaertner, F.C.; Kessler, H.; Wester, H.-J.; Schwaiger, M.; Beer, A.J. Radiolabelled RGD peptides for imaging and therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 126–138. [Google Scholar] [CrossRef]
- Liu, S. Radiolabeled cyclic rgd peptide bioconjugates as radiotracers targeting multiple integrins. Bioconjug. Chem. 2015, 26, 1413–1438. [Google Scholar] [CrossRef]
- Futaki, S.; Nakase, I. Cell-surface interactions on arginine-rich cell-penetrating peptides allow for multiplex modes of inter-nalization. Acc. Chem. Res. 2017, 50, 2449–2456. [Google Scholar] [CrossRef] [PubMed]

| Compound | Retention Time (min) 1 |
|---|---|
| Cy5-SE | 22.6 |
| sulfoCy5-SE | 9.3 |
| Cy3-SE | 20.8 |
| sulfoCy3-SE | 7.3 |
| Cy5-peptide A | 14.1 |
| sulfoCy5-peptide A | 6.8 |
| Cy3-peptide A | 12.8 |
| sulfoCy3-peptide A | 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takakura, H.; Sato, H.; Nakajima, K.; Suzuki, M.; Ogawa, M. In Vitro and In Vivo Cell Uptake of a Cell-Penetrating Peptide Conjugated with Fluorescent Dyes Having Different Chemical Properties. Cancers 2021, 13, 2245. https://doi.org/10.3390/cancers13092245
Takakura H, Sato H, Nakajima K, Suzuki M, Ogawa M. In Vitro and In Vivo Cell Uptake of a Cell-Penetrating Peptide Conjugated with Fluorescent Dyes Having Different Chemical Properties. Cancers. 2021; 13(9):2245. https://doi.org/10.3390/cancers13092245
Chicago/Turabian StyleTakakura, Hideo, Honoka Sato, Kohei Nakajima, Motofumi Suzuki, and Mikako Ogawa. 2021. "In Vitro and In Vivo Cell Uptake of a Cell-Penetrating Peptide Conjugated with Fluorescent Dyes Having Different Chemical Properties" Cancers 13, no. 9: 2245. https://doi.org/10.3390/cancers13092245
APA StyleTakakura, H., Sato, H., Nakajima, K., Suzuki, M., & Ogawa, M. (2021). In Vitro and In Vivo Cell Uptake of a Cell-Penetrating Peptide Conjugated with Fluorescent Dyes Having Different Chemical Properties. Cancers, 13(9), 2245. https://doi.org/10.3390/cancers13092245





