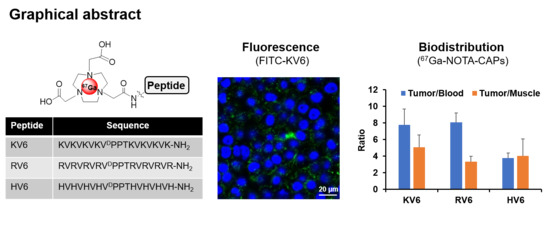
Synthesis and Characterization of Radiogallium-Labeled Cationic Amphiphilic Peptides as Tumor Imaging Agents
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
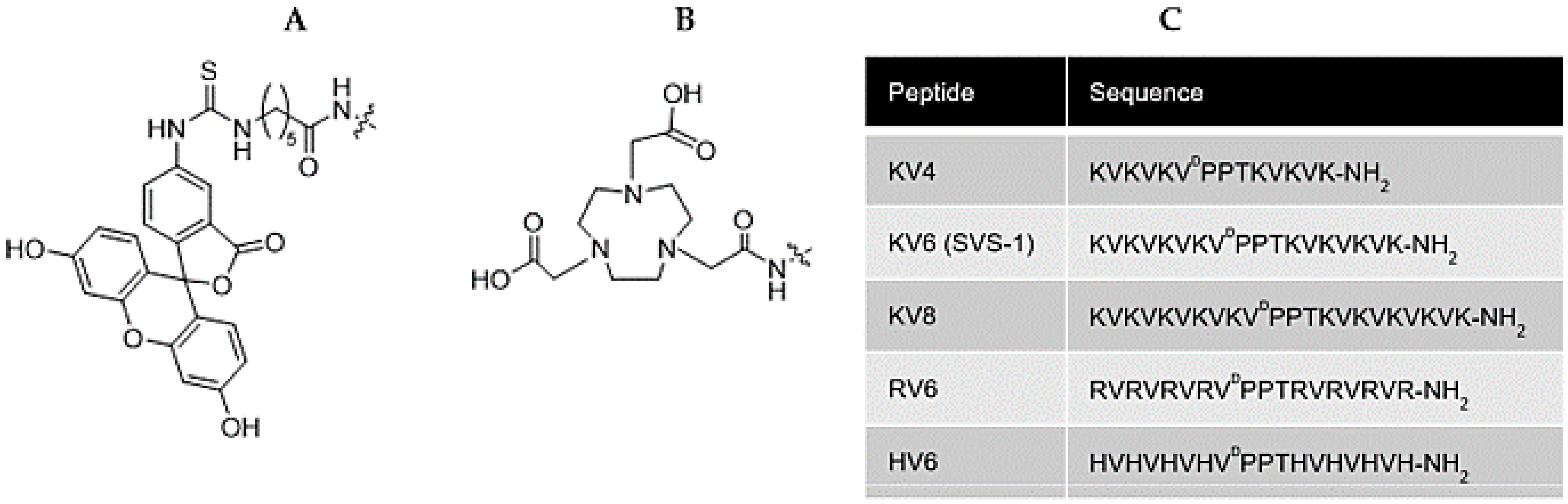
2.2. Peptide Synthesis
2.3. Synthesis of Ga-NOTA-CAPs
2.4. Synthesis of 67Ga-NOTA-CAPs
2.5. Preparation of Liposomes
2.6. Measurement of Zeta Potential of the CAP–Liposome Mixtures
2.7. CD Spectroscopy of the CAP–Liposome Mixture
2.8. In Vitro Stability of 67Ga-NOTA-CAPs in Phosphate-Buffered Saline and Mouse Plasma
2.9. Cell Cultures
2.10. Measurement of Zeta Potential of Cell Cultures
2.11. Cellular Uptake Study
2.12. Confocal Fluorescence Microscopic Imaging of Cells Exposed to 67Ga-NOTA-CAPs
2.13. Evaluation of the Internalization Pathway of CAPs
2.14. Tumor Xenograft Model
2.15. Biodistribution of 67Ga-CAPs in Tumor-Bearing Mice
2.16. Statistical Analysis
3. Results
3.1. In Vitro Binding of FITC-Labeled CAPs to Cancer Cells and Healthy Cells
3.2. Zeta Potential and CD Spectra of NOTA-CAPs in the Presence of a Lipid Membrane
3.3. Radiosynthesis and In Vitro Stability of 67Ga-NOTA-CAPs
3.4. In Vitro Cellular Uptake of 67Ga-NOTA-CAPs
3.5. Evaluation of the Internalization Pathway for CAPs
3.6. In Vivo Biodistribution Studies of 67Ga-NOTA-CAPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bevers, E.M.; Comfurius, P.; Zwaal, R.F. Regulatory mechanisms in maintenance and modulation of transmembrane lipid asymmetry: Pathophysiological implications. Lupus 1996, 5, 480–487. [Google Scholar] [CrossRef]
- Utsugi, T.; Schroit, A.J.; Connor, J.; Bucana, C.D.; Fidler, I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991, 51, 3062–3066. [Google Scholar]
- Dobrzyńska, I.; Szachowicz-Petelska, B.; Sulkowski, S.; Figaszewski, Z. Changes in electric charge and phospholipids composition in human colorectal cancer cells. Mol. Cell. Biochem. 2005, 276, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Burdick, M.D.; Harris, A.; Reid, C.J.; Iwamura, T.; Hollingsworth, M.A. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J. Biol. Chem. 1997, 272, 24198–24202. [Google Scholar] [CrossRef]
- Yoon, W.H.; Park, H.D.; Lim, K.; Hwang, B.D. Effect of O-glycosylated mucin on invasion and metastasis of HM7 human colon cancer cells. Biochem. Biophys. Res. Commun. 1996, 222, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Cazet, A.; Julien, S.; Bobowski, M.; Krzewinski-Recchi, M.A.; Harduin-Lepers, A.; Groux-Degroote, S.; Delannoy, P. Consequences of the expression of sialylated antigens in breast cancer. Carbohydr. Res. 2010, 345, 1377–1383. [Google Scholar] [CrossRef]
- Wang, F.L.; Cui, S.X.; Sun, L.P.; Qu, X.J.; Xie, Y.Y.; Zhou, L.; Mu, Y.L.; Tang, W.; Wang, Y.S. High expression of alpha 2, 3-linked sialic acid residues is associated with the metastatic potential of human gastric cancer. Cancer Detect. Prev. 2009, 32, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Park, S.M.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; England, C.G.; Chen, F.; Cai, W. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Adv. Drug Deliv. Rev. 2017, 113, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Sinthuvanich, C.; Veiga, A.S.; Gupta, K.; Gaspar, D.; Blumenthal, R.; Schneider, J.P. Anticancer beta-hairpin peptides: Membrane-induced folding triggers activity. J. Am. Chem. Soc. 2012, 134, 6210–6217. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.H.; Schneider, J.P. Cancer cell surface induced peptide folding allows intracellular translocation of drug. J. Control. Release Off. J. Control. Release Soc. 2015, 209, 317–326. [Google Scholar] [CrossRef]
- Kapur, A.; Medina, S.H.; Wang, W.; Palui, G.; Schneider, J.P.; Mattoussi, H. Intracellular Delivery of Gold Nanocolloids Promoted by a Chemically Conjugated Anticancer Peptide. ACS Omega 2018, 3, 12754–12762. [Google Scholar] [CrossRef]
- Kapur, A.; Medina, S.H.; Wang, W.; Palui, G.; Ji, X.; Schneider, J.P.; Mattoussi, H. Enhanced Uptake of Luminescent Quantum Dots by Live Cells Mediated by a Membrane-Active Peptide. ACS Omega 2018, 3, 17164–17172. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Sinthuvanich, C.; Schneider, J.P.; Castanho, M.A. Anticancer peptide SVS-1: Efficacy precedes membrane neutralization. Biochemistry 2012, 51, 6263–6265. [Google Scholar] [CrossRef][Green Version]
- Ishikawa, K.; Medina, S.H.; Schneider, J.P.; Klar, A.J.S. Glycan Alteration Imparts Cellular Resistance to a Membrane-Lytic Anticancer Peptide. Cell Chem. Biol. 2017, 24, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.H.; Miller, S.E.; Keim, A.I.; Gorka, A.P.; Schnermann, M.J.; Schneider, J.P. An Intrinsically Disordered Peptide Facilitates Non-Endosomal Cell Entry. Angew. Chem. Int. Ed. Engl. 2016, 55, 3369–3372. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Tsuji, K.; Abrams, R.P.M.; Burke, T.R.; Schneider, J.P. Uncoupling the Folding-Function Paradigm of Lytic Peptides to Deliver Impermeable Inhibitors of Intracellular Protein–Protein Interactions. J. Am. Chem. Soc. 2020, 142, 19950–19955. [Google Scholar] [CrossRef] [PubMed]
- Touchefeu, Y.; Bailly, C.; Frampas, E.; Eugène, T.; Rousseau, C.; Bourgeois, M.; Bossard, C.; Faivre-Chauvet, A.; Rauscher, A.; Masson, D.; et al. Promising clinical performance of pretargeted immuno-PET with anti-CEA bispecific antibody and gallium-68-labelled IMP-288 peptide for imaging colorectal cancer metastases: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, R.; Sharkey, R.M.; Goldenberg, D.M.; Franssen, G.; McBride, W.J.; Rossi, E.A.; Chang, C.H.; Laverman, P.; Disselhorst, J.A.; Eek, A.; et al. Pretargeted immuno-positron emission tomography imaging of carcinoembryonic antigen-expressing tumors with a bispecific antibody and a68Ga- And18F-labeled hapten peptide in mice with human tumor xenografts. Mol. Cancer Ther. 2010, 9, 1019–1027. [Google Scholar] [CrossRef]
- Iwasaki, T.; Tokuda, Y.; Kotake, A.; Okada, H.; Takeda, S.; Kawano, T.; Nakayama, Y. Cellular uptake and in vivo distribution of polyhistidine peptides. J. Control. Release 2015, 210, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Huang, Y.W.; Chiou, S.H.; Aronstam, R.S. Polyhistidine facilitates direct membrane translocation of cell-penetrating peptides into cells. Sci. Rep. 2019, 9, 9398. [Google Scholar] [CrossRef]
- Velikyan, I. 68Ga-Based radiopharmaceuticals: Production and application relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef]
- Velikyan, I. Prospective of ⁶⁸Ga-radiopharmaceutical development. Theranostics 2013, 4, 47–80. [Google Scholar] [CrossRef] [PubMed]
- Fuchigami, T.; Ono, H.; Oyadomari, K.; Iwatake, M.; Hayasaka, D.; Akbari, M.; Yui, K.; Nishi, K.; Kudo, T.; Yoshida, S.; et al. Development of a68Ge/68Ga Generator System Using Polysaccharide Polymers and Its Application in PET Imaging of Tropical Infectious Diseases. ACS Omega 2017, 2, 1400–1407. [Google Scholar] [CrossRef]
- Ebenhan, T.; Chadwick, N.; Sathekge, M.M.; Govender, P.; Govender, T.; Kruger, H.G.; Marjanovic-Painter, B.; Zeevaart, J.R. Peptide synthesis, characterization and (6)(8)Ga-radiolabeling of NOTA-conjugated ubiquicidin fragments for prospective infection imaging with PET/CT. Nucl. Med. Biol. 2014, 41, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Guérin, B.; Ait-Mohand, S.; Tremblay, M.C.; Dumulon-Perreault, V.; Fournier, P.; Bénard, F. Total solid-phase synthesis of NOTA-functionalized peptides for PET imaging. Org. Lett. 2010, 12, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Effendi, N.; Mishiro, K.; Shiba, K.; Kinuya, S.; Ogawa, K. Development of Radiogallium-Labeled Peptides for Platelet-Derived Growth Factor Receptor β (PDGFRβ) Imaging: Influence of Different Linkers. Molecules 2020, 26, 41. [Google Scholar] [CrossRef]
- Beinat, C.; Haywood, T.; Chen, Y.S.; Patel, C.B.; Alam, I.S.; Murty, S.; Gambhir, S.S. The Utility of [(18)F]DASA-23 for Molecular Imaging of Prostate Cancer with Positron Emission Tomography. Mol. Imaging Biol. 2018, 20, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Kronauge, J.F.; Noska, M.A.; Davison, A.; Holman, B.L.; Jones, A.G. Interspecies variation in biodistribution of technetium (2-carbomethoxy-2-isocyanopropane)6+. J. Nucl. Med. 1992, 33, 1357–1365. [Google Scholar] [PubMed]
- West, M.A.; Bretscher, M.S.; Watts, C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 1989, 109, 2731–2739. [Google Scholar] [CrossRef]
- Anderson, H.A.; Chen, Y.; Norkin, L.C. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 1996, 7, 1825–1834. [Google Scholar] [CrossRef]
- Prata, M.I.; Santos, A.C.; Geraldes, C.F.; de Lima, J.J. Structural and in vivo studies of metal chelates of Ga(III) relevant to biomedical imaging. J. Inorg. Biochem. 2000, 79, 359–363. [Google Scholar] [CrossRef][Green Version]
- Reid, K.A.; Davis, C.M.; Dyer, R.B.; Kindt, J.T. Binding, folding and insertion of a beta-hairpin peptide at a lipid bilayer surface: Influence of electrostatics and lipid tail packing. Biochim. Biophys. Acta Biomembr. 2018, 1860, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Wu, C.S.; Yang, J.T. Circular dichroic analysis of protein conformation: Inclusion of the beta-turns. Anal. Biochem. 1978, 91, 13–31. [Google Scholar] [CrossRef]
- Delehanty, J.B.; Medintz, I.L.; Pons, T.; Brunel, F.M.; Dawson, P.E.; Mattoussi, H. Self-Assembled Quantum Dot-Peptide Bioconjugates for Selective Intracellular Delivery. Bioconjugate Chem. 2006, 17, 920–927. [Google Scholar] [CrossRef]
- Heuser, J.E.; Anderson, R.G. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 1989, 108, 389–400. [Google Scholar] [CrossRef]
- Liscano, Y.; Oñate-Garzón, J.; Delgado, J.P. Peptides with dual antimicrobial–anticancer activity: Strategies to overcome peptide limitations and rational design of anticancer peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Jia, X.L.; Qi, J.L.; Yang, L.P.; Sun, W.H.; Yan, X.; Yang, S.K.; Cao, D.Y.; Du, Q.; Qi, X.R. Enhancing siRNA-based cancer therapy using a new pH-responsive activatable cell-penetrating peptide-modified liposomal system. Int. J. Nanomed. 2017, 12, 2385–2405. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ma, Y.; Zhang, W.; Li, L.; Zhang, Y.; Zhang, L.; Liu, H.; Ni, J.; Wang, R. Design of new acid-activated cell-penetrating peptides for tumor drug delivery. PeerJ 2017, 5, e3429. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Niwa, M.; Takeuchi, T.; Sonomura, K.; Kawabata, N.; Koike, Y.; Takehashi, M.; Tanaka, S.; Ueda, K.; Simpson, J.C.; et al. Cellular uptake of arginine-rich peptides: Roles for macropinocytosis and actin rearrangement. Mol. Ther. J. Am. Soc. Gene Ther. 2004, 10, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Downes, A.; Thorpe, P.E. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002, 62, 6132–6140. [Google Scholar]
- Sarko, D.; Beijer, B.; Garcia Boy, R.; Nothelfer, E.-M.; Leotta, K.; Eisenhut, M.; Altmann, A.; Haberkorn, U.; Mier, W. The Pharmacokinetics of Cell-Penetrating Peptides. Mol. Pharm. 2010, 7, 2224–2231. [Google Scholar] [CrossRef]
- Jae, M.J.; Mee, K.H.; Young, S.C.; Lee, Y.S.; Young, J.K.; Gi, J.C.; Dong, S.L.; Chung, J.K.; Myung, C.L. Preparation of a promising angiogenesis PET imaging agent: 68Ga-labeled c(RGDyK)-isothiocyanatobenzyl-1,4,7-triazacyclononane-1, 4,7-triacetic acid and feasibility studies in mice. J. Nucl. Med. 2008, 49, 830–836. [Google Scholar] [CrossRef]
- Gourni, E.; Demmer, O.; Schottelius, M.; D’Alessandria, C.; Schulz, S.; Dijkgraaf, I.; Schumacher, U.; Schwaiger, M.; Kessler, H.; Wester, H.J. PET of CXCR4 expression by a 68Ga-labeled highly specific targeted contrast agent. J. Nucl. Med. 2011, 52, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, J.; Rejtar, T.; Sawant, R.; Wang, Z.; Torchilin, V.P. TAT Peptide and Its Conjugates: Proteolytic Stability. Bioconjugate Chem. 2009, 20, 1531–1537. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchigami, T.; Chiga, T.; Yoshida, S.; Oba, M.; Fukushima, Y.; Inoue, H.; Matsuura, A.; Toriba, A.; Nakayama, M. Synthesis and Characterization of Radiogallium-Labeled Cationic Amphiphilic Peptides as Tumor Imaging Agents. Cancers 2021, 13, 2388. https://doi.org/10.3390/cancers13102388
Fuchigami T, Chiga T, Yoshida S, Oba M, Fukushima Y, Inoue H, Matsuura A, Toriba A, Nakayama M. Synthesis and Characterization of Radiogallium-Labeled Cationic Amphiphilic Peptides as Tumor Imaging Agents. Cancers. 2021; 13(10):2388. https://doi.org/10.3390/cancers13102388
Chicago/Turabian StyleFuchigami, Takeshi, Takeshi Chiga, Sakura Yoshida, Makoto Oba, Yu Fukushima, Hiromi Inoue, Akari Matsuura, Akira Toriba, and Morio Nakayama. 2021. "Synthesis and Characterization of Radiogallium-Labeled Cationic Amphiphilic Peptides as Tumor Imaging Agents" Cancers 13, no. 10: 2388. https://doi.org/10.3390/cancers13102388
APA StyleFuchigami, T., Chiga, T., Yoshida, S., Oba, M., Fukushima, Y., Inoue, H., Matsuura, A., Toriba, A., & Nakayama, M. (2021). Synthesis and Characterization of Radiogallium-Labeled Cationic Amphiphilic Peptides as Tumor Imaging Agents. Cancers, 13(10), 2388. https://doi.org/10.3390/cancers13102388