Targeting BTK Signaling in the Microenvironment of Solid Tumors as a Feasible Cancer Therapy Option
Abstract
Simple Summary
Abstract
1. Introduction
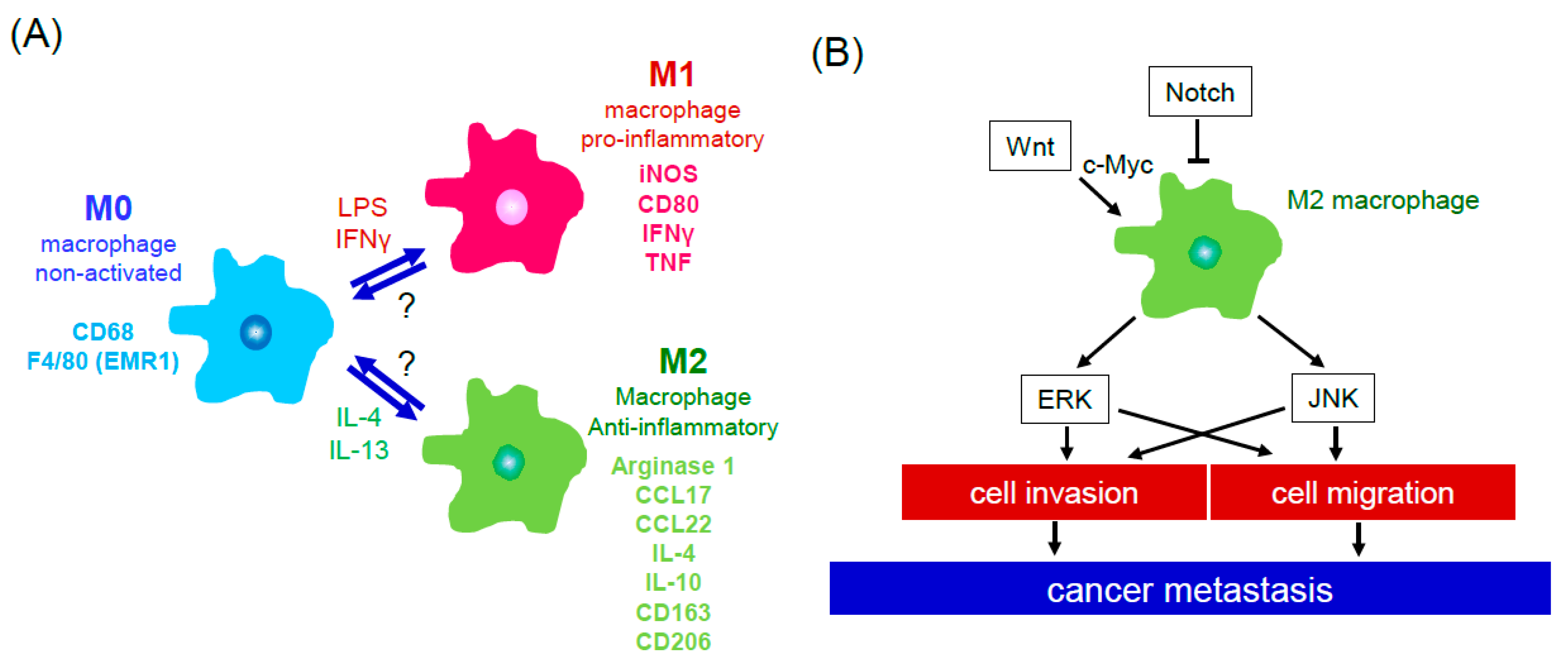
2. Cells of the Tumor Microenvironment
3. Cell Signaling in Tumor Microenvironment
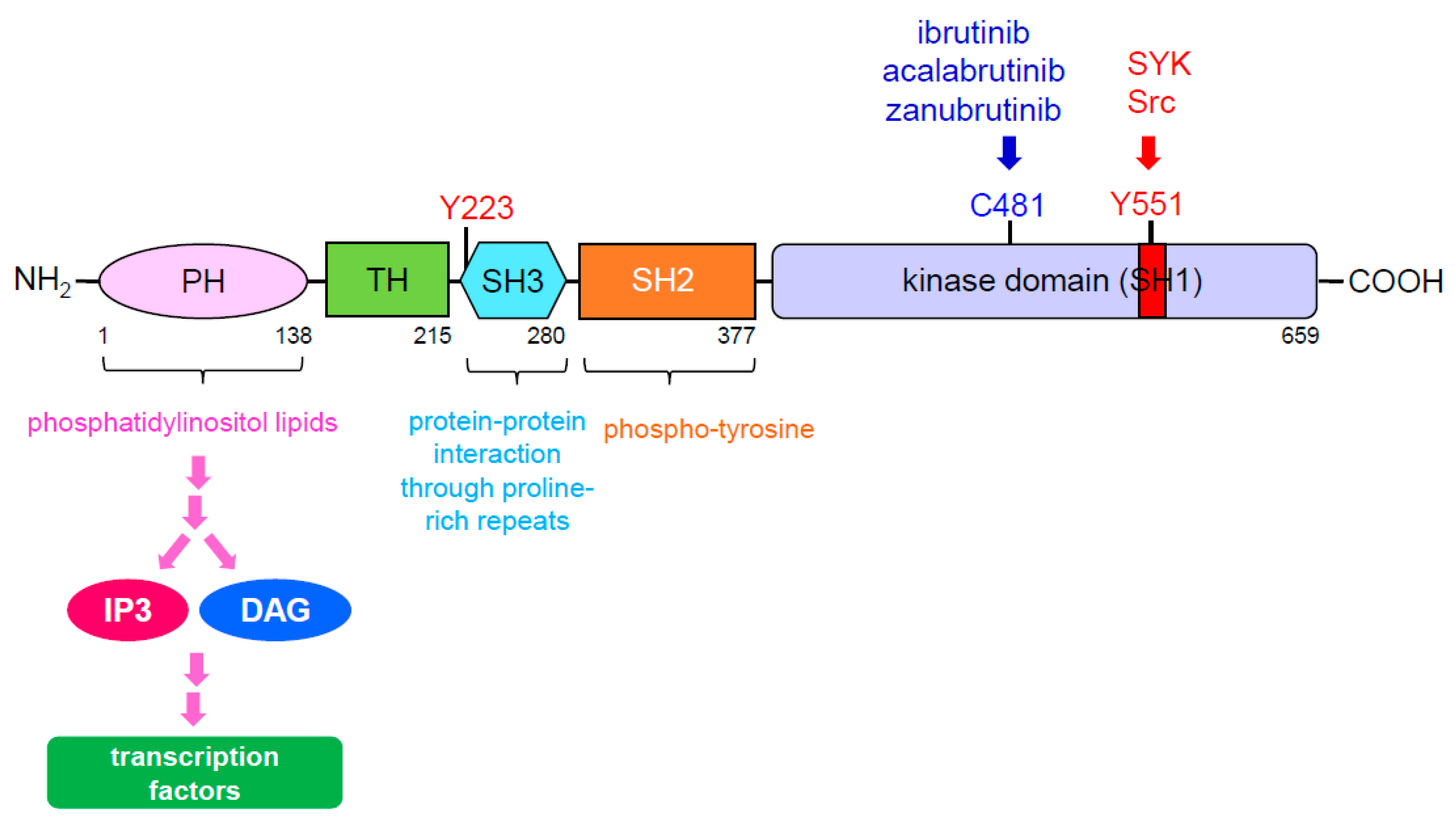
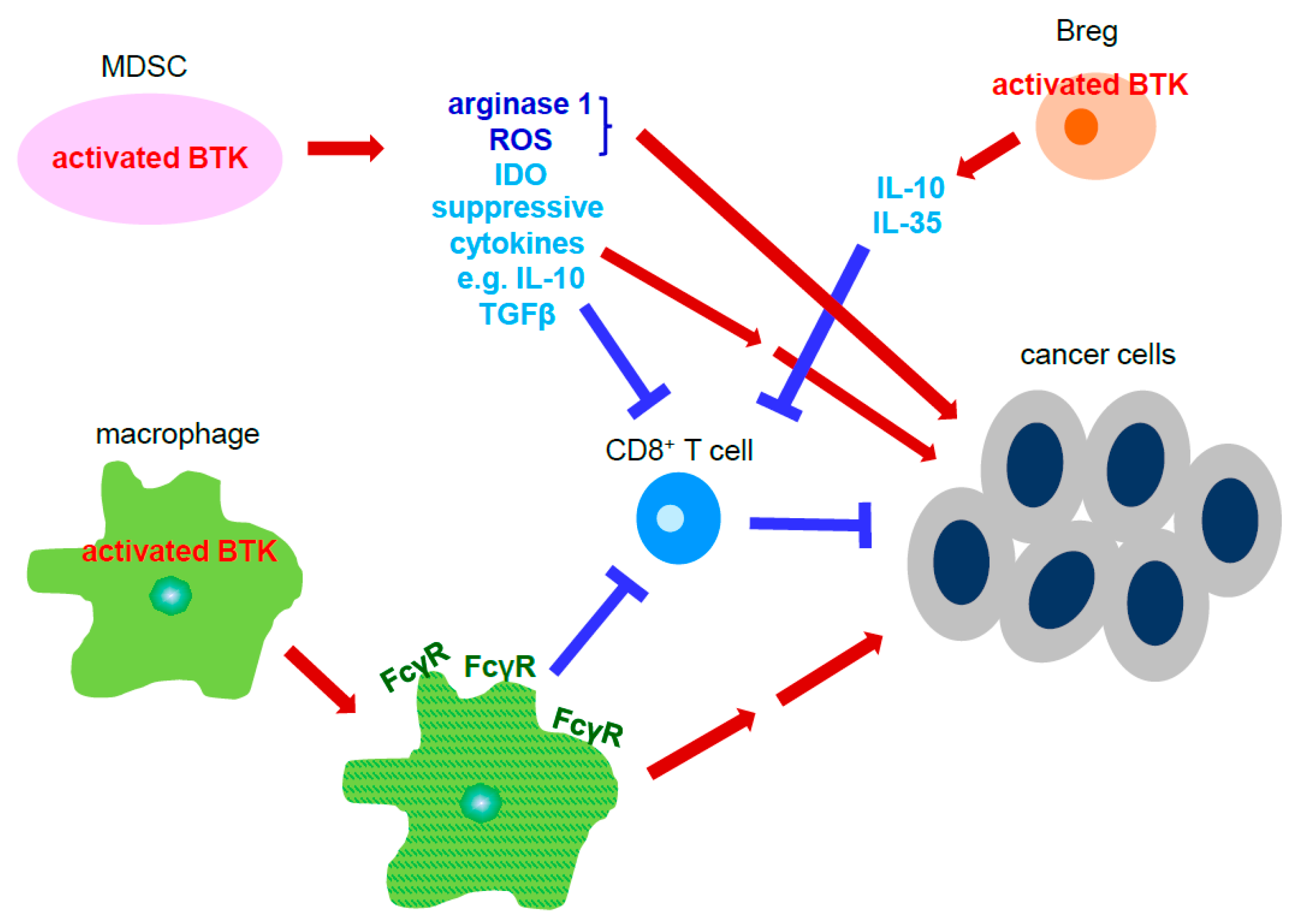
4. BTK Signaling
5. BTK Inhibitors and Their Clinic Application for Cancer Therapy
6. Concluding Remarks
Funding
Conflicts of Interest
References
- Catalano, V.; Turdo, A.; di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and Its Microenvironment: A Synergistic Interplay. In Seminars in Cancer Biology; Elsevier: London, UK, 2013; Volume 23, pp. 522–532. [Google Scholar]
- Ozdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef]
- Whiteside, T.L. The Tumor Microenvironment and Its Role in Promoting Tumor Growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Vetrie, D.; Vorechovsky, I.; Sideras, P.; Holland, J.; Davies, A.; Flinter, F.; Hammarstrom, L.; Kinnon, C.; Levinsky, R.; Bobrow, M.; et al. The Gene Involved in X-linked Agammaglobulinaemia is a Member of the SRC Family of Protein-Tyrosine Kinases. Nature 1993, 361, 226–233. [Google Scholar] [CrossRef]
- Tsukada, S.; Saffran, D.C.; Rawlings, D.J.; Parolini, O.; Allen, R.C.; Klisak, I.; Sparkes, R.S.; Kubagawa, H.; Mohandas, T.; Quan, S.; et al. Deficient Expression of a B Cell Cytoplasmic Tyrosine Kinase in Human X-linked Agammaglobulinemia. Cell 1993, 72, 279–290. [Google Scholar] [CrossRef]
- Bauer, J.; Emon, M.A.B.; Staudacher, J.J.; Thomas, A.L.; Zessner-Spitzenberg, J.; Mancinelli, G.; Krett, N.; Saif, M.T.; Jung, B. Increased Stiffness of the Tumor Microenvironment in Colon Cancer Stimulates Cancer Associated Fibroblast-Mediated Prometastatic Activin a Signaling. Sci. Rep. 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pei, H.; Tan, F. Matrix Stiffness and Colorectal Cancer. OncoTargets Ther. 2020, 13, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Day, S.D.; Enos, R.T.; McClellan, J.L.; Steiner, J.L.; Velazquez, K.T.; Murphy, E.A. Linking Inflammation to Tumorigenesis in a Mouse Model of High-Fat-Diet-Enhanced Colon Cancer. Cytokine 2013, 64, 454–462. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbuhler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFalpha and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef]
- Barcellos-de-Souza, P.; Comito, G.; Pons-Segura, C.; Taddei, M.L.; Gori, V.; Becherucci, V.; Bambi, F.; Margheri, F.; Laurenzana, A.; Del Rosso, M.; et al. Mesenchymal Stem Cells are Recruited and Activated into Carcinoma-Associated Fibroblasts by Prostate Cancer Microenvironment-Derived TGF-beta1. Stem Cells 2016, 34, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qian, J.; Zeng, F.; Li, S.; Guo, W.; Chen, L.; Li, G.; Zhang, Z.; Wang, Q.J.; Deng, F.; et al. Protein Kinase Ds Promote Tumor Angiogenesis through Mast Cell Recruitment and Expression of Angiogenic Factors in Prostate Cancer Microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, T.; Wang, Z. Analysis of Non-Small Cell Lung Cancer Microenvironment Indicates Preponderance of T Cell Exhaustion Marker Expression. Exp. Cell Res. 2017, 360, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, I.; Stelmaszczyk-Emmel, A.; Polubiec-Kownacka, M.; Dziedzic, D.; Domagala-Kulawik, J. Elevated Regulatory T Cells, Surface and Intracellular CTLA-4 Expression and Interleukin-17 in the Lung Cancer Microenvironment in Humans. Cancer Immunol. Immunother. 2017, 66, 161–170. [Google Scholar] [CrossRef]
- Faget, J.; Groeneveld, S.; Boivin, G.; Sankar, M.; Zangger, N.; Garcia, M.; Guex, N.; Zlobec, I.; Steiner, L.; Piersigilli, A.; et al. Neutrophils and Snail Orchestrate the Establishment of a Pro-tumor Microenvironment in Lung Cancer. Cell Rep. 2017, 21, 3190–3204. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Cheng, H.C.; Wang, J.; Wang, S.W.; Tai, H.C.; Lin, C.W.; Tang, C.H. Prostate Cancer-Derived CCN3 Induces M2 Macrophage Infiltration and Contributes to Angiogenesis in Prostate Cancer Microenvironment. Oncotarget 2014, 5, 1595–1608. [Google Scholar] [CrossRef]
- Mantovani, A.; Germano, G.; Marchesi, F.; Locatelli, M.; Biswas, S.K. Cancer-Promoting Tumor-Associated Macrophages: New Vistas and Open Questions. Eur. J. Immunol. 2011, 41, 2522–2525. [Google Scholar] [CrossRef]
- Mantovani, A.; Schioppa, T.; Porta, C.; Allavena, P.; Sica, A. Role of Tumor-Associated Macrophages in Tumor Progression and Invasion. Cancer Metastasis Rev. 2006, 25, 315–322. [Google Scholar] [CrossRef]
- Puig-Kroger, A.; Sierra-Filardi, E.; Dominguez-Soto, A.; Samaniego, R.; Corcuera, M.T.; Gomez-Aguado, F.; Ratnam, M.; Sanchez-Mateos, P.; Corbi, A.L. Folate Receptor Beta is Expressed by Tumor-Associated Macrophages and Constitutes a Marker for M2 Anti-Inflammatory/Regulatory Macrophages. Cancer Res. 2009, 69, 9395–9403. [Google Scholar] [CrossRef]
- De Robertis, M.; Massi, E.; Poeta, M.L.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V.M. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011, 10, 9. [Google Scholar]
- Dong, P.; Ma, L.; Liu, L.; Zhao, G.; Zhang, S.; Dong, L.; Xue, R.; Chen, S. CD86(+)/CD206(+), Diametrically Polarized Tumor-Associated Macrophages, Predict Hepatocellular Carcinoma Patient Prognosis. Int. J. Mol. Sci. 2016, 17, 320. [Google Scholar] [CrossRef]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The Cellular and Molecular Origin of Tumor-Associated Macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, L.; Lai, W.; Zeng, Y.; Xu, H.; Lan, Q.; Su, P.; Chu, Z. Interaction with Tumorassociated Macrophages Promotes PRL3induced Invasion of Colorectal Cancer Cells via MAPK Pathwayinduced EMT and NFkappaB Signalinginduced Angiogenesis. Oncol. Rep. 2019, 41, 2790–2802. [Google Scholar]
- Yang, Y.; Ye, Y.C.; Chen, Y.; Zhao, J.L.; Gao, C.C.; Han, H.; Liu, W.C.; Qin, H.Y. Crosstalk between Hepatic Tumor Cells and Macrophages via Wnt/Beta-Catenin Signaling Promotes M2-Like Macrophage Polarization and Reinforces Tumor Malignant Behaviors. Cell Death Dis. 2018, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef]
- Donkor, M.K.; Sarkar, A.; Savage, P.A.; Franklin, R.A.; Johnson, L.K.; Jungbluth, A.A.; Allison, J.P.; Li, M.O. T Cell Surveillance of Oncogene-Induced Prostate Cancer is Impeded by T Cell-Derived TGF-Beta1 Cytokine. Immunity 2011, 35, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Gabhann, J.; Hams, E.; Smith, S.; Wynne, C.; Byrne, J.C.; Brennan, K.; Spence, S.; Kissenpfennig, A.; Johnston, J.A.; Fallon, P.G.; et al. Btk Regulates Macrophage Polarization in Response to Lipopolysaccharide. PLoS ONE 2014, 9, e85834. [Google Scholar] [CrossRef]
- Singh, S.P.; Dammeijer, F.; Hendriks, R.W. Role of Bruton’s Tyrosine Kinase in B Cells and Malignancies. Mol. Cancer 2018, 17, 57. [Google Scholar] [CrossRef]
- Park, H.; Wahl, M.I.; Afar, D.E.; Turck, C.W.; Rawlings, D.J.; Tam, C.; Scharenberg, A.M.; Kinet, J.P.; Witte, O.N. Regulation of Btk Function by a Major Autophosphorylation Site within the SH3 Domain. Immunity 1996, 4, 515–525. [Google Scholar] [CrossRef]
- Rawlings, D.J.; Scharenberg, A.M.; Park, H.; Wahl, M.I.; Lin, S.; Kato, R.M.; Fluckiger, A.-C.; Witte, O.N.; Kinet, J.-P. Activation of BTK by a Phosphorylation Mechanism Initiated by SRC Family Kinases. Science 1996, 271, 822–825. [Google Scholar] [CrossRef]
- Solvason, N.; Wu, W.W.; Kabra, N.; Lund-Johansen, F.; Roncarolo, M.G.; Behrens, T.W.; Grillot, D.A.; Nunez, G.; Lees, E.; Howard, M.; et al. Transgene Expression of bcl-xL Permits Anti-Immunoglobulin (Ig)-Induced Proliferation in Xid B Cells. J. Exp. Med. 1998, 187, 1081–1091. [Google Scholar] [CrossRef]
- Craxton, A.; Jiang, A.; Kurosaki, T.; Clark, E.A. Syk and Bruton’s Tyrosine Kinase are Required for B Cell Antigen Receptor-Mediated Activation of the Kinase Akt. J. Biol. Chem. 1999, 274, 30644–30650. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Ngo, V.N.; Ekland, E.H.; Forster, R.; Lipp, M.; Littman, D.R.; Cyster, J.G. Chemokine Requirements for B Cell Entry to Lymph Nodes and Peyer’s Patches. J. Exp. Med. 2002, 196, 65–75. [Google Scholar] [CrossRef] [PubMed]
- De Gorter, D.J.; Beuling, E.A.; Kersseboom, R.; Middendorp, S.; van Gils, J.M.; Hendriks, R.W.; Pals, S.T.; Spaargaren, M. Bruton’s Tyrosine Kinase and Phospholipase Cgamma2 Mediate Chemokine-Controlled B Cell Migration and Homing. Immunity 2007, 26, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Alugupalli, K.R.; Akira, S.; Lien, E.; Leong, J.M. MyD88-and Bruton’s Tyrosine Kinase-Mediated Signals are Essential for T Cell-Independent Pathogen-Specific IgM Responses. J. Immunol. 2007, 178, 3740–3749. [Google Scholar] [CrossRef]
- Mitsuiki, N.; Yang, X.; Bartol, S.J.; Grosserichter-Wagener, C.; Kosaka, Y.; Takada, H.; Imai, K.; Kanegane, H.; Mizutani, S.; van der Burg, M.; et al. Mutations in Bruton’s Tyrosine Kinase Impair IgA Responses. Int. J. Hematol. 2015, 101, 305–313. [Google Scholar] [CrossRef]
- Ng, Y.S.; Wardemann, H.; Chelnis, J.; Cunningham-Rundles, C.; Meffre, E. Bruton’s Tyrosine Kinase is Essential for Human B Cell Tolerance. J. Exp. Med. 2004, 200, 927–934. [Google Scholar] [CrossRef]
- Rajaiya, J.; Hatfield, M.; Nixon, J.C.; Rawlings, D.J.; Webb, C.F. Bruton’s Tyrosine Kinase Regulates Immunoglobulin Promoter Activation in Association with the Transcription Factor Bright. Mol. Cell Biol. 2005, 25, 2073–2084. [Google Scholar] [CrossRef]
- Mundy-Bosse, B.L.; Lesinski, G.B.; Jaime-Ramirez, A.C.; Benninger, K.; Khan, M.; Kuppusamy, P.; Guenterberg, K.; Kondadasula, S.V.; Chaudhury, A.R.; La Perle, K.M.; et al. Myeloid-Derived Suppressor Cell Inhibition of the IFN Response in Tumor-Bearing Mice. Cancer Res. 2011, 71, 5101–5110. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Kaneda, M.M.; Tsujikawa, T.; Nguyen, A.V.; Affara, N.I.; Ruffell, B.; Gorjestani, S.; Liudahl, S.M.; Truitt, M.; Olson, P.; et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 2016, 6, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Bar-Sagi, D. BTK Signaling Drives CD1d(hi)CD5(+) Regulatory B-Cell Differentiation to Promote Pancreatic Carcinogenesis. Oncogene 2019, 38, 3316–3324. [Google Scholar] [CrossRef]
- Kawakami, Y.; Inagaki, N.; Salek-Ardakani, S.; Kitaura, J.; Tanaka, H.; Nagao, K.; Kawakami, Y.; Xiao, W.; Nagai, H.; Croft, M.; et al. Regulation of Dendritic Cell Maturation and Function by Bruton’s Tyrosine Kinase via IL-10 and Stat3. Proc. Natl. Acad. Sci. USA 2006, 103, 153–158. [Google Scholar] [CrossRef]
- Natarajan, G.; Oghumu, S.; Terrazas, C.; Varikuti, S.; Byrd, J.C.; Satoskar, A.R. A Tec Kinase BTK Inhibitor Ibrutinib Promotes Maturation and Activation of Dendritic Cells. Oncoimmunology 2016, 5, e1151592. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Kohli, P.B.; Katewa, A.; Gogol, E.; Belmont, L.D.; Choy, R.; Penuel, E.; Burton, L.; Eigenbrot, C.; Yu, C.; et al. Battling Btk Mutants With Noncovalent Inhibitors That Overcome Cys481 and Thr474 Mutations. ACS Chem. Biol. 2016, 11, 2897–2907. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Tsui, S.T.; Liu, D. Second-Generation Inhibitors of Bruton Tyrosine Kinase. J. Hematol. Oncol. 2016, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R. Ibrutinib (PCI-32765), the First BTK (Bruton’s Tyrosine Kinase) Inhibitor in Clinical Trials. Curr. Hematol. Malig. Rep. 2013, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Buggy, J.J. Bruton Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765). Leuk. Lymphoma 2013, 54, 2385–2391. [Google Scholar] [CrossRef]
- Sivina, M.; Kreitman, R.J.; Arons, E.; Ravandi, F.; Burger, J.A. The Bruton Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Blocks Hairy Cell Leukaemia Survival, Proliferation and B Cell Receptor Signalling: A New Therapeutic Approach. Br. J. Haematol. 2014, 166, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, L.A.; Smith, A.M.; Sirisawad, M.; Verner, E.; Loury, D.; Chang, B.; Li, S.; Pan, Z.; Thamm, D.H.; Miller, R.A.; et al. The Bruton Tyrosine Kinase Inhibitor PCI-32765 Blocks B-Cell Activation and is Efficacious in Models of Autoimmune Disease and B-Cell Malignancy. Proc. Natl. Acad. Sci. USA 2010, 107, 13075–13080. [Google Scholar] [CrossRef]
- Herman, S.E.; Gordon, A.L.; Hertlein, E.; Ramanunni, A.; Zhang, X.; Jaglowski, S.; Flynn, J.; Jones, J.; Blum, K.A.; Buggy, J.J.; et al. Bruton Tyrosine Kinase Represents a Promising Therapeutic Target for Treatment of Chronic Lymphocytic Leukemia and is Effectively Targeted by PCI-32765. Blood 2011, 117, 6287–6296. [Google Scholar] [CrossRef]
- De Rooij, M.F.; Kuil, A.; Geest, C.R.; Eldering, E.; Chang, B.Y.; Buggy, J.J.; Pals, S.T.; Spaargaren, M. The Clinically Active BTK Inhibitor PCI-32765 Targets B-Cell Receptor-and Chemokine-Controlled Adhesion and Migration in Chronic Lymphocytic Leukemia. Blood 2012, 119, 2590–2594. [Google Scholar] [CrossRef]
- Ponader, S.; Chen, S.S.; Buggy, J.J.; Balakrishnan, K.; Gandhi, V.; Wierda, W.G.; Keating, M.J.; O’Brien, S.; Chiorazzi, N.; Burger, J.A.; et al. The Bruton Tyrosine Kinase Inhibitor PCI-32765 Thwarts Chronic Lymphocytic Leukemia Cell Survival and Tissue Homing in Vitro and in Vivo. Blood 2012, 119, 1182–1189. [Google Scholar] [CrossRef]
- Thorp, B.C.; Badoux, X. Atrial Fibrillation as a Complication of Ibrutinib Therapy: Clinical Features and Challenges of Management. Leuk. Lymphoma. 2018, 59, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Kriegsmann, K.; Kriegsmann, M.; Witzens-Harig, M. Acalabrutinib, A Second-Generation Bruton’s Tyrosine Kinase Inhibitor. Recent Results Cancer Res. 2018, 212, 285–294. [Google Scholar] [PubMed]
- Wu, J.; Zhang, M.; Liu, D. Acalabrutinib (ACP-196): A Selective Second-Generation BTK Inhibitor. J. Hematol. Oncol. 2016, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Harrington, B.; O’Brien, S.; Jones, J.A.; Schuh, A.; Devereux, S.; Chaves, J.; Wierda, W.G.; Awan, F.T.; Brown, J.R.; et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Heather, B.K.H.; Gardner, L.; Raquel, I.; Ahmed, H.; Allard, K.; Bart, V.L.; Cheryl, A.; London, J.C.B.; Amy, J.J.; William, C.K. ACP-196: A Second Generation Btk Inhibitor Demonstrates Biological Activity in a Canine Model of B-Cell Non-Hodgkin Lymphoma. In Proceedings of the 105th Annual Meeting of the American Association for Cancer Research, San Diego, CA, USA, 5–9 April 2014; Volume 74. [Google Scholar]
- Niemann, C.U.; Montraveta, A.; Herman, S.E.M.; Ingallinera, T.; Barf, T.; Colomer, D.; Wiestner, A. The Novel Bruton’s Tyrosine Kinase Inhibitor ACP-196 Shows in Vivo Efficacy Against Human Chronic Lymphocytic Leukemia Cells Xenografted to the NSG Mouse Model. Cancer Res. 2014, 74, 2624. [Google Scholar]
- Herman, S.E.; Sun, X.; McAuley, E.M.; Hsieh, M.M.; Pittaluga, S.; Raffeld, M.; Liu, D.; Keyvanfar, K.; Chapman, C.M.; Chen, J.; et al. Modeling Tumor-Host Interactions of Chronic Lymphocytic Leukemia in Xenografted Mice to Study Tumor Biology and Evaluate Targeted Therapy. Leukemia 2013, 27, 2311–2321. [Google Scholar] [CrossRef]
- Herman, S.E.M.; Montraveta, A.; Niemann, C.U.; Mora-Jensen, H.; Gulrajani, M.; Krantz, F.; Mantel, R.; Smith, L.L.; McClanahan, F.; Harrington, B.K.; et al. The Bruton Tyrosine Kinase (BTK) Inhibitor Acalabrutinib Demonstrates Potent On-Target Effects and Efficacy in Two Mouse Models of Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2017, 23, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Lucas, D.M.; Muthusamy, N.; Smith, L.L.; Edwards, R.B.; De Lay, M.D.; Croce, C.M.; Grever, M.R.; Byrd, J.C. Characterization of the TCL-1 Transgenic Mouse as a Preclinical Drug Development Tool for Human Chronic Lymphocytic Leukemia. Blood 2006, 108, 1334–1338. [Google Scholar] [CrossRef]
- Skarzynski, M.; Niemann, C.U.; Lee, Y.S.; Martyr, S.; Maric, I.; Salem, D.; Stetler-Stevenson, M.; Marti, G.E.; Calvo, K.R.; Yuan, C.; et al. Interactions between Ibrutinib and Anti-CD20 Antibodies: Competing Effects on the Outcome of Combination Therapy. Clin. Cancer Res. 2016, 22, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Varikuti, S.; Singh, B.; Volpedo, G.; Ahirwar, D.K.; Jha, B.K.; Saljoughian, N.; Viana, A.G.; Verma, C.; Hamza, O.; Halsey, G.; et al. Ibrutinib Treatment Inhibits Breast Cancer Progression and Metastasis by Inducing Conversion of Myeloid-Derived Suppressor Cells to Dendritic Cells. Br. J. Cancer 2020, 122, 1005–1013. [Google Scholar] [CrossRef]
- Hong, D.; Rasco, D.; Veeder, M.; Luke, J.J.; Chandler, J.; Balmanoukian, A.; George, T.J.; Munster, P.; Berlin, J.D.; Gutierrez, M.; et al. A Phase 1b/2 Study of the Bruton Tyrosine Kinase Inhibitor Ibrutinib and the PD-L1 Inhibitor Durvalumab in Patients with Pretreated Solid Tumors. Oncology 2019, 97, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.; Javle, M.; Davis, R.E.; Vats, P.; Kumar-Sinha, C.; Xiao, L.; Mettu, N.B.; Parra, E.R.; Benson, A.B.; Lopez, C.D.; et al. Randomized Phase II Study of the Bruton Tyrosine Kinase Inhibitor Acalabrutinib, Alone or with Pembrolizumab in Patients with Advanced Pancreatic Cancer. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.; Oh, D.Y.; Tabernero, J.; Reni, M.; Van Cutsem, E.; Hendifar, A.; Waldschmidt, D.T.; Starling, N.; Bachet, J.B.; Chang, H.M.; et al. Ibrutinib in Combination with Nab-Paclitaxel and Gemcitabine for First-Line Treatment of Patients with Metastatic Pancreatic Adenocarcinoma: Phase III RESOLVE Study. Ann. Oncol. 2021, 32, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Harrison, M.R.; O’Donnell, P.H.; Alva, A.S.; Hahn, N.M.; Appleman, L.J.; Cetnar, J.; Burke, J.M.; Fleming, M.T.; Milowsky, M.I.; et al. A Randomized Phase 2 Trial of Pembrolizumab Versus Pembrolizumab and Acalabrutinib in Patients with Platinum-Resistant Metastatic Urothelial Cancer. Cancer 2020, 126, 4485–4497. [Google Scholar] [CrossRef] [PubMed]
| BTK Inhibitor | Study Type | Cancer Type |
|---|---|---|
| ibrutinib | in vitro | B cell lymphoma |
| chronic lymphocytic leukemia | ||
| pancreatic cancer | ||
| in vivo | chronic lymphocytic leukemia | |
| pancreatic cancer | ||
| colorectal cancer | ||
| breast cancer | ||
| acalabrutinib | in vitro | chronic lymphocytic leukemia |
| in vivo | B cell lymphoma | |
| chronic lymphocytic leukemia | ||
| pancreatic cancer * | ||
| urothelial cancer * | ||
| tirabrutinib | in vivo | pancreatic cancer |
| zanubrutinib | in vivo | mantle cell lymphoma * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messex, J.K.; Liou, G.-Y. Targeting BTK Signaling in the Microenvironment of Solid Tumors as a Feasible Cancer Therapy Option. Cancers 2021, 13, 2198. https://doi.org/10.3390/cancers13092198
Messex JK, Liou G-Y. Targeting BTK Signaling in the Microenvironment of Solid Tumors as a Feasible Cancer Therapy Option. Cancers. 2021; 13(9):2198. https://doi.org/10.3390/cancers13092198
Chicago/Turabian StyleMessex, Justin K., and Geou-Yarh Liou. 2021. "Targeting BTK Signaling in the Microenvironment of Solid Tumors as a Feasible Cancer Therapy Option" Cancers 13, no. 9: 2198. https://doi.org/10.3390/cancers13092198
APA StyleMessex, J. K., & Liou, G.-Y. (2021). Targeting BTK Signaling in the Microenvironment of Solid Tumors as a Feasible Cancer Therapy Option. Cancers, 13(9), 2198. https://doi.org/10.3390/cancers13092198






