Clinical Effectiveness of Oncological Treatment in Metastatic Colorectal Cancer Is Independent of Comorbidities and Age
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
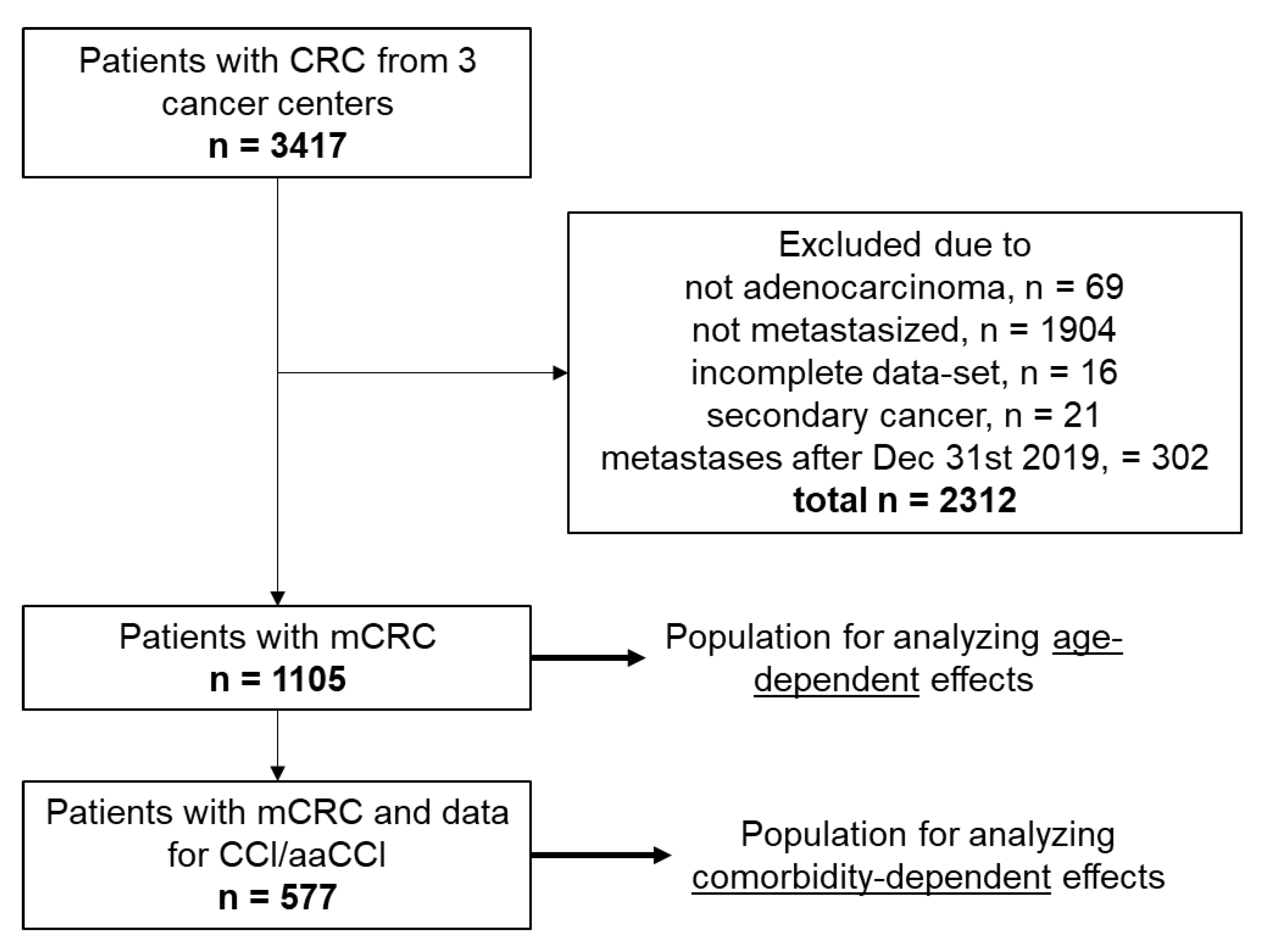
2.1. Patient Population
2.2. Statistical Analysis
3. Results
3.1. Patients
3.2. Prognostic Value of CCI and aaCCI
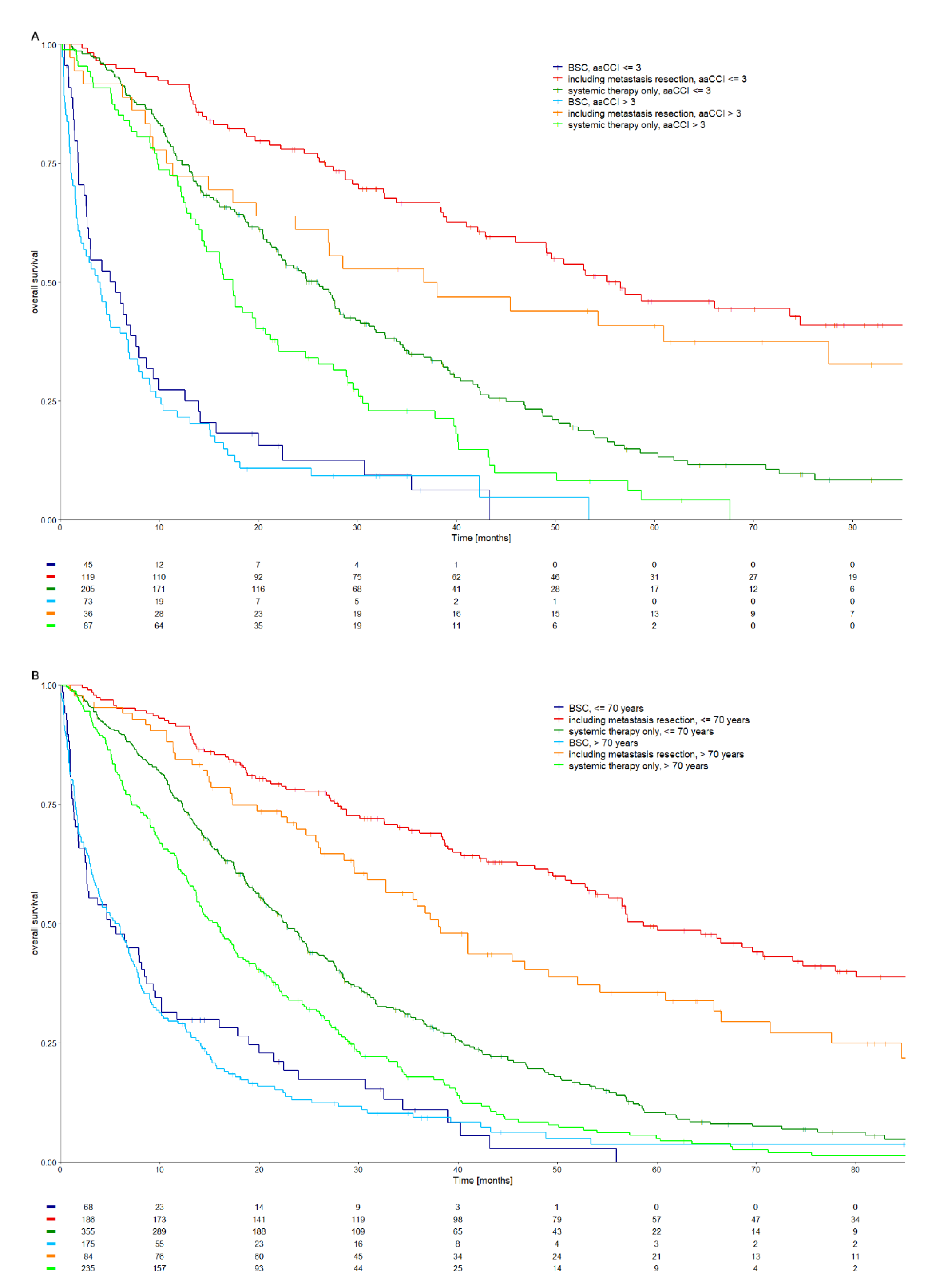
3.3. Treatment Outcomes According to Comorbidities and Age
3.4. Treatment Differences in Comorbid and Elderly Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Effects for Age | HR | z | CI 95% | p |
| Age > 70 years | 0.91 | −0.62 | (0.68–1.22) | 0.53 |
| Effect of resection of metastases | 0.13 | −12.39 | (0.09–0.18) | 0.00 |
| Effect of systemic treatment only | 0.36 | −7.25 | (0.27–0.47) | 0.00 |
| Resection of metastases in age > 70 years | 1.68 | 2.32 | (1.08–2.61) | 0.02 |
| Sysystemic treatment only in age > 70 | 1.58 | 2.57 | (1.11–2.23) | 0.01 |
| Effects for aaCCI | ||||
| aaCCI 4–9 | 1.16 | 0.74 | (0.79–1.70) | 0.46 |
| Effect of resection of metastases | 0.11 | −10.48 | (0.07–0.17) | 0.00 |
| Effect of systemic treatment only | 0.28 | −7.19 | (0.20–0.39) | 0.00 |
| Resection of metastases in aaCCI 4–9 | 1.22 | 0.64 | (0.81–2.08) | 0.52 |
| Sysystemic treatment only in aaCCI 4–9 | 1.29 | 1.07 | (0.68–1.22) | 0.29 |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.L.; Hallas, J.; Friis, S.; Herrstedt, J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br. J. Cancer 2012, 106, 1353–1360. [Google Scholar] [CrossRef]
- Yancik, R.; Havlik, R.J.; Wesley, M.N.; Ries, L.; Long, S.; Rossi, W.K.; Edwards, B.K. Cancer and comorbidity in older patients: A descriptive profile. Ann. Epidemiol. 1996, 6, 399–412. [Google Scholar] [CrossRef]
- Lewis, J.H.; Kilgore, M.L.; Goldman, D.P.; Trimble, E.L.; Kaplan, R.; Montello, M.J.; Housman, M.G.; Escarce, J.J. Participation of patients 65 years of age or older in cancer clinical trials. J. Clin. Oncol. 2003, 21, 1383–1389. [Google Scholar] [CrossRef]
- Hutchins, L.F.; Unger, J.M.; Crowley, J.J.; Coltman, C.A.; Albain, K.S. Underrepresentation of Patients 65 Years of Age or Older in Cancer-Treatment Trials. N. Engl. J. Med. 1999, 341, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Zulman, D.M.; Sussman, J.B.; Chen, X.; Cigolle, C.T.; Blaum, C.S.; Hayward, R.A. Examining the evidence: A systematic review of the inclusion and analysis of older adults in randomized controlled trials. J. Gen. Intern. Med. 2011, 26, 783–790. [Google Scholar] [CrossRef]
- Cremolini, C.; Schirripa, M.; Antoniotti, C.; Moretto, R.; Salvatore, L.; Masi, G.; Falcone, A.; Loupakis, F. First-line chemotherapy for mCRC-a review and evidence-based algorithm. Nat. Rev. Clin. Oncol. 2015, 12, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer a randomized clinical trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Pfeiffer, P.; Cavalli-Björkman, N.; Qvortrup, C.; Holsen, M.H.; Wentzel-Larsen, T.; Glimelius, B. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer 2009, 15, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Doat, S.; Thiébaut, A.; Samson, S.; Ricordeau, P.; Guillemot, D.; Mitry, E. Elderly patients with colorectal cancer: Treatment modalities and survival in France. National data from the ThInDiT cohort study. Eur. J. Cancer 2014, 50, 1276–1283. [Google Scholar] [CrossRef]
- Razenberg, L.G.E.M.; Creemers, G.J.; Beerepoot, L.V.; Vos, A.H.; van de Wouw, A.J.; Maas, H.A.A.M.; Lemmens, V.E.P.P. Age-related systemic treatment and survival of patients with metachronous metastases from colorectal cancer. Acta Oncol. 2016, 55, 1443–1449. [Google Scholar] [CrossRef]
- Tomita, Y.; Karapetis, C.S.; Ullah, S.; Townsend, A.R.; Roder, D.; Beeke, C.; Roy, A.C.; Padbury, R.; Price, T.J. Survival improvements associated with access to biological agents: Results from the South Australian (SA) metastatic colorectal cancer (mCRC) registry. Acta Oncol. 2016, 55, 480–485. [Google Scholar] [CrossRef]
- Van Erning, F.N.; Van Steenbergen, L.N.; Lemmens, V.E.P.P.; Rutten, H.J.T.; Martijn, H.; Van Spronsen, D.J.; Janssen-Heijnen, M.L.G. Conditional survival for long-term colorectal cancer survivors in the Netherlands: Who do best? Eur. J. Cancer 2014, 50, 1731–1739. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Moore, M.R.; Harker, G.; Villa, L.; Rinaldi, D.; Hecht, J.R. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J. Clin. Oncol. 2003, 21, 807–814. [Google Scholar] [CrossRef]
- Figer, A.; Perez-Staub, N.; Carola, E.; Tournigand, C.; Lledo, G.; Flesch, M.; Barcelo, R.; Cervantes, A.; André, T.; Colin, P.; et al. FOLFOX in patients aged between 76 and 80 years with metastatic colorectal cancer: An exploratory cohort of the OPTIMOX1 study. Cancer 2007, 110, 2666–2671. [Google Scholar] [CrossRef] [PubMed]
- Kozloff, M.F.; Berlin, J.; Flynn, P.J.; Kabbinavar, F.; Ashby, M.; Dong, W.; Sing, A.P.; Grothey, A. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: Results from the BRiTE observational cohort study. Oncology 2010, 78, 329–339. [Google Scholar] [CrossRef]
- Cunningham, D.; Lang, I.; Marcuello, E.; Lorusso, V.; Ocvirk, J.; Shin, D.B.; Jonker, D.; Osborne, S.; Andre, N.; Waterkamp, D.; et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial. Lancet Oncol. 2013, 14, 1077–1085. [Google Scholar] [CrossRef]
- Sastre, J.; Grávalos, C.; Rivera, F.; Massuti, B.; Valladares-Ayerbes, M.; Marcuello, E.; Manzano, J.L.; Benavides, M.; Hidalgo, M.; Díaz-Rubio, E.; et al. First-Line Cetuximab Plus Capecitabine in Elderly Patients with Advanced Colorectal Cancer: Clinical Outcome and Subgroup Analysis According to KRAS Status from a Spanish TTD Group Study. Oncologist 2012, 17, 339–345. [Google Scholar] [CrossRef]
- Papamichael, D.; Audisio, R.A.; Glimelius, B.; de Gramont, A.; Glynne-Jones, R.; Haller, D.; Köhne, C.H.; Rostoft, S.; Lemmens, V.; Mitry, E.; et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann. Oncol. 2015, 26, 463–476. [Google Scholar] [CrossRef]
- Aparicio, T.; Francois, E.; Cristol-Dalstein, L.; Carola, E.; Maillard, E.; Paillaud, E.; Retornaz, F.; Faroux, R.; André, T.; Bedenne, L.; et al. PRODIGE 34—FFCD 1402—ADAGE: Adjuvant chemotherapy in elderly patients with resected stage III colon cancer: A randomized phase 3 trial. Dig. Liver Dis. 2016, 48, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Folprecht, G.; Seymour, M.T.; Saltz, L.; Douillard, J.Y.; Hecker, H.; Stephens, R.J.; Maughan, T.S.; Van Cutsem, E.; Rougier, P.; Mitry, E.; et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: Combined analysis of 2,691 patients in randomized controlled trials. J. Clin. Oncol. 2008, 26, 1443–1451. [Google Scholar] [CrossRef]
- Jackson, N.A.; Barrueco, J.; Soufi-Mahjoubi, R.; Marshall, J.; Mitchell, E.; Zhang, X.; Meyerhardt, J. Comparing safety and efficacy of first-line irinotecan/fluoropyrimidine combinations in elderly versus nonelderly patients with metastatic colorectal cancer: Findings from the bolus, infusional, or capecitabine with camptostar-celecoxib study. Cancer 2009, 115, 2617–2629. [Google Scholar] [CrossRef]
- Venderbosch, S.; Doornebal, J.; Teerenstra, S.; Lemmens, W.; Punt, C.J.A.; Koopman, M. Outcome of first line systemic treatment in elderly compared to younger patients with metastatic colorectal cancer: A retrospective analysis of the CAIRO and CAIRO2 studies of the Dutch Colorectal Cancer Group (DCCG). Acta Oncol. 2012, 51, 831–839. [Google Scholar] [CrossRef]
- Mitry, E.; Rougier, P. Review article: Benefits and risks of chemotherapy in elderly patients with metastatic colorectal cancer. Aliment. Pharmacol. Ther. 2009, 2, 161–171. [Google Scholar] [CrossRef]
- Lieu, C.H.; Renfro, L.A.; De Gramont, A.; Meyers, J.P.; Maughan, T.S.; Seymour, M.T.; Saltz, L.; Goldberg, R.M.; Sargent, D.J.; Eckhardt, S.G.; et al. Association of age with survival in patients with metastatic colorectal cancer: Analysis from the ARCAD clinical trials program. J. Clin. Oncol. 2014, 32, 2975–2982. [Google Scholar] [CrossRef]
- Lee, L.; Cheung, W.Y.; Atkinson, E.; Krzyzanowska, M.K. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review. J. Clin. Oncol. 2011, 29, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, M.; Thomsen, R.W.; Bossen, K.S.; Sørensen, H.T.; Nørgaard, M. The impact of comorbidity on cancer survival: A review. Clin. Epidemiol. 2013, 5, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Nardo, B.; Serafini, S.; Ruggiero, M.; Grande, R.; Fugetto, F.; Zullo, A.; Novello, M.; Rizzuto, A.; Bonaiuto, E.; Vaccarisi, S.; et al. Liver resection for metastases from colorectal cancer in very elderly patients: New surgical horizons. Int. J. Surg. 2016, 33, S135–S141. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Prioletta, M.; Di Nuzzo, D.; Cipollone, G.; Camplese, P.; Mucilli, F. Pulmonary metastasectomy in elderly colorectal cancer patients: A retrospective single center study. Updates Surg. 2016, 68, 357–367. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Koppert, L.B.; Lemmens, V.E.P.P.; Coebergh, J.W.W.; Steyerberg, E.W.; Wijnhoven, B.P.L.; Tilanus, H.W.; Janssen-Heijnen, M.L.G. Impact of age and co-morbidity on surgical resection rate and survival in patients with oesophageal and gastric cancer. Br. J. Surg. 2012, 99, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, V.E.P.P.; Janssen-Heijnen, M.L.G.; Verheij, C.D.G.W.; Houterman, S.; Repelaer Van Driel, O.J.; Coebergh, J.W.W. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br. J. Surg. 2005, 92, 615–623. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Patterson, H.D.; Belsley, D.A.; Kuh, E.; Welsch, R.E. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Biometrics 1981, 37, 862. [Google Scholar] [CrossRef]
- Morishima, T.; Matsumoto, Y.; Koeda, N.; Shimada, H.; Maruhama, T.; Matsuki, D.; Nakata, K.; Ito, Y.; Tabuchi, T.; Miyashiro, I. Impact of comorbidities on survival in gastric, colorectal, and lung cancer patients. J. Epidemiol. 2019, 29, 110–115. [Google Scholar] [CrossRef]
- Piccirillo, J.F.; Tierney, R.M.; Costas, I.; Grove, L.; Spitznagel, E.L. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004, 291, 2441–2447. [Google Scholar] [CrossRef]
- Wu, C.C.; Hsu, T.W.; Chang, C.M.; Yu, C.H.; Lee, C.C. Age-adjusted charlson comorbidity index scores as predictor of survival in colorectal cancer patients who underwent surgical resection and chemoradiation. Medicine 2015, 94, e431. [Google Scholar] [CrossRef]
- Lund, C.M.; Vistisen, K.K.; Dehlendorff, C.; Rønholt, F.; Johansen, J.S.; Nielsen, D.L. Age-dependent differences in first-line chemotherapy in patients with metastatic colorectal cancer: The DISCO study. Acta Oncol. 2018, 57, 1445–1454. [Google Scholar] [CrossRef]
- Sarfati, D.; Hill, S.; Blakely, T.; Robson, B.; Purdie, G.; Dennett, E.; Cormack, D.; Dew, K. The effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: A retrospective cohort study. BMC Cancer 2009, 9, 1–10. [Google Scholar] [CrossRef]
- Gross, C.P.; McAvay, G.J.; Krumholz, H.M.; Paltiel, A.D.; Bhasin, D.; Tinetti, M.E. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: Implications for screening. Ann. Intern. Med. 2006, 145, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Boakye, D.; Walter, V.; Jansen, L.; Martens, U.M.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Magnitude of the age-advancement effect of comorbidities in colorectal cancer prognosis. JNCCN J. Natl. Compr. Cancer Netw. 2020, 18, 59–68. [Google Scholar] [CrossRef]
- Glimelius, B.; Pfeiffer, P. Do we make progress in elderly patients with metastatic colorectal cancer? Acta Oncol. 2018, 57, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Nelson, R.; Sanchez, J.; Lee, W.; Uyeno, L.; Garcia-Aguilar, J.; Hurria, A.; Kim, J. Elderly patients with colon cancer have unique tumor characteristics and poor survival. Cancer 2013, 119, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Lund, C.M.; Vistisen, K.K.; Dehlendorff, C.; Rønholt, F.; Johansen, J.S.; Nielsen, D.L. The effect of geriatric intervention in frail elderly patients receiving chemotherapy for colorectal cancer: A randomized trial (GERICO). BMC Cancer 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Signal, V.; Jackson, C.; Signal, L.; Hardie, C.; Holst, K.; McLaughlin, M.; Steele, C.; Sarfati, D. Improving management of comorbidity in patients with colorectal cancer using comprehensive medical assessment: A pilot study. BMC Cancer 2020, 20, 1–10. [Google Scholar] [CrossRef]
| Variable | Category | N (%) |
|---|---|---|
| Patients (n) | number | 1105 |
| Age (Median (IQR)) | years | 69.00 (60.00, 76.00) |
| Sex | female | 413 (37.4) |
| male | 692 (62.6) | |
| CCI | 0 | 357 (63.0) |
| 1 to 2 | 178 (31.4) | |
| >2 | 32 (5.6) | |
| aaCCI | 0 to 1 | 123 (21.7) |
| 2 to 4 | 261 (46.0) | |
| >4 | 183 (32.3) | |
| Type of treatment | sytemic treatment only | 590 (53.4) |
| resection of metastases +/− systemic treatment | 270 (24.4) | |
| Treated or not treated | treated (resection and/or systemic therapy) | 860 (77.8) |
| no treatment (BSC) | 245 (22.2) | |
| First line treatment | no | 342 (31.0) |
| yes | 763 (69.0) | |
| Second line treatment | no | 613 (55.5) |
| yes | 492 (44.5) | |
| Third line treatment | no | 819 (74.1) |
| yes | 286 (25.9) | |
| Location of primary tumor | right colon | 195 (18.1) |
| left colon | 499 (46.2) | |
| rectum | 385 (35.7) | |
| Time to metastasis | synchronous | 771 (69.8) |
| metachronous | 334 (30.2) | |
| BRAF status | BRAF-mut | 41 (3.7) |
| BRAF-wt | 365 (33.0) | |
| not analyzed | 699 (63.3) | |
| RAS status | RAS-mut | 453 (41.0) |
| RAS-wt | 426 (38.6) | |
| not analyzed | 226 (20.5) | |
| Organs with metastases | >1 organ with metastases | 322 (29.2) |
| single organ metastasis | 781 (70.8) |
| Patient Groups | Treatment Groups | HR | CI 90% | OS (Months) | CI 95% |
|---|---|---|---|---|---|
| All patients (n = 1105) | BSC | 1 | 5.58 | (3.88–7.17) | |
| incl. metastasis resection | 0.16 | (0.13–0.20) | 54.3 | (45.96–65.80) | |
| systemic therapy only | 0.45 | (0.38–0.52) | 19.74 | (17.67–22.04) | |
| aaCCI ≤ 3 (n = 370) | BSC | 1 | 5.58 | (2.73–9.4) | |
| incl. metastasis resection | 0.12 | (0.08–0.17) | 56.54 | (45.96–100.5) | |
| systemic therapy only | 0.3 | (0.22–0.39) | 26.02 | (22.01–29.9) | |
| aaCCI > 3 (n = 197) | BSC | 1 | 3.93 | (1.97–6.87) | |
| incl. metastasis resection | 0.16 | (0.10–0.24) | 36.7 | (19.81–96.06) | |
| systemic therapy only | 0.39 | (0.29–0.51) | 17.44 | (14.29–21.22) | |
| Age ≤ 70 years (n = 609) | BSC | 1 | 5.03 | (2.66–9.4) | |
| incl. metastasis resection | 0.12 | (0.09–0.15) | 58.64 | (53.02–78.0) | |
| systemic therapy only | 0.36 | (0.28–0.45) | 22.77 | (20.47–24.9) | |
| Age > 70 years (n = 496) | BSC | 1 | 5.91 | (3.88–7.33) | |
| incl. metastasis resection | 0.23 | (0.18–0.30) | 38.04 | (30.91–54.30) | |
| systemic therapy only | 0.56 | (0.47–0.66) | 15.8 | (13.70–17.84) |
| Variable | Category | aaCCI | p (Exact) | |
| aaCCI ≤ 3 | aaCCI > 3 | |||
| n= 370 | n= 197 | |||
| Type and intensity of treatment | Receiving best supportive care | 46 (12.4) | 74 (37.6) | <0.001 |
| Metastasis resection only | 27 (7.3) | 17 (8.6) | 0.622 | |
| Metastasis resection and systemic treatment | 92 (24.9) | 19 (9.6) | <0.001 | |
| Receiving systemic treatment only | 205 (55.4) | 87 (44.2) | 0.013 | |
| Receiving first line therapy | 297 (80.3) | 106 (53.8) | <0.001 | |
| Receiving second line therapy | 189 (51.1) | 50 (25.4) | <0.001 | |
| Receiving third line or more therapy | 91 (24.6) | 27 (13.7) | 0.002 | |
| 1 treatment line only | 108 (29.2) | 57 (28.9) | 1.000 | |
| 2 treatment lines only | 98 (26.5) | 23 (11.7) | <0.001 | |
| 3 or more treatment lines | 91 (24.6) | 26 (13.2) | 0.001 | |
| Location of primary tumor | Right sided colon cancer | 53 (14.6) | 39 (20.2) | 0.010 |
| Left sided colon cancer | 160 (44.0) | 98 (50.8) | ||
| Rectum cancer | 151 (41.5) | 56 (29.0) | ||
| Prognostic features | RAS analyzed | 325 (87.8) | 129 (65.5) | <0.001 |
| Synchronous disease | 266 (71.9) | 141 (71.6) | 1.000 | |
| >1 organ with metastases | 136 (36.8) | 59 (30.1) | 0.115 | |
| Age [Years] | p (Exact) | |||
| ≤70 | >70 | |||
| n= 609 | n= 496 | |||
| Type and intensity of treatment | Receiving best supportive care | 68 (11.2) | 177 (35.7) | <0.001 |
| Metastasis resection only | 58 (9.5) | 39 (7.9) | 0.339 | |
| Metastasis resection and systemic treatment | 128 (21.0) | 45 (9.1) | <0.001 | |
| Receiving systemic treatment only | 355 (58.3) | 235 (47.4) | <0.001 | |
| Receiving first line therapy | 483 (79.3) | 280 (56.5) | <0.001 | |
| Receiving second line therapy | 344 (56.5) | 148 (29.8) | <0.001 | |
| Receiving third line or more therapy | 204 (33.5) | 82 (16.5) | <0.001 | |
| 1 treatment line only | 142 (23.3) | 132 (26.6) | 0.208 | |
| 2 treatment lines only | 140 (23.0) | 66 (13.3) | <0.001 | |
| 3 or more treatment lines | 201 (33.0) | 82 (16.5) | <0.001 | |
| Location of primary tumor | Right sided colon cancer | 87 (14.6) | 108 (22.3) | <0.001 |
| Left sided colon cancer | 270 (45.5) | 229 (47.2) | ||
| Rectum cancer | 237 (39.9) | 148 (30.5) | ||
| Prognostic features | RAS analyzed | 521 (85.6) | 358 (72.2) | <0.001 |
| Synchronous disease | 425 (69.8) | 346 (69.8) | 1.000 | |
| >1 organ with metastases | 190 (31.2) | 132 (26.7) | 0.097 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedersüß-Beke, D.; Orlinger, M.; Falch, D.; Heiler, C.; Piringer, G.; Thaler, J.; Hilbe, W.; Petzer, A.; Rumpold, H. Clinical Effectiveness of Oncological Treatment in Metastatic Colorectal Cancer Is Independent of Comorbidities and Age. Cancers 2021, 13, 2091. https://doi.org/10.3390/cancers13092091
Niedersüß-Beke D, Orlinger M, Falch D, Heiler C, Piringer G, Thaler J, Hilbe W, Petzer A, Rumpold H. Clinical Effectiveness of Oncological Treatment in Metastatic Colorectal Cancer Is Independent of Comorbidities and Age. Cancers. 2021; 13(9):2091. https://doi.org/10.3390/cancers13092091
Chicago/Turabian StyleNiedersüß-Beke, Dora, Manuel Orlinger, David Falch, Cordula Heiler, Gudrun Piringer, Josef Thaler, Wolfgang Hilbe, Andreas Petzer, and Holger Rumpold. 2021. "Clinical Effectiveness of Oncological Treatment in Metastatic Colorectal Cancer Is Independent of Comorbidities and Age" Cancers 13, no. 9: 2091. https://doi.org/10.3390/cancers13092091
APA StyleNiedersüß-Beke, D., Orlinger, M., Falch, D., Heiler, C., Piringer, G., Thaler, J., Hilbe, W., Petzer, A., & Rumpold, H. (2021). Clinical Effectiveness of Oncological Treatment in Metastatic Colorectal Cancer Is Independent of Comorbidities and Age. Cancers, 13(9), 2091. https://doi.org/10.3390/cancers13092091






