Excluding Lung Tissue from the PTV during Internal Mammary Irradiation. A Safe Technique for OAR-Sparing?
Abstract
Simple Summary
Abstract
1. Introduction
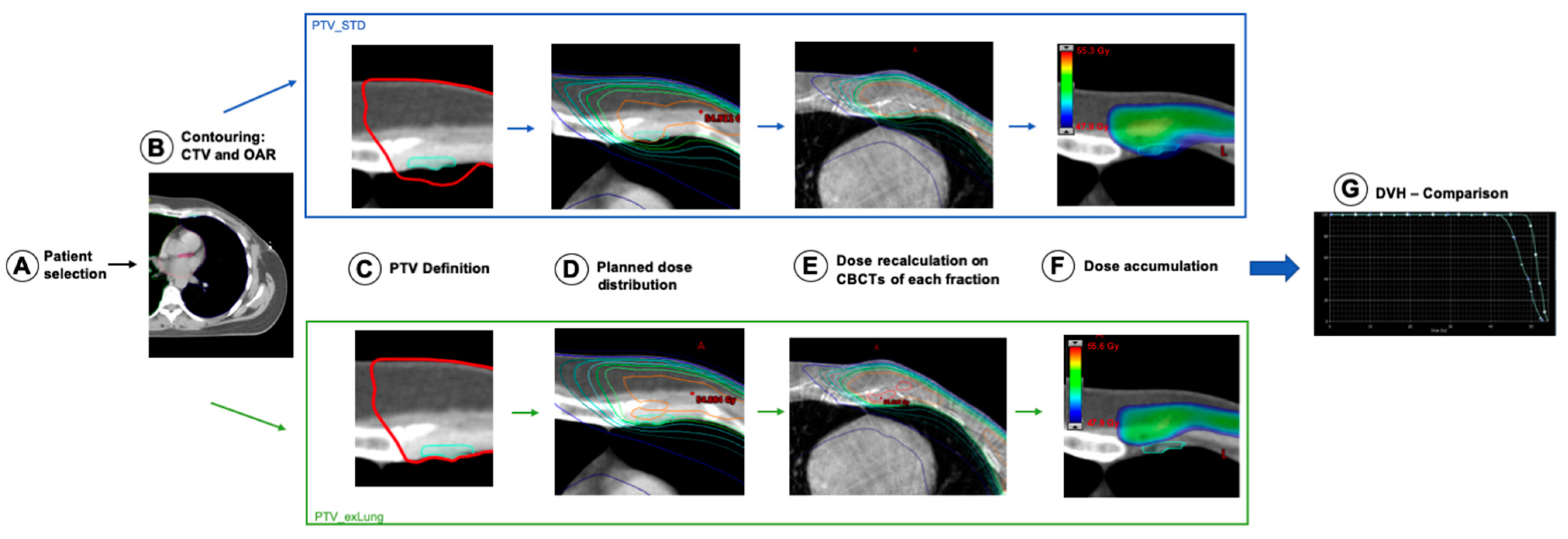
2. Materials and Methods
2.1. Patients
2.2. Radiotherapy Planning
- (1)
- a standard PTV with a 5 mm CTV-PTV safety margin around the lymph node areas including the IMN
- (2)
- a modified PTV with a 5 mm CTV-PTV safety margin around the lymph node areas including the IMN excluding the overlapping lung volumes from the PTV
- (1)
- a standard treatment plan (STD) based on the standard PTV
- (2)
- an alternative treatment plan (ExLung) based on the PTV excluding the overlapping lung tissue
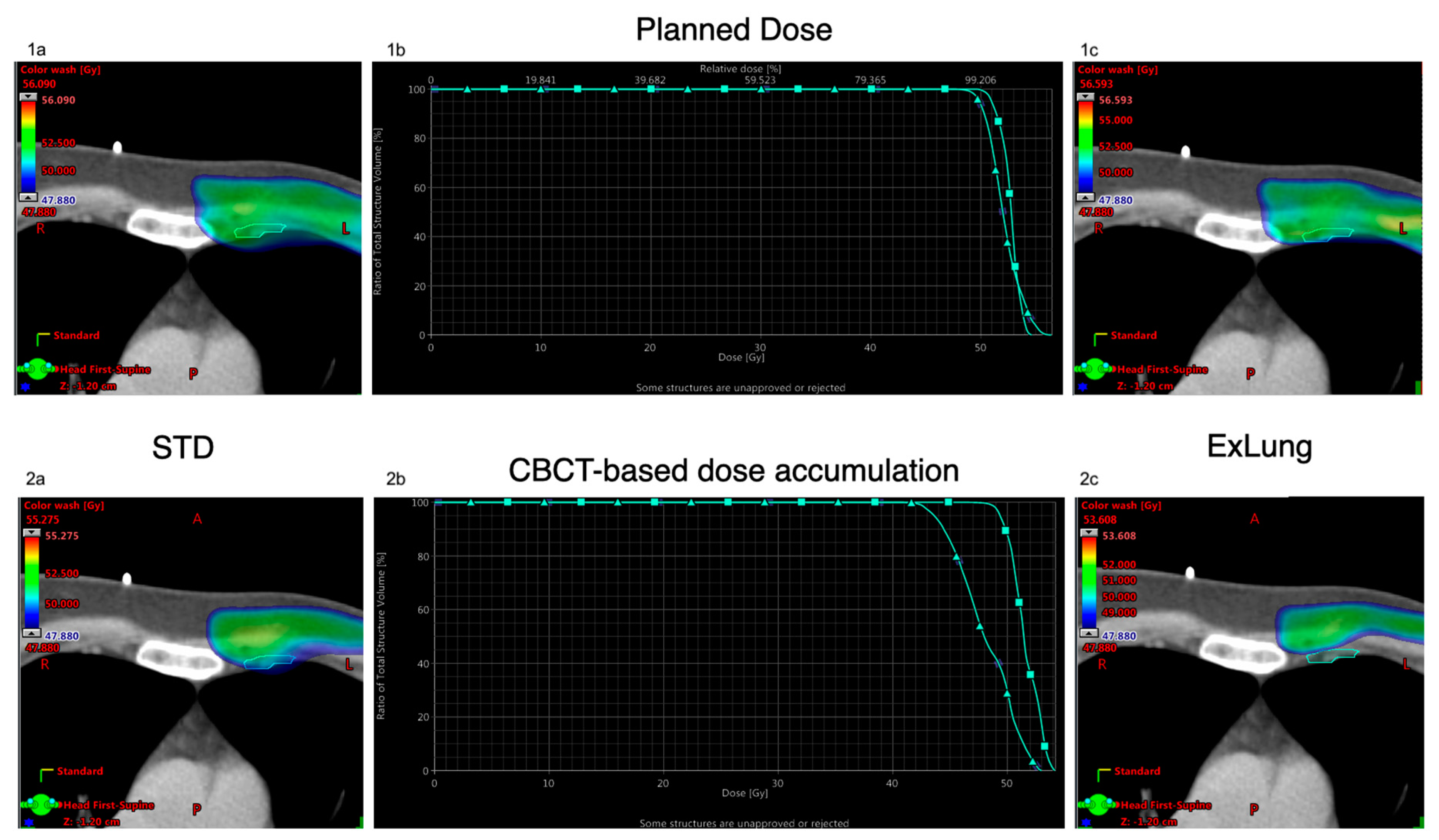
2.3. Dose Analyses
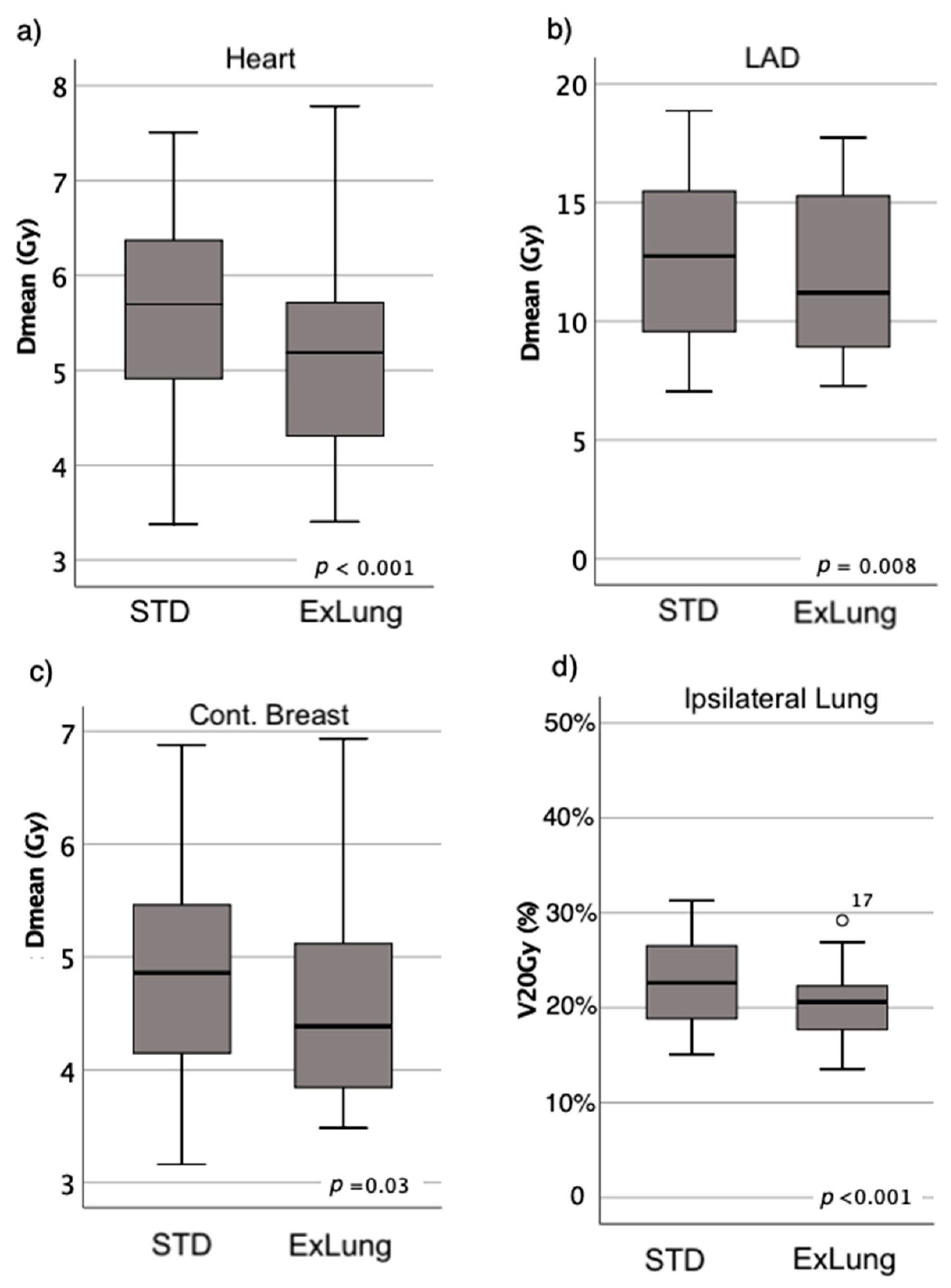
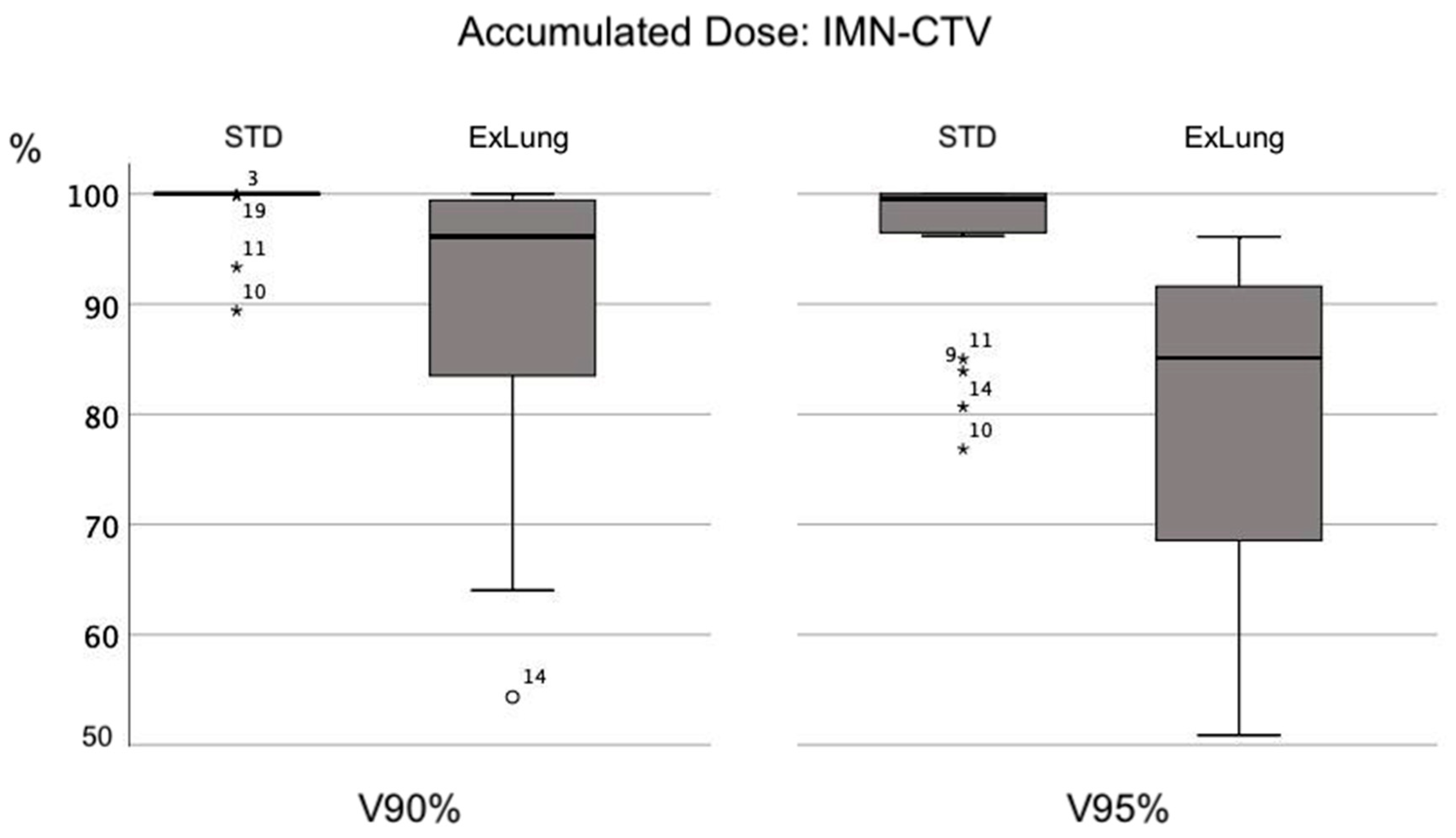
3. Results
3.1. OAR Dose
3.2. IMN-CTV Coverage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poortmans, P.M.; Collette, S.; Kirkove, C.; van Limbergen, E.; Budach, V.; Struikmans, H.; Collette, L.; Fourquet, A.; Maingon, P.; Valli, M.; et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N. Engl. J. Med. 2015, 373, 317–327. [Google Scholar] [CrossRef]
- Whelan, T.J.; Olivotto, I.A.; Parulekar, W.R.; Ackerman, I.; Chua, B.H.; Nabid, A.; Vallis, K.A.; White, J.R.; Rousseau, P.; Fortin, A.; et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N. Engl. J. Med. 2015, 373, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Borm, K.J.; Voppichler, J.; Dusberg, M.; Oechsner, M.; Vag, T.; Weber, W.; Combs, S.E.; Duma, M.N. FDG/PET-CT based lymph node atlas in breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 574–582. [Google Scholar] [CrossRef]
- Riegger, C.; Koeninger, A.; Hartung, V.; Otterbach, F.; Kimmig, R.; Forsting, M.; Bockisch, A.; Antoch, G.; Heusner, T.A. Comparison of the diagnostic value of FDG-PET/CT and axillary ultrasound for the detection of lymph node metastases in breast cancer patients. Acta Radiol. 2012, 53, 1092–1098. [Google Scholar] [CrossRef]
- Akashi-Tanaka, S.; Fukutomi, T.; Sato, N.; Miyakawa, K. The role of computed tomography in the selection of breast cancer treatment. Breast Cancer 2003, 10, 198–203. [Google Scholar] [CrossRef]
- Chen, R.C.; Lin, N.U.; Golshan, M.; Harris, J.R.; Bellon, J.R. Internal mammary nodes in breast cancer: Diagnosis and implications for patient management--a systematic review. J. Clin. Oncol. 2008, 26, 4981–4989. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Zheng, K.; Yin, X.D.; Xing, L.; Zhang, A.J.; Shi, Y.; Kong, L.Q.; Li, F.; Ma, B.L.; et al. Predictors of internal mammary lymph nodes (IMLN) metastasis and disease-free survival comparison between IMLN-positive and IMLN-negative breast cancer patients: Results from Western China Clinical Cooperation Group (WCCCG) database (CONSORT). Medicine (Baltimore) 2018, 97, 29. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, J.; Budach, W.; Tamaskovics, B.; Bolke, E.; Corradini, S.; Djiepmo-Njanang, F.J.; Kammers, K.; Matuschek, C. Which target volume should be considered when irradiating the regional nodes in breast cancer? Results of a network-meta-analysis. Radiat. Oncol. 2019, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Bossard, N.; Servagi-Vernat, S.; Maingon, P.; Dubois, J.B.; Datchary, J.; Carrie, C.; Roullet, B.; Suchaud, J.P.; Teissier, E.; et al. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 860–866. [Google Scholar] [CrossRef]
- Stemmer, S.M.; Rizel, S.; Hardan, I.; Adamo, A.; Neumann, A.; Goffman, J.; Brenner, H.J.; Pfeffer, M.R. The role of irradiation of the internal mammary lymph nodes in high-risk stage II to IIIA breast cancer patients after high-dose chemotherapy: A prospective sequential nonrandomized study. J. Clin. Oncol. 2003, 21, 2713–2718. [Google Scholar] [CrossRef]
- Thorsen, L.B.; Offersen, B.V.; Dano, H.; Berg, M.; Jensen, I.; Pedersen, A.N.; Zimmermann, S.J.; Brodersen, H.J.; Overgaard, M.; Overgaard, J. DBCG-IMN: A Population-Based Cohort Study on the Effect of Internal Mammary Node Irradiation in Early Node-Positive Breast Cancer. J. Clin. Oncol. 2016, 34, 314–320. [Google Scholar] [CrossRef]
- Committee, A.B. Recommendations 2020. Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. 2020.
- National Comprehensive Cancer Network. Breast Cancer Version 3.2020. 25 October 2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 1 December 2020).
- Borm, K.J.; Simonetto, C.; Kundrat, P.; Eidemuller, M.; Oechsner, M.; Dusberg, M.; Combs, S.E. Toxicity of internal mammary irradiation in breast cancer. Are concerns still justified in times of modern treatment techniques? Acta Oncol. 2020, 59, 1201–1209. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Bronnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.J.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R.; et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J. Clin. Oncol. 2017, 35, 1641–1649. [Google Scholar] [CrossRef]
- Ranger, A.; Dunlop, A.; Hutchinson, K.; Convery, H.; Maclennan, M.K.; Chantler, H.; Twyman, N.; Rose, C.; McQuaid, D.; Amos, R.A.; et al. A Dosimetric Comparison of Breast Radiotherapy Techniques to Treat Locoregional Lymph Nodes Including the Internal Mammary Chain. Clin. Oncol. (R Coll. Radiol.) 2018, 30, 346–353. [Google Scholar] [CrossRef]
- RTOG. Breast Cancer Atlas for Radiation Therapy Planning. Available online: https://www.rtog.org/LinkClick.aspx?fileticket=vzJFhPaBipE%3d&tabid=236 (accessed on 1 December 2020).
- Offersen, B.V.; Boersma, L.J.; Kirkove, C.; Hol, S.; Aznar, M.C.; Sola, A.B.; Kirova, Y.M.; Pignol, J.P.; Remouchamps, V.; Verhoeven, K.; et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother. Oncol. 2016, 118, 205–208. [Google Scholar] [CrossRef]
- Borm, K.J.; Kessel, K.; Devecka, M.; Muench, S.; Straube, C.; Schiller, K.; Schuttrumpf, L.; Dapper, H.; Woller, B.; Pigorsch, S.; et al. Variability in lymph node irradiation in patients with breast cancer-results from a multi-center survey in German-speaking countries. Strahlenther. Onkol. 2020, 196, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Surmann, K.; van der Leer, J.; Branje, T.; van der Sangen, M.; van Lieshout, M.; Hurkmans, C.W. Elective breast radiotherapy including level I and II lymph nodes: A planning study with the humeral head as planning risk volume. Radiat. Oncol. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Takahashi, K.; Morota, M.; Kagami, Y.; Okamoto, H.; Sekii, S.; Inaba, K.; Murakami, N.; Igaki, H.; Ito, Y.; Uno, T.; et al. Prospective study of postoperative whole breast radiotherapy for Japanese large-breasted women: A clinical and dosimetric comparisons between supine and prone positions and a dose measurement using a breast phantom. BMC Cancer 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashimoyo, H.; Omura, M.; Matsui, K.; Mukai, Y.; Hongo, H.; Yamakabe, W.; Saito, K.; Yoshida, M. Tangent field technique of TomoDirect improves dose distribution for whole-breast irradiation. J. Appl. Clin. Med. Phys. 2015, 16, 225–232. [Google Scholar] [CrossRef]
- Chen, G.P.; Liu, F.; White, J.; Vicini, F.A.; Freedman, G.M.; Arthur, D.W.; Li, X.A. A planning comparison of 7 irradiation options allowed in RTOG 1005 for early-stage breast cancer. Med. Dosim. 2015, 40, 21–25. [Google Scholar] [CrossRef]
- Hodapp, N. [The ICRU Report 83: Prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT)]. Strahlenther. Onkol. 2012, 188, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Remick, J.; Amin, N.P. Postmastectomy Breast Cancer Radiation Therapy; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Wang, W.; Zhang, Y.; Xu, M.; Shao, Q.; Sun, T.; Yu, T.; Liu, X.; Li, J. Postmastectomy radiotherapy using three different techniques: A retrospective evaluation of the incidental dose distribution in the internal mammary nodes. Cancer Manag. Res. 2019, 11, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.H.; Gerry, E.; Chmura, S.J.; Hasan, Y.; Al-Hallaq, H.A. An image-guided study of setup reproducibility of postmastectomy breast cancer patients treated with inverse-planned intensity modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.P.; Dvorak, T.; Curry, M.S.; Buchholz, D.J.; Meeks, S.L. Clinical evaluation of interfractional variations for whole breast radiotherapy using 3-dimensional surface imaging. Pract. Radiat. Oncol. 2013, 3, 16–25. [Google Scholar] [CrossRef]
- Cong, B.B.; Cao, X.S.; Cao, L.; Zhu, H.; Yu, Y.S.; Yu, J.M.; Wang, Y.S. Internal mammary lymph nodes radiotherapy of breast cancer in the era of individualized medicine. Oncotarget 2017, 8, 81583–81590. [Google Scholar] [CrossRef][Green Version]
- Thorsen, L.B.; Thomsen, M.S.; Overgaard, M.; Overgaard, J.; Offersen, B.V.; Danish Breast Cancer Cooperative Group Radiotherapy Committee. Quality assurance of conventional non-CT-based internal mammary lymph node irradiation in a prospective Danish Breast Cancer Cooperative Group trial: The DBCG-IMN study. Acta Oncol. 2013, 52, 1526–1534. [Google Scholar] [CrossRef]
- Borm, K.J.; Oechsner, M.; Dusberg, M.; Buschner, G.; Weber, W.; Combs, S.E.; Duma, M.N. Irradiation of regional lymph node areas in breast cancer-Dose evaluation according to the Z0011, AMAROS, EORTC 10981-22023 and MA-20 field design. Radiother. Oncol. 2019, 142, 195–201. [Google Scholar] [CrossRef]
- Yang, K.; Kim, H.; Choi, D.H.; Park, W.; Noh, J.M.; Cho, W.K. Optimal radiotherapy for patients with internal mammary lymph node metastasis from breast cancer. Radiat. Oncol. 2020, 15, 1–12. [Google Scholar] [CrossRef]
- Reitz, D.; Carl, G.; Schonecker, S.; Pazos, M.; Freislederer, P.; Niyazi, M.; Ganswindt, U.; Alongi, F.; Reiner, M.; Belka, C.; et al. Real-time intra-fraction motion management in breast cancer radiotherapy: Analysis of 2028 treatment sessions. Radiat. Oncol. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Duma, M.N.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; et al. Heart-sparing radiotherapy techniques in breast cancer patients: A recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO). Strahlenther. Onkol. 2019, 195, 861–871. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borm, K.J.; Hofmann, C.; Düsberg, M.; Oechsner, M.; Dapper, H.; Devecka, M.; Combs, S.E. Excluding Lung Tissue from the PTV during Internal Mammary Irradiation. A Safe Technique for OAR-Sparing? Cancers 2021, 13, 1951. https://doi.org/10.3390/cancers13081951
Borm KJ, Hofmann C, Düsberg M, Oechsner M, Dapper H, Devecka M, Combs SE. Excluding Lung Tissue from the PTV during Internal Mammary Irradiation. A Safe Technique for OAR-Sparing? Cancers. 2021; 13(8):1951. https://doi.org/10.3390/cancers13081951
Chicago/Turabian StyleBorm, Kai J., Christopher Hofmann, Mathias Düsberg, Markus Oechsner, Hendrik Dapper, Michal Devecka, and Stephanie E. Combs. 2021. "Excluding Lung Tissue from the PTV during Internal Mammary Irradiation. A Safe Technique for OAR-Sparing?" Cancers 13, no. 8: 1951. https://doi.org/10.3390/cancers13081951
APA StyleBorm, K. J., Hofmann, C., Düsberg, M., Oechsner, M., Dapper, H., Devecka, M., & Combs, S. E. (2021). Excluding Lung Tissue from the PTV during Internal Mammary Irradiation. A Safe Technique for OAR-Sparing? Cancers, 13(8), 1951. https://doi.org/10.3390/cancers13081951




