A Comprehensive Prospective Comparison of Acute Skin Toxicity after Hypofractionated and Normofractionated Radiation Therapy in Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Dose Distribution PTV
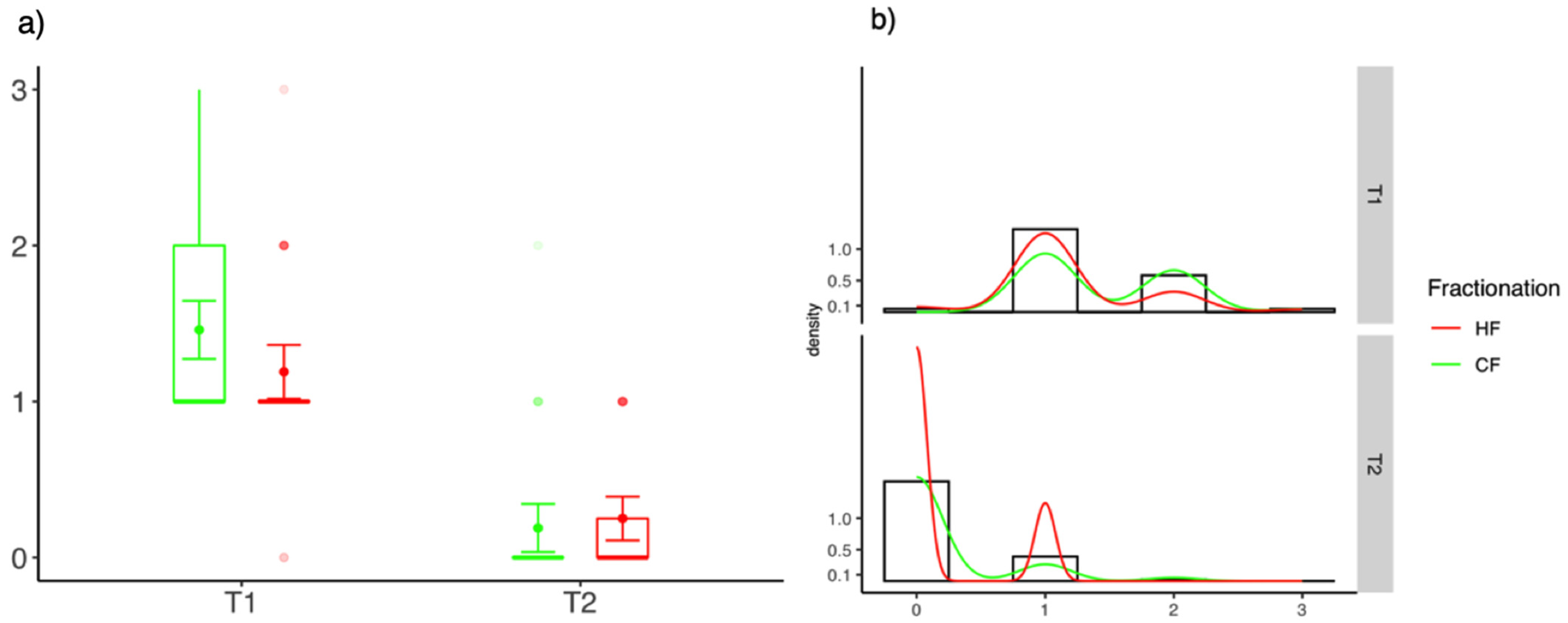
3.2. Differences between CTCAE Scores
3.3. Differences between Skindex-16
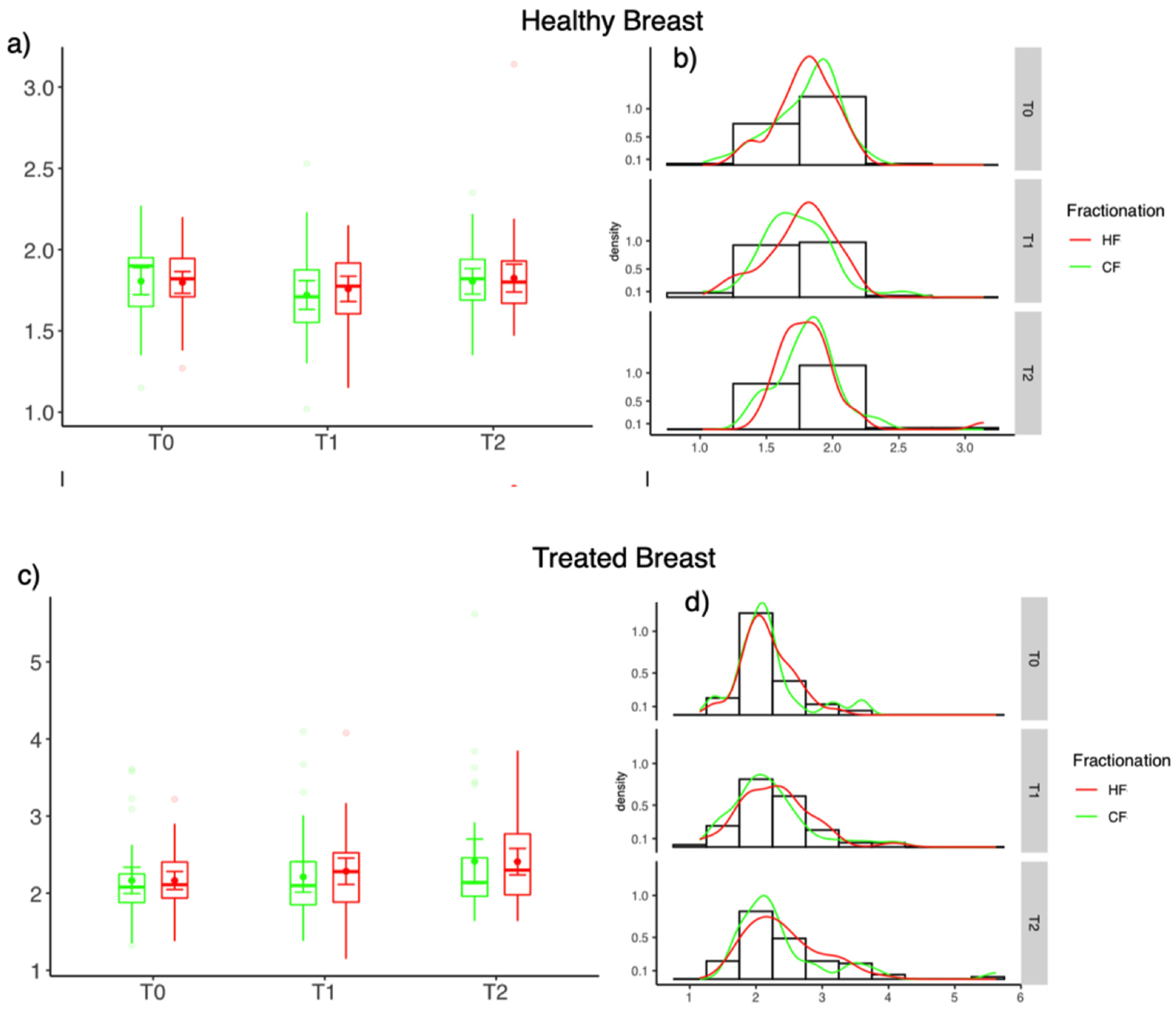
3.4. Differences of Skin Thickness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Early Breast Cancer Trialists’ Collaborative Group; Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [PubMed] [Green Version]
- Duma, M.N.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; et al. Heart-sparing radiotherapy techniques in breast cancer patients: A recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO). Strahlenther Onkol. 2019, 195, 861–871. [Google Scholar] [CrossRef]
- López, E.; Núñez, M.I.; Guerrero, M.R.; Del Moral, R.; Luna, J.D.D.; Rodríguez, M.D.M.; Valenzuela, M.T.; Villalobos, M.; De Almodóvar, J.M.R. Breast cancer acute radiotherapy morbidity evaluated by different scoring systems. Breast Cancer Res. Treat. 2002, 73, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Schnur, J.B.; Ouellette, S.C.; DiLorenzo, T.A.; Green, S.; Montgomery, G.H. A qualitative analysis of acute skin toxicity among breast cancer radiotherapy patients. Psychooncology 2011, 20, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsenault, J.; Parpia, S.; Goldberg, M.; Rakovitch, E.; Reiter, H.; Doherty, M.; Lukka, H.; Sussman, J.; Wright, J.; Julian, J.; et al. Acute Toxicity and Quality of Life of Hypofractionated Radiation Therapy for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 943–948. [Google Scholar] [CrossRef] [PubMed]
- START Trialists’ Group; Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.; Barrett, J.M.; Barrett-Lee, P.J.; Bliss, J.M.; Brown, J.; Dewar, J.A.; Dobbs, H.J.; et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. 2008, 9, 331–341. [Google Scholar] [PubMed] [Green Version]
- Offersen, B.V.; Alsner, J.; Nielsen, H.M.; Jakobsen, E.H.; Nielsen, M.H.; Krause, M.; Stenbygaard, L.; Mjaaland, I.; Schreiber, A.; Kasti, U.-M.; et al. Hypofractionated Versus Standard Fractionated Radiotherapy in Patients With Early Breast Cancer or Ductal Carcinoma In Situ in a Randomized Phase III Trial: The DBCG HYPO Trial. J. Clin. Oncol. 2020, 38, 3615–3625. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, L.C.; Koch, D.; Schmeel, F.C.; Röhner, F.; Schoroth, F.; Bücheler, B.M.; Mahlmann, B.; Leitzen, C.; Schüller, H.; Tschirner, S.; et al. Acute radiation-induced skin toxicity in hypofractionated vs. conventional whole-breast irradiation: An objective, randomized multicenter assessment using spectrophotometry. Radiother. Oncol. 2020, 146, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Tortorelli, G.; Di Murro, L.; Barbarino, R.; Cicchetti, S.; Di Cristino, D.; Falco, M.D.; Fedele, D.; Ingrosso, G.; Janniello, D.; Morelli, P.; et al. Standard or hypofractionated radiotherapy in the postoperative treatment of breast cancer: A retrospective analysis of acute skin toxicity and dose inhomogeneities. BMC Cancer 2013, 13, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnur, J.B.; Love, B.; Scheckner, B.L.; Green, S.; Gabriella, A.; Montgomery, G.H. A systematic review of patient-rated measures of radiodermatitis in breast cancer radiotherapy. Am. J. Clin. Oncol. 2011, 34, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Chren, M.M.; Lasek, R.J.; Sahay, A.P.; Sands, L.P. Measurement properties of Skindex-16: A brief quality-of-life measure for patients with skin diseases. J. Cutan. Med. Surg. 2001, 5, 105–110. [Google Scholar] [CrossRef] [PubMed]
- De Langhe, S.; Mulliez, T.; Veldeman, L.; Remouchamps, V.; Van Greveling, A.; Gilsoul, M.; De Schepper, E.; De Ruyck, K.; De Neve, W.; Thierens, H. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer 2014, 14, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzepecki, A.; Birnbaum, M.; Ohri, N.; Daily, J.; Fox, J.; Bodner, W.; Kabarriti, R.; Garg, M.; Mehta, K.; Kalnicki, S.; et al. Characterizing the Effects of Radiation Dermatitis on Quality of Life: A Prospective Survey-Based Study. J. Am. Acad. Dermatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mayinger, M.; Straube, C.; Habermehl, D.; Duma, M.; Combs, S. Hypo- vs. normofractionated radiation therapy in breast cancer: A patterns of care analysis in German speaking countries. Rep. Pract. Oncol. Radiother. 2020, 25, 775–779. [Google Scholar] [CrossRef]
- Qi, X.S.; White, J.; Li, X.A. Is alpha/beta for breast cancer really low? Radiother. Oncol. 2011, 100, 282–288. [Google Scholar] [CrossRef]
- Borm, K.J.; Loos, M.; Oeschsner, M.; Mayinger, M.; Paepke, D.; Kiechle, M.B.; Combs, S.E.; Duma, M.N. Acute radiodermatitis in modern adjuvant 3D conformal radiotherapy for breast cancer–The impact of dose distribution and patient related factors. Radiat. Oncol. 2018, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-F.; Chen, W.-C.; Lai, C.-H.; Hung, C.-H.; Liu, K.-C.; Cheng, Y.-H. Predictive factors of radiation-induced skin toxicity in breast cancer patients. BMC Cancer 2010, 10, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaitelman, S.F.; Schlembach, P.J.; Arzu, I.; Ballo, M.T.; Bloom, E.S.; Buchholz, D.; Chronowski, G.M.; Dvorak, T.; Grade, E.; Hoffman, K.E.; et al. Acute and Short-term Toxic Effects of Conventionally Fractionated vs Hypofractionated Whole-Breast Irradiation: A Randomized Clinical Trial. JAMA Oncol. 2015, 1, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, P.; Haviland, J.; Sumo, G.; Mills, J.; Bliss, J.; Yarnold, J.R. Comparison of patient-reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised Standardisation of Breast Radiotherapy (START) trials. Lancet Oncol. 2010, 11, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhou, J.; Osterman, K.S.; Zhang, P.; Woodhouse, S.A.; Schiff, P.B.; Kutcher, G.J. Measurements of Radiation-Induced Skin Changes in Breast-Cancer Radiation Therapy Using Ultrasonic Imaging. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2, 718–722. [Google Scholar] [PubMed] [Green Version]
- National Comprehensive Cancer Network. Breast Cancer- Version 8.2021. 25 October 2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 1 August 2021).
| Variable | CF | HF | Total | |
|---|---|---|---|---|
| n (%) | 38 (47.5%) | 42 (52.5%) | 80 (100.0%) | |
| Age | ||||
| Mean (SD) | 55.6 (11.9) | 61.3 (13.0) | 58.6 (12.8) | |
| Body Mass Index | ||||
| Mean (SD) | 25.1 (3.8) | 24.7 (4.5) | 24.9 (4.2) | |
| Diabetes Mellitus | ||||
| No | 37 (97.4%) | 42 (100.0%) | 79 (98.8%) | |
| Smoker | ||||
| No | 36 (94.8%) | 42 (100.0%) | 78 (97.5%) | |
| Cup-Size | ||||
| A | 1 (2.6%) | 8 (19.1%) | 9 (11.3%) | |
| B | 19 (50.0%) | 13 (31.0.%) | 32 (40.0%) | |
| C | 10 (26.3%) | 10 (23.8%) | 20 (25.0%) | |
| D | 4 (10.5%) | 7 (16.7%) | 11 (13.8%) | |
| E | 1 (2.6%) | 1 (2.4%) | 2 (2.5%) | |
| F | 0 (0.0%) | 1 (2.4%) | 1 (1.3%) | |
| Side | ||||
| Right | 25 (65.8%) | 23 (54.8%) | 48 (60.0%) | |
| Left | 13 (34.2%) | 19 (45.3%) | 32 (40.0%) | |
| Topic Cremes | ||||
| Bepanthen | 16 (42.1%) | 16 (38.1%) | 32 (40.0%) | |
| No Creme | 15 (39.5%) | 18 (42.9%) | 33 (41.3%) | |
| Anthyllis | 4 (10.5%) | 4 (9.5%) | 8 (10.0%) | |
| Both | 3 (7.9%) | 4 (9.5%) | 7 (8.8%) | |
| Surgery Typ | ||||
| BET | 30 (79.0%) | 41 (97.6%) | 71 (88.8%) | |
| Mastectomy | 7 (18.4%) | 1 (2.4%) | 8 (10.0%) | |
| others | 1 (2.6%) | 0 (0.0%) | 1 (1.3%) | |
| Lymph Node surgery | ||||
| SLNB | 16 (42.1%) | 39 (92.9%) | 55 (68.8%) | |
| None | 12 (31.6%) | 0 (0.0%) | 12 (15.0%) | |
| ALND | 10 (26.3%) | 3 (7.1%) | 13 (16.3%) | |
| Chemotherapy | ||||
| None | 29 (76.3%) | 38 (90.5%) | 67 (83.8%) | |
| Neo-Adjuvant | 8 (21.1%) | 4 (9.5%) | 12 (15.0%) | |
| Adjuvant | 1 (2.6%) | 0 (0.0%) | 1 (1.3%) | |
| Lymph node irradiation | ||||
| 0 | 24 (63.2%) | 40 (95.2%) | 64 (80.0%) | |
| 1 | 14 (36.8%) | 1 (2.4%) | 15 (18.8%) | |
| Photon Energy | ||||
| 6 MV | 22 (57.9%) | 21 (50.0%) | 43 (53.8%) | |
| 6/15 MV | 12 (31.6%) | 15 (35.7%) | 27 (33.8%) | |
| 6/10 MV | 4 (10.53%) | 1 (2.38%) | 5 (6.25%) | |
| Boost | ||||
| Yes | 20 (52.6%) | 29 (69.1%) | 49 (61.3%) | |
| No | 18 (47.4%) | 13 (31.0%) | 31 (38.8%) | |
| Variable | T | CF | HF | CF vs. HF |
|---|---|---|---|---|
| Emotions | ||||
| T0 | 0 (8.9) | 0 (4.8) | 0 (0; 0) p = 0.68 | |
| T1 | 19.1 (25.0) | 7.1 (27.4) | 11.9 (0; 14.3) p = 0.05 | |
| T2 | 0.0 (7.1) | 0 (13.1) | 0.0 (0; 0.0) p = 0.76 | |
| Symptoms | ||||
| T0 | 0 (12.5) | 4.2 (12.5) | −4.2 (0; 0) p = 0.79 | |
| T1 | 33.3 (41.7) | 29.2 (41.7) | 4.2 (−0.0; 20.8) p = 0.11 | |
| T2 | 8.3 (17.7) | 4.17 (25.0) | 4.2 (0; 4.2) p = 0.55 | |
| Functioning | ||||
| T0 | 0 (6.67) | 0 (0.0) | 0 (0; 0) p = 0.13 | |
| T1 | 6.7 (16.67) | 0 (10.0) | 6.7 (0; 6.7) p = 0.15 | |
| T2 | 0 (6.7) | 0 (3.3) | 0 (0; 0) p = 0.57 | |
| Total | ||||
| T0 | 1.0 (8.3) | 2.1 (6.3) | −1.0 (−1.0; 1.04) p = 0.84 | |
| T1 | 20.8 (25.8) | 8.3 (27.1) | 12.5 (−1.0; 14.6) p = 0.04 | |
| T2 | 3.7 (7.6) | 2.1 (9.6) | 1.6 (−2.1; 3.1) p = 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borm, K.J.; Kleine Vennekate, J.; Vagedes, J.; Islam, M.O.A.; Duma, M.N.; Loos, M.; Combs, S.E.; Schiller, K.; Klusen, S.; Paepke, S.; et al. A Comprehensive Prospective Comparison of Acute Skin Toxicity after Hypofractionated and Normofractionated Radiation Therapy in Breast Cancer. Cancers 2021, 13, 5826. https://doi.org/10.3390/cancers13225826
Borm KJ, Kleine Vennekate J, Vagedes J, Islam MOA, Duma MN, Loos M, Combs SE, Schiller K, Klusen S, Paepke S, et al. A Comprehensive Prospective Comparison of Acute Skin Toxicity after Hypofractionated and Normofractionated Radiation Therapy in Breast Cancer. Cancers. 2021; 13(22):5826. https://doi.org/10.3390/cancers13225826
Chicago/Turabian StyleBorm, Kai J., Johanne Kleine Vennekate, Jan Vagedes, Mohammad O. A. Islam, Marciana N. Duma, Maximilian Loos, Stephanie E. Combs, Kilian Schiller, Sophie Klusen, Stefan Paepke, and et al. 2021. "A Comprehensive Prospective Comparison of Acute Skin Toxicity after Hypofractionated and Normofractionated Radiation Therapy in Breast Cancer" Cancers 13, no. 22: 5826. https://doi.org/10.3390/cancers13225826
APA StyleBorm, K. J., Kleine Vennekate, J., Vagedes, J., Islam, M. O. A., Duma, M. N., Loos, M., Combs, S. E., Schiller, K., Klusen, S., Paepke, S., Kiechle, M. B., & Paepke, D. (2021). A Comprehensive Prospective Comparison of Acute Skin Toxicity after Hypofractionated and Normofractionated Radiation Therapy in Breast Cancer. Cancers, 13(22), 5826. https://doi.org/10.3390/cancers13225826







