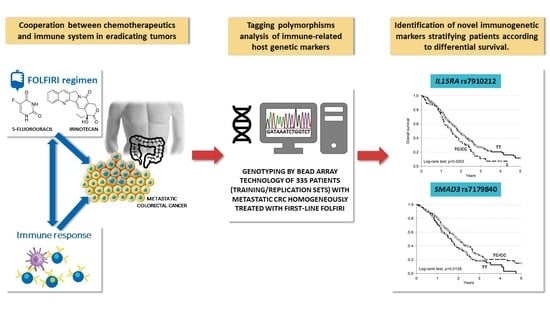
IL15RA and SMAD3 Genetic Variants Predict Overall Survival in Metastatic Colorectal Cancer Patients Treated with FOLFIRI Therapy: A New Paradigm
Abstract
Simple Summary
Abstract
1. Introduction
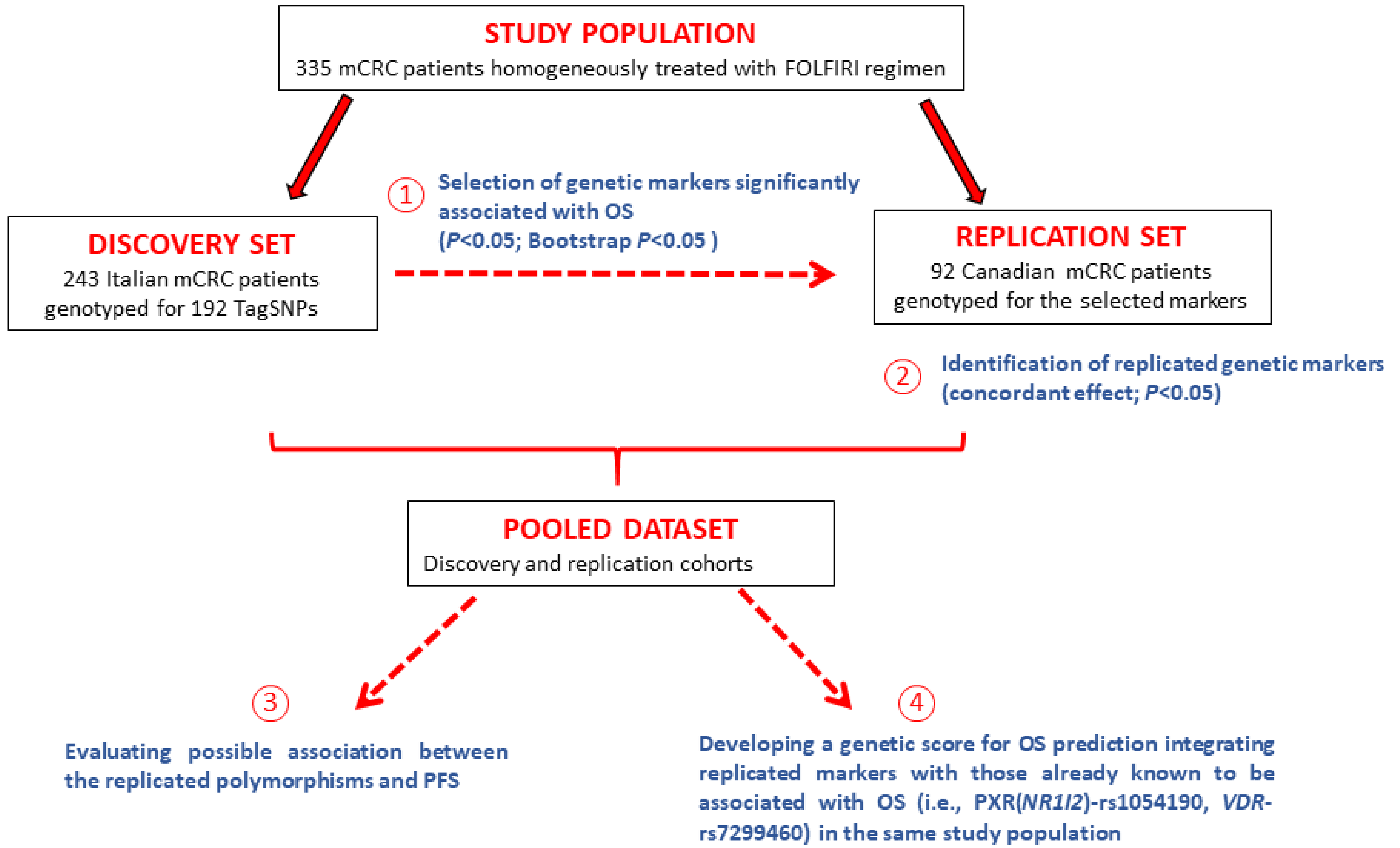
2. Patients and Methods
2.1. Patient Cohorts and Treatment
2.2. Marker Selection
2.3. Genetic Analysis
2.3.1. Discovery Cohort
2.3.2. Replication Cohort
2.4. Bioinformatic Analysis
2.5. Statistical Analysis
3. Results
3.1. Patients and Genotyping
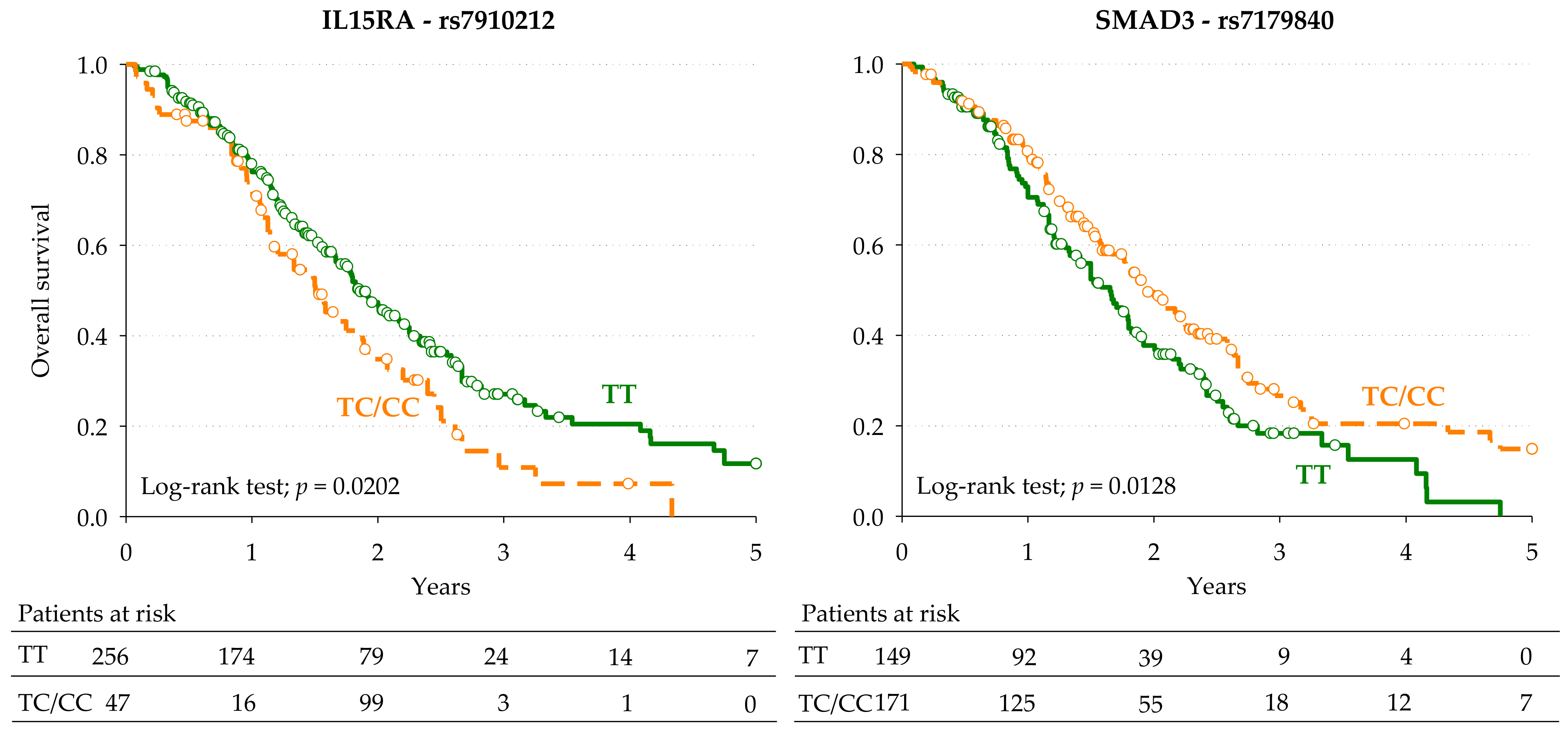
3.2. Markers of Overall Survival
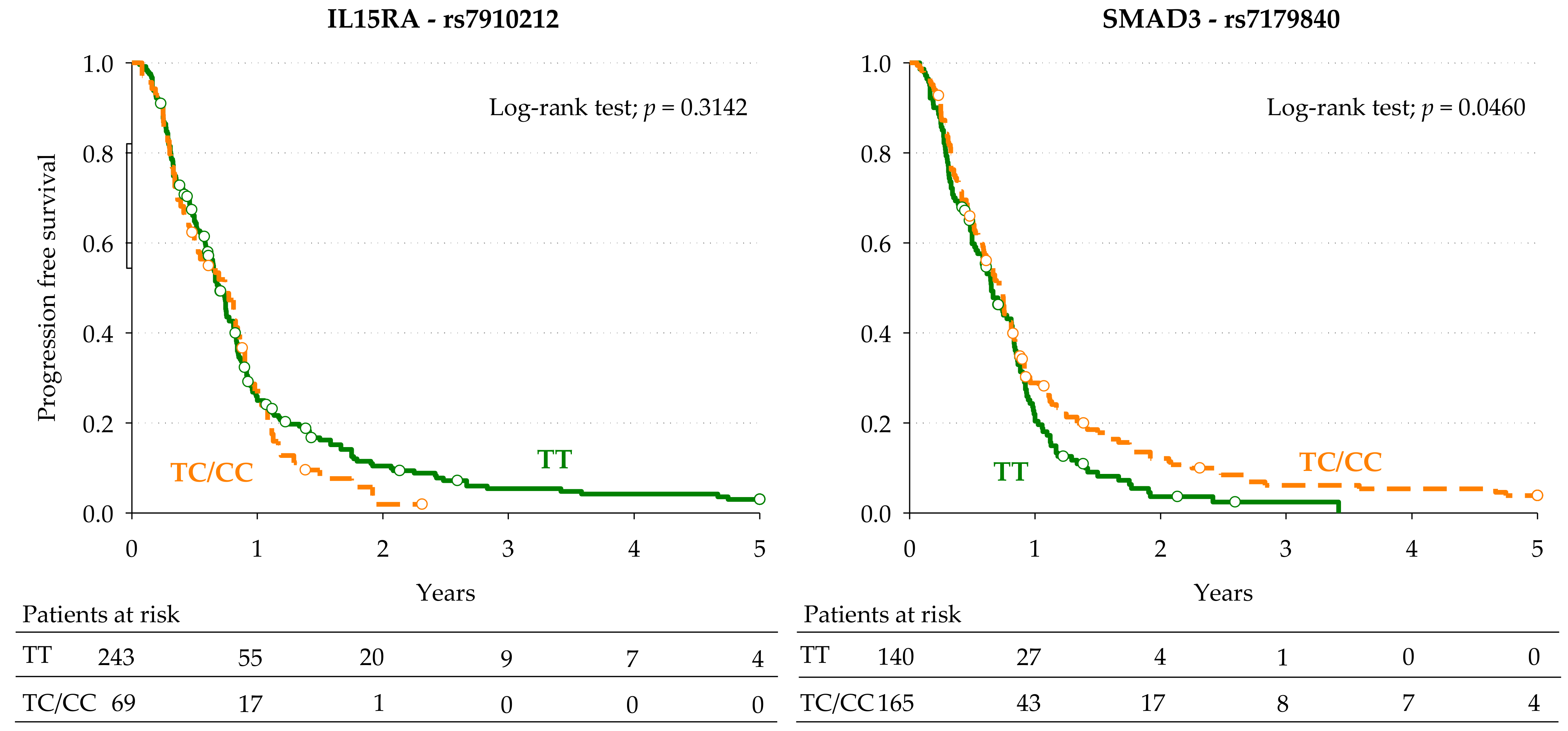
3.3. Markers of Progression-Free Survival
3.4. Prognostic Score for Overall Survival
3.5. Bioinformatic Analysis of IL15RA-rs7910212 and SMAD3-rs7179840
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Caro, G.; Marchesi, F.; Laghi, L.; Grizzi, F. Immune cells: Plastic players along colorectal cancer progression. J. Cell. Mol. Med. 2013, 17, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Fletcher, R.; Yu, J.; Zhang, L. Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis. 2018, 5, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Asleh, K.; Brauer, H.A.; Sullivan, A.; Lauttia, S.; Lindman, H.; Nielsen, T.O.; Joensuu, H.; Thompson, E.A.; Chumsri, S. Predictive Biomarkers for Adjuvant Capecitabine Benefit in Early-Stage Triple-Negative Breast Cancer in the FinXX Clinical Trial. Clin. Cancer Res. 2020, 26, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, W.H. Fluoropyrimidine Modulation of the Anti-Tumor Immune Response―Prospects for Improved Colorectal Cancer Treatment. Cancers 2020, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Kanterman, J.; Sade-Feldman, M.; Biton, M.; Ish-Shalom, E.; Lasry, A.; Goldshtein, A.; Hubert, A.; Baniyash, M. Adverse Immunoregulatory Effects of 5FU and CPT11 Chemotherapy on Myeloid-Derived Suppressor Cells and Colorectal Cancer Outcomes. Cancer Res. 2014, 74, 6022–6035. [Google Scholar] [CrossRef]
- Maeda, K.; Hazama, S.; Tokuno, K.; Kan, S.; Maeda, Y.; Watanabe, Y.; Kamei, R.; Shindo, Y.; Maeda, N.; Yoshimura, K.; et al. Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res. 2011, 31, 4569–4574. [Google Scholar]
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rébé, C.; Ghiringhelli, F. 5-Fluorouracil Selectively Kills Tumor-Associated Myeloid-Derived Suppressor Cells Resulting in Enhanced T Cell–Dependent Antitumor Immunity. Cancer Res. 2010, 70, 3052–3061. [Google Scholar] [CrossRef]
- Cecchin, E.; De Mattia, E.; Toffoli, G. Nuclear receptors and drug metabolism for the personalization of cancer therapy. Expert Opin. Drug Metab. Toxicol. 2016, 12, 291–306. [Google Scholar] [CrossRef]
- De Mattia, E.; Cecchin, E.; Roncato, R.; Toffoli, G. Pregnane X receptor, constitutive androstane receptor and hepatocyte nuclear factors as emerging players in cancer precision medicine. Pharmacogenomics 2016, 17, 1547–1571. [Google Scholar] [CrossRef]
- De Mattia, E.; Dreussi, E.; Cecchin, E.; Toffoli, G. Pharmacogenetics of the nuclear hormone receptors: The missing link between environment and drug effects? Pharmacogenomics 2013, 14, 2035–2054. [Google Scholar] [CrossRef]
- De Mattia, E.; Cecchin, E.; Montico, M.; Labriet, A.; Guillemette, C.; Dreussi, E.; Roncato, R.; Bignucolo, A.; Buonadonna, A.; D’Andrea, M.; et al. Association of STAT-3 rs1053004 and VDR rs11574077 with FOLFIRI-Related Gastrointestinal Toxicity in Metastatic Colorectal Cancer Patients. Front. Pharmacol. 2018, 9, 367. [Google Scholar] [CrossRef]
- De Mattia, E.; Polesel, J.; Roncato, R.; Labriet, A.; Bignucolo, A.; Dreussi, E.; Romanato, L.; Guardascione, M.; Buonadonna, A.; D’Andrea, M.; et al. Germline Polymorphisms in the Nuclear Receptors PXR and VDR as Novel Prognostic Markers in Metastatic Colorectal Cancer Patients Treated With FOLFIRI. Front. Oncol. 2019, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Labriet, A.; De Mattia, E.; Cecchin, E.; Lévesque, É.; Jonker, D.; Couture, F.; Buonadonna, A.; D’Andrea, M.; Villeneuve, L.; Toffoli, G.; et al. Improved Progression-Free Survival in Irinotecan-Treated Metastatic Colorectal Cancer Patients Carrying the HNF1A Coding Variant p.I27L. Front. Pharmacol. 2017, 8, 712. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Gherman, A.; Balacescu, L.; Gheorghe-Cetean, S.; Vlad, C.; Balacescu, O.; Irimie, A.; Lisencu, C. Current and New Predictors for Treatment Response in Metastatic Colorectal Cancer. The Role of Circulating miRNAs as Biomarkers. Int. J. Mol. Sci. 2020, 21, 2089. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Gil-Delgado, M.A.; Guinet, F.; Castaing, D.; Adam, R.; Coeffic, D.; Durrani, A.K.S.; Bismuth, H.; Khayat, D. Prospective Phase II Trial of Irinotecan, 5-Fluorouracil, and Leucovorin in Combination as Salvage Therapy for Advanced Colorectal Cancer. Am. J. Clin. Oncol. 2001, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Douillard, J.; Pirotta, N.; Alakl, M.; Gruia, G.; Awad, L.; Elfring, G.L.; Locker, P.K.; Miller, L.L. Irinotecan Plus Fluorouracil/Leucovorin for Metastatic Colorectal Cancer: A New Survival Standard. Oncology 2001, 6, 81–91. [Google Scholar] [CrossRef]
- Cecchin, E.; De Mattia, E.; Ecca, F.; Toffoli, G. Host genetic profiling to increase drug safety in colorectal cancer from discovery to implementation. Drug Resist. Updat. 2018, 39, 18–40. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, E.; Cecchin, E.; Toffoli, G. Pharmacogenomics of intrinsic and acquired pharmacoresistance in colorectal cancer: Toward targeted personalized therapy. Drug Resist. Updat. 2015, 20, 39–70. [Google Scholar] [CrossRef]
- Olivera, G.; Sendra, L.; Herrero, M.J.; Puig, C.; Aliño, S.F. Colorectal cancer: Pharmacogenetics support for the correct drug prescription. Pharmacogenomics 2019, 20, 741–763. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Marjaneh, R.; Khazaei, M.; Seifi, S.; Hassanian, S.M.; Ferns, G.A.; Avan, A. Pharmacogenetics of Anticancer Drug Sensitivity and Toxicity in Colorectal Cancer. Curr. Pharm. Des. 2018, 24, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Palmirotta, R.; Carella, C.; Silvestris, E.; Cives, M.; Stucci, S.L.; Tucci, M.; Lovero, D.; Silvestris, F. SNPs in predicting clinical efficacy and toxicity of chemotherapy: Walking through the quicksand. Oncotarget 2018, 9, 25355–25382. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, P.; Gou, M.; Yang, F.; Qian, N.; Dai, G. The Role of Serum CEA and CA19-9 in Efficacy Evaluations and Progression-Free Survival Predictions for Patients Treated with Cetuximab Combined with FOLFOX4 or FOLFIRI as a First-Line Treatment for Advanced Colorectal Cancer. Dis. Markers 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Suenaga, M.; Matsusaka, S.; Ueno, M.; Yamamoto, N.; Shinozaki, E.; Mizunuma, N.; Yamaguchi, T.; Hatake, K. Predictors of the efficacy of FOLFIRI plus bevacizumab as second-line treatment in metastatic colorectal cancer patients. Surg. Today 2011, 41, 1067–1074. [Google Scholar] [CrossRef]
- Shen, H.; Yang, J.; Huang, Q.; Jiang, M.-J.; Tan, Y.-N.; Fu, J.-F.; Zhu, L.-Z.; Fang, X.-F.; Yuan, Y. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J. Gastroenterol. 2015, 21, 6470–6478. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, E.; Innocenti, F.; D’Andrea, M.; Corona, G.; De Mattia, E.; Biason, P.; Buonadonna, A.; Toffoli, G. Predictive Role of the UGT1A1, UGT1A7, and UGT1A9 Genetic Variants and Their Haplotypes on the Outcome of Metastatic Colorectal Cancer Patients Treated with Fluorouracil, Leucovorin, and Irinotecan. J. Clin. Oncol. 2009, 27, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, G.; Cecchin, E.; Corona, G.; Russo, A.; Buonadonna, A.; D’Andrea, M.; Pasetto, L.M.; Pessa, S.; Errante, D.; De Pangher, V.; et al. The Role of UGT1A1*28 Polymorphism in the Pharmacodynamics and Pharmacokinetics of Irinotecan in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2006, 24, 3061–3068. [Google Scholar] [CrossRef]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef]
- Lévesque, É.; Bélanger, A.-S.; Harvey, M.; Couture, F.; Jonker, D.; Innocenti, F.; Cecchin, E.; Toffoli, G.; Guillemette, C. Refining theUGT1AHaplotype Associated with Irinotecan-Induced Hematological Toxicity in Metastatic Colorectal Cancer Patients Treated with 5-Fluorouracil/Irinotecan-Based Regimens. J. Pharmacol. Exp. Ther. 2013, 345, 95–101. [Google Scholar] [CrossRef]
- De Mattia, E.; Dreussi, E.; Montico, M.; Gagno, S.; Zanusso, C.; Quartuccio, L.; De Vita, S.; Guardascione, M.; Buonadonna, A.; D’Andrea, M.; et al. A Clinical-Genetic Score to Identify Surgically Resected Colorectal Cancer Patients Benefiting From an Adjuvant Fluoropyrimidine-Based Therapy. Front. Pharmacol. 2018, 9, 1101. [Google Scholar] [CrossRef]
- Illumina Website. Available online: https://illumina.com (accessed on 1 December 2020).
- HaploReg v4.1. Available online: https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php (accessed on 1 December 2020).
- RegulomeDB v2.0. Available online: https://regulomedb.org/regulome-search/) (accessed on 1 December 2020).
- Ensembl’s Variant Effect Predictor. Available online: https://www.ensembl.org/info/docs/tools/vep/index.html) (accessed on 1 December 2020).
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI)-dbSNP Database. Available online: http://www.ncbi.nlm.nih.gov/snp (accessed on 1 December 2020).
- Fumagalli, M.; Pozzoli, U.; Cagliani, R.; Comi, G.P.; Riva, S.; Clerici, M.; Bresolin, N.; Sironi, M. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J. Exp. Med. 2009, 206, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Grizzi, F.; Bianchi, P.; Malesci, A.; Laghi, L. Prognostic value of innate and adaptive immunity in colorectal cancer. World J. Gastroenterol. 2013, 19, 174–184. [Google Scholar] [CrossRef]
- Lasry, A.; Zinger, A.; Ben-Neriah, A.L.A.Z.Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef]
- Markman, J.L.; Shiao, S.L. Impact of the immune system and immunotherapy in colorectal cancer. J. Gastrointest. Oncol. 2015, 6, 208–223. [Google Scholar] [PubMed]
- Tuomisto, E.A.; Mäkinen, M.J.; Väyrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.C.; Waldmann, T.A.; Morris, J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012, 33, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luan, L.; Patil, N.K.; Sherwood, E.R. Immunobiology of the IL-15/IL-15Rα complex as an antitumor and antiviral agent. Cytokine Growth Factor Rev. 2017, 38, 10–21. [Google Scholar] [CrossRef]
- Bergh, J.M.V.D.; Lion, E.; Van Tendeloo, V.F.; Smits, E.L. IL-15 receptor alpha as the magic wand to boost the success of IL-15 antitumor therapies: The upswing of IL-15 transpresentation. Pharmacol. Ther. 2017, 170, 73–79. [Google Scholar] [CrossRef]
- Cui, F.; Qu, D.; Sun, R.; Zhang, M.; Nan, K. NK cell-produced IFN-γ regulates cell growth and apoptosis of colorectal cancer by regulating IL-15. Exp. Ther. Med. 2019, 19, 1400–1406. [Google Scholar] [CrossRef]
- Yu, P.; Steel, J.C.; Zhang, M.; Morris, J.C.; Waldmann, T.A. Simultaneous Blockade of Multiple Immune System Inhibitory Checkpoints Enhances Antitumor Activity Mediated by Interleukin-15 in a Murine Metastatic Colon Carcinoma Model. Clin. Cancer Res. 2010, 16, 6019–6028. [Google Scholar] [CrossRef]
- Kobayashi, H.; Dubois, S.; Sato, N.; Sabzevari, H.; Sakai, Y.; Waldmann, T.A.; Tagaya, Y. Role of trans-cellular IL-15 presentation in the activation of NK cell–mediated killing, which leads to enhanced tumor immunosurveillance. Blood 2005, 105, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Black, J.D.; Troutt, A.B.; Rustum, Y.M. Interleukin 15 offers selective protection from irinotecan-induced intestinal toxicity in a preclinical animal model. Cancer Res. 1998, 58, 3270–3274. [Google Scholar] [PubMed]
- Cao, S.; Troutt, A.B.; Rustum, Y.M. Interleukin 15 protects against toxicity and potentiates antitumor activity of 5-fluorouracil alone and in combination with leucovorin in rats bearing colorectal cancer. Cancer Res. 1998, 58, 1695–1699. [Google Scholar] [PubMed]
- Shinohara, H.; Bucana, C.D.; Killion, J.J.; Fidler, I.J. Intensified Regression of Colon Cancer Liver Metastases in Mice Treated with Irinotecan and the Immunomodulator JBT 3002. J. Immunother. 2000, 23, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Forootan, F.S.; Esfahani, M.H.N.; Ghaedi, K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Batlle, E. Targeting the Microenvironment in Advanced Colorectal Cancer. Trends Cancer 2016, 2, 495–504. [Google Scholar] [CrossRef]
- Liu, X.; Ji, Q.; Fan, Z.; Li, Q. Cellular signaling pathways implicated in metastasis of colorectal cancer and the associated targeted agents. Future Oncol. 2015, 11, 2911–2922. [Google Scholar] [CrossRef]
- Romano, G.; Santi, L.; Bianco, M.R.; Giuffrè, M.R.; Pettinato, M.; Bugarin, C.; Garanzini, C.; Savarese, L.; Leoni, S.; Cerrito, M.G.; et al. The TGF-β pathway is activated by 5-fluorouracil treatment in drug resistant colorectal carcinoma cells. Oncotarget 2016, 7, 22077–22091. [Google Scholar] [CrossRef]
- Jung, B.; Staudacher, J.J.; Beauchamp, D. Transforming Growth Factor β Superfamily Signaling in Development of Colorectal Cancer. Gastroenterology 2017, 152, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Herrick, J.S.; Lundgreen, A.; Wolff, R.K. Genetic Variation in the TGF-β Signaling Pathway and Colon and Rectal Cancer Risk. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Lundgreen, A. The Influence of the CHIEF Pathway on Colorectal Cancer-Specific Mortality. PLoS ONE 2014, 9, e116169. [Google Scholar] [CrossRef]
- Huang, M.-Y.; Lin, C.-H.; Huang, C.-M.; Tsai, H.-L.; Huang, C.-W.; Yeh, Y.-S.; Chai, C.-Y.; Wang, J.-Y. Relationships Between SMAD3 Expression and Preoperative Fluoropyrimidine-Based Chemoradiotherapy Response in Locally Advanced Rectal Cancer Patients. World J. Surg. 2015, 39, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.U.; Kang, M.H.; Sung, J.H.; Kim, J.W.; Lee, J.O.; Kim, Y.J.; Lee, K.W.; Bang, S.M.; Lee, J.S.; Kim, J.H. Effect of Smad3/4 on chemotherapeutic drug sensitivity in colorectal cancer cells. Oncol. Rep. 2014, 33, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Juliá, E.P.; Mordoh, J.; Levy, E.M. Cetuximab and IL-15 Promote NK and Dendritic Cell Activation In Vitro in Triple Negative Breast Cancer. Cells 2020, 9, 1573. [Google Scholar] [CrossRef]
- Pinette, A.; McMichael, E.; Courtney, N.B.; Duggan, M.; Benner, B.N.; Choueiry, F.; Yu, L.; Abood, D.; Mace, T.A.; Carson, W.E. An IL-15-based superagonist ALT-803 enhances the NK cell response to cetuximab-treated squamous cell carcinoma of the head and neck. Cancer Immunol. Immunother. 2019, 68, 1379–1389. [Google Scholar] [CrossRef]
- Rocca, Y.S.; Roberti, M.P.; Juliá, E.P.; Pampena, M.B.; Bruno, L.; Rivero, S.; Huertas, E.; Loria, F.S.; Pairola, A.; Caignard, A.; et al. Phenotypic and Functional Dysregulated Blood NK Cells in Colorectal Cancer Patients Can Be Activated by Cetuximab Plus IL-2 or IL-15. Front. Immunol. 2016, 7, 413. [Google Scholar] [CrossRef]
- Yegodayev, K.M.; Novoplansky, O.; Golden, A.; Prasad, M.; Levin, L.; Jagadeeshan, S.; Zorea, J.; Dimitstein, O.; Joshua, B.-Z.; Cohen, L.; et al. TGF-Beta-Activated Cancer-Associated Fibroblasts Limit Cetuximab Efficacy in Preclinical Models of Head and Neck Cancer. Cancers 2020, 12, 339. [Google Scholar] [CrossRef]
- Yu, W.-D.; Sun, G.; Li, J.; Xu, J.; Wang, X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019, 452, 66–70. [Google Scholar] [CrossRef]
| Discovery | Replication | ||||
|---|---|---|---|---|---|
| N | (%) | N | (%) | ||
| Gender | |||||
| Male | 158 | (65.0) | 61 | (66.3) | |
| Female | 85 | (35.0) | 31 | (33.7) | p = 0.8255 |
| Age (years) | |||||
| <55 | 61 | (25.1) | 23 | (25.0) | |
| 55–59 | 32 | (13.2) | 18 | (19.6) | |
| 60–64 | 52 | (21.4) | 17 | (18.5) | |
| ≥65 | 98 | (40.3) | 34 | (37.0) | p = 0.5128 |
| Cancer site | |||||
| Right colon | 76 | (31.3) | 23 | (25.0) | |
| Left colon/Rectum | 167 | (68.7) | 61 | (66.3) | |
| Colon, NOS | 0 | (0.0) | 8 | (8.7) | p = 0.5030 b |
| Stage at cancer diagnosis | |||||
| I–II | 24 | (9.9) | |||
| III | 64 | (26.3) | |||
| IV | 155 | (63.8) | |||
| Radical surgery | |||||
| No | 50 | (20.6) | |||
| Yes | 193 | (79.4) | |||
| Adjuvant therapy | |||||
| No | 163 | (67.1) | |||
| Yes | 80 | (32.9) | |||
| Overall survival (95% CI) | |||||
| 1 year | 74.5% (68.5–80.0%) | 72.1% (61.3–80.3%) | |||
| 2 years | 41.6% (34.0–48.9%) | 41.9% (31.4–52.0%) | |||
| 3 years | 26.5% (18.4–35.4%) | 20.1% (13.1–30.0%) | |||
| 5 years | 9.2% (1.1–28.6%) | 8.1% (3.6–15.1%) | p = 0.4867 | ||
| Discovery Cohort | Replication Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genes | SNP | Base Change | Model | HR (95% CI) a | p-Value | Bootstrap | HR (95% CI) b | p-Value c | |
| HR | p-Value | ||||||||
| FAS | rs983751 | G > T | Dominant | 1.60 (1.03–2.47) | 0.0366 | 1.65 | 0.0319 | 1.82 (0.52–6.40) | 0.1760 |
| FAS | rs9658706 | A > G | Dominant | 1.84 (1.18–2.87) | 0.0075 | 1.88 | 0.0094 | 0.88 (0.42–1.85) | |
| FOXO3 | rs9384683 | T > G | Recessive | 4.39 (1.31–14.67) | 0.0163 | 4.53 | 0.0185 | --- | --- |
| MIF | rs738806 | G > A | Dominant | 1.55 (1.08–2.22) | 0.0184 | 1.56 | 0.0217 | 1.06 (0.65–1.72) | 0.4044 |
| IFNGR2 | rs1532 | C > T | Dominant | 0.53 (0.37–0.75) | 0.0005 | 0.51 | 0.0005 | 1.05 (0.66–1.67) | |
| IFNGR2 | rs9808753 | A > G | Additive | 1.57 (1.06–2.33) | 0.0249 | 1.66 | 0.0165 | 1.13 (0.69–1.83) | 0.3159 |
| IL15RA | rs1998521 | G > A | Additive | 0.72 (0.54–0.95) | 0.0211 | 0.71 | 0.0215 | 1.03 (0.73–1.46) | |
| IL15RA | rs2228059 | A > C | Additive | 1.45 (1.12–1.88) | 0.0051 | 1.49 | 0.0042 | 0.99 (0.71–1.39) | |
| IL15RA | rs3136626 | T > C | Dominant | 1.56 (1.07–2.26) | 0.0196 | 1.60 | 0.0169 | 1.02 (0.64–1.63) | 0.4639 |
| IL15RA | rs7910212 | T > C | Dominant | 1.57 (1.04–2.39) | 0.0327 | 1.62 | 0.0280 | 1.71 (0.93–3.12) | 0.0411 |
| SMAD3 | rs11636161 | G > A | Additive | 1.32 (1.03–1.70) | 0.0282 | 1.34 | 0.0297 | 1.27 (0.89–1.80) | 0.0963 |
| SMAD3 | rs1545161 | T > C | Dominant | 0.57 (0.40–0.83) | 0.0029 | 0.56 | 0.0036 | 0.79 (0.48–1.31) | 0.1809 |
| SMAD3 | rs3743343 | T > C | Recessive | 3.72 (1.10–12.55) | 0.0345 | 3.79 | 0.0479 | 0.42 (0.08–2.23) | |
| SMAD3 | rs7179840 | T > C | Dominant | 0.65 (0.45–0.93) | 0.0202 | 0.64 | 0.0203 | 0.61 (0.37–0.99) | 0.0216 |
| SMAD3 | rs718663 | A > G | Dominant | 1.75 (1.03–2.97) | 0.0391 | 1.76 | 0.0502 | 1.43 (0.77–2.64) | 0.1280 |
| STAT3 | rs17405722 | G > A | Recessive | 35.31 (4.14–300.87) | 0.0011 | 39.62 | 0.0015 | 0.78 (0.10–6.09) | |
| STAT3 | rs3744483 | T > C | Dominant | 0.61 (0.41–0.90) | 0.0125 | 0.60 | 0.0144 | 1.23 (0.74–2.05) | |
| STAT5A | rs7217728 | T > C | Dominant | 0.69 (0.48–0.99) | 0.0463 | 0.67 | 0.0404 | 0.95 (0.61–1.49) | 0.4129 |
| STAT6 | rs167769 | C > T | Dominant | 1.81 (1.25–2.64) | 0.0019 | 1.87 | 0.0019 | 0.97 (0.59–1.60) | |
| TGFBR2 | rs12487185 | A > G | Dominant | 0.63 (0.44–0.92) | 0.0152 | 0.64 | 0.0226 | 1.03 (0.64–1.65) | |
| TGFBR2 | rs4583693 | T > C | Recessive | 3.14 (1.47–6.71) | 0.0031 | 3.33 | 0.0027 | 1.36 (0.40–4.56) | 0.3115 |
| TGFBR2 | rs5020833 | C > G | Dominant | 0.62 (0.43–0.90) | 0.0125 | 0.62 | 0.0154 | 1.16 (0.72–1.85) | |
| TLR10 | rs11466657 | T > C | Additive | 0.51 (0.30–0.88) | 0.0206 | 0.50 | 0.0159 | 0.97 (0.28–3.41) | 0.4822 |
| Gene-SNP | HR (95% CI) a | HR (95% CI) b | Score Points |
|---|---|---|---|
| IL15RA-rs7910212 (TC/CC vs. TT) | 1.55 (1.12–2.15) | 1.66 (1.19–2.31) | 1 |
| SMAD3-rs7179840 (TT vs. TC/CC) | 1.53 (1.15–2.04) | 1.54 (1.15–2.06) | 1 |
| VDR-rs7299460 (CC vs. CT/TT) | 1.53 (1.15–2.03) | 1.48 (1.10–1.99) | 1 |
| NR1I2-rs1054190 (TT vs. CC/CT) | 4.61 (1.97–10.81) | 4.31 (1.82–10.20) | 4 |
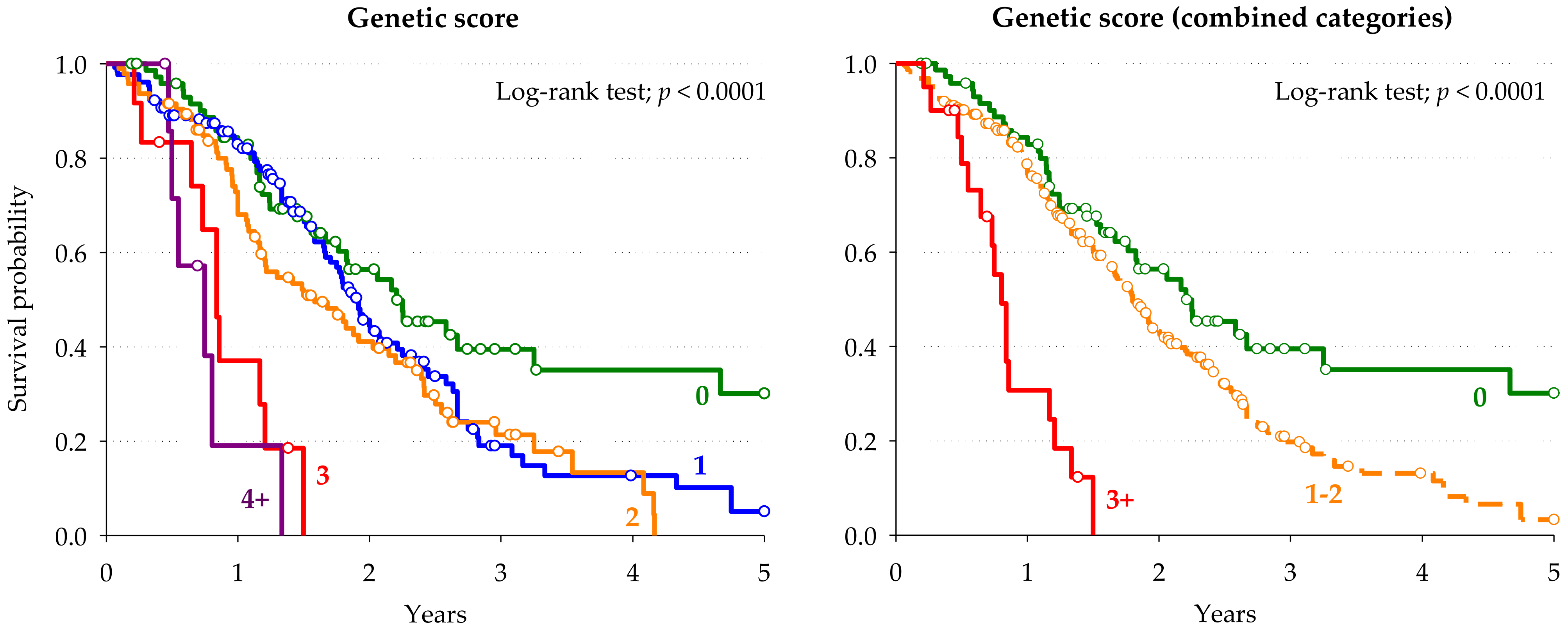
| Genetic Score | Genetic Score (Combined Categories) | |||||||
|---|---|---|---|---|---|---|---|---|
| Score Points | Patients | HR (95% CI) a | Score Points | Patients | HR (95% CI) a | p-Value | ||
| n | (%) | n | (%) | |||||
| 0 | 77 | (24.1) | Reference | 0 | 77 | (24.1) | Reference | |
| 1 | 129 | (40.3) | 1.72 (1.15–2.57) | 1–2 | 223 | (69.7) | 1.90 (1.31–2.76) | 0.0007 |
| 2 | 94 | (29.4) | 2.21 (1.45–3.38) | |||||
| 3 | 12 | (3.8) | 6.93 (3.34–14.39) | ≥3 | 20 | (6.3) | 7.37 (3.93–13.84) | <0.0001 |
| ≥4 | 8 | (2.5) | 8.57 (3.43–21.36) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Mattia, E.; Polesel, J.; Roncato, R.; Labriet, A.; Bignucolo, A.; Gagno, S.; Buonadonna, A.; D’Andrea, M.; Lévesque, E.; Jonker, D.; et al. IL15RA and SMAD3 Genetic Variants Predict Overall Survival in Metastatic Colorectal Cancer Patients Treated with FOLFIRI Therapy: A New Paradigm. Cancers 2021, 13, 1705. https://doi.org/10.3390/cancers13071705
De Mattia E, Polesel J, Roncato R, Labriet A, Bignucolo A, Gagno S, Buonadonna A, D’Andrea M, Lévesque E, Jonker D, et al. IL15RA and SMAD3 Genetic Variants Predict Overall Survival in Metastatic Colorectal Cancer Patients Treated with FOLFIRI Therapy: A New Paradigm. Cancers. 2021; 13(7):1705. https://doi.org/10.3390/cancers13071705
Chicago/Turabian StyleDe Mattia, Elena, Jerry Polesel, Rossana Roncato, Adrien Labriet, Alessia Bignucolo, Sara Gagno, Angela Buonadonna, Mario D’Andrea, Eric Lévesque, Derek Jonker, and et al. 2021. "IL15RA and SMAD3 Genetic Variants Predict Overall Survival in Metastatic Colorectal Cancer Patients Treated with FOLFIRI Therapy: A New Paradigm" Cancers 13, no. 7: 1705. https://doi.org/10.3390/cancers13071705
APA StyleDe Mattia, E., Polesel, J., Roncato, R., Labriet, A., Bignucolo, A., Gagno, S., Buonadonna, A., D’Andrea, M., Lévesque, E., Jonker, D., Couture, F., Guillemette, C., Cecchin, E., & Toffoli, G. (2021). IL15RA and SMAD3 Genetic Variants Predict Overall Survival in Metastatic Colorectal Cancer Patients Treated with FOLFIRI Therapy: A New Paradigm. Cancers, 13(7), 1705. https://doi.org/10.3390/cancers13071705