Succinate Pathway in Head and Neck Squamous Cell Carcinoma: Potential as a Diagnostic and Prognostic Marker
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Measurement of Circulating Succinate
2.3. Gene Expression Analysis
2.4. Histology
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Patients Included in the Study
3.2. Circulating Levels of the Oncometabolite Succinate Are Elevated in Patients with HNSCC
3.3. SUCNR1 Is Predominantly Expressed in Tumoral Mucosa and Positively Associates with Plasma Succinate
3.4. SUCNR1 and SDHA Gene Expression in Tumoral Tissue Is Associated with Locoregional Disease-Free Survival
3.5. Relationship between SUCNR1/SDHA Profiles and Clinicopathological Variables
3.6. SUCNR1/SDHA Profiles Are Independent Predictors of HNSCC Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Orit, K.-P.; Ziv, G.; Salem, B. Precision medicine in head and neck cancer. Drug Resist. Updates 2018, 40, 13–16. [Google Scholar]
- Dando, I.; Pozza, E.D.; Ambrosini, G.; Torrens-Mas, M.; Butera, G.; Mullappilly, N.; Pacchiana, R.; Palmieri, M.; Donadelli, M. Oncometabolites in cancer aggressiveness and tumour repopulation. Biol. Rev. 2019, 94, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Li, J.T.; Qu, J.; Yin, M.; Lei, Q.Y. Metabolite sensing and signaling in cancer. J. Biol. Chem. 2020, 295, 11938–11946. [Google Scholar] [CrossRef]
- Eijkelenkamp, K.; Osinga, T.E.; Links, T.P.; van der Horst-Schrivers, A.N.A. Clinical implications of the oncometabolite succinate in SDHx-mutation carriers. Clin. Genet. 2020, 97, 39–53. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- La Vecchia, S.; Sebastián, C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin. Cell Dev. Biol. 2020, 98, 63–70. [Google Scholar] [CrossRef]
- Nanda, C.; Venkateswaran, S.V.; Patani, N.; Yuneva, M. Defining a metabolic landscape of tumours: Genome meets metabolism. Br. J. Cancer 2020, 122, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Stewart, G.D.; Frezza, C. Oncometabolites in renal cancer. Nat. Rev. Nephrol. 2020, 16, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feng, F.; Guo, Q.H.; Wang, Y.-P.; Zhao, R. Role of succinate dehydrogenase deficiency and oncometabolites in gastrointestinal stromal tumors. World J Gastroenterol. 2020, 26, 5074–5089. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, T.; Lleonart, M.E.; Takaori-Kondo, A.; Inagaki, N.; Yokode, M.; Kondoh, H. Dysregulated glycolysis as an oncogenic event. Cell Mol. Life Sci. 2015, 72, 1881–1892. [Google Scholar] [CrossRef]
- Schaefer, I.M.; Hornick, J.L.; Bovée, J.V.M.G. The role of metabolic enzymes in mesenchymal tumors and tumor syndromes: Genetics, pathology, and molecular mechanisms. Lab. Investig. 2018, 98, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Masiá, D.; Gisbert-Ferrándiz, L.; Bauset, C.; Coll, S.; Mamie, C.; Scharl, M.; Esplugues, J.V.; Alós, R.; Navarro, F.; Cosín-Roger, J.; et al. Succinate Activates EMT in Intestinal Epithelial Cells through SUCNR1: A Novel Protagonist in Fistula Development. Cells 2020, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhao, T.; Xu, C.; Shi, W.; Geng, B.; Shen, J.; Zhang, C.; Pan, J.; Yang, J.; Hu, S.; et al. Oncometabolite succinate promotes angiogenesis by upregulating VEGF expression through GPR91-mediated STAT3 and ERK activation. Oncotarget 2017, 8, 13174–13185. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Huang, T.W.; Hsieh, Y.T.; Wang, Y.F.; Yen, C.C.; Lee, G.L.; Yeh, C.C.; Peng, Y.J.; Kuo, Y.Y.; Wen, H.T.; et al. Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol. Cell 2020, 77, 213–227.e5. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Yang, Y.; Wang, Q. Association Between Succinate Receptor SUCNR1 Expression and Immune Infiltrates in Ovarian Cancer. Front. Mol. Biosci. 2020, 7, 150. [Google Scholar] [CrossRef]
- Serena, C.; Ceperuelo-Mallafré, V.; Keiran, N.; Queipo-Ortuño, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Pérez-Brocal, V.; Andrés-Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef]
- Chiesa, F.; Tradati, N.; Mauri, S.; Calabrese, L.; Grigolato, R.; Giugliano, G.; Zurrida, S.; Chiesa, E.; Squadrelli, M.; Viale, G.; et al. Prognostic factors in head and neck oncology: A critical appraisal for use in clinical practice. Anticancer Res. 1998, 18, 4769–4776. [Google Scholar]
- Ceperuelo-Mallafre, V.; Llaurado, G.; Keiran, N.; Benaiges, E.; Astiarraga, B.; Martínez, L.; Pellitero, S.; González-Clemente, J.M.; Rodríguez, A.; Fernández-Real, J.M.; et al. Preoperative circulating succinate levels as a biomarker for diabetes remission after bariatric surgery. Diabetes Care 2019, 42, 1956–1965. [Google Scholar] [CrossRef]
- Fernández-Veledo, S.; Vendrell, J. Gut microbiota-derived succinate: Friend or foe in human metabolic diseases? Rev. Endocr. Metab. Disord. 2019, 20, 439–447. [Google Scholar] [CrossRef]
- Li, X.; Xie, L.; Qu, X.; Zhao, B.; Fu, W.; Wu, B.; Wu, J. GPR91, a critical signaling mechanism in modulating pathophysiologic processes in chronic illnesses. FASEB J. 2020, 34, 13091–13105. [Google Scholar] [CrossRef] [PubMed]
- Lussey-Lepoutre, C.; Bellucci, A.; Morin, A.; Buffet, A.; Amar, L.; Janin, M.; Ottolenghi, C.; Zinzindohoué, F.; Autret, G.; Burnichon, N.; et al. In vivo detection of succinate by magnetic resonance spectroscopy as a hallmark of SDHx mutations in paraganglioma. Clin. Cancer Res. 2016, 22, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Yokota, A.; Harada, H.; Huang, G. Hypoxia/pseudohypoxia-mediated activation of hypoxia-inducible factor-1α in cancer. Cancer Sci. 2019, 110, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, T.; Du, X.; Wu, Q.; Le, Y.Z. G protein-coupled receptor 91 signaling in diabetic retinopathy and hypoxic retinal diseases. Vis. Res. 2017, 139, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Moreno, C.; Santos, R.M.; Burns, R.; Zhang, W.C. Succinate dehydrogenase and ribonucleic acid networks in cancer and other diseases. Cancers 2020, 12, 3237. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, J.; Seong, K.C.; Bisambar, C.; Madhu, B.; Allinson, K.; Marker, A.; Warren, A.; Park, S.M.; Giger, O.; Challis, B.G.; et al. A review of the tumour spectrum of germline succinate dehydrogenase gene mutations: Beyond phaeochromocytoma and paraganglioma. Clin. Endocrinol. 2020, 93, 528–538. [Google Scholar] [CrossRef]
- Li, J.; Liang, N.; Long, X.; Zhao, J.; Yang, J.; Du, X.; Yang, T.; Yuan, P.; Huang, X.; Zhang, J.; et al. SDHC-related deficiency of SDH complex activity promotes growth and metastasis of hepatocellular carcinoma via ROS/NFκB signaling. Cancer Lett. 2019, 461, 44–55. [Google Scholar] [CrossRef]
- Schöpf, B.; Weissensteiner, H.; Schäfer, G.; Fazzini, F.; Charoentong, P.; Naschberger, A.; Rupp, B.; Fendt, L.; Bukur, V.; Giese, I.; et al. OXPHOS remodeling in high-grade prostate cancer involves mtDNA mutations and increased succinate oxidation. Nat. Commun. 2020. [Google Scholar] [CrossRef]
- Dalla Pozza, E.; Dando, I.; Pacchiana, R.; Liboi, E.; Scupoli, M.; Donadelli, M.; Palmieri, M. Regulation of succinate dehydrogenase and role of succinate in cancer. Semin. Cell Dev. Biol. 2020, 98, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ristic, B.; Bhutia, Y.D.; Ganapathy, V. Cell-surface G-protein-coupled receptors for tumor-associated metabolites: A direct link to mitochondrial dysfunction in cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Pamplona, R.; Berenguer, A.; Cordero, D.; Molleví, D.G.; Crous-Bou, M.; Sole, X.; Paré-Brunet, L.; Guino, E.; Salazar, R.; Santos, C.; et al. Aberrant gene expression in mucosa adjacent to tumor reveals a molecular crosstalk in colon cancer. Mol. Cancer 2008, 13, 28–30. [Google Scholar] [CrossRef]
- Ikeda, Y.; Suzuki, T.; Saitou, H.; Ogane, S.; Hashimoto, K.; Takano, N.; Nomura, T. Usefulness of fluorescence visualization-guided surgery for early-stage tongue squamous cell carcinoma compared to iodine vital staining. Int. J. Clin. Oncol. 2020, 25, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Wozny, A.S.; Alphonse, G.; Cassard, A.; Malésys, C.; Louati, S.; Beuve, M.; Lalle, P.; Ardail, D.; Nakajima, T.; Rodriguez-Lafrasse, C. Impact of hypoxia on the double-strand break repair after photon and carbon ion irradiation of radioresistant HNSCC cells. Sci. Rep. 2020, 10, 21357. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Reoyo, S.; Roig-Lopez, J.L.; Yang, E.S. Potential biomarkers for radiosensitivity in head and neck cancers. Ann. Transl. Med. 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yan, W. Succinate in the cancer–immune cycle. Cancer Lett. 2017, 390, 45–47. [Google Scholar] [CrossRef]
- Xiang, K.; Jendrossek, V.; Matschke, J. Oncometabolites and the response to radiotherapy. Radiat. Oncol. 2020, 15, 197. [Google Scholar] [CrossRef] [PubMed]
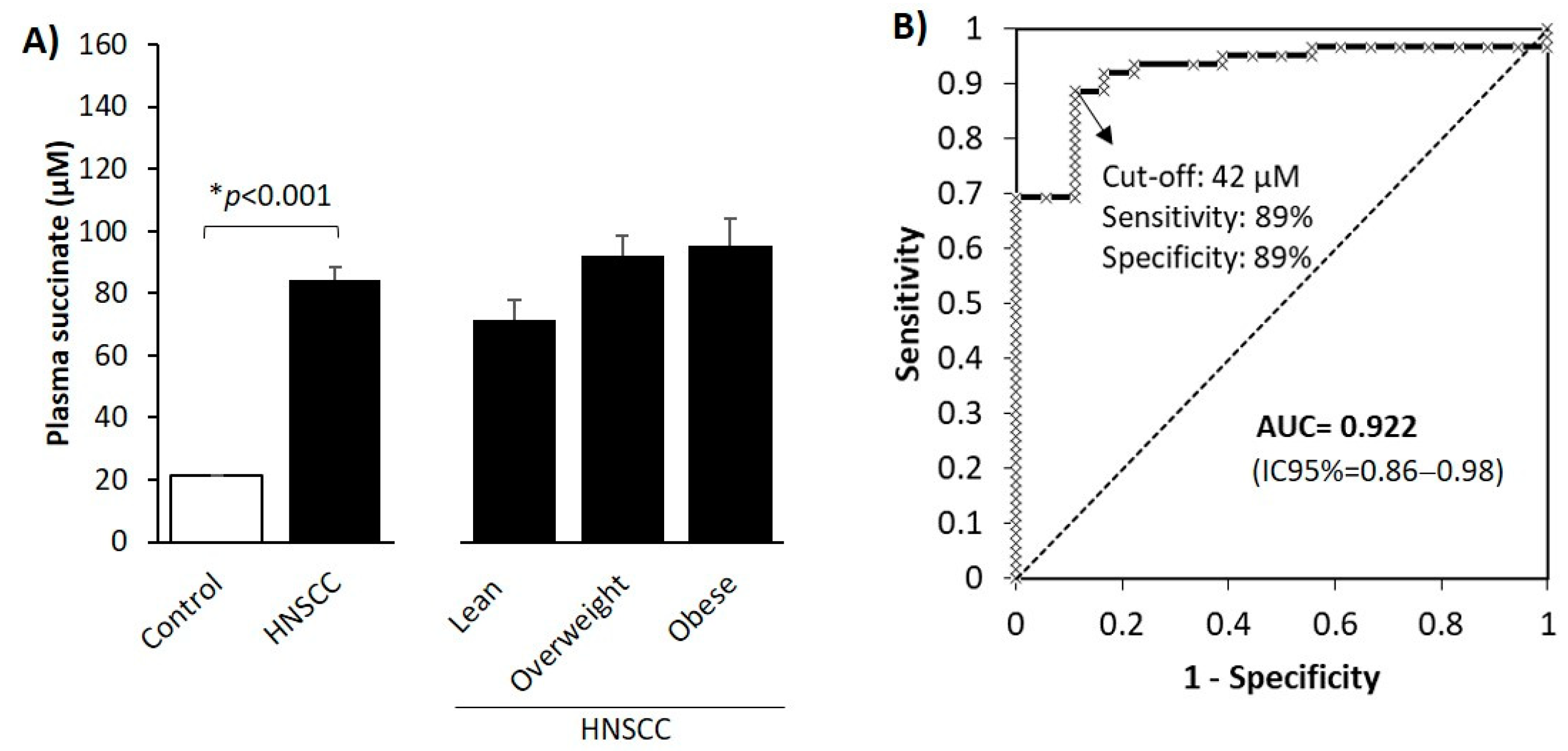
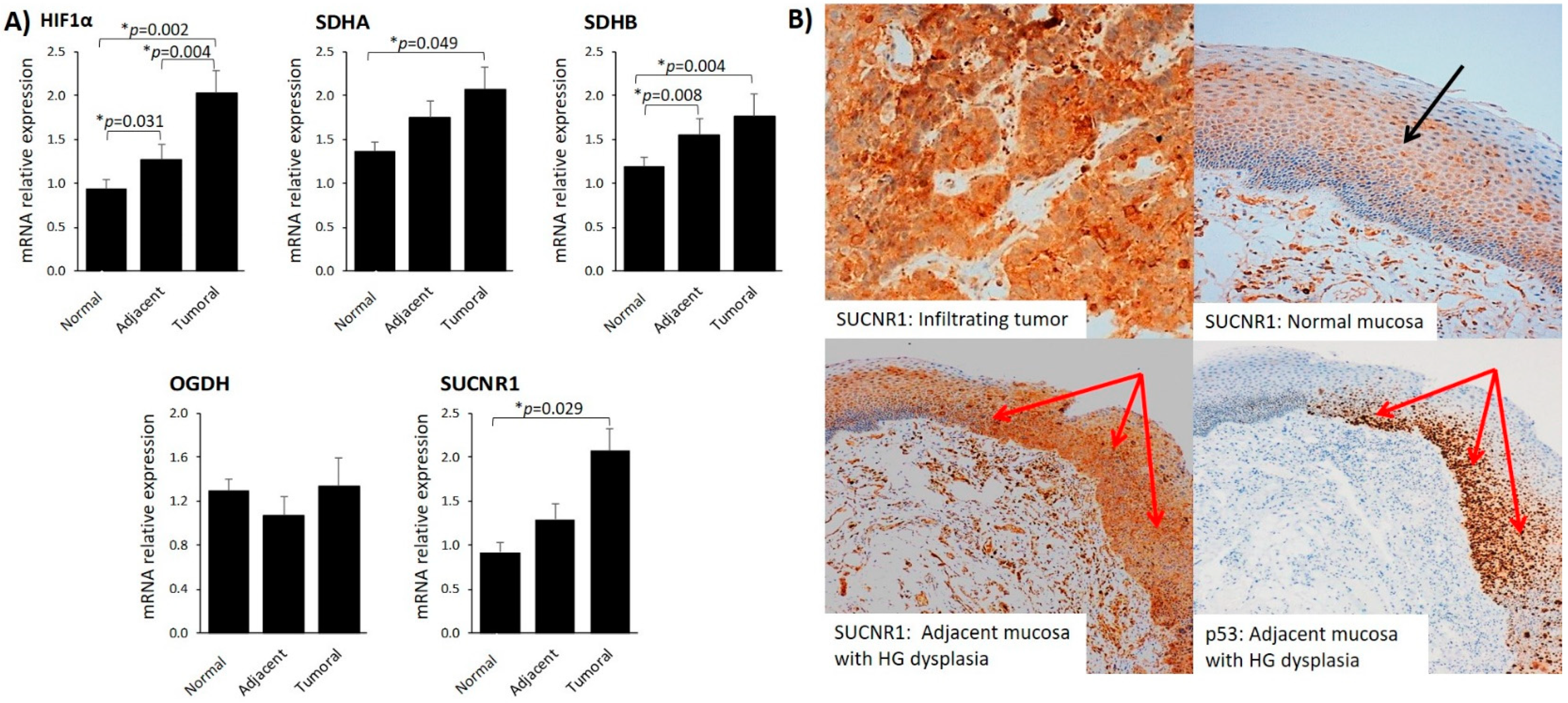
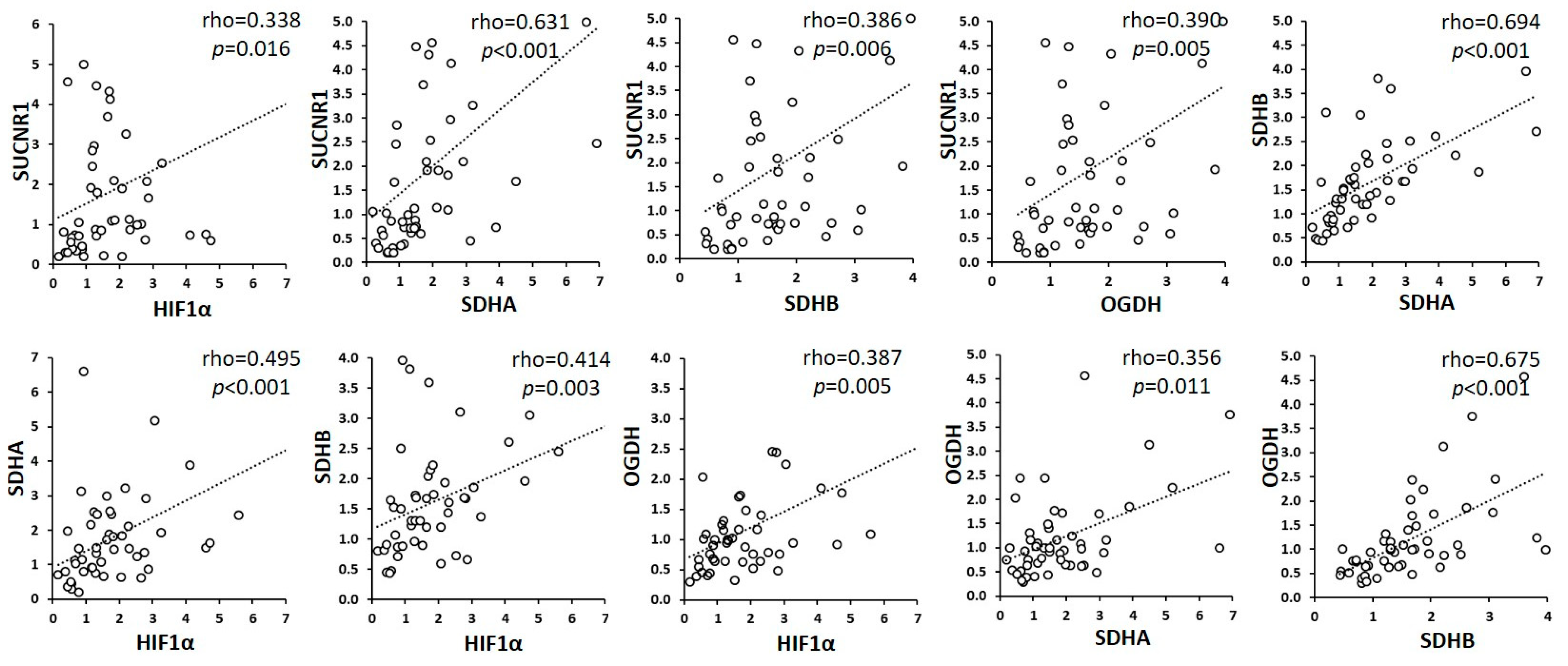
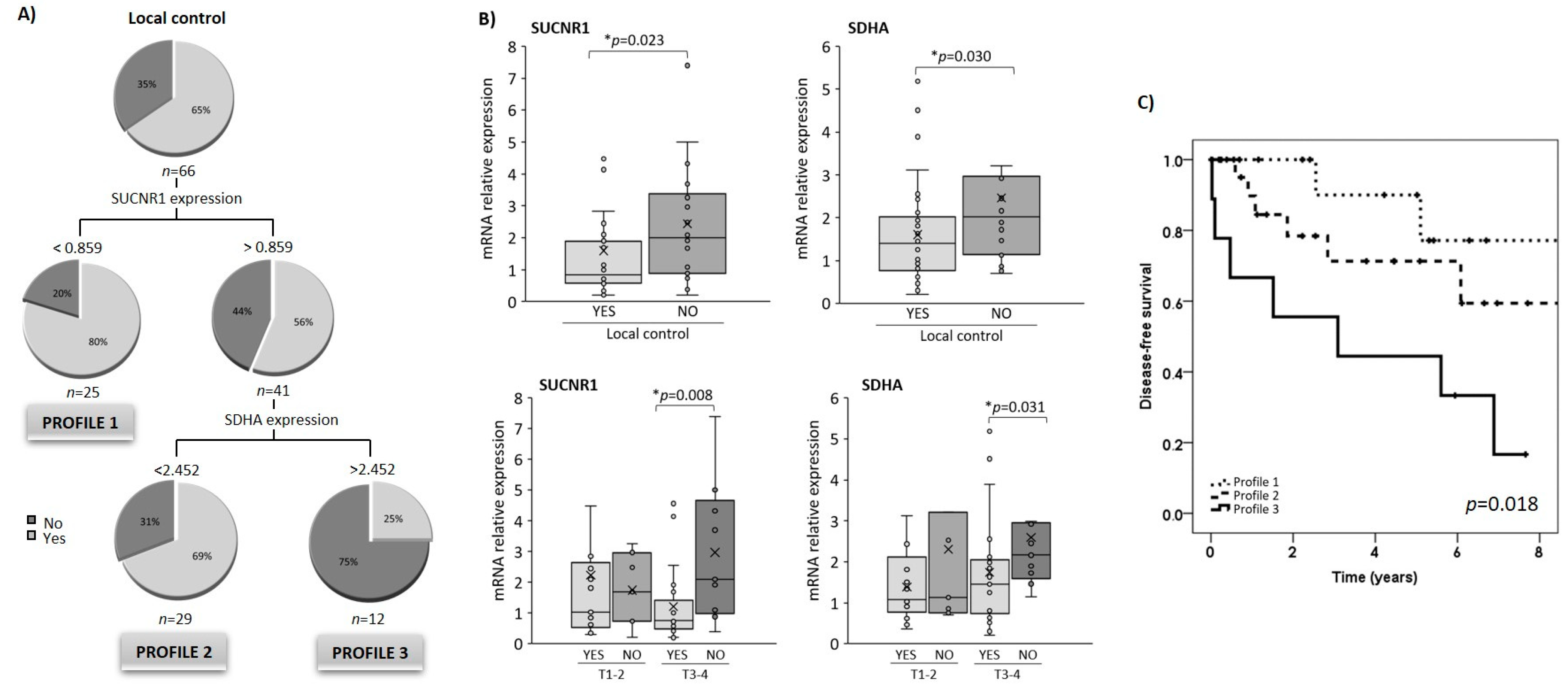
| Characteristics | Num. Patients (%) | Disease-Free Survival % (95% CI) | p-Value |
|---|---|---|---|
| Age (years) | 0.256 | ||
| <50 | 10 (15.2) | 60.0 (14.6–94.7) | |
| 50–60 | 23 (34.8) | 52.6 (28.8–75.5) | |
| 60–70 | 21 (31.8) | 76.0 (54.8–90.6) | |
| >70 | 12 (18.2) | 64.7 (38.3–85.8) | |
| Sex | * 0.039 | ||
| Male | 63 (95.5) | 68.2 (55.3–79.4) | |
| Female | 3 (4.5) | 0.0 (0.0–70.7) | |
| Tobacco consumption | 0.383 | ||
| Never | 9 (13.6) | 55.5 (21.2–86.3) | |
| <1 pack-year | 0 | - | |
| >1 pack-year | 57 (86.4) | 66.6 (52.9–78.5) | |
| BMI (kg/m2) | 0.966 | ||
| <25 (Lean) | 28 (42.4) | 68.0 (46.4–85.1) | |
| 25–30 (Overweight) | 28 (42.4) | 64.0 (42.5–82.0) | |
| >30 (Obese) | 10 (15.2) | 66.7 (29.9–92.5) | |
| Alcohol consumption | 0.395 | ||
| Never | 18 (27.3) | 72.2 (46.5–90.3) | |
| Mild-moderate | 6 (9.1) | 83.3 (35.9–99.5) | |
| Severe | 42 (63.9) | 59.5 (43.2–74.3) | |
| ECOG Index | 0.148 | ||
| 0 | 28 (42.4) | 75.0 (55.1–89.3) | |
| >0 | 38 (57.6) | 60.5 (43.3–75.9) | |
| Tumor location | 0.131 | ||
| Oral cavity-oropharynx | 15 (22.7) | 46.6 (21.2–73.4) | |
| Larynx-hypopharynx | 51 (77.3) | 70.5 (56.1–82.5) | |
| T category | 0.439 | ||
| T1-T2 | 26 (39.4) | 69.2 (48.2–85.7) | |
| T3-T4 | 40 (60.6) | 65.0 (48.3–79.3) | |
| N category | * 0.039 | ||
| N0 | 35 (53.0) | 77.1 (59.8–89.5) | |
| N+ | 31 (47.0) | 53.3 (34.3–71.6) | |
| Stage | 0.185 | ||
| I–II | 21 (31.8) | 77.1 (59.8–89.5) | |
| III–IV | 45 (68.2) | 53.3 (34.3–71.6) | |
| Tumor differentiation | 0.371 | ||
| Good | 9 (13.6) | 70.0 (34.7–93.3) | |
| Moderate | 49 (74.2) | 63.2 (48.3–76.5) | |
| Poor | 8 (12.1) | 28.5 (3.6–70.9) | |
| Treatment | * 0.014 | ||
| RT or ChRT | 41 (62.1) | 61.3 (42.2–78.2) | |
| Surgery | 15 (22.7) | 93.8 (69.8–99.8) | |
| Surgery and RT/ChRT | 10 (15.2) | 45.5 (16.7–76.6) |
| Variables | Succinate | |
|---|---|---|
| HIF-1α | rho | 0.289 |
| p-value | * 0.049 | |
| SDHA | rho | 0.406 |
| p-value | * 0.005 | |
| SDHB | rho | 0.371 |
| p-value | * 0.010 | |
| OGDH | rho | 0.248 |
| p-value | 0.092 | |
| SUCNR1 | rho | 0.296 |
| p-value | * 0.044 | |
| Clinic-Pathological Variables | SUCNR1-SDHA Expression Profiles | |||
|---|---|---|---|---|
| Profile 1 (%) | Profile 2 (%) | Profile 3 (%) | p-Value | |
| Tumor stage | ||||
| I–II | 83.3 | 77.8 | 0.0 | 0.064 |
| III–IV | 86.7 | 63.6 | 37.5 | 0.053 |
| N category | ||||
| N0 | 91.7 | 83.3 | 33.3 | * 0.017 |
| N+ | 77.8 | 50.0 | 25.0 | 0.181 |
| Tumor location | ||||
| Oral cavity-oropharynx | 71.4 | 0.0 | 0.0 | 0.117 |
| Larynx-hypopharynx | 92.9 | 73.7 | 37.5 | * 0.019 |
| Treatment | ||||
| RT or ChRT | 80.0 | 77.8 | 16.7 | * 0.021 |
| Surgery | 100.0 | 80.0 | 100.0 | 0.466 |
| Surgery and RT/ChRT | 50.0 | 66.7 | 50.0 | 0.907 |
| BMI (kg/m2) | ||||
| Lean (<25) | 100.0 | 66.7 | 0.0 | * 0.001 |
| Overweight (25–30) | 71.4 | 71.4 | 17.6 | 0.270 |
| Obese (>30) | 100.0 | 60.0 | 0.0 | 0.476 |
| Variables | Categories | HR | 95% CI | p-Value |
|---|---|---|---|---|
| Univariate Models | ||||
| SUCNR1/SDHA profile | 2 vs. 1 | 2.46 | 0.50–12.20 | 0.272 |
| 3 vs. 1 | 6.63 | 1.37–32.07 | * 0.019 | |
| Stage | III–IV vs. I–II | 1.79 | 0.65–4.90 | 0.258 |
| ECOG | >0 vs. 0 | 2.27 | 0.92–5.61 | 0.075 |
| N category | N+ vs. N0 | 2.45 | 1.01–5.92 | 0.047 |
| Treatment | Surgery vs. RT or ChRT | 1.844 | 0.68–5.02 | 0.230 |
| Surgery vsSurgery and RT/ChRT | 0.163 | 0.02–1.26 | 0.082 | |
| Tumor location | LH vs. OCO | 0.53 | 0.21–1.32 | 0.171 |
| Multivariate Mode | ||||
| SUCNR1-SDHA profile | 2 vs. 1 | 2.75 | 0.55–13.79 | 0.218 |
| 3 vs. 1 | 7.02 | 1.45–33.96 | * 0.015 | |
| N category | N+ vs. N0 | 2.18 | 0.78–6.08 | 0.135 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terra, X.; Ceperuelo-Mallafré, V.; Merma, C.; Benaiges, E.; Bosch, R.; Castillo, P.; Flores, J.C.; León, X.; Valduvieco, I.; Basté, N.; et al. Succinate Pathway in Head and Neck Squamous Cell Carcinoma: Potential as a Diagnostic and Prognostic Marker. Cancers 2021, 13, 1653. https://doi.org/10.3390/cancers13071653
Terra X, Ceperuelo-Mallafré V, Merma C, Benaiges E, Bosch R, Castillo P, Flores JC, León X, Valduvieco I, Basté N, et al. Succinate Pathway in Head and Neck Squamous Cell Carcinoma: Potential as a Diagnostic and Prognostic Marker. Cancers. 2021; 13(7):1653. https://doi.org/10.3390/cancers13071653
Chicago/Turabian StyleTerra, Ximena, Victoria Ceperuelo-Mallafré, Carla Merma, Ester Benaiges, Ramon Bosch, Paola Castillo, Joan Carles Flores, Xavier León, Izaskun Valduvieco, Neus Basté, and et al. 2021. "Succinate Pathway in Head and Neck Squamous Cell Carcinoma: Potential as a Diagnostic and Prognostic Marker" Cancers 13, no. 7: 1653. https://doi.org/10.3390/cancers13071653
APA StyleTerra, X., Ceperuelo-Mallafré, V., Merma, C., Benaiges, E., Bosch, R., Castillo, P., Flores, J. C., León, X., Valduvieco, I., Basté, N., Cámara, M., Lejeune, M., Gumà, J., Vendrell, J., Vilaseca, I., Fernández-Veledo, S., & Avilés-Jurado, F. X. (2021). Succinate Pathway in Head and Neck Squamous Cell Carcinoma: Potential as a Diagnostic and Prognostic Marker. Cancers, 13(7), 1653. https://doi.org/10.3390/cancers13071653









