Immuno-Oncological Biomarkers for Squamous Cell Cancer of the Head and Neck: Current State of the Art and Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
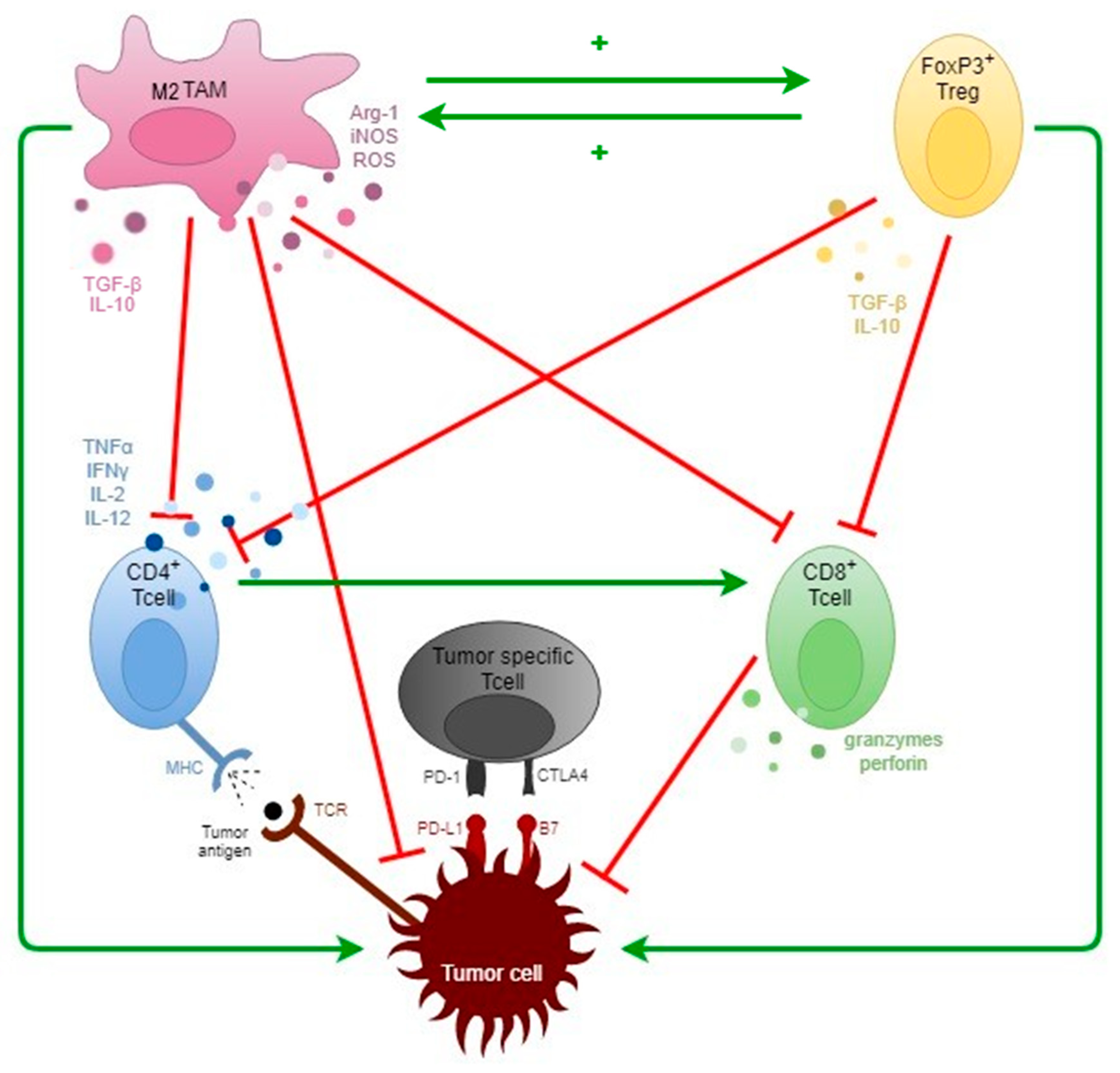
2. General Concepts of Tumor Immunology
3. The TME in SCCHN
3.1. Tissue-Based Biomarkers
3.1.1. HPV/P16 Status
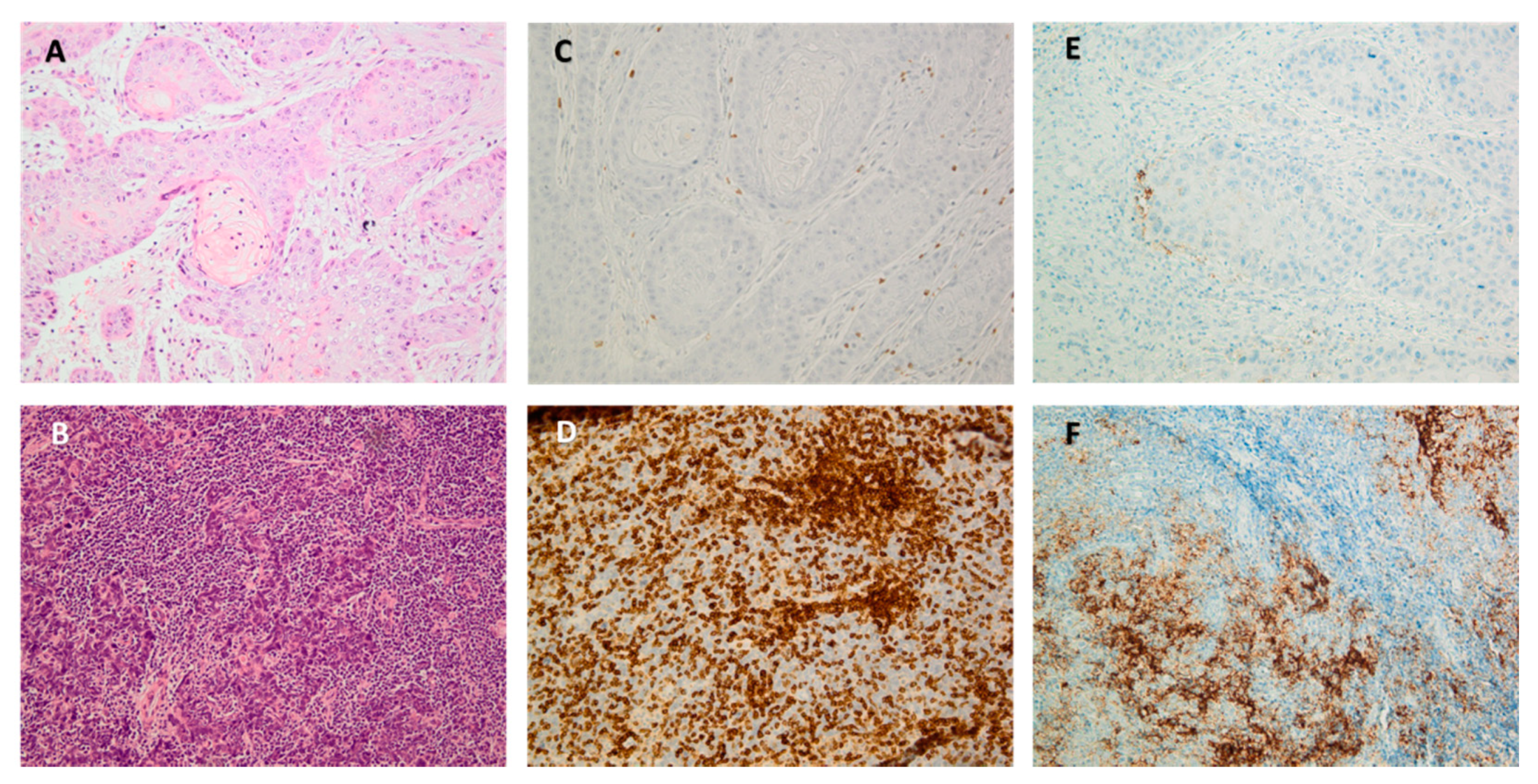
3.1.2. Tumor Immune Infiltration: Subtyping and Quantification
Tumor Infiltrating Lymphocytes (TILs)
Tumor-Associated Macrophages (TAMs)
Other Immune Cells
- -
- Neutrophils and NK cells are innate effector cells recruited as first line of defense in case of tissue damage. Although they have been well-described in blood as a marker for inflammation, few data exist regarding their anti-tumor function. Generally, tumor-associated neutrophils or TANs are subdivided in anti-tumorigenic (N1) or pro-tumorigenic (N2) [99]. Two papers viewed high infiltration of polymorphonuclear cells in SCCHN being generally associated with advanced disease, cancer progression, and lower OS [100,101]. NK-cells are lymphocytes that engage in both the innate immunity as an effector cell, and as a regulator of the adaptive immunity due to their IFNγ secretion [102]. Karpathiou et al. [103] investigated NK-infiltration in 152 SCCHN tissue slides using the CD57 protein surface marker. High CD57+ cell density in SCCHN was correlated to a lower rate of metastasis and better survival by means of OS and DFS. These CD57+ cells were mostly present in OPSCC subsites. Additionally, based on the NK-related transcriptome from the TCGA database, it seems CD56dim marked NK cells are a major part of the immune infiltrate in SCCHN, and has been correlated to increased OS [28]. Reports regarding NK infiltration in SCCHN seems limited; thus, this topic requires further investigation.
- -
- Dendritic cells (DC) are myeloid cells functioning as antigen-presenting cells for inducing T-cell activation. General discordance exists about the prognostic role of DC within the immune landscape of SCCHN. Some papers linked higher DC infiltration with positive HPV-status, though this needs to be further elaborated [104]. MDSCs function as suppressors of the native and adaptive immune system. One study depicted MDSC’s are more prevalent in SCCHN-tissue samples than healthy oral tissue via an IHC-based human MDSC marker, MPO [100]. In addition, the number of MPO-stained MDSC also linearly increased according to pathological stage [105]. The value of these myeloid cells, next to TAMs, needs to be further elucidated in SCCHN.
- -
- Although B-lymphocytes are key-players in humoral immunity through immunoglobin production, its role in tumor progression is ill-defined. B cells can participate in anti-tumor immunity by enhancing cytotoxic T-cell responses or anti-neoplastic cytokine production, while also being capable of inducing cancer immune evasion [106]. This translates in discrepant findings in literature regarding its prognostic role, for which we refer reader to Wondergem et al. [52]. To this end, we concur that these immune cells are understudied in all types of solid carcinoma.
3.1.3. PD-L1 Status
3.2. Genetic Biomarkers
3.2.1. Micro-Satellite Instability (MSI)
3.2.2. Genetic Screening
- Type of material
- Tumor mutational burden (TMB)
- Gene signatures
3.3. Circulating Blood Cells
3.4. Oral Microbiota
4. General Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015. JAMA Oncol. 2017, 3, 524. [Google Scholar] [CrossRef] [PubMed]
- European Cancer Information System. Cancer Burden Statistics and Trends across Europe. 2020. Available online: https://ecis.jrc.ec.europa.eu/index.php (accessed on 14 December 2020).
- Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; Anderson, B.O.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016 a systematic analysis for the global burden of disease study global burden o. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [PubMed]
- Data Explorer|ECIS. Available online: https://ecis.jrc.ec.europa.eu/explorer.php (accessed on 11 January 2021).
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer. 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Canning, M.; Guo, G.; Yu, M.; Myint, C.; Groves, M.W.; Byrd, J.K.; Cui, Y. Heterogeneity of the head and neck squamous cell carcinoma immune landscape and its impact on immunotherapy. Front. Cell Dev. Biol. 2019, 7, 52. [Google Scholar] [CrossRef]
- Lechien, J.R.; Seminerio, I.; Descamps, G.; Mat, Q.; Mouawad, F.; Hans, S.; Julieron, M.; Dequanter, D.; Vanderhaegen, T.; Journe, F.; et al. Impact of HPV Infection on the Immune System in Oropharyngeal and Non-Oropharyngeal Squamous Cell Carcinoma: A Systematic Review Jerome. Cells 2019, 8, 1061. [Google Scholar] [CrossRef]
- Lill, C.; Kornek, G.; Bachtiary, B.; Selzer, E.; Schopper, C.; Mittlboeck, M.; Burian, M.; Wrba, F.; Thurnher, D. Survival of patients with HPV-positive oropharyngeal cancer after radiochemotherapy is significantly enhanced. Wien Klin Wochenschr. 2011, 123, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Trigo, J.; Hitt, R.; Koralewski, P.; Diaz-Rubio, E.; Rolland, F.; Knecht, R.; Amellal, N.; Schueler, A.; Baselga, J. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J. Clin. Oncol. 2007, 25, 2171–2177. [Google Scholar] [CrossRef]
- Palucka, A.K.; Coussens, L.M. The Basis of Oncoimmunology. Cell 2016, 164, 1233–1247. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26967289 (accessed on 22 July 2020). [CrossRef]
- Kerkar, S.P.; Restifo, N.P. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012, 72, 3125–3130. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, H.; Kaanders, J.H.; Wheeler, D.L.; Van Der Kogel, A.J.; Verheijen, M.M.; Waaijer, S.J.; Iida, M.; Grénman, R.; Span, P.N.; Bussink, J. Activation of AKT by hypoxia: A potential target for hypoxic tumors of the head and neck. BMC Cancer 2012, 12, 463. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23046567 (accessed on 2 August 2020). [CrossRef] [PubMed]
- Drake, C.G.; Lipson, E.J.; Brahmer, J.R. Breathing new life into immunotherapy: Review of melanoma, lung and kidney cancer. Nat. Rev. Clin. Oncol. 2014, 11, 24–37. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24247168 (accessed on 25 March 2020). [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. Available online: https://pubmed.ncbi.nlm.nih.gov/26115796/ (accessed on 8 November 2020). [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Merlano, M.C.; Denaro, N.; Garrone, O. Immune escape mechanisms in head and neck squamous cell carcinoma and implication for new immunotherapy approach. Curr. Opin. Oncol. 2020, 32, 203–209. [Google Scholar] [CrossRef]
- Oliva, M.; Spreafico, A.; Taberna, M.; Alemany, L.; Coburn, B.; Mesia, R.; Siu, L. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann. Oncol. 2019, 30, 57–67. [Google Scholar] [CrossRef]
- Chen, Y.P.; Wang, Y.Q.; Lv, J.W.; Li, Y.Q.; Chua, M.L.K.; Le, Q.T. Identification and validation of novel microenvironment-based immune molecular subgroups of head and neck squamous cell carcinoma: Implications for immunotherapy. Ann. Oncol. 2019, 30, 68–75. [Google Scholar] [CrossRef]
- Puntmann, V.O. How-to guide on biomarkers: Biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad Med. J. 2009, 85, 538–545. [Google Scholar] [CrossRef]
- Oldenhuis, C.N.A.M.; Oosting, S.F.; Gietema, J.A.; de Vries, E.G.E. Prognostic versus predictive value of biomarkers in oncology. Eur. J. Cancer 2008, 44, 946–953. [Google Scholar] [CrossRef]
- Wang, H.C.; Yeh, T.J.; Chan, L.P.; Hsu, C.M.; Cho, S.F. Exploration of feasible immune biomarkers for immune checkpoint inhibitors in head and neck squamous cell carcinoma treatment in real world clinical practice. Int. J. Mol. Sci. 2020, 21, 7621. [Google Scholar] [CrossRef]
- Goossens, N.; Nakagawa, S.; Sun, X.; Hoshida, Y. Cancer biomarker discovery and validation. Transl. Cancer Res. 2015, 4, 256–269. [Google Scholar]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J.; Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—Elimination, equilibrium and escape. Current opinion in immunology. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef]
- Sun, W.; Wei, F.-Q.; Li, W.-J.; Wei, J.-W.; Zhong, H.; Wen, Y.-H.; Lei, W.-B.; Chen, L.; Li, H.; Lin, H.-Q.; et al. A positive-feedback loop between tumour infiltrating activated Treg cells and type 2-skewed macrophages is essential for progression of laryngeal squamous cell carcinoma. Br. J. Cancer 2017, 117, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef]
- Raudenska, M.; Sztalmachova, M.; Gumulec, J.; Fojtu, M.; Polanska, H.; Balvan, J.; Feith, M.; Binkova, H.; Horakova, Z.; Kostrica, R.; et al. Prognostic significance of the tumour-adjacent tissue in head and neck cancers. Tumor Biol. 2015, 36, 9929–9939. [Google Scholar] [CrossRef] [PubMed]
- Watnick, R.S. The Role of the Tumor Microenvironment in Regulating Angiogenesis. Cold Spring Harb. Perspect. Med. 2012, 2, a006676. [Google Scholar] [CrossRef]
- Terry, S.; Savagner, P.; Ortiz-Cuaran, S.; Mahjoubi, L.; Saintigny, P.; Thiery, J.-P.; Chouaib, S. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 2017, 11, 824–846. [Google Scholar] [CrossRef] [PubMed]
- Hyun-Tak, J.; Rafi, A.; Taku, O. Role of PD-1 in Regulating T-Cell Immunity. Curr. Top. Microbiol. Immunol. 2011, 350, 17–37. [Google Scholar]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways Similarities, Differences, and Implications of Their Inhibition. 2015. Available online: www.amjclinicaloncology.com (accessed on 7 January 2019).
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Vandecasteele, K.; Rottey, S.; Ferdinande, L. TILs in Head and Neck Cancer: Ready for Clinical Implementation and Why (Not)? Head Neck Pathol. 2017, 11, 354–363. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Horton, J.D.; Knochelmann, H.M.; Day, T.A.; Paulos, C.M.; Neskey, D.M. Immune Evasion by Head and Neck Cancer: Foundations for Combination Therapy. Trends Cancer 2019, 5, 208–232. [Google Scholar] [CrossRef]
- Perri, F.; Ionna, F.; Longo, F.; Scarpati, G.D.V.; De Angelis, C.; Ottaiano, A.; Botti, G.; Caponigro, F. Immune Response Against Head and Neck Cancer: Biological Mechanisms and Implication on Therapy. Transl. Oncol. 2020, 13, 262–274. [Google Scholar] [CrossRef]
- Desrichard, A.; Kuo, F.; Chowell, D.; Lee, K.-W.; Riaz, N.; Wong, R.J.; Chan, T.A.; Morris, L.G.T. Tobacco smoking-associated alterations in the immune microenvironment of squamous cell carcinomas. J. Natl. Cancer Inst. 2018, 110, 1386–1392. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R. Europe PMC Funders Group Signatures of mutational processes in human cancer. Nature 2014, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.S.; Kim, B.J. MET inhibitors in advanced non-small-cell lung cancer: A metaanalysis and review. Oncotarget 2017, 8, 75500–75508. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.A. Editorial: Advances in head and neck cancer immunology and immunotherapy. Front. Oncol. 2019, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.; Machiels, J.P. Targeting the Tumor Environment in Squamous Cell Carcinoma of the Head and Neck. Curr. Treat. Opt. Oncol. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Elrefaey, S.; Massaro, M.A.; Chiocca, S.; Chiesa, F.; Ansarin, M. HPV in oropharyngeal cancer: The basics to know in clinical practice. Acta Otorhinolaryngol. Ital. 2014, 34, 299–309. [Google Scholar] [PubMed]
- Tanaka, T.I.; Alawi, F. Human Papillomavirus and Oropharyngeal Cancer. Dent. Clin. N. Am. 2018, 62, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Wai, K.C.; Strohl, M.P.; van Zante, A.; Ha, P.K. Molecular Diagnostics in Human Papillomavirus-Related Head and Neck Squamous Cell Carcinoma. Cells 2020, 9, 500. [Google Scholar] [CrossRef]
- Ajila, V.; Shetty, H.; Babu, S.; Shetty, V.; Hegde, S. Human Papilloma Virus Associated Squamous Cell Carcinoma of the Head and Neck. J. Sex. Transm. Dis. 2015. [Google Scholar] [CrossRef]
- Prigge, E.-S.; Arbyn, M.; von Knebel Doeberitz, M.; Reuschenbach, M. Diagnostic accuracy of p16 INK4a immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int. J. Cancer 2017, 140, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Zafereo, M.E.; Xu, L.; Dahlstrom, K.R.; Viamonte, C.A.; El-Naggar, A.K.; Wei, Q.; Li, G.; Sturgis, E.M. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol. 2016, 56, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.K.; Divine, G.; Chen, K.M.; Chitale, D.; Havard, S.; Worsham, M.J. Significance of p16 in Site-specific HPV Positive and HPV Negative Head and Neck Squamous Cell Carcinoma. Cancer Clin. Oncol. 2013, 2, 51–61. [Google Scholar]
- Wondergem, N.E.; Nauta, I.H.; Muijlwijk, T.; Leemans, C.R.; van de Ven, R. The Immune Microenvironment in Head and Neck Squamous Cell Carcinoma: On Subsets and Subsites. Curr. Oncol. Rep. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Lechien, J.R.; Descamps, G.; Seminerio, I.; Furgiuele, S.; Dequanter, D.; Mouawad, F.; Julieron, M.; Dequanter, D. HPV involvement in the tumor microenvironment and immune treatment in head and neck squamous cell carcinomas. Cancers 2020, 12, 1060. [Google Scholar] [CrossRef]
- Lechner, A.; Schlößer, H.; Rothschild, S.I.; Thelen, M.; Reuter, S.; Zentis, P.; Shimabukuro-Vornhagen, A.; Therurich, S.; Wennhold, K.; Garcia-Marquez, M.; et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 44418–44433. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Wang, D.; Rubenstein, L.M.; Morris, W.A.; Turek, L.P.; Haugen, T.H. Association between p53 and Human Papillomavirus in Head and Neck Cancer Survival. Ann. Oncol. 2019, 30, 56–67. [Google Scholar] [CrossRef]
- Scheel, A.; Bellile, E.; McHugh, J.B.; Walline, H.; Prince, M.E.; Urba, S.; Wolf, G.T.; Eisbruch, A.; Worden, F.; Carey, T.E.; et al. Classification of TP53 Mutations and HPV Predict Survival in Advanced Larynx Cancer. Laryngoscope 2016, 126, E292–E299. [Google Scholar] [CrossRef]
- Andersen, A.S.; Koldjaer Sølling, A.S.; Ovesen, T.; Rusan, M. The interplay between HPV and host immunity in head and neck squamous cell carcinoma. Int. J. Cancer 2014, 134, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Bauml, J.; Seiwert, T.Y.; Pfister, D.G.; Worden, F.; Liu, S.V.; Gilbert, J.; Saba, N.F.; Weiss, J.; Wirth, L.; Sukari, A.; et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: Results from a single-arm, phase II study. J. Clin. Oncol. 2017, 35, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.H.; Ou, S.H.I.; Balmanoukian, A.; Fury, M.G.; Massarelli, E.; Brahmer, J.R.; Weiss, J.; Schöffski, P.; Antonia, S.J.; Massard, C.; et al. Safety and efficacy of durvalumab in patients with head and neck squamous cell carcinoma: Results from a phase I/II expansion cohort. Eur. J. Cancer 2019, 109, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Harrington, K.J.; Tahara, M.; Schulten, J.; Chomette, P.; Castro, A.F.; Licitra, L. Evidence-Based Treatment Options in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. Front. Oncol. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Balermpas, P.; Michel, Y.; Wagenblast, J.; Seitz, O.; Weiss, C.; Rödel, F.; Rödel, C.; Fokas, E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br. J. Cancer 2014, 110, 501–509. [Google Scholar] [CrossRef]
- Balermpas, P.; Rödel, F.; Rödel, C.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Gkika, E.; et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int. J. Cancer 2016, 138, 171–181. [Google Scholar] [CrossRef]
- Schneider, K.; Marbaix, E.; Bouzin, C.; Hamoir, M.; Mahy, P.; Bol, V.; Grégoire, V. Acta Oncologica Immune cell Infiltration in Head and Neck Squamous Cell Carcinoma and Patient Outcome: A Retrospective Study Immune Cell Infiltration in Head and Neck Squamous Cell Carcinoma and Patient Outcome: A Retrospective Study. 2018. Available online: https://www.tandfonline.com/action/journalInformation?journalCode=ionc20 (accessed on 10 July 2020).
- Talmadge, J.E.; Donkor, M.; Scholar, E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007, 26, 373–400. [Google Scholar] [CrossRef] [PubMed]
- Pretscher, D.; Distel, L.V.; Grabenbauer, G.G.; Wittlinger, M.; Buettner, M.; Niedobitek, G. Distribution of immune cells in head and neck cancer: CD8+T-cells and CD20+B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer 2009, 9, 292. [Google Scholar] [CrossRef]
- Nordfors, C.; Grün, N.; Tertipis, N.; Ährlund-Richter, A.; Haeggblom, L.; Sivars, L.; Du, J.; Nyberg, T.; Marklund, L.; Dalianis, T.; et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer 2013, 49, 2522–2530. [Google Scholar] [CrossRef]
- Nä sman, A.; Romanitan, M.; Nordfors, C.; Grü, N.; Johansson, H.; Hammarstedt, L.; Marklund, L.; Munck-Wikland, E.; Dalianis, E.; Ramqvist, T. Tumor Infiltrating CD8 + and Foxp3 + Lymphocytes Correlate to Clinical Outcome and Human Papillomavirus (HPV) Status in Tonsillar Cancer. Available online: www.plosone.org (accessed on 15 May 2020).
- Gooden, M.; Lampen, M.; Jordanova, E.S.; Leffers, N.; Trimbos, J.B.; Van Der Burg, S.H.; Nijman, H.; Van Hall, T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8 + T lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10656–10661. [Google Scholar] [CrossRef] [PubMed]
- Zancope, E.; Costa, N.L.; Junqueira-Kipnis, A.P.; Valadares, M.C.; Silva, T.A.; Leles, C.R.; Mendonça, E.F.; Batista, A.C. Differential infiltration of CD8 + and NK cells in lip and oral cavity squamous cell carcinoma. J. Oral Pathol. Med. 2010, 39, 162–167. [Google Scholar] [CrossRef]
- Watanabe, Y.; Katou, F.; Ohtani, H.; Nakayama, T.; Yoshie, O.; Hashimoto, K. Tumor-infiltrating lymphocytes, particularly the balance between CD8+ T cells and CCR4+ regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 744–752. [Google Scholar] [CrossRef]
- Wansom, D.; Light, E.; Thomas, D.; Worden, F.; Prince, M.; Urba, S.; Chepeha, D.; Kumar, B.; Cordell, K.; Eisbruch, A.; et al. UM Head and Neck SPORE Program. Infiltrating lymphocytes and human papillomavirus-16-associated oropharyngeal cancer. Laryngoscope 2012, 122, 121–127. [Google Scholar] [CrossRef] [PubMed]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Deron, P.; Duprez, F.; Laukens, D.; Van Dorpe, J.; Ferdinande, L.; Rottey, S. Tumor PD-L1 status and CD8 + tumor-infiltrating T cells: Markers of improved prognosis in oropharyngeal cancer. Oncotarget 2017, 8, 80443. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. OncoImmunology 2017, 6, e1356148. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the “Immunoscore” in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhao, H.; Wang, Y.; Bai, S.; Yang, Z.; Wei, F.; Ren, X. Prognostic Value of the Neo-Immunoscore in Renal Cell Carcinoma. Front. Oncol. 2019, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Economopoulou, P.; de Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic tumor markers in head and neck squamous cell carcinoma (HNSCC) in the clinical setting. Front. Oncol. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.B.; Suchak, K.; Webb, A.A.R.; Hampton, C.L.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef] [PubMed]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Zwaenepoel, K.; Deron, P.; Duprez, F. Prognostic markers in oropharyngeal squamous cell carcinoma: Focus on CD70 and tumour infiltrating lymphocytes. Pathology 2017, 49, 397–404. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Meng, X.; Kong, L.; Yu, J. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: A systematic review. Cancer Lett. 2018, 414, 166–173. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, N.; Zhou, Y.; Chen, J.; Wei, Q.; Han, M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B 2020, 10, 2156–2170. [Google Scholar] [CrossRef]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 1–34. [Google Scholar] [CrossRef]
- Li, Z.; Maeda, D.; Yoshida, M.; Umakoshi, M.; Nanjo, H.; Shiraishi, K.; Saito, M.; Kohno, T.; Konno, H.; Saito, H.; et al. The intratumoral distribution influences the prognostic impact of CD68- and CD204-positive macrophages in non-small cell lung cancer. Lung Cancer 2018, 123, 127–135. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Tumor-associated macrophages. Curr. Biol. 2020, 30, R246–R248. [Google Scholar] [CrossRef]
- Zhang, M.; He, Y.; Sun, X.; Li, Q.; Wang, W.; Zhao, A.; Di, W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 2014, 7, 1–16. [Google Scholar] [CrossRef]
- Jackute, J.; Žemaitis, M.; Pranys, D.; Sitkauskiene, B.; Miliauskas, S.; Vaitkiene, S.; Sakalauskas, R. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wei, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Dou, R.; Xiong, B. Elevated CD163+/CD68+ Ratio at Tumor Invasive Front is Closely Associated with Aggressive Phenotype and Poor Prognosis in Colorectal Cancer. Int. J. Biol. Sci. 2019, 15, 984–998. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Caponio, V.C.A.; Adipietro, I.; Tepedino, M.; Santoro, R.; Laino, L.; Lo Russo, L.; Cirillo, N.; Lo Muzio, L. Prognostic significance of CD68+ and CD163+ tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2019, 93, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shintani, S.; Terakado, N.; Nakashiro, K.I.; Hamakawa, H. Infiltration of tumor-associated macrophages in human oral squamous cell carcinoma. Oncol. Rep. 2002, 9, 1219–1223. [Google Scholar] [CrossRef]
- Badoual, C.; Hans, S.; Rodriguez, J.; Peyrard, S.; Klein, C.; Agueznay, N.E.H.; Mosseri, V.; Laccourreye, O.; Bruneval, P.; Fridman, W.H.; et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin. Cancer Res. 2006, 12, 465–472. [Google Scholar] [CrossRef]
- Wolf, G.T.; Chepeha, D.B.; Bellile, E.; Nguyen, A.; Thomas, D.; Mchugh, J.; The University of Michigan Head and Neck SPORE Program. Tumor Infiltrating Lymphocytes (TIL) and Prognosis in Oral Cavity Squamous Carcinoma: A Preliminary Study. Oral Oncol. 2015, 51, 90–95. [Google Scholar] [CrossRef]
- Weber, M.; Wehrhan, F.; Baran, C.; Agaimy, A.; Büttner-Herold, M.; Öztürk, H.; Neubauer, K.; Wickenhauser, C.; Kesting, M.; Ries, J. Malignant transformation of oral leukoplakia is associated with macrophage polarization. J. Transl. Med. 2020, 18, 1–18. [Google Scholar] [CrossRef]
- Weber, M.; Büttner-Herold, M.; Hyckel, P.; Moebius, P.; Distel, L.; Ries, J.; Amann, K.; Neukam, F.W.; Wehrhan, F. Small oral squamous cell carcinomas with nodal lymphogenic metastasis show increased infiltration of M2 polarized macrophages—An immunohistochemical analysis. J. Cranio-Maxillofacial Surg. 2014, 42, 1087–1094. [Google Scholar] [CrossRef]
- Haque, A.S.M.R.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Moebius, P.; Büttner-Herold, M.; Amann, K.; Preidl, R.; Neukam, F.W.; Wehrhan, F. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas-an immunohistochemical study. Br. J. Cancer 2015, 113, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Sánchez, F.J.; Lequerica-Fernández, P.; Suárez-Canto, J.; Rodrigo, J.P.; Rodriguez-Santamarta, T.; Domínguez-Iglesias, F.; García-Pedrero, J.M.; De Vicente, J.C. Macrophages in Oral Carcinomas: Relationship with Cancer Stem Cell Markers and PD-L1 Expression. Cancers 2020, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Trellakis, S.; Bruderek, K.; Dumitru, C.A.; Gholaman, H.; Gu, X.; Bankfalvi, A.; Scherag, A.; Hütte, J.; Dominas, N.; Lehnerdt, G.F.; et al. Polymorphonuclear granulocytes in human head and neck cancer: Enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int. J. Cancer 2011, 129, 2183–2193. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Bankfalvi, A.; Gu, X.; Eberhardt, W.E.; Zeidler, R.; Lang, S.; Brandau, S. Neutrophils activate tumoral CORTACTIN to enhance progression of orohypopharynx carcinoma. Front. Immunol. 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Karpathiou, G.; Casteillo, F.; Giroult, J.-B.; Forest, F.; Fournel, P.; Monaya, A.; Froudarakis, M.; Dumollard, J.M.; Prades, J.M.; Peoc’H, M. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: Immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget 2017, 8, 19310–19322. [Google Scholar] [CrossRef]
- Kindt, N.; Descamps, G.; Seminerio, I.I.; Bellier, J.J.; Lechien, J.R.; Pottier, C.C.; Larsimont, D.; Journé, F.; Delvenne, P.; Saussez, S. Langerhans cell number is a strong and independent prognostic factor for head and neck squamous cell carcinomas. Oral Oncol. 2016, 62, 1–10. [Google Scholar] [CrossRef]
- Ma, X.; Sheng, S.; Wu, J.; Jiang, Y.; Gao, X.; Cen, X.; Wu, J.; Wang, S.; Tang, Y.; Tang, Y. LncRNAs as an intermediate in HPV16 promoting myeloid-derived suppressor cell recruitment of head and neck squamous cell carcinoma. Oncotarget 2017, 8, 42061–42075. [Google Scholar] [CrossRef]
- Largeot, A.; Pagano, G.; Gonder, S.; Moussay, E.; Paggetti, J. The B-Side of Cancer Immunity: The Underrated Tune. Cells 2019, 8, 449. [Google Scholar] [CrossRef]
- Oguejiofor, K.; Hall, J.; Slater, C.; Betts, G.; Hall, G.; Slevin, N. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br. J. Cancer. 2015, 113, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wong, M.C.M.; Thomson, P.J.; Li, K.-Y.; Su, Y. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2018, 86, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-A.; Yoon, H.-J.; Lee, J.-I.; Hong, S.-P.; Hong, S.-D. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011, 47, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Canteli, M.; Granda-Díaz, R.; del Rio-Ibisate, N.; Allonca, E.; López-Alvarez, F.; Agorreta, J.; Garmendia, I.; Montunega, L.M.; Garcia-Pedrero, J.M.; Rodrigo, J.P. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas. Cancer Immunol. Immunother. 2020, 69, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Ngamphaiboon, N.; Chureemas, T.; Siripoon, T.; Arsa, L.; Trachu, N.; Jiarpinitnun, C.; Pattaranutaporn, P.; Sirachainan, E.; Larbcharoensub, N. Characteristics and impact of programmed death-ligand 1 expression, CD8+ tumor-infiltrating lymphocytes, and p16 status in head and neck squamous cell carcinoma. Med. Oncol. 2019, 36, 1–10. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; De Castro, G. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Chen, S.-W.; Li, S.-H.; Shi, D.-B.; Jiang, W.-M.; Song, M.; Yang, A.-K.; Li, Y.-D.; Bei, J.-X.; Chen, W.-K.; Zhang, Q. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int. J. Biol. Mark. 2019, 34, 398–405. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1: PD-L1/2 pathway from discovery to clinical implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, X.; Sun, L.; Mao, Y. Correlation between PD-L2 Expression and Clinical Outcome in Solid Cancer Patients: A Meta-Analysis. Front. Oncol. 2019, 9, 47. [Google Scholar] [CrossRef]
- Maleki Vareki, S.; Garrigós, C.; Duran, I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit. Rev. Oncol. Hematol. 2017, 116, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Poropatich, K.; Hernandez, D.; Fontanarosa, J.; Brown, K.; Woloschak, G.; Paintal, A.; Raparia, K.; Samant, S. Peritumoral cuffing by T-cell tumor-infiltrating lymphocytes distinguishes HPV-related oropharyngeal squamous cell carcinoma from oral cavity squamous cell carcinoma. J. Oral Pathol. Med. 2017, 46, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Long-Mira, E.; Bence, C.; Butori, C.; Lassalle, S.; Bouhlel, L.; Fazzalari, L.; Zahaf, K.; Lalvée, S.; Washetine, K.; et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: A potential issue for anti-PD-L1 therapeutic strategies. Ann. Oncol. 2016, 27, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Gradecki, S.E.; Grange, J.S.; Stelow, E.B. Concordance of PD-L1 Expression between Core Biopsy and Resection Specimens of Non–Small Cell Lung Cancer. Am. J. Surg. Pathol. 2018, 42, 1090–1094. [Google Scholar] [CrossRef]
- Paintal, A.S.; Brockstein, B.E. PD-L1 CPS Scoring Accuracy in Small Biopsies and Aspirate Cell Blocks from Patients with Head and Neck Squamous Cell Carcinoma. Head Neck Pathol. 2020, 14, 657–665. [Google Scholar] [CrossRef]
- Badoual, C.; Hans, S.; Merillon, N.; Van Ryswick, C.; Ravel, P.; Benhamouda, N.; Levionnois, E.; Nizard, M.; Si-Mohamed, A.; Besnier, N.; et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-Associated head and neck cancer. Cancer Res. 2013, 73, 128–138. [Google Scholar] [CrossRef]
- Colevas, A.; Bahleda, R.; Braiteh, F.; Balmanoukian, A.; Brana, I.; Chau, N.; Sarkar, I.; Molinero, L.; Grossman, W.; Kabbinavar, F.; et al. Safety and clinical activity of atezolizumab in head and neck cancer: Results from a phase I trial. Ann. Oncol. 2018, 29, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.S.; Kerr, K.M.; Kockx, M.; Beasley, M.-B.; Borczuk, A.C.; Botling, J.; Bubendorf, L.; Chirieac, L.; Chen, G.; Chou, T.-Y.; et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J. Thorac. Oncol. 2018, 13, 1302–1311. [Google Scholar] [CrossRef]
- Rasmussen, J.H.; Lelkaitis, G.; Håkansson, K.; Vogelius, I.R.; Johannesen, H.H.; Fischer, B.M.; Bentzer, S.M.; Specht, L.; Krustensen, C.A.; von Buchwald, C.; et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br. J. Cancer. 2019, 120, 1003–1006. [Google Scholar] [CrossRef]
- Straub, M.; Drecoll, E.; Pfarr, N.; Weichert, W.; Langer, R.; Hapfelmeier, A.; Götz, C.; Wolf, K.D.; Kolk, A.; Specht, K. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget 2016, 7, 12024–12034. [Google Scholar] [CrossRef] [PubMed]
- Stenzinger, A.; Allen, J.D.; Maas, J.; Stewart, M.D.; Merino, D.M.; Wempe, M.M.; Dietel, M. Tumor mutational burden standardization initiatives: Recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosom. Cancer 2019, 58, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chang, M.; Chang, H.M.; Chang, F. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Appl. Immunohistochem. Mol. Morphol. 2018, 26, e15–e21. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Chang, M.-F.; Chung, M.-Y.; Chang, C.-S.; Kao, S.-Y.; Liu, C.-J.; Chang, K.-W. Frequent microsatellite alterations of chromosome locus 4q13.1 in oral squamous cell carcinomas. J. Oral Pathol. Med. 2005, 34, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.-Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Yalniz, Z.; Demokan, S.; Suoglu, Y.; Ulusan, M.; Dalay, N. Assessment of microsatellite instability in head and neck cancer using consensus markers. Mol. Biol. Rep. 2010, 37, 3541–3545. [Google Scholar] [CrossRef]
- García Martínez, J.; Pérez-Escuredo, J.; García-Carracedo, D.; Alonso-Guervós, M.; Suárez-Nieto, C.; Luis Llorente-Pendás, J.; Alvarez-Marcos, C.; Hermsen, M. Analysis of Microsatellite Instability in Laryngeal Squamous Cell Carcinoma. Acta Otorrinolaringol. 2012, 63, 79–84. [Google Scholar] [CrossRef][Green Version]
- De Schutter, H.; Spaepen, M.; Mc Bride, W.H.; Nuyts, S. The clinical relevance of microsatellite alterations in head and neck squamous cell carcinoma: A critical review. Eur. J. Hum. Genet 2007, 15, 734–741. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Tardy, M.P.; Di Mauro, I.; Ebran, N.; Refae, S.; Bozec, A.; Benezery, K.; Peyrade, F.; Guigay, J.; Sudaka-Bahadoran, A.; Badoual, C.; et al. Microsatellite instability associated with durable complete response to PD-L1 inhibitor in head and neck squamous cell carcinoma. Oral Oncol. 2018, 80, 104–107. [Google Scholar] [CrossRef]
- Meng, Y.; Bian, L.; Zhang, M.; Bo, F.; Lu, X.; Dong, L. Liquid biopsy and their application progress in head and neck cancer: Focus on biomarkers CTCs, cfDNA, ctDNA and EVs. Biomark. Med. 2020, 14, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.P.; De Melo, J.B.; Carreira, I.M. Head and neck cancer: Searching for genomic and epigenetic biomarkers in body fluids—The state of art. Mol. Cytogenet. 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Schrock, A.B.; Fabrizio, D.; Frampton, G.M.; Sun, J.; He, J.; Gowen, K.; Johnson, M.L.; Bauer, T.M.; Kalemkerian, G.P.; et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J. Clin. Oncol. 2016, 34 (Suppl. 15), 9017. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.-J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.-C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-cell–inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 2019, 37, 318–327. [Google Scholar] [CrossRef]
- Hanna, G.J.; Lizotte, P.; Cavanaugh, M.; Kuo, F.C.; Shivdasani, P.; Frieden, A.; Chau, N.G.; Schoenfeld, J.D.; Lorch, H.L.; Uppaluri, R.; et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- She, Y.; Kong, X.; Ge, Y.; Yin, P.; Liu, Z.; Chen, J.; Gao, F.; Fang, S. Immune-related gene signature for predicting the prognosis of head and neck squamous cell carcinoma. Cancer Cell Int. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Huo, M.; Zhang, Y.; Chen, Z.; Zhang, S.; Bao, Y.; Li, T. Tumor microenvironment characterization in head and neck cancer identifies prognostic and immunotherapeutically relevant gene signatures. Sci. Rep. 2020, 10, 11163. [Google Scholar] [CrossRef]
- Fang, R.; Chen, L.; Liao, J.; Luo, J.; Zhang, C.; Wei, F.; Zhang, Z.; Wen, W.; Sun, W. A Novel Comprehensive Immune-Related Gene Signature as a Promising Survival Predictor for Head and Neck Squamous Cell Carcinoma. 2020. Available online: https://www.researchsquare.com/article/rs-26402/v1 (accessed on 22 October 2020).
- Wu, S.; Dai, X.; Xie, D. Identification and Validation of an Immune-Related RNA Signature to Predict Survival of Patients With Head and Neck Squamous Cell Carcinoma. Front. Genet. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Bai, S.; Yan, Y.-B.; Chen, W.; Zhang, P.; Zhang, T.-M.; Tian, Y.-Y.; Liu, H. Bioinformatic Analysis Reveals an Immune/Inflammatory-Related Risk Signature for Oral Cavity Squamous Cell Carcinoma. J. Oncol. 2019, 2019, 1–10. Available online: https://www.hindawi.com/journals/jo/2019/3865279/ (accessed on 23 October 2020). [CrossRef] [PubMed]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. The “cancer immunogram” Visualizing the state of cancer–immune system interactions may spur personalized therapy. Science 2016, 352, 658–660. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network, T.C.G.A.N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Zuo, Z.; Keck, M.K.; Khattri, A.; Pedamallu, C.S.; Stricker, T.; Brown, C.; Pugh, T.J.; Stojanov, P.; Cho, J.; et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin. Cancer Res. 2015, 21, 632–641. [Google Scholar] [CrossRef]
- Hammerman, P.S.; Neil Hayes, D.; Grandis, J.R. Therapeutic insights from genomic studies of head and neck squamous cell carcinomas. Cancer Discov. 2015, 5, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Harbison, R.A.; Kubik, M.; Konnick, E.Q.; Zhang, Q.; Lee, S.-G.; Park, H.; Zhang, J.; Carlson, C.S.; Chen, C.; Schwartz, S.M.; et al. The mutational landscape of recurrent versus nonrecurrent human papillomavirus-related oropharyngeal cancer. JCI Insight 2018, 3. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30046007 (accessed on 23 October 2020). [CrossRef] [PubMed]
- Lim, S.M.; Choi, J.G.; Cho, S.H.; Kang, E.J.; Hwang, I.G.; Yun, T. Investigating the feasibility of targeted next-generation sequencing to guide the treatment of head and neck squamous cell carcinoma. Cancer Res. Treat. 2019, 51, 300–312. [Google Scholar] [CrossRef]
- Song, Y.; Pan, Y.; Liu, J. The relevance between the immune response-related gene module and clinical traits in head and neck squamous cell carcinoma. Cancer Manag. Res. 2019, 11, 7455–7472. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, P.F.; Ascierto, P.A.; Pigozzo, J.; Del Vecchio, M.; Maio, M.; Cappellini, G.C.A.; Guidoboni, M.; Queirolo, P.; Savoia, P.; Mandalà, M.; et al. Baseline neutrophils and derived neutrophilto-lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann. Oncol. 2016, 27, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; Wistuba-Hamprecht, K.; Yuan, J.; Postow, M.A.; Wong, P.; Capone, M.; Madonna, G.; Khammari, A.; Schilling, B.; Sucker, A.; et al. Increases in absolute lymphocytes and circulating CD4+ and CD8+ T cells are associated with positive clinical outcome of melanoma patients treated with ipilimumab. Clin. Cancer Res. 2016, 22, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Tham, T.; Olson, C.; Khaymovich, J.; Herman, S.W.; Costantino, P.D. The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: A systematic review and meta-analysis. Eur. Arch Oto-Rhino-Laryngol. 2018, 275, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Oya, R.; Kitamiura, T.; Ashida, N.; Shimizu, K.; Takemura, K.; Yamamoto, Y.; Uno, A. Platelet count and platelet-lymphocyte ratio as prognostic markers for head and neck squamous cell carcinoma: Meta-analysis. Head Neck 2018, 40, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, C.; Pang, G.; Wang, P. Emerging Blood-Based Biomarkers for Predicting Response to Checkpoint Immunotherapy in Non-Small-Cell Lung Cancer. Front. Immunol. 2020, 11, 2731. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Yarchoan, M.; Hopkins, A.; Mehra, R.; Grossman, S.; Kang, H. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J. Immunother. Cancer 2018, 6, 84. [Google Scholar] [CrossRef]
- Young, Y.K.; Bolt, A.M.; Ahn, R.; Mann, K.K. Analyzing the tumor microenvironment by flow cytometry. In Methods in Molecular Biology; The Tumor Microenvironment: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1458, pp. 95–110. Available online: http://link.springer.com/10.1007/978-1-4939-3801-8_8 (accessed on 17 December 2020).
- Quan, H.; Shan, Z.; Liu, Z.; Liu, S.; Yang, L.; Fang, X.; Li, K.; Wang, B.; Deng, Z.; Hu, Y.; et al. The repertoire of tumor-infiltrating lymphocytes within the microenvironment of oral squamous cell carcinoma reveals immune dysfunction. Cancer Immunol. Immunother. 2020, 69, 465–476. [Google Scholar] [CrossRef]
- Takahashi, H.; Sakakura, K.; Mito, I.; Ida, S.; Chikamatsu, K. Dynamic changes in immune cell profile in head and neck squamous cell carcinoma: Immunomodulatory effects of chemotherapy. Cancer Sci. 2016, 107, 1065–1071. [Google Scholar] [CrossRef]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Functional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the FoxP3 Transcription Factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef]
- Turksma, A.; Bontkes, H.; van den Heuvel, H.; De Gruijl, T.; Von Blomberg, B.; Braakhuis, B.; Leemans, C.R.; Bloemena, E.; Meiijer, C.J.L.M.; Hooijberg, E. Effector memory T-cell frequencies in relation to tumour stage, location and HPV status in HNSCC patients. Oral Dis. 2013, 19, 577–584. [Google Scholar] [CrossRef]
- Boucek, J.; Mrkvan, T.; Chovanec, M.; Kuchar, M.; Betka, J.; Boucek, V.; Hladikova, M.; Betka, J.; Eckschlager, T.; Rihova, B. Regulatory T cells and their prognostic value for patients with squamous cell carcinoma of the head and neck. J. Cell Mol. Med. 2010, 14, 426–433. [Google Scholar] [CrossRef]
- Hu, G.; Wang, S. Tumor-infiltrating CD45RO + Memory T Lymphocytes Predict Favorable Clinical Outcome in Solid Tumors. Sci. Rep. 2017, 7, 10376. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.C.; Ferreira, S.B.P.; Gonçalves, L.C.; De-Paula, A.M.B.; De Faria, E.S.; Teixeira-Carvalho, A.; Martins-Filho, O.A. Cell surface markers for T and B lymphocytes activation and adhesion as putative prognostic biomarkers for head and neck squamous cell carcinoma. Hum. Immunol. 2013, 74, 1563–1574. [Google Scholar] [CrossRef]
- Molling, J.W.; Langius, J.A.E.; Langendijk, J.A.; Leemans, C.R.; Bontkes, H.J.; Van Der Vliet, H.J.J.; von Blomberg, M.E.; Scheper, R.J.; van den Eertwegh, A.J.M. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J. Clin. Oncol. 2007, 25, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J.; Van Waes, C.; Allen, C.T. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 2016, 58, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Chikamatsu, K.; Sakakura, K.; Toyoda, M.; Takahashi, K.; Yamamoto, T.; Masuyama, K. Immunosuppressive activity of CD14 + HLA-DR—cells in squamous cell carcinoma of the head and neck. Cancer Sci. 2012, 103, 976–983. [Google Scholar] [CrossRef]
- Vasquez-Dunddel, D.; Pan, F.; Zeng, Q.; Gorbounov, M.; Albesiano, E.; Fu, J.; Blosser, R.L.; Tam, A.J.; Bruno, T.; Zhang, H.; et al. STAT3 regulates arginase-i in myeloid-derived suppressor cells from cancer patients. J. Clin. Investig. 2013, 123, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Lai, C.H.; Chuang, H.C.; Lin, P.Y.; Chen, M.F. Inflammation-induced myeloid-derived suppressor cells associated with squamous cell carcinoma of the head and neck. Head Neck 2017, 39, 347–355. [Google Scholar] [CrossRef]
- Weed, D.T.; Vella, J.L.; Reis, I.M.; De La Fuente, A.C.; Gomez, C.; Sargi, Z.; Nazarian, R.; Califano, J.; Borrello, I.; Serafini, P. Tadalafil reduces myeloid-derived suppressor cells and regulatory t cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 39–48. [Google Scholar] [CrossRef]
- Suzuki, S.; Ogawa, T.; Sano, R.; Takahara, T.; Inukai, D.; Akira, S.; Tsuchida, H.; Yoshikawa, K.; Ueda, R.; Tsuzuki, T. Immune-checkpoint molecules on regulatory T-cells as a potential therapeutic target in head and neck squamous cell cancers. Cancer Sci. 2020, 111, 1943–1957. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L.; Clinic, C.; Clinic, C.; Clinic, C. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2018, 120, 1183–1196. [Google Scholar] [CrossRef]
- Jia, G.; Zhi, A.; Lai, P.F.H.; Wang, G.; Xia, Y.; Xiong, Z.; Zhang, H.; Che, N.; Ai, L. The oral microbiota—A mechanistic role for systemic diseases. Br. Dent. J. 2018, 224, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.Y.; Yu, J.; Wong, S.H.; Peppelenbosch, M.P.; Fuhler, G.M. The gastrointestinal microbiota and its role in oncogenesis. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.C.H.; Sung, J.J.Y.; Yu, J. Gut microbiota: Impacts on gastrointestinal cancer immunotherapy. Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Thomas, A.M.; Gleber-netto, F.O.; Fernandes, G.R.; Amorim, M.; Barbosa, L.F.; Lúcia, A.; Guerra, A.; Carlos, J.; Kowalski, L.P.; Nunes, D.N. Alcohol and tobacco consumption affects bacterial richness in oral cavity mucosa biofilms. BMC Microb. 2014, 14, 250. [Google Scholar] [CrossRef]
- Shin, J.M.; Luo, T.; Kamarajan, P.; Fenno, J.C.; Alexander, H.; Kapila, Y.L. Microbial Communities Associated with Primary and Metastatic Head and Neck Squamous Cell Carcinoma—A High Fusobacterial and Low Streptococcal Signature. Sci. Rep. 2017, 9934. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Harrington, K.; Fayette, J.; Guigay, J.; Coleva, A.D.; Licitra, L.; Vokes, E.; Gillison, M.; Even, C.; et al. Evaluation of oral microbiome profiling as a response biomarker in squamous cell carcin- oma of the head and neck: Analyses from CheckMate 141. Cancer Res. 2017, 77 (Suppl. 13), CT022. [Google Scholar]
| ICI Agent | Complement PD-L1 Ab | Ab Host Species | Platform | Detection System | Diagnostic Cut-Off |
|---|---|---|---|---|---|
| Pembrolizumab [59,112] | 22C3 | murine | Dako autostainer Link 48 | EnVision FLEX visualization system | TC or IC ≥1% (CPS) |
| Nivolumab [18] | 28-8 | rabbit | Dako Autostainer Link 48 | OptiView DAB IHC Detection Kit | TC >1%, TC >5% |
| Atezolizumab [123] | SP142 | rabbit | Ventana Benchmark Ultra | OptiView DAB IHC Detection Kit | TC: ≥ 5%, IC: ≥ 5% |
| Durvalumab [60] | SP263 | rabbit | Ventana Benchmark Ultra | OptiView DAB IHC Detection Kit/ OptiView Amplification Kit | TC: ≥ 25% |
| Avelumab [124] | 73-10 | rabbit | Dako autostainer Link 48 | OptiView DAB IHC Detection Kit | N/A |
| Study Reference | Associated Genes |
|---|---|
| [7] | AJUBA (−), CASP8 (−), CD56 (+), CD8 (+), CDKN2A (−), EGFR (−), FAT1 (−), FGFR2 (+), HRAS (−), LAG3 (+), NOTCH1 (−/+), PIK3CA (−/+), TP53 (+), TP63 (−/+), TRAF3 (+) |
| [140] | CCL5, CD27, CD274, CD276, CD8a, CMKLR1, CXCL9, CXCR6, HLA-DOA, HLA-DRB1, HLA-E, IDO1, LAG3, NKG7, PDCD1GL2, PSMB10, STAT1, TIGIT |
| [143] | AVPR2, BTC, CCL22, CCR6, CHGB, DKK1, HBEGF, HRG, ICOS, IL20RA, INHBB, KLRK1, LCNL1, MASP1, OLR1, PDGFA, PTX3, RBP4, RFXAP, ROBO1, RORB, SH3BP2, TMSB4Y, TNFRSF4, TNFRSF18, TNFRSF25, ULBP1 |
| [147] | BATF, CCL11, CCR4, CCR7, CD27, CD79B, CMA1, CNR2, CTLA4, CTSG, GZMM, IL16, IL19, MASP1, PGLYRP4, SAA1, TNFAIP3, TREML1 |
| [149] | AJUBA (−), CASP8 (−), CCND1 (−), CDKN2A (−), EGFR (−/+), FAT1 (−), FGFR1 (−), FGFR3 (+), HLA-A (−/+), HRAS (−), KMT2D (−), MYC (−), NOTCH1 (−/+), NSD1 (−), PIK3CA (−/+), TP53 (−), TP63 (−/+), TRAF3 (+) |
| [150] | CDKN2A (−), CUL3 (−), FGFR3 (+), FLG (−/+), MLL2 (−/+), MLL3 (+), NOTCH1 (−/+), NOTCH2 (−), NSD1 (−), PIK3CA (−/+), TP53 (−), UBR5 (−) |
| [151] | AJUBA (−), B2M (+), CCND1 (−), CDK4 (−), CDK6 (−), CDKN2A (−), CUL3 (−), E2F1 (+), FAT1 (−), FGFR2 (+), FGFR3 (+), HLA (+), HRAS (−), KEAP1 (−), KRAS, NF1, NF1 (+), NFE2L2 (−), NOTCH1 (−/+), NRAS, PIK3CA (−/+), RB1 (−), TP53 (−), TP63 (−/+), TRAF3 (+) |
| [152] | GZMA (+), GZMB (+), IDO1 (+), IFNG (+), LAG3 (+), PRF1 (+) |
| [153] | CYLD (+), EP300 (+), FGFR3 (+), KMT2D (+), NFE2L2 (+), PEG3 (+), PIK3CA (+), RB1 (+), STAT3 (+), TSC2 (+) |
| [154] | ADGRV1 (−), CCND1, CDKN2A (−), CDKN2B (−), EGFR (−), FAT1 (−), FAT2 (−), FAT4 (−), KMT2C (−/+), KMT2D (−), NFE2L2 (−), NOTCH1 (−), PIK3CA (−/+), RELN (−), TP53 (−) |
| [155] | AKNA, ARHGAP9, CCR7, CORO1A, GIMAP4, GIMAP7, IL10RA, ITGAL, ITK, P2RY8, PPP1R16B, PRKCB, SASH3, SP140, TBC1D10C, TRAF3IP3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Keukeleire, S.J.; Vermassen, T.; Hilgert, E.; Creytens, D.; Ferdinande, L.; Rottey, S. Immuno-Oncological Biomarkers for Squamous Cell Cancer of the Head and Neck: Current State of the Art and Future Perspectives. Cancers 2021, 13, 1714. https://doi.org/10.3390/cancers13071714
De Keukeleire SJ, Vermassen T, Hilgert E, Creytens D, Ferdinande L, Rottey S. Immuno-Oncological Biomarkers for Squamous Cell Cancer of the Head and Neck: Current State of the Art and Future Perspectives. Cancers. 2021; 13(7):1714. https://doi.org/10.3390/cancers13071714
Chicago/Turabian StyleDe Keukeleire, Stijn J., Tijl Vermassen, Elien Hilgert, David Creytens, Liesbeth Ferdinande, and Sylvie Rottey. 2021. "Immuno-Oncological Biomarkers for Squamous Cell Cancer of the Head and Neck: Current State of the Art and Future Perspectives" Cancers 13, no. 7: 1714. https://doi.org/10.3390/cancers13071714
APA StyleDe Keukeleire, S. J., Vermassen, T., Hilgert, E., Creytens, D., Ferdinande, L., & Rottey, S. (2021). Immuno-Oncological Biomarkers for Squamous Cell Cancer of the Head and Neck: Current State of the Art and Future Perspectives. Cancers, 13(7), 1714. https://doi.org/10.3390/cancers13071714







