Increased Visceral Adipose Tissue and Hyperinsulinemia Raise the Risk for Recurrence of Non-B Non-C Hepatocellular Carcinoma after Curative Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients, Treatment, and Determination of Recurrence
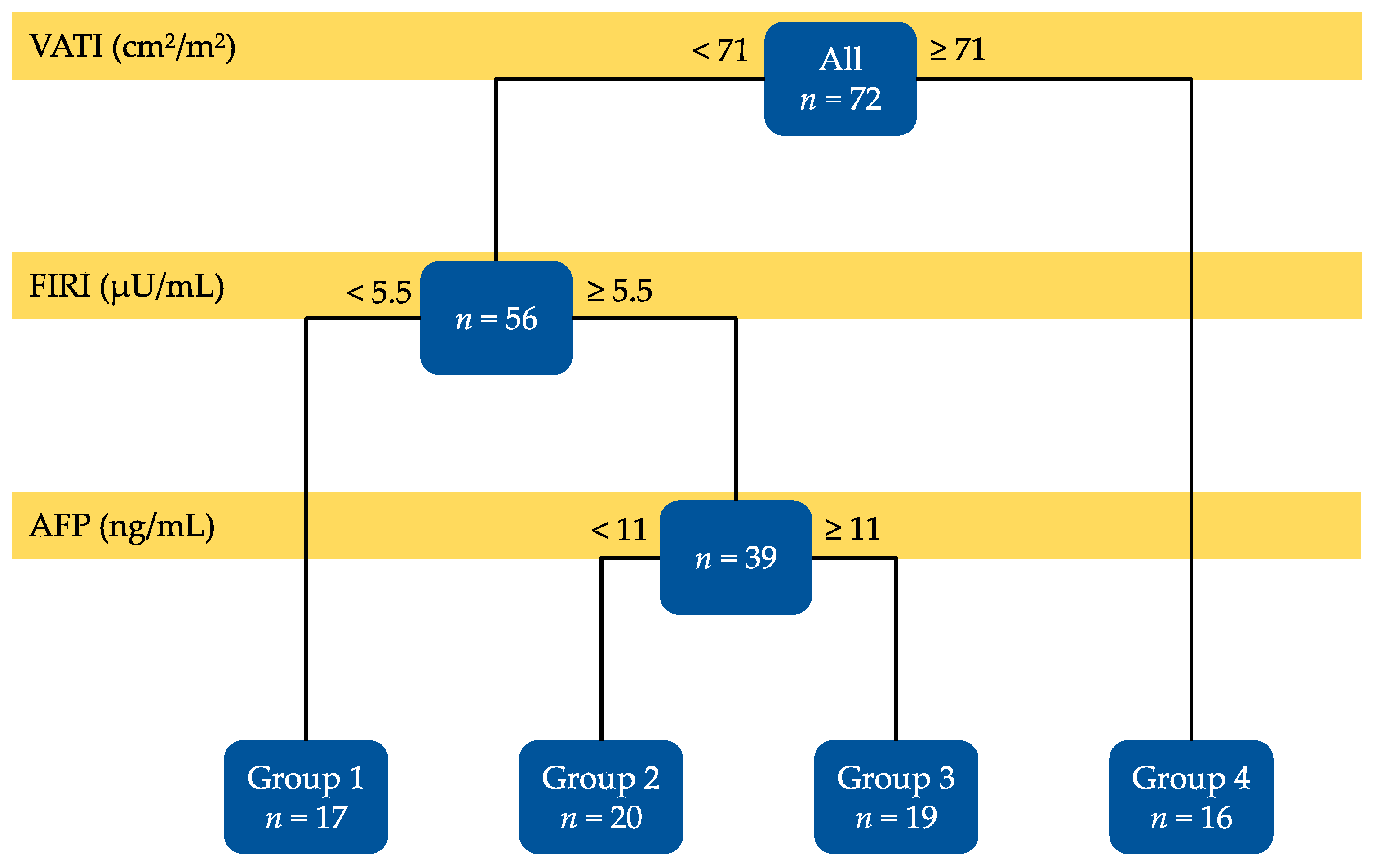
2.2. Decision-Tree Analysis of Risk Factors Affecting the Recurrence of Non-B Non-C HCC
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics and Laboratory Data of Enrolled Patients
3.2. Possible Risk Factors Affecting Recurrence-Free Survival of Non-B Non-C HCC Patients
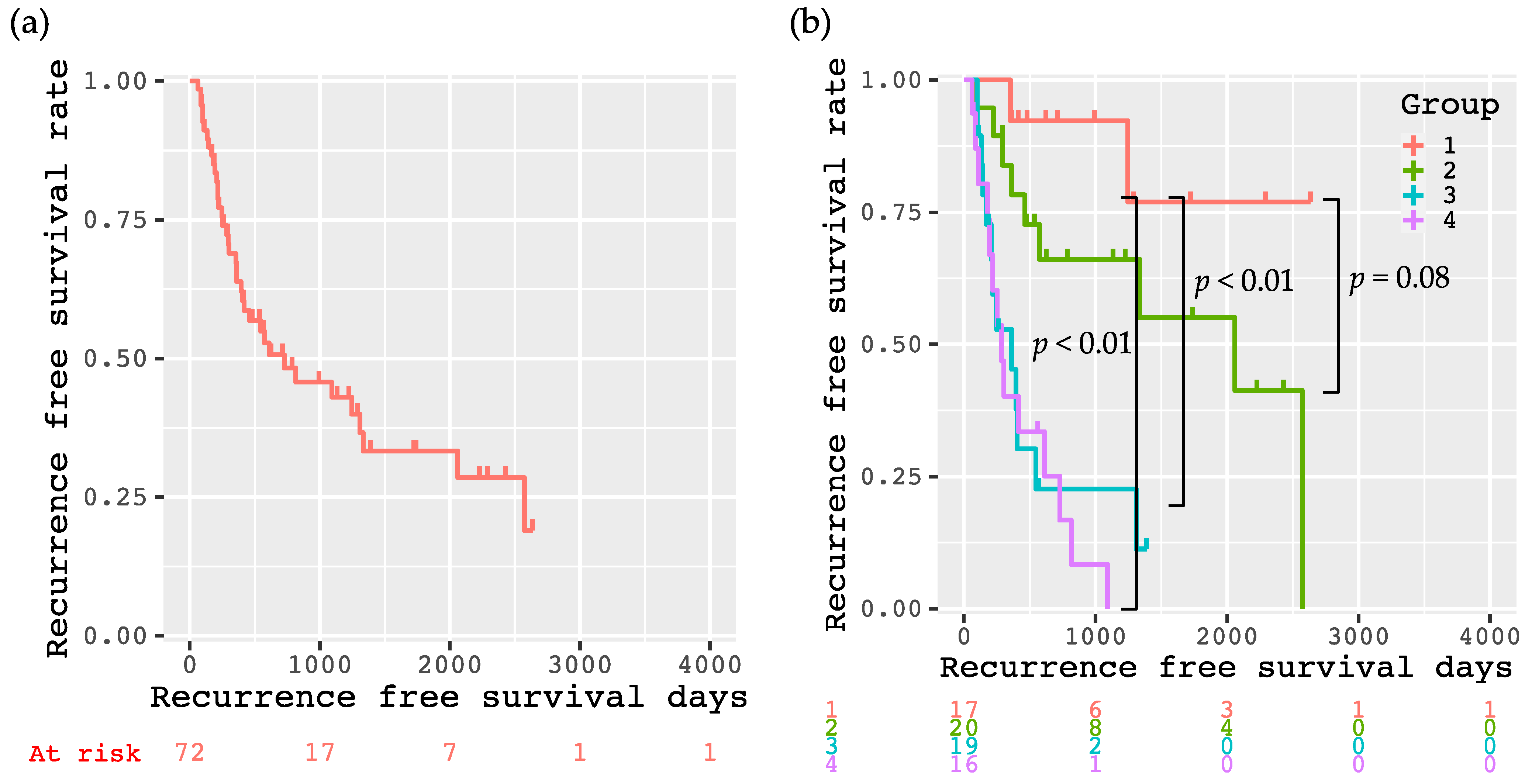
3.3. Recurrence-Free Survival Rates of the Enrolled Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular carcinoma: An epidemiologic view. J. Clin. Gastroenterol. 2002, 35, S72–S78. [Google Scholar] [CrossRef]
- Bertuccio, P.; Turati, F.; Carioli, G.; Rodriguez, T.; La Vecchia, C.; Malvezzi, M.; Negri, E. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 2017, 67, 302–309. [Google Scholar] [CrossRef]
- Imai, K.; Takai, K.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Sustained virological response by direct-acting antivirals reduces the recurrence risk of hepatitis C-related hepatocellular carcinoma after curative treatment. Mol. Clin. Oncol. 2019, 111–116. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Sung, J.J.Y.; Chow, W.C.; Farrell, G.; Lee, C.Z.; Yuen, H.; Tanwandee, T.; Tao, Q.M.; Shue, K.; Keene, O.N.; et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 2004. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, R.; Uchino, K.; Fujiwara, N.; Takehara, T.; Okanoue, T.; Seike, M.; Yoshiji, H.; Yatsuhashi, H.; Shimizu, M.; Torimura, T.; et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J. Gastroenterol. 2019, 54, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Karagozian, R.; Derdak, Z.; Baffy, G. Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism 2014, 63, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T. Prevention of recurrence after resection of hepatocellular carcinoma: A daunting challenge. Hepatology 2011, 54, 757–759. [Google Scholar] [CrossRef]
- Nagashima, I.; Hamada, C.; Naruse, K.; Osada, T.; Nagao, T.; Kawano, N.; Muto, T. Surgical resection for small hepatocellular carcinoma. Surgery 1996, 119, 40–45. [Google Scholar] [CrossRef]
- Koike, Y.; Shiratori, Y.; Sato, S.; Obi, S.; Teratani, T.; Imamura, M.; Hamamura, K.; Imai, Y.; Yoshida, H.; Shiina, S.; et al. Risk factors for recurring hepatocellular carcinoma differ according to infected hepatitis virus-an analysis of 236 consecutive patients with a single lesion. Hepatology 2000, 32, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Saitoh, S.; Tsubota, A.; Arase, Y.; Chayama, K.; Kumada, H.; Watanabe, G.; Tsurumaru, M. Risk factors for tumor recurrence and prognosis after curative resection of hepatocellular carcinoma. Cancer 1993, 71, 19–25. [Google Scholar] [CrossRef]
- Adachi, E.; Maeda, T.; Matsumata, T.; Shirabe, K.; Kinukawa, N.; Sugimachi, K.; Tsuneyoshi, M. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology 1995, 108, 768–775. [Google Scholar] [CrossRef]
- Imai, K.; Takai, K.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Homeostatic Model Assessment of Insulin Resistance for Predicting the Recurrence of Hepatocellular Carcinoma after Curative Treatment. Int. J. Mol. Sci. 2019, 20, 605. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Takai, K.; Imai, K.; Shimizu, M.; Naiki, T.; Nagaki, M.; Moriwaki, H. Increased levels of serum leptin are a risk factor for the recurrence of stage I/II hepatocellular carcinoma after curative treatment. J. Clin. Biochem. Nutr. 2011, 49, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Imai, K.; Takai, K.; Hanai, T.; Hayashi, H.; Naiki, T.; Nishigaki, Y.; Tomita, E.; Shimizu, M.; Moriwaki, H. Hepatocellular carcinoma patients with increased oxidative stress levels are prone to recurrence after curative treatment: A prospective case series study using the d-ROM test. J. Cancer Res. Clin. Oncol. 2013, 139, 845–852. [Google Scholar] [CrossRef]
- Imai, K.; Takai, K.; Maeda, T.; Watanabe, S.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Increased visceral fat volume raises the risk for recurrence of hepatocellular carcinoma after curative treatment. Oncotarget 2018, 9, 14058–14067. [Google Scholar] [CrossRef]
- Tanaka, T.; Kurosaki, M.; Lilly, L.B.; Izumi, N.; Sherman, M. Identifying candidates with favorable prognosis following liver transplantation for hepatocellular carcinoma: Data mining analysis. J. Surg. Oncol. 2015, 112, 72–79. [Google Scholar] [CrossRef]
- Feng, L.H.; Sun, H.C.; Zhu, X.D.; Liu, X.F.; Zhang, S.Z.; Li, X.L.; Li, Y.; Tang, Z.Y. Prognostic nomograms and risk classifications of outcomes in very early-stage hepatocellular carcinoma patients after hepatectomy. Eur. J. Surg. Oncol. 2020, 47, 681–689. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef]
- Quinlan, J.R. Induction of decision trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef]
- Imai, K.; Takai, K.; Miwa, T.; Taguchi, D.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Rapid depletion of subcutaneous adipose tissue during sorafenib treatment predicts poor survival in patients with hepatocellular carcinoma. Cancers 2020, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Wang, Y.C.; McPherson, K.; Marsh, T.; Gortmaker, S.L.; Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011, 378, 815–825. [Google Scholar] [CrossRef]
- Samanic, C.; Chow, W.H.; Gridley, G.; Jarvholm, B.; Fraumeni, J.F. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control 2006. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Poklepovic, A.; Moyneur, E.; Barghout, V. Population-based risk factors and resource utilization for HCC: US perspective. Curr. Med. Res. Opin. 2010. [Google Scholar] [CrossRef]
- Shimizu, M.; Tanaka, T.; Moriwaki, H. Obesity and hepatocellular carcinoma: Targeting obesity-related inflammation for chemoprevention of liver carcinogenesis. Semin. Immunopathol. 2013, 35, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol. Res. 2006, 35, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Miwa, T.; Taguchi, D.; Hanai, T.; Suetsugu, A. Rapid Depletions of Subcutaneous Fat Mass and Skeletal Muscle Mass Predict Worse Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers 2019, 11, 1206. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Anti-diabetic medications and the risk of hepatocellular cancer: A systematic review and meta-analysis. Am. J. Gastroenterol. 2013, 108, 881–891, quiz 892. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhu, G.Q.; Liu, T.; Zheng, J.N.; Cheng, Z.; Zou, T.T.; Braddock, M.; Fu, S.W.; Zheng, M.H. Systematic Review with Network Meta-Analysis: Antidiabetic Medication and Risk of Hepatocellular Carcinoma. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, E. Sample sizes based on the log-rank statistic in complex clinical trials. Biometrics 1988, 44, 229–241. [Google Scholar] [CrossRef] [PubMed]

| Variables | (n = 72) |
|---|---|
| Sex (male/female) | 49/23 |
| Age (years) | 72.6 ± 9.1 |
| Etiology (NASH/alcohol/others) | 48/18/6 |
| BMI (kg/m2) | 24.7 ± 3.5 |
| SMI (cm2/m2) | 45.5 ± 6.9 |
| SATI (cm2/m2) | 46.8 ± 28.0 |
| VATI (cm2/m2) | 55.1 ± 26.5 |
| Drinking habit (yes/no) | 18/54 |
| DM (yes/no) | 44/28 |
| Hypertension (yes/no) | 39/33 |
| Hyperlipidemia (yes/no) | 17/55 |
| FPG | 119.3 ± 36.4 |
| FIRI | 15.0 ± 25.0 |
| HOMA-IR | 5.1 ± 11.2 |
| HbA1c (%) | 6.4 ± 1.3 |
| Child-Pugh score (5/6/7/8/9/10) | 49/16/4/1/1/1 |
| ALBI score | −2.57 ± 0.44 |
| Underlying liver disease (CH/LC) | 18/54 |
| M2BPGi | 1.9 ± 1.8 |
| Stage (I/II/III/IV) | 20/13/28/0 |
| AFP (ng/mL) | 878 ± 3175 |
| PIVKA-II | 18,269 ± 80,487 |
| Degree of differentiation (well/moderate/poor/unknown) | 6/33/7/26 |
| Capsule formation (yes/no/unknown) | 32/11/29 |
| Vascular invasion (yes/no) | 9/63 |
| Initial treatment (resection/RFA) | 47/25 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Sex (male vs. female) | 0.398 (0.699–2.795) | 0.343 | ||
| Age (years) | 0.999 (0.968–1.031) | 0.940 | ||
| Drinking habit (yes vs. no) | 1.440 (0.711–2.919) | 0.311 | ||
| DM (yes vs. no) | 1.923 (0.964–3.835) | 0.063 | ||
| Hyperlipidemia (yes vs. no) | 1.100 (0.503–2.407) | 0.811 | ||
| Hypertension (yes vs. no) | 1.248 (0.658–2.370) | 0.498 | ||
| VATI (≥71 vs. <71 (cm2/m2)) | 2.718 (1.381–5.349) | 0.004 | 2.556 (1.191–5.486) | 0.016 |
| Child-Pugh score | 1.104 (0.787–1.548) | 0.568 | ||
| PLT (× 104/mL) | 1.001 (0.961–1.043) | 0.947 | ||
| FIRI (≥5.5 vs. <5.5 (µU/mL)) | 2.805 (1.065–7.388) | 0.037 | 3.149 (1.156–8.575) | 0.025 |
| LC (yes vs. no) | 1.422 (0.623–3.242) | 0.403 | ||
| AFP (≥11 vs. <10 (ng/mL)) | 2.171 (1.131–4.166) | 0.020 | 3.362 (1.550–7.288) | 0.002 |
| Degree of differentiation (moderate vs. well) (poor vs. well) | 2.794 (0.646–12.08) 3.471 (0.626–19.26) | 0.169 0.155 | ||
| Vascular invasion (yes vs. no) | 2.237 (0.972–5.152) | 0.058 | ||
| Initial treatment (RFA vs. Resection) | 0.923 (0.467–1.824) | 0.818 | ||
| Variables | Group 1 (n = 17) | Group 2 (n = 20) | Group 3 (n = 19) | Group 4 (n = 16) | P Value |
|---|---|---|---|---|---|
| Sex (male/female) | 12/5 | 13/7 | 12/7 | 12/4 | 0.873 |
| Age (years) | 72.2±12.3 | 72.3 ± 10.5 | 71.4 ± 6.6 | 74.8 ± 5.7 | 0.729 |
| Etiology (NASH/alcohol/others) | 12/5/0 | 14/4/2 | 9/8/2 | 13/1/2 | 0.105 |
| BMI (kg/m2) | 21.4 ± 2.7 | 25.0 ± 2.1 | 25.6 ± 3.6 | 26.9 ± 3.3 | <0.001 |
| SMI (cm2/m2) | 40.0 ± 3.6 | 44.8 ± 7.6 | 48.1 ± 6.6 | 48.9 ± 5.6 | <0.001 |
| SATI (cm2/m2) | 34.5 ± 18.4 | 48.7 ± 21.4 | 50.5 ± 29.1 | 53.1 ± 38.6 | 0.209 |
| VATI (cm2/m2) | 34.2 ± 14.2 | 50.9 ± 14.0 | 48.2 ± 17.4 | 90.7 ± 23.8 | <0.001 |
| Drinking habit (yes/no) | 5/12 | 4/16 | 8/11 | 1/15 | 0.093 |
| DM (yes/no) | 8/9 | 11/9 | 13/6 | 12/4 | 0.327 |
| Hypertension (yes/no) | 8/9 | 8/12 | 13/6 | 10/6 | 0.265 |
| Hyperlipidemia (yes/no) | 4/13 | 2/18 | 6/13 | 5/11 | 0.356 |
| FPG | 113.6 ± 43.8 | 117.1 ± 24.1 | 113.1 ± 28.6 | 135.3 ± 46.1 | 0.250 |
| FIRI | 3.9 ± 0.9 | 22.8 ± 33.0 | 19.8 ± 30.8 | 11.7 ± 13.0 | 0.124 |
| HOMA-IR | 1.1 ± 0.6 | 6.7 ± 10.3 | 7.3 ± 17.1 | 4.7 ± 8.5 | 0.389 |
| HbA1c (%) | 6.3 ± 1.5 | 6.4 ± 1.1 | 6.2 ± 1.0 | 7.0 ± 1.6 | 0.277 |
| Child-Pugh score (5/6/7/8/9/10) | 12/3/1/0/0/1 | 12/6/2/0/0/0 | 12/4/1/1/1/0 | 13/3/0/0/0/0 | 0.686 |
| ALBI score | −2.52 ± 0.44 | –2.56 ± 0.45 | –2.58 ± 0.47 | −2.64 ± 0.45 | 0.890 |
| Underlying liver disease (CH/LC) | 7/10 | 4/16 | 4/15 | 3/13 | 0.372 |
| M2BPGi | 1.4 ± 0.6 | 1.6 ± 0.9 | 2.7 ± 3.1 | 1.8 ± 1.3 | 0.408 |
| Stage (I/II/III/IV) | 2/10/3/2 | 8/9/3/0 | 4/6/8/1 | 3/6/7/0 | 0.141 |
| AFP (ng/mL) | 1686 ± 3782 | 5.6 ± 2.3 | 541 ± 997 | 1510 ± 5379 | 0.331 |
| PIVKA-II | 12375 ± 48613 | 3242 ± 8843 | 18528 ± 72059 | 40205 ± 140137 | 0.609 |
| Degree of differentiation (well/moderate/poor/unknown) | 2/7/2/6 | 3/6/1/10 | 1/10/1/7 | 0/10/3/3 | 0.419 |
| Capsule formation (yes/no/unknown) | 9/2/6 | 7/3/10 | 7/3/9 | 9/3/4 | 0.914 |
| Vascular invasion (yes/no) | 3/14 | 0/20 | 3/16 | 3/13 | 0.258 |
| Initial treatment (resection/RFA) | 11/6 | 11/9 | 13/6 | 12/4 | 0.662 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, K.; Takai, K.; Miwa, T.; Maeda, T.; Hanai, T.; Shiraki, M.; Suetsugu, A.; Shimizu, M. Increased Visceral Adipose Tissue and Hyperinsulinemia Raise the Risk for Recurrence of Non-B Non-C Hepatocellular Carcinoma after Curative Treatment. Cancers 2021, 13, 1542. https://doi.org/10.3390/cancers13071542
Imai K, Takai K, Miwa T, Maeda T, Hanai T, Shiraki M, Suetsugu A, Shimizu M. Increased Visceral Adipose Tissue and Hyperinsulinemia Raise the Risk for Recurrence of Non-B Non-C Hepatocellular Carcinoma after Curative Treatment. Cancers. 2021; 13(7):1542. https://doi.org/10.3390/cancers13071542
Chicago/Turabian StyleImai, Kenji, Koji Takai, Takao Miwa, Toshihide Maeda, Tatsunori Hanai, Makoto Shiraki, Atsushi Suetsugu, and Masahito Shimizu. 2021. "Increased Visceral Adipose Tissue and Hyperinsulinemia Raise the Risk for Recurrence of Non-B Non-C Hepatocellular Carcinoma after Curative Treatment" Cancers 13, no. 7: 1542. https://doi.org/10.3390/cancers13071542
APA StyleImai, K., Takai, K., Miwa, T., Maeda, T., Hanai, T., Shiraki, M., Suetsugu, A., & Shimizu, M. (2021). Increased Visceral Adipose Tissue and Hyperinsulinemia Raise the Risk for Recurrence of Non-B Non-C Hepatocellular Carcinoma after Curative Treatment. Cancers, 13(7), 1542. https://doi.org/10.3390/cancers13071542







