Interstitial HDR Brachytherapy in the Treatment of Non-Melanocytic Skin Cancers around the Eye
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Group Characteristics
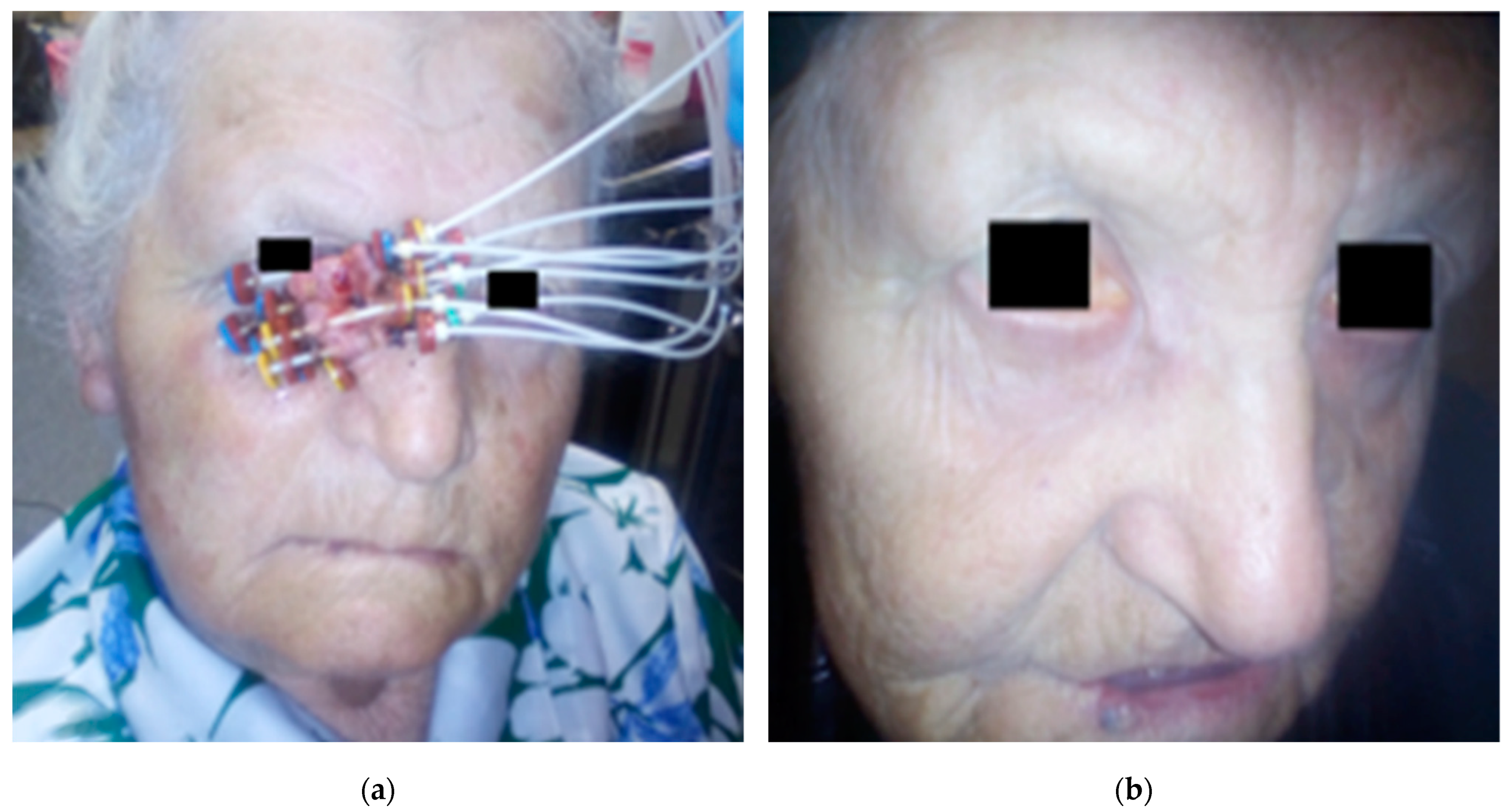
2.2. Application Procedure
2.3. Application Procedure
2.4. Post-Treatment Surveillance
3. Results
3.1. Dosage and Treatment Planning
3.2. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frakulli, R.; Galuppi, A.; Cammelli, S.; Macchia, G.; Cima, S.; Gambacorta, M.A.; Cafaro, I.; Tagliaferri, L.; Perrucci, E.; Buwenge, M.; et al. Brachytherapy in Non Melanoma Skin Cancer of Eyelid: A Systematic Review. J. Contemp. Brachytherapy 2015, 7, 497–502. [Google Scholar] [CrossRef]
- Petsuksiri, J.; Frank, S.J.; Garden, A.S.; Ang, K.K.; Morrison, W.H.; Chao, K.S.C.; Rosenthal, D.I.; Schwartz, D.L.; Ahamad, A.; Esmaeli, B. Outcomes after Radiotherapy for Squamous Cell Carcinoma of the Eyelid. Cancer 2008, 112, 111–118. [Google Scholar] [CrossRef]
- Chung, S. Basal Cell Carcinoma. Arch. Plast. Surg. 2012, 39, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.R.; Zens, M.S.; Celaya, M.O.; Riddle, B.L.; Karagas, M.R.; Peacock, J.L. Survival after Squamous Cell and Basal Cell Carcinoma of the Skin: A Retrospective Cohort Analysis. Int. J. Cancer 2015, 137, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Moscarella, E.; Lallas, A.; Longo, C.; Alfano, R.; Argenziano, G. Five-Point Checklist for Skin Cancer Detection in Primary Care. G. Ital. Dermatol. Venereol. 2019, 154, 523–528. [Google Scholar] [CrossRef]
- Neagu, N.; Lallas, K.; Maskalane, J.; Salijuma, E.; Papageorgiou, C.; Gkentsidi, T.; Spyridis, I.; Morariu, S.H.; Apalla, Z.; Lallas, A. Minimizing the Dermatoscopic Morphologic Overlap between Basal and Squamous Cell Carcinoma: A Retrospective Analysis of Initially Misclassified Tumours. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Delishaj, D.; Rembielak, A.; Manfredi, B.; Ursino, S.; Pasqualetti, F.; Laliscia, C.; Orlandi, F.; Morganti, R.; Fabrini, M.G.; Paiar, F. Non-Melanoma Skin Cancer Treated with High-Dose-Rate Brachytherapy: A Review of Literature. J. Contemp. Brachytherapy 2016, 8, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Sosman, J.A.; Chandra, S. Current Therapy for Basal Cell Carcinoma and the Potential Role for Immunotherapy with Checkpoint Inhibitors. Clin. Ski. Cancer 2017, 2, 59–65. [Google Scholar] [CrossRef]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef]
- Villani, A.; Cinelli, E.; Fabbrocini, G.; Lallas, A.; Scalvenzi, M. Hedgehog Inhibitors in the Treatment of Advanced Basal Cell Carcinoma: Risks and Benefits. Expert Opin. Drug Saf. 2020, 19, 1585–1594. [Google Scholar] [CrossRef]
- Jeganathan, V.S.E.; Wirth, A.; MacManus, M.P. Ocular Risks from Orbital and Periorbital Radiation Therapy: A Critical Review. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 650–659. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed on 20 February 2021).
- RTOG.org. Available online: https://www.rtog.org/ResearchAssociates/AdverseEventReporting/CooperativeGroupCommonToxicityCriteria.aspx (accessed on 20 February 2021).
- Laskar, S.G.; Basu, T.; Chaudhary, S.; Chaukar, D.; Nadkarni, M.; Gn, M. Postoperative Interstitial Brachytherapy in Eyelid Cancer: Long Term Results and Assessment of Cosmesis after Interstitial Brachytherapy Scale. J. Contemp. Brachytherapy 2015, 6, 350–355. [Google Scholar] [CrossRef]
- Mareco, V.; Bujor, L.; Abrunhosa-Branquinho, A.N.; Ferreira, M.R.; Ribeiro, T.; Vasconcelos, A.L.; Ferreira, C.R.; Jorge, M. Interstitial High-Dose-Rate Brachytherapy in Eyelid Cancer. Brachytherapy 2015, 14, 554–564. [Google Scholar] [CrossRef]
- Azad, S.; Choudhary, V. Treatment Results of High Dose Rate Interstitial Brachytherapy in Carcinoma of Eye Lid. J. Cancer Res. Ther. 2011, 7, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Rio, E.; Bardet, E.; Ferron, C.; Peuvrel, P.; Supiot, S.; Campion, L.; de Montreuil, C.B.; Mahe, M.A.; Dreno, B. Interstitial Brachytherapy of Periorificial Skin Carcinomas of the Face: A Retrospective Study of 97 Cases. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, A.; Riva, G.; Durante, S.; Fodor, C.; Comi, S.; Cambria, R.; Cattani, F.; Spadola, G.; Orecchia, R.; Jereczek-Fossa, B.A. Mould-Based Surface High-Dose-Rate Brachytherapy for Eyelid Carcinoma. J. Contemp. Brachytherapy 2019, 11, 443–448. [Google Scholar] [CrossRef]
- Daly, N.J.; De Lafontan, B.; Combes, P.F. Results of the Treatment of 165 Lid Carcinomas by Iridium Wire Implant. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 455–459. [Google Scholar] [CrossRef]
- Conill, C.; Sánchez-Reyes, A.; Molla, M.; Vilalta, A. Brachytherapy with 192Ir as Treatment of Carcinoma of the Tarsal Structure of the Eyelid. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Ducassou, A.; David, I.; Filleron, T.; Rives, M.; Bonnet, J.; Delannes, M. Retrospective Analysis of Local Control and Cosmetic Outcome of 147 Periorificial Carcinomas of the Face Treated with Low-Dose Rate Interstitial Brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 726–731. [Google Scholar] [CrossRef]
- Krengli, M.; Masini, L.; Comoli, A.M.; Negri, E.; Deantonio, L.; Filomeno, A.; Gambaro, G. Interstitial Brachytherapy for Eyelid Carcinoma. Outcome Analysis in 60 Patients. Strahlenther. Onkol. 2014, 190, 245–249. [Google Scholar] [CrossRef]
- Lederman, M. Radiation treatment of cancer of the eyelids. Br. J. Ophthalmol. 1976, 60, 794–805. [Google Scholar] [CrossRef][Green Version]
- Eftekhari, K.; Anderson, R.L.; Suneja, G.; Bowen, A.; Oberg, T.J.; Bowen, G.M. Local Recurrence and Ocular Adnexal Complications Following Electronic Surface Brachytherapy for Basal Cell Carcinoma of the Lower Eyelid. JAMA Dermatol. 2015, 151, 1002–1004. [Google Scholar] [CrossRef][Green Version]
- Schlienger, P.; Brunin, F.; Desjardins, L.; Laurent, M.; Haye, C.; Vilcoq, J.R. External radiotherapy for carcinoma of the eyelid: Report of 850 cases treated. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 277–287. [Google Scholar] [CrossRef]
- Bertelsen, K.; Gadeberg, C. Carcinoma of the eyelid. Acta Radiol. Oncol. Radiat. Phys. Biol. 1978, 17, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Berking, C.; Hauschild, A.; Kölbl, O.; Mast, G.; Gutzmer, R. Basal cell carcinoma-treatments for the commonest skin cancer. Dtsch. Ärzteblatt Int. 2014, 111, 389–395. [Google Scholar] [CrossRef]
- Gill, H.S.; Moscato, E.E.; Seiff, S.R. Eyelid Margin Basal Cell Carcinoma Managed with Full-Thickness En-Face Frozen Section Histopathology. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 15–19. [Google Scholar] [CrossRef]
- Castley, A.J.; Theile, D.R.; Lambie, D. The Use of Frozen Section in the Excision of Cutaneous Malignancy: A Queensland Experience. Ann. Plast. Surg. 2013, 71, 386–389. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Lee, C.T.; Zhang, E.; Galloway, T.J. Skin CanceR Brachytherapy vs External Beam Radiation Therapy (SCRiBE) Meta-Analysis. Radiother. Oncol. 2018, 126, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Merriam, G.R.; Szechter, A.; Focht, E.F. The Effects of Ionizing Radiations on the Eye1; Columbia University: New York, NY, USA, 2015; pp. 346–385. [Google Scholar]
- Emami, B.; Lyman, J.; Brown, A.; Cola, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of Normal Tissue to Therapeutic Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Eric, J.; Hall, A.J.G. Radiobiology for the Radiologist; Lippincott: New York, NY, USA, 1988; Volume 12–13, pp. 439–442. [Google Scholar]
- Deeg, H.J.; Flournoy, N.; Sullivan, K.M.; Sheehan, K.; Buckner, C.D.; Sanders, J.E.; Storb, R.; Witherspoon, R.P.; Thomas, E.D. Cataracts after Total Body Irradiation and Marrow Transplantation: A Sparing Effect of Dose Fractionation. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 957–964. [Google Scholar] [CrossRef]
- Takeda, A.; Shigematsu, N.; Suzuki, S.; Fujii, M.; Kawata, T.; Kawaguchi, O.; Uno, T.; Takano, H.; Kubo, A.; Ito, H. Late Retinal Complications of Radiation Therapy for Nasal and Paranasal Malignancies: Relationship between Irradiated-Dose Area and Severity. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 599–605. [Google Scholar] [CrossRef]
- Evans, J.R.; Sivagnanavel, V.; Chong, V. Radiotherapy for Neovascular Age-Related Macular Degeneration. Cochrane Database Syst. Rev. 2010, 2010, CD004004. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Char, D.H.; Sagerman, R.H. Late Effects of Radiation on the Eye and Ocular Adnexa. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1123–1139. [Google Scholar] [CrossRef]
- Barabino, S.; Raghavan, A.; Loeffler, J.; Dana, R. Radiotherapy-Induced Ocular Surface Disease. Cornea 2005, 24, 909–914. [Google Scholar] [CrossRef] [PubMed]
| Clinical and Histopathological Factors | Number/Median (Range) |
|---|---|
| age | 82 (64–96) |
| Sex | |
| Men | 17 |
| Women | 11 |
| Histopathological type | |
| Basal cell carcinoma | 24 |
| G1 squamous cell carcinoma | 2 |
| G2 squamous cell carcinoma | 2 |
| Stage | |
| T1N0M0 | 23 |
| T2N0M0 | 5 |
| Location | |
| Lower eyelid | 14 |
| Upper eyelid | 1 |
| Medial canthus | 11 |
| Lateral canthus | 1 |
| Lower eyelid and medial canthus | 1 |
| Number of applicators | 5 (3–11) |
| Fractionation Scheme | Median | Mean | Min | Max | |
|---|---|---|---|---|---|
| 3.5 Gy/49 Gy/BID (two times a day) | D90 | 51.8 | 54.6 | 49.0 | 60.2 |
| BED D90 | 71.0 | 75.9 | 66.2 | 86.1 | |
| EQD2 D90 | 59.1 | 63.3 | 55.1 | 71.7 | |
| D100 | 37.8 | 36.4 | 28.0 | 44.8 | |
| BED D100 | 48.0 | 45.9 | 33.6 | 59.1 | |
| EQD2 D100 | 40.0 | 38.2 | 28.0 | 49.3 | |
| 5 Gy/45 Gy/BID | D90 | 48.6 | 49.5 | 45.0 | 54.0 |
| BED D90 | 74.8 | 76.7 | 67.5 | 86.4 | |
| EQD2 D90 | 62.4 | 63.9 | 56.3 | 72.0 | |
| D100 | 33.3 | 34.2 | 27.0 | 41.4 | |
| BED D100 | 45.6 | 47.2 | 35.1 | 60.4 | |
| EQD2 D100 | 38.02 | 39.3 | 29.3 | 50.4 |
| Fractionation Scheme | Median | Mean | Min | Max | |
|---|---|---|---|---|---|
| 3.5 Gy/49 Gy/BID | Dmax lens | 18.2 | 19.1 | 9.8 | 32.4 |
| BED Dmax | 25.5 | 28.8 | 12.1 | 55.7 | |
| EQD2 Dmax | 15.3 | 17.3 | 7.3 | 33.4 | |
| Dmax eyeball | 48.6 | 48.9 | 43.4 | 57.8 | |
| Dmax retina | 12.6 | 11.2 | 7.0 | 24.9 | |
| Dmax nerve | 10.8 | 9.8 | 5.6 | 22.1 | |
| 5 Gy/45 Gy/BID | Dmax lens | 17.1 | 17.2 | 9.9 | 34.2 |
| BED Dmax | 27.9 | 28.2 | 13.5 | 77.5 | |
| EQD2 Dmax | 16.8 | 16.9 | 8.2 | 46.5 | |
| Dmax eyeball | 44.1 | 45.2 | 36.9 | 53.1 | |
| Dmax retina | 11.7 | 12.9 | 9.9 | 35.1 | |
| Dmax nerve | 9.0 | 9.8 | 3.6 | 24.3 | |
| Complications from the Application Procedure | CTCAE/number (Percentage) of Patients | RTOG/number (Percentage) of Patients |
| oedema | CTCAE 1–28 (100%) | - |
| hematoma | CTCAE 1–3 (11%) | - |
| Early complications | CTCAE/number (percentage) of patients | RTOG/number (percentage) of patients |
| skin | - | RTOG 1–18 (64%) |
| RTOG 2–9 (32%) | ||
| RTOG 3–1 (3%) | ||
| Conjunctivitis | CTCAE 1–20 (71%) | - |
| CTCAE 2–1 (3%) | ||
| Late complications | CTCA/number (percentage) of patients | RTOG/number (percentage) of patients |
| Eyelid deformity | CTCAE 1–6 (30%) | - |
| CTCAE 2–1 (5%) | ||
| Dry eye syndrome | CTCAE 1–11 (55%) | - |
| Skin lesions (discoloration, thinning, telangiectasia) | - | RTOG 1–16 (80%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisek, P.; Kieszko, D.; Bilski, M.; Dębicki, R.; Grywalska, E.; Hrynkiewicz, R.; Bębnowska, D.; Kordzińska-Cisek, I.; Rolińska, A.; Niedźwiedzka-Rystwej, P.; et al. Interstitial HDR Brachytherapy in the Treatment of Non-Melanocytic Skin Cancers around the Eye. Cancers 2021, 13, 1425. https://doi.org/10.3390/cancers13061425
Cisek P, Kieszko D, Bilski M, Dębicki R, Grywalska E, Hrynkiewicz R, Bębnowska D, Kordzińska-Cisek I, Rolińska A, Niedźwiedzka-Rystwej P, et al. Interstitial HDR Brachytherapy in the Treatment of Non-Melanocytic Skin Cancers around the Eye. Cancers. 2021; 13(6):1425. https://doi.org/10.3390/cancers13061425
Chicago/Turabian StyleCisek, Paweł, Dariusz Kieszko, Mateusz Bilski, Radomir Dębicki, Ewelina Grywalska, Rafał Hrynkiewicz, Dominika Bębnowska, Izabela Kordzińska-Cisek, Agnieszka Rolińska, Paulina Niedźwiedzka-Rystwej, and et al. 2021. "Interstitial HDR Brachytherapy in the Treatment of Non-Melanocytic Skin Cancers around the Eye" Cancers 13, no. 6: 1425. https://doi.org/10.3390/cancers13061425
APA StyleCisek, P., Kieszko, D., Bilski, M., Dębicki, R., Grywalska, E., Hrynkiewicz, R., Bębnowska, D., Kordzińska-Cisek, I., Rolińska, A., Niedźwiedzka-Rystwej, P., & Grzybowska-Szatkowska, L. (2021). Interstitial HDR Brachytherapy in the Treatment of Non-Melanocytic Skin Cancers around the Eye. Cancers, 13(6), 1425. https://doi.org/10.3390/cancers13061425









