Merkel Cell Carcinoma: New Trends
Abstract
Simple Summary
Abstract
1. Introduction
2. Etiology, Pathology, and Tumor Biology
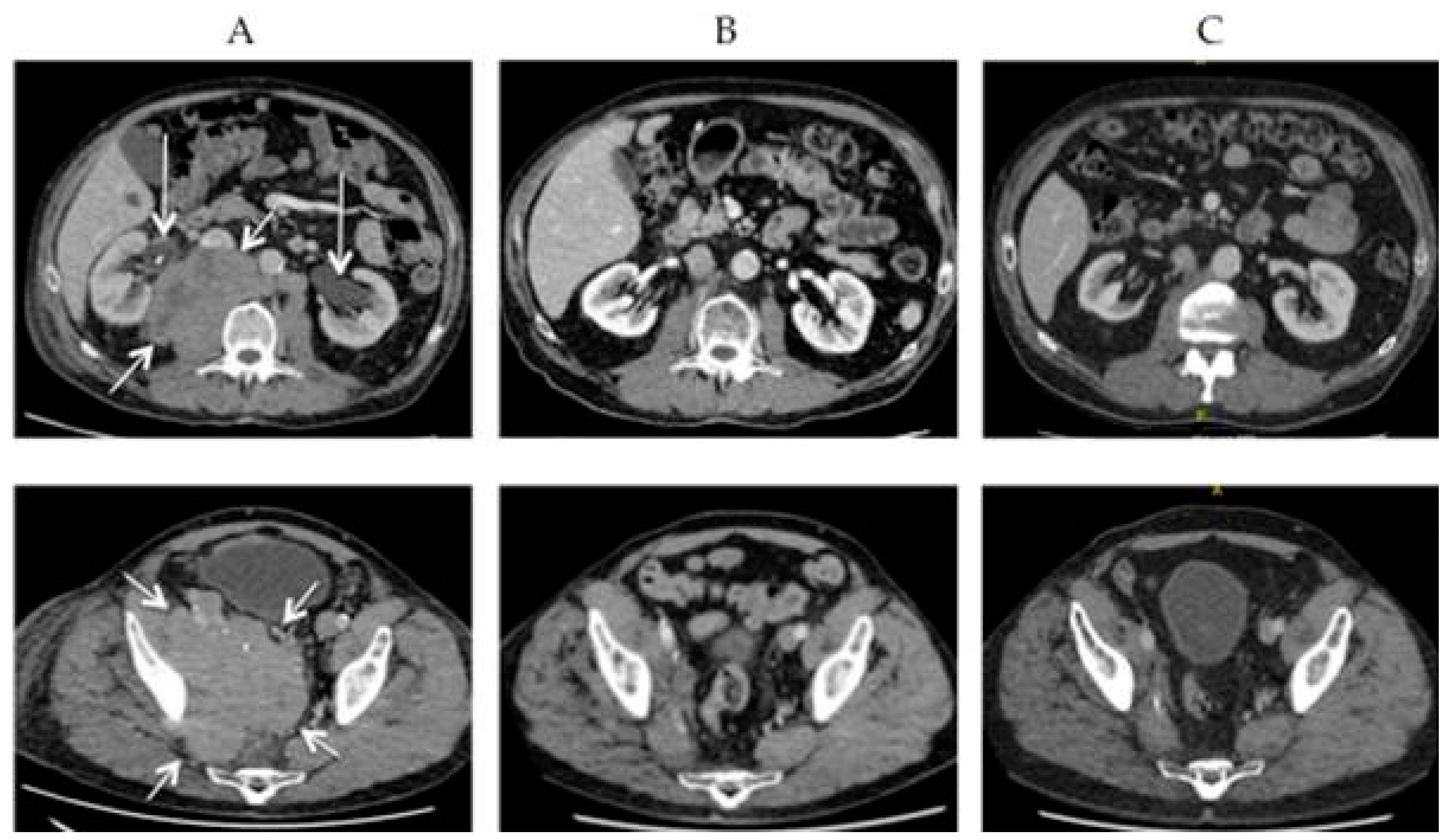
3. Diagnostics and Staging
4. Treatment
4.1. Surgery and Adjuvant Radiotherapy
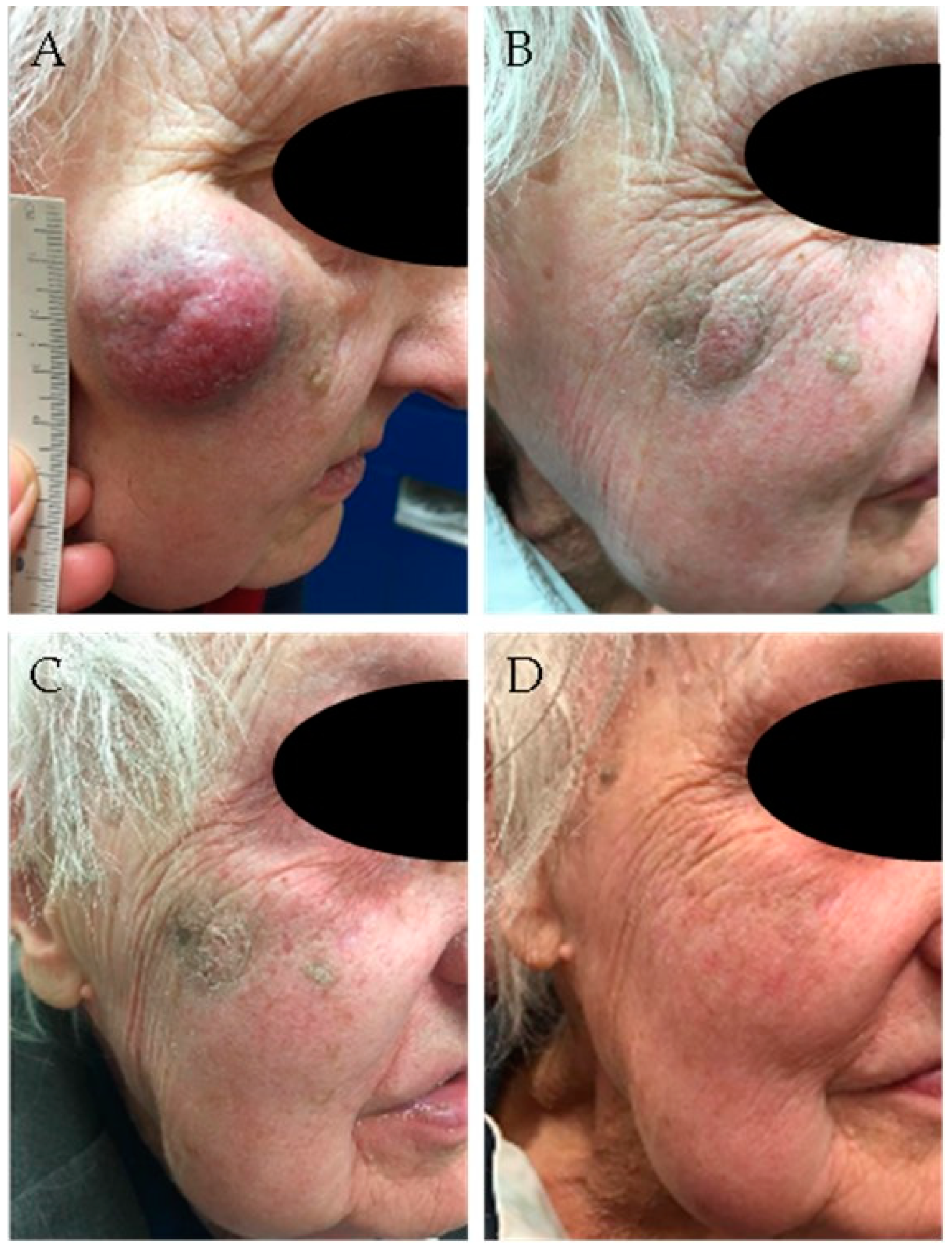
4.2. Definitive Radiotherapy
4.3. Systemic Treatment
5. Biomarkers
6. Multi-Disciplinary and Expertise
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albores-Saavedra, J.; Batich, K.; Chable-Montero, F.; Sagy, N.; Schwartz, A.M.; Henson, D.A. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: A population based study. J. Cutan. Pathol. 2010, 37, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Reichgelt, B.A.; Visser, O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population-based study of 808 cases in 1993–2007. Eur. J. Cancer 2011, 47, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.E.; Brewer, J.D. Merkel cell carcinoma in immunosuppressed patients. Cancers 2014, 6, 1328–1350. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.D.; Shanafelt, T.D.; Otley, C.C.; Roenigk, R.K.; Cerhan, J.R.; Kay, N.E.; Weaver, A.L.; Call, T.G. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a SEER population-based study. J Clin. Oncol. 2012, 30, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.K.; Ward, C.; Gaber, C.E.; Strassle, P.D.; Ollila, D.W.; Laks, S. Decreased survival and increased recurrence in Merkel cell carcinoma significantly linked with immunosuppression. J. Surg. Oncol. 2020, 122, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Andea, A.A.; Coit, D.G.; Amin, B.; Busam, K.J. Merkel cell carcinoma: Histologic features and prognosis. Cancer 2008, 113, 2549–2558. [Google Scholar] [CrossRef]
- Farah, M.; Reuben, A.; Spassova, I.; Yang, R.K.; Kubat, L.; Nagarajan, P.; Ning, J.; Li, W.; Aung, P.P.; Curry, J.L.; et al. T-cell repertoire in combination with T-cell density predicts clinical outcomes in patients with Merkel cell carcinoma. J. Investig. Dermatol. 2020, 140, 2146–2156. [Google Scholar] [CrossRef]
- Becker, J.; Houben, R.; Ugurel, S.; Trefzer, U.; Pföhler, C.; Schrama, D. Polyomavirus is frequently present in Merkel cell carcinoma of European patients. J. Investig. Dermatol. 2009, 129, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Becker, J.C.; Nghiem, P.; Ferlay, J. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: An international assessment. Eur. J. Cancer 2018, 94, 47–60. [Google Scholar] [CrossRef]
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463. [Google Scholar] [CrossRef]
- Garbutcheon-Singh, K.B.; Curchin, D.J.; McCormack, C.J.; Smith, S.D. Trends in the incidence of Merkel cell carcinoma in Victoria, Australia, between 1986 and 2016. Australas. J. Dermatol. 2020, 61, e34–e38. [Google Scholar] [CrossRef]
- Pinault, L.; Bushnik, T.; Fioletov, V.; Peters, C.E.; King, W.D.; Tjepkema, M. The risk of melanoma associated with ambient summer ultraviolet radiation. Health Rep. 2017, 28, 3–11. [Google Scholar]
- Harms, K.L.; Healy, M.A.; Nghiem, P.; Sober, A.J.; Johnson, T.M.; Bichakjian, C.K.; Wong, S.L. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann. Surg. Oncol. 2016, 23, 3564–3571. [Google Scholar] [CrossRef] [PubMed]
- Iyer, J.G.; Storer, B.E.; Paulson, K.G.; Lemos, B.; Philips, J.L.; Bichakjian, C.K.; Zeitouni, N.; Gershenwald, J.E.; Sondak, V.; Otley, C.C.; et al. Relationship among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J. Am. Acad. Dermatol. 2014, 70, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Storer, B.E.; Iyer, J.G.; Moshiri, A.; Parvathaneni, U.; Byrd, D.; Sober, A.J.; Sondak, V.K.; Gershenwald, J.E.; Nghiem, P. Adjuvant radiation therapy and chemotherapy in Merkel cell carcinoma: Survival analyses of 6908 cases from the National Cancer Data Base. J. Natl. Cancer Inst. 2016, 108, djw042. [Google Scholar] [CrossRef] [PubMed]
- Van Veenendaal, L.M.; van Akkooi, A.C.J.; Verhoef, C.; Grünhagen, D.J.; Klop, W.M.C.; Valk, G.D.; Tesselaar, M.E.T. Merkel cell carcinoma: Clinical outcome and prognostic factors in 351 patients. J. Surg. Oncol. 2018, 117, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Farley, C.R.; Perez, M.C.; Soelling, S.J.; Delman, K.A.; Harit, A.; Wuthrick, E.J.; Messina, J.L.; Sondak, V.K.; Zager, J.S.; Lowe, M.C. Merkel cell carcinoma outcomes: Does AJCC8 underestimate survival? Ann. Surg. Oncol. 2020, 27, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Harms, K.L.; Moore, P.S.; DeCaprio, J.A.; Nghiem, P.; Wong, M.K.K.; Brownell, I.; on behalf of the International Workshop on Merkel Cell Carcinoma Research (IWMCC) Working Group. The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat. Rev. Clin. Oncol. 2018, 15, 763–776. [Google Scholar] [CrossRef]
- Kervarrec, T.; Tallet, A.; Miquelestorena-Standley, E.; Houben, R.; Schrama, D.; Gambichler, T.; Berthon, P.; Le Corre, Y.; Hainaut-Wierzbicka, E.; Aubin, F.; et al. Morphologic and immunophenotypical features distinguishing Merkel cell polyomavirus-positive and negative Merkel cell carcinoma. Mod. Pathol. 2019, 32, 1605–1616. [Google Scholar] [CrossRef]
- Moran, J.M.T.; Biecek, P.; Donizy, P.; Wu, C.-L.; Kopczynski, M.D.; Pieniazek, M.; Rys, J.; Hoang, M.P. Large nuclear size correlated with better overall survival, Merkel cell polyomavirus positivity, and terminal deoxynucleotidyl transferase expression in Merkel cell carcinoma. J. Am. Acad. Dermatol. 2021, 84, 550–552. [Google Scholar] [CrossRef]
- Kervarrec, T.; Samimi, M.; Guyétant, S.; Sarma, B.; Chéret, J.; Blanchard, E.; Berthon, P.; Schrama, D.; Houben, R.; Touzé, A. Histogenesis of Merkel cell carcinoma: A comprehensive review. Front. Oncol. 2019, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Tolstov, Y.L.; Pastrana, D.V.; Feng, H.; Becker, J.C.; Jenkins, F.J.; Moschos, S.; Chang, Y.; Buck, C.B.; Moore, P.S. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int. J. Cancer 2009, 125, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Touzé, A.; Gaitan, J.; Arnold, F.; Cazal, R.; Fleury, M.J.; Combelas, N.; Sizaret, P.-Y.; Guyetant, S.; Maruani, A.; Baay, M.; et al. Generation of Merkel Cell Polyomavirus (MCV)-like particles and their application to detection of MCV antibodies. J. Clin. Microbiol. 2010, 48, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Coursaget, P.; Samimi, M.; Nicol, J.T.J.; Gardair, C.; Touzé, A. Human Merkel cell polyomavirus: Virological background and clinical implications. APMIS 2013, 121, 755–769. [Google Scholar] [CrossRef]
- Moshiri, A.S.; Doumani, R.; Yelistratova, L.; Blom, A.; Lachance, K.; Shinohara, M.M.; Delaney, M.; Chang, O.; McArdle, S.; Thomas, H.; et al. Polyomavirus-negative Merkel cell carcinoma: A more aggressive subtype based on analysis of 282 cases using multimodal tumor virus detection. J. Investig. Dermatol. 2017, 137, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Righi, A.; Ambrosi, F.; Gibertoni, D.; Maletta, F.; Uccella, S.; Sessa, F.; Asioli, S.; Pellilli, M.; Maragliano, R.; et al. Prognostic impact of MCPyV and TIL subtyping in Merkel cell carcinoma: Evidence from a large European cohort of 95 patients. Endocr. Pathol. 2020, 31, 21–32. [Google Scholar] [CrossRef]
- Schrama, D.; Sarosi, E.M.; Adam, C.; Ritter, C.; Kaemmerer, U.; Klopocki, E.; König, E.M.; Utikal, J.; Becker, J.C.; Houben, R. Characterization of six Merkel cell polyomavirus-positive Merkel cell carcinoma cell lines: Integration pattern suggest that large T antigen truncating events occur before or during integration. Int. J. Cancer 2019, 145, 1020–1032. [Google Scholar] [CrossRef]
- Afanasiev, O.K.; Yelistratova, L.; Miller, N.; Nagase, K.; Paulson, K.; Iyer, J.G.; Ibrani, D.; Koelle, D.M.; Nghiem, P. Merkel polyomavirus-specific T cells fluctuate with Merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin. Cancer Res. 2013, 19, 5351–5360. [Google Scholar] [CrossRef]
- Iyer, J.G.; Afanasiev, O.K.; McClurkan, C.; Paulson, K.; Nagase, K.; Jing, L.; Marshak, J.O.; Dong, L.; Carter, J.; Lai, I.; et al. Merkel cell polyomavirus-specific CD8+ and CD4+ T-cell responses identified in Merkel cell carcinomas and blood. Clin. Cancer Res. 2011, 17, 6671–6680. [Google Scholar] [CrossRef]
- Friedlaender, M.M.; Rubinger, D.; Rosenbaum, B.; Amir, G.; Siguencia, E. Temporary regression of Merkel cell carcinoma metastases after cessation of cyclosporine. Transplantation 2002, 73, 1849–1850. [Google Scholar] [CrossRef]
- Sais, G.; Admella, C.; Soler, T. Spontaneous regression in primary cutaneous neuroendocrine (Merkel cell) carcinoma: A rare immune phenomenon? J. Eur. Acad. Derm. Venereol. 2002, 16, 82–83. [Google Scholar] [CrossRef]
- Connelly, T.J.; Cribier, B.; Brown, T.J.; Yanguas, I. Complete spontaneous regression of Merkel cell carcinoma: A review of 10 reported cases. Dermatol. Surg. 2000, 26, 853–856. [Google Scholar] [CrossRef]
- Bertolotti, A.; Conte, H.; Francois, L.; Dutriaux, C.; Ezzedine, K.; Mélard, P.; Vergier, B.; Taieb, A.; Jouary, T. Merkel cell carcinoma: Complete clinical remission associated with disease progression. JAMA Dermatol. 2013, 149, 501–502. [Google Scholar] [CrossRef]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Blitzblau, R.; Bowen, G.M.; Contreras, C.M.; Daniels, G.A.; Decker, R.; et al. Merkel cell carcinoma, Version 1.2018. NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 742–774. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Alexander, N.A.; Lachance, K.; Lewis, C.W.; McEvoy, A.; Akaike, G.; Byrd, D.; Behnia, S.; Bhatia, S.; Paulson, K.G.; et al. Clinical benefit of baseline imaging in Merkel cell carcinoma: Analysis of 584 patients. J. Am. Acad. Dermatol. 2021, 84, 330–339. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Sidiropoulou, P.; Hassel, J.C.; Drakoulis, N.; Dimitrakopoulou-Strauss, A. Positron emission tomography in Merkel cell carcinoma. Cancers 2020, 12, 2897. [Google Scholar] [CrossRef] [PubMed]
- Lebbe, C.; Becker, J.C.; Grob, J.-J.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Saiag, P.; Middleton, M.R.; Bastholt, L.; et al. Diagnosis and treatment of Merkel cell carcinoma. European consensus-based interdisciplinary guideline. Eur. J. Cancer. 2015, 51, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Steiniche, T.; Ladekarl, M.; Bønnelykke-Behrndtz, M.L.; Hölmich, L.R.; Langer, S.W.; Venzo, A.; Tabaksblat, E.; Klausen, S.; Skaarup Larsen, M.; et al. Management recommendations for Merkel cell carcinoma-a Danish perspective. Cancers 2020, 12, 554. [Google Scholar] [CrossRef] [PubMed]
- Cassler, N.M.; Merrill, D.; Bichakjian, C.K.; Brownell, I. Merkel cell carcinoma therapeutic update. Curr. Treat. Options Oncol. 2016, 17, 36. [Google Scholar] [CrossRef]
- Allen, P.J.; Browne, W.B.; Jaques, D.P.; Brennan, M.F.; Busam, K.; Coit, D.G. Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. J. Clin. Oncol. 2005, 23, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.L.; Griffith, K.A.; Lowe, L.; Wong, S.L.; McLean, S.A.; Fullen, D.R.; Lao, C.D.; Hayman, J.A.; Bradford, C.R.; Rees, R.S.; et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J. Clin. Oncol. 2011, 29, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Grotz, T.E.; Pockaj, B.A.; Joseph, R.W.; Foote, R.L.; Otley, C.C.; Weaver, A.L.; Jakub, J.W.; Price, D.L. Sentinel lymph node biopsy in Merkel cell carcinoma: The Mayo Clinic experience of 150 patients. Surg. Oncol. 2018, 27, 11–17. [Google Scholar] [CrossRef]
- Smith, F.O.; Yue, B.; Marzban, S.S.; Walls, B.L.; Carr, M.; Jackson, R.S.; Puleo, C.A.; Padhya, T.; Cruse, C.W.; Gonzalez, R.J.; et al. Both tumor depth and diameter are predictive of sentinel node status and survival in Merkel cell carcinoma. Cancer 2015, 121, 3252–3260. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, V.A.; Camp, E.R.; Lentsch, E.J. Sentinel lymph node status in Merkel cell carcinoma of the head and neck: Not a predictor of survival. Head Neck. 2014, 36, 571–579. [Google Scholar] [CrossRef]
- Conic, R.R.Z.; Ko, J.; Saridakis, S.; Damiani, G.; Funchain, P.; Vidimos, A.; Gastman, B.R. Sentinel lymph node biopsy in Merkel cell carcinoma: Predictors of sentinel lymph node positivity and association with overall survival. J. Am. Acad. Dermatol. 2019, 81, 364–372. [Google Scholar] [CrossRef]
- Harounian, J.A.; Molin, N.; Galloway, T.J.; Ridge, D.; Bauman, J.; Farma, J.; Reddy, S.; Lango, M.N. Effect of sentinel lymph node biopsy and LVI on Merkel cell carcinoma prognosis and treatment. Laryngoscope 2021, 131, E828–E835. [Google Scholar] [CrossRef]
- Gunaratne, D.A.; Howle, J.A.A.R.; Veness, M.J. Sentinel lymph node biopsy in Merkel cell carcinoma; a 15 year institutional experience and statistical analysis of 721 reported cases. Br. J. Dermatol. 2016, 174, 273–281. [Google Scholar] [CrossRef]
- Karunaratne, Y.G.; Gunaratne, D.A.; Veness, M.J. Systematic review of sentinel lymph node biopsy in Merkel cell carcinoma of the head and neck. Head Neck. 2018, 40, 2704–2713. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Bononi, I.; Puozzo, A.; Govoni, M.; Foschi, V.; Lanza, G.; Gafà, R.; Gaboriaud, P.; Touzé, F.A.; Selvatici, R.; et al. Merkel cell carcinomas arising in autoimmune disease affected patients treated with biologic drugs, including anti-TNF. Clin. Cancer Res. 2017, 23, 3929–3934. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, W.J.; Brodland, D.G. Merkel cell carcinoma. Dermatol. Surg. 1996, 22, 262–267. [Google Scholar] [CrossRef]
- Yiengpruksawan, A.; Coit, D.G.; Thaler, H.T.; Urmacher, C.; Knapper, W.K. Merkel cell carcinoma. Prognosis and management. Arch. Surg. 1991, 126, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Haag, M.L.; Glass, L.F.; Fenske, N.A. Merkel cell carcinoma. Diagnosis and treatment. Dermatol. Surg. 1995, 21, 669–683. [Google Scholar] [CrossRef]
- Perez, M.C.; de Pinho, F.R.; Holstein, A.; Oliver, D.E.; Naqvi, S.M.H.; Kim, Y.; Messina, J.L.; Burke, E.; Gonzalez, R.J.; Sarnaik, A.A.; et al. Resection margins in Merkel cell carcinoma: Is a 1-cm margin wide enough? Ann. Surg. Oncol. 2018, 25, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Mojica, P.; Smith, D.; Ellenhorn, J.D.I. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J. Clin. Oncol. 2007, 25, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Roman, S.A.; Sosa, J.A.; Judson, B.L. The role of adjuvant therapy in the management of head and neck Merkel cell carcinoma: An analysis of 4815 patients. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 137–141. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, A.; Torchio, M.; Prinzi, N.; Trevisan, F.; Dallera, P.; De Stefani, A.; Russo, A.; Vitali, E.; Bruschieri, L.; et al. Adjuvant radiotherapy for Merkel cell carcinoma: A systematic review and meta-analysis. Radiother. Oncol. 2019, 134, 211–219. [Google Scholar] [CrossRef]
- Frohm, M.L.; Griffith, K.A.; Harms, K.L.; Hayman, J.A.; Fullen, D.R.; Nelson, C.C.; Wong, S.L.; Schwartz, J.L.; Bichakjian, C.K. Recurrence and survival in patients with Merkel cell carcinoma undergoing surgery without adjuvant radiation therapy to the primary site. JAMA Dermatol. 2016, 152, 1001–1007. [Google Scholar] [CrossRef]
- Strom, T.; Carr, M.; Zager, J.S.; Naghavi, A.; Smith, F.O.; Cruse, C.W.; Messina, J.L.; Russell, J.; Rao, N.G.; Fulp, W.; et al. Radiation therapy is associated with improved outcomes in Merkel cell carcinoma. Ann. Surg. Oncol. 2016, 23, 3572–3578. [Google Scholar] [CrossRef]
- Henderson, M.A.; Burmeister, B.H.; Ainslie, J.; Fisher, R.; Di Iulio, J.; Smithers, B.M.; Hong, A.; Shannon, K.; Scolyer, R.A.; Carruthers, S.; et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 2015, 16, 1049–1060. [Google Scholar] [CrossRef]
- Poulsen, M. Radiation therapy rather than surgery for merkel cell carcinoma: The advantages of radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 100, 14–15. [Google Scholar] [CrossRef]
- Alexander, N.; Schaub, S.; Hippe, D.; Lachance, K.; Liao, J.J.; Apisarnthanarax, S.; Bhatia, S.; Tseng, Y.D.; Nghiem, P.T.; Parvathaneni, U. Merkel cell carcinoma recurrence risk increases with the delay op post-operative radiation therapy. In Proceedings of the First International Symposium on Merkel Cell Carcinoma, Tampa, FL, USA, 21–22 October 2019. [Google Scholar]
- Zwijnenburg, E.M.; Lubeek, S.F.; Werner, J.E.; Torres-Rivas, J.; Span, P.N.; Takes, R.J.; Weijs, W.L.; Kaanders, J.H. Merkel cell carcinoma is an oncologic emergency. In Proceedings of the Proceedings First International Symposium on Merkel Cell Carcinoma, Tampa, FL, USA, 21–22 October 2019. [Google Scholar]
- Tsang, G.; O’Brien, P.; Robertson, R.; Hamilton, C.; Wratten, C.; Denham, J. All delays before radiotherapy risk progression of Merkel cell carcinoma. Australas. Radiol. 2004, 48, 371–375. [Google Scholar] [CrossRef]
- Hong, A.M.; Stretch, J.R.; Thompson, J.F. Treatment of primary Merkel cell carcinoma: Radiotherapy can be an effective, less morbid alternative to surgery. Eur. J. Surg. Oncol. 2021, 47, 483–485. [Google Scholar] [CrossRef]
- Wright, G.P.; Holtzman, M.P. Surgical resection improves median overall survival with marginal improvement in long-term survival when compared with definitive radiotherapy in Merkel cell carcinoma: A propensity score matched analysis of the National Cancer Database. Am. J. Surg. 2018, 215, 384–387. [Google Scholar] [CrossRef]
- Hui, A.C.; Stillie, A.L.; Seel, M.; Ainslie, J. Merkel cell carcinoma: 27-year experience at the Peter MacCallum Cancer Centre. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1430–1435. [Google Scholar] [CrossRef]
- Santamaria-Barria, J.A.; Boland, G.M.; Yeap, B.Y.; Nardi, V.; Dias-Santagata, D.; Cusack, J.C., Jr. Merkel cell carcinoma: 30-year experience from a single institution. Ann. Surg. Oncol. 2013, 20, 1365–1373. [Google Scholar] [CrossRef]
- Bishop, A.J.; Garden, A.S.; Gunn, G.B.; Rosenthal, D.I.; Beadle, B.M.; Fuller, C.D.; Levy, L.B.; Gillenwater, A.M.; Kies, M.S.; Esmaeli, B.; et al. Merkel cell carcinoma of the head and neck: Favorable outcomes with radiotherapy. Head Neck 2016, 38 (Suppl. 1), E452–E458. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Morris, C.G.; Kirwan, J.M.; Amdur, R.J.; Shaw, C.; Dziegielewski, P.T. Management of cutaneous Merkel cell carcinoma. Acta Oncol. 2018, 57, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Pape, E.; Rezvoy, N.; Penel, N.; Salleron, J.; Martinot, V.; Guerresschi, P.; Dziwniel, V.; Darras, S.; Mirabel, X.; Mortier, L. Radiotherapy alone for Merkel cell carcinoma: A comparative and retrospective study of 25 patients. J. Am. Acad. Dermatol. 2011, 65, 983–990. [Google Scholar] [CrossRef]
- Veness, M.; Foote, M.; Gebski, V.; Poulsen, M. The role of radiotherapy alone in patients with Merkel cell carcinoma: Reporting the Australian experience of 43 patients. Int. J. Radiat. Oncol. 2010, 78, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.; Kwan, W. Outcomes of Merkel cell carcinoma treated with radiotherapy without radical surgical excision. Ann. Surg. Oncol. 2014, 21, 3401–3405. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, D.A.; Howle, J.R.; Veness, M.J. Definitive radiotherapy for Merkel cell carcinoma confers clinically meaningful in-field locoregional control: A review and analysis of the literature. J. Am. Acad. Dermatol. 2017, 77, 142–148.e1. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.H.; Ramsey, J.R.; Kearsley, J.H.; Birrell, G.W. Radiation sensitivity of Merkel cell carcinoma cells. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1401–1407. [Google Scholar] [PubMed]
- Kok, D.L.; Wang, A.; Xu, W.; Chua, M.S.T.; Guminski, A.; Veness, M.; Howle, J.; Tothill, R.; Kichendasse, G.; Poulsen, M.; et al. The changing paradigm of managing Merkel cell carcinoma in Australia: An expert commentary. Asia Pac. J. Clin. Oncol. 2020, 16, 312–319. [Google Scholar] [CrossRef]
- Tello, T.L.; Coggshall, K.; Yom, S.S.; Yu, S.S. Merkel cell carcinoma: An update and review: Current and future therapy. J. Am. Acad. Dermatol. 2018, 78, 445–454. [Google Scholar] [CrossRef]
- Boyerinas, B.; Jochems, C.; Fantini, M.; Heery, C.R.; Gulley, J.L.; Tsang, K.Y.; Schlom, J. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol. Res. 2015, 3, 1148–1157. [Google Scholar]
- D’Angelo, S.P.; Bhatia, S.; Brohl, A.S.; Hamid, O.; Mehnert, J.M.; Terheyden, P.; Shih, K.C.; Brownell, I.; Lebbé, C.; Lewis, K.D.; et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: Long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J. Immunother. Cancer 2020, 8, e000674. [Google Scholar] [CrossRef]
- Levy, S.; Aarts, M.J.B.; Eskens, F.A.L.M.; Keymeulen, K.B.M.I.; Been, L.B.; Grünhagen, D.; van Akkooi, A.; Jalving, M.; Tesselaar, M.E.T. Avelumab for advanced Merkel cell carcinoma in the Netherlands: A real-world cohort. J. Immunother. Cancer 2020, 8, e001076. [Google Scholar] [CrossRef]
- Walker, J.W.; Lebbé, C.; Grignani, G.; Nathan, P.; Dirix, L.; Fenig, E.; Ascierto, P.A.; Sandhu, S.; Munhoz, R.; Benincasa, E.; et al. Efficacy and safety of avelumab treatment in patients with metastatic Merkel cell carcinoma: Experience from a global expanded access program. J. Immunother. Cancer 2020, 8, e000313. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. J. Clin. Oncol. 2019, 37, 693–702. [Google Scholar] [CrossRef]
- Topalian, S.L.; Bhatia, S.; Amin, A.; Kudchadkar, R.R.; Sharfman, W.H.; Lebbé, C.; Delord, J.-P.; Dunn, L.A.; Shinohara, M.M.; Kulikauskas, R.; et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the Checkmate 538 Trial. J. Clin. Oncol. 2020, 38, 2476–2487. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Wu, S.; Daud, A.I.; Yu, S.S.; Yom, S.S. In-field and abscopal response after short-course radiation therapy in patients with metastatic Merkel cell carcinoma progressing on PD-1 checkpoint blockade: A case series. J. Immunother. Cancer 2018, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Arina, A.; Gutiontov, S.I.; Weichselbaum, R.R. Radiotherapy and immunotherapy for cancer: From “systemic” to “multisite”. Clin. Cancer Res. 2020, 26, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Elia, G.; Ragusa, F.; Ruffilli, I.; Patrizio, A.; Galdiero, M.R.; Baldini, E.; Ulisse, S.; Marone, G.; et al. Autoimmune endocrine dysfunctions associated with cancer immunotherapies. Int. J. Mol. Sci. 2019, 20, 2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kong, D.; Wang, C.; Chen, J.; Li, J.; Liu, Z.; Li, X.; Wang, Z.; Yao, G.; Wang, X. A systematic review and meta-analysis of immune-related adverse events of anti-PD-1 drugs in randomized controlled trials. Technol. Cancer Res. Treat. 2020, 19, 1533033820967454. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Longino, N.V.; Miller, N.J.; Kulikauskas, R.; Iyer, J.G.; Ibrani, D.; Blom, A.; Byrd, D.R.; Parvathaneni, U.; Twitty, C.G.; et al. Intratumoral delivery of plasmid IL12 via electroporation leads to regression of injected and noninjected tumors in Merkel cell carcinoma. Clin. Cancer Res. 2020, 26, 598–607. [Google Scholar] [CrossRef]
- Lyngaa, R.; Pedersen, N.W.; Schrama, D.; Thrue, C.A.; Ibrani, D.; Met, O.; Thor Straten, P.; Nghiem, P.; Becker, J.C.; Hadrup, S.R. T-cell responses to oncogenic merkel cell polyomavirus proteins distinguish patients with Merkel cell carcinoma from healthy donors. Clin. Cancer Res. 2014, 20, 1768–1778. [Google Scholar] [CrossRef]
- Ciudad, C.; Avilés, J.A.; Alfageme, F.; Lecona, M.; Suárez, R.; Lázaro, P. Spontaneous regression in Merkel cell carcinoma: Report of two cases with a description of dermoscopic features and review of the literature. Dermatol. Surg. 2010, 36, 687–693. [Google Scholar] [CrossRef]
- Tabachnick-Cherny, S.; Pulliam, T.; Church, C.; Koelle, D.M.; Nghiem, P. Polyomavirus-driven Merkel cell carcinoma: Prospects for therapeutic vaccine development. Mol. Carcinog. 2020, 59, 807–821. [Google Scholar]
- Longino, N.V.; Yang, J.; Iyer, J.G.; Ibrani, D.; Chow, I.T.; Laing, K.J.; Campbell, V.L.; Paulson, K.G.; Kulikauskas, R.M.; Church, C.D.; et al. Human CD4(+) T cells specific for Merkel cell polyomavirus localize to Merkel cell carcinomas and target a required oncogenic domain. Cancer Immunol. Res. 2019, 7, 1727–1739. [Google Scholar] [CrossRef]
- Zeng, Q.; Gomez, B.P.; Viscidi, R.P.; Peng, S.; He, L.; Ma, B.; Wu, T.C.; Hung, C.F. Development of a DNA vaccine targeting Merkel cell polyomavirus. Vaccine 2012, 30, 1322–1329. [Google Scholar] [CrossRef]
- Gerer, K.F.; Erdmann, M.; Hadrup, S.R.; Lyngaa, R.; Martin, L.M.; Voll, R.E.; Schuler-Thurner, B.; Schuler, G.; Schaft, N.; Hoyer, S.; et al. Preclinical evaluation of NF-κB-triggered dendritic cells expressing the viral oncogenic driver of Merkel cell carcinoma for therapeutic vaccination. Ther. Adv. Med. Oncol. 2017, 9, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Park, D.E.; Cheng, J.; McGrath, J.P.; Lim, M.Y.; Cushman, C.; Swanson, S.K.; Tillgren, M.L.; Paulo, J.A.; Gokhale, P.C.; Florens, L.; et al. Merkel cell polyomavirus activates LSD1-mediated blockade of non-canonical BAF to regulate transformation and tumorigenesis. Nat. Cell. Biol. 2020, 22, 603–615. [Google Scholar] [CrossRef]
- Mauri, F.; Blanpain, C. Targeting the epigenetic addiction of Merkel cell carcinoma. EMBO Mol. Med. 2020, 12, e13347. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.H.; et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 2018, 174, 549–563.e19. [Google Scholar] [CrossRef]
- Bretz, A.C.; Parnitzke, U.; Kronthaler, K.; Dreker, T.; Bartz, R.; Hermann, F.; Ammendola, A.; Wulff, T.; Hamm, S. Domatinostat favors the immunotherapy response by modulating the tumor immune microenvironment (TIME). J. Immunother. Cancer 2019, 7, 294. [Google Scholar] [CrossRef]
- Song, L.; Bretz, A.C.; Gravemeyer, J.; Spassova, I.; Muminova, S.; Gambichler, T.; Sriram, A.; Ferrone, S.; Becker, J.C. The HDAC Inhibitor domatinostat promotes cell-cycle arrest, induces apoptosis, and increases immunogenicity of Merkel cell carcinoma cells. J. Invest. Dermatol. 2020, 141, 903–912.e4. [Google Scholar] [CrossRef]
- Samimi, M.; Gardair, C.; Nicol, J.T.; Arnold, F.; Touzé, A.; Coursaget, P. Merkel cell polyomavirus in Merkel cell carcinoma: Clinical and therapeutic perspectives. Semin. Oncol. 2015, 42, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Polak, J.M.; Van Noorden, S.; Pearse, A.G.; Marangos, P.J.; Azzopardi, J.G. Immunostaining of neuron-specific enolase as a diagnostic tool for Merkel cell tumors. Cancer 1983, 52, 1039–1043. [Google Scholar] [CrossRef]
- Van Veenendaal, L.M.; Bertolli, E.; Korse, C.M.; Klop, W.M.C.; Tesselaar, M.E.T.; van Akkooi, A.C.J. The clinical utility of Neuron-Specific Enolase (NSE) serum levels as a biomarker for Merkel cell carcinoma (MCC). Ann. Surg. Oncol. 2021, 28, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, M.R.; Daily, K.; Hoffmann, J.; Brune, M.; Enk, A.; Brownell, I. Evaluating blood levels of neuron specific enolase, chromogranin A, and circulating tumor cells as Merkel cell carcinoma biomarkers. Oncotarget 2015, 6, 26472–26482. [Google Scholar] [CrossRef] [PubMed]
- Knepper, T.C.; Montesion, M.; Russell, J.S.; Sokol, E.S.; Frampton, G.M.; Miller, V.A.; Albacker, L.A.; McLeod, H.L.; Eroglu, Z.; Khushalani, N.I.; et al. The genomic landscape of Merkel cell carcinoma and clinicogenomic biomarkers of response to immune checkpoint inhibitor therapy. Clin. Cancer Res. 2019, 25, 5961–5971. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.J.; Luu, M.; Freeman, M.; Essner, R.; Gharavi, N.M.; Shiao, S.L.; Mallen-St. Clair, J.; Hamid, O.; Ho, A.S.; Zumsteg, Z.S. The association between facility volume and overall survival in patients with Merkel cell carcinoma. J. Surg. Oncol. 2020, 122, 254–262. [Google Scholar] [CrossRef] [PubMed]
| ClinicalTrials. Gov Identifier | Type of Study | Investigational Drug | Mode of Action | Eligibility | Recruitment Status |
|---|---|---|---|---|---|
| NCT02584829 | Phase I/II | Avelumab * | PD-L1 inhibition | Stage IV | active, not recruiting |
| NCT04160065 | Phase I | IFx-Hu2.0 (intratumoral) | Emm55 protein expression | Advanced | recruiting |
| NCT04291885 | Phase II, randomized | Avelumab | PD-L1 inhibition | Stage I-III | recruiting |
| NCT03271372 | Phase III, randomized | Avelumab | PD-L1 inhibition | Stage III | recruiting |
| NCT03988647 | Phase II | Pembrolizumab * | PD-1 inhibition | Stage IV | recruiting |
| NCT03798639 | Phase I, randomized | Nivolumab * ipilimumab | PD-1 inhibition CTLA-4 inhibition | pathol. Stage IIIA-B | recruiting |
| NCT03712605 | Phase III, randomized | Pembrolizumab | PD-1 inhibition | Stage I-III | recruiting |
| NCT03304639 | Phase II, randomized | Pembrolizumab * | PD-1 inhibition | Stage III-IV | active, not recruiting |
| NCT04261855 | Phase Ib/II | Avelumab * | PD-L1 inhibition | Stage IV | recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwijnenburg, E.M.; Lubeek, S.F.K.; Werner, J.E.M.; Amir, A.L.; Weijs, W.L.J.; Takes, R.P.; Pegge, S.A.H.; van Herpen, C.M.L.; Adema, G.J.; Kaanders, J.H.A.M. Merkel Cell Carcinoma: New Trends. Cancers 2021, 13, 1614. https://doi.org/10.3390/cancers13071614
Zwijnenburg EM, Lubeek SFK, Werner JEM, Amir AL, Weijs WLJ, Takes RP, Pegge SAH, van Herpen CML, Adema GJ, Kaanders JHAM. Merkel Cell Carcinoma: New Trends. Cancers. 2021; 13(7):1614. https://doi.org/10.3390/cancers13071614
Chicago/Turabian StyleZwijnenburg, Ellen M., Satish F.K. Lubeek, Johanna E.M. Werner, Avital L. Amir, Willem L.J. Weijs, Robert P. Takes, Sjoert A.H. Pegge, Carla M.L. van Herpen, Gosse J. Adema, and Johannes H. A. M. Kaanders. 2021. "Merkel Cell Carcinoma: New Trends" Cancers 13, no. 7: 1614. https://doi.org/10.3390/cancers13071614
APA StyleZwijnenburg, E. M., Lubeek, S. F. K., Werner, J. E. M., Amir, A. L., Weijs, W. L. J., Takes, R. P., Pegge, S. A. H., van Herpen, C. M. L., Adema, G. J., & Kaanders, J. H. A. M. (2021). Merkel Cell Carcinoma: New Trends. Cancers, 13(7), 1614. https://doi.org/10.3390/cancers13071614








