A Potential Renewed Use of Very Heavy Ions for Therapy: Neon Minibeam Radiation Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
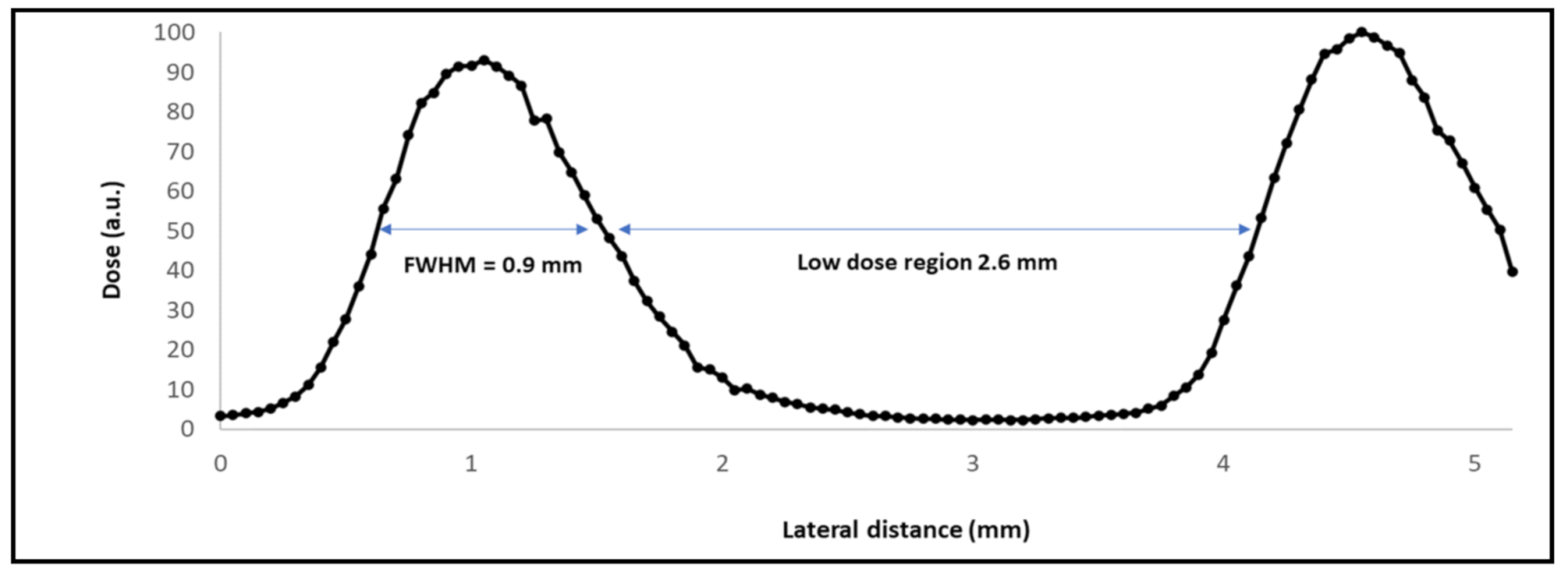
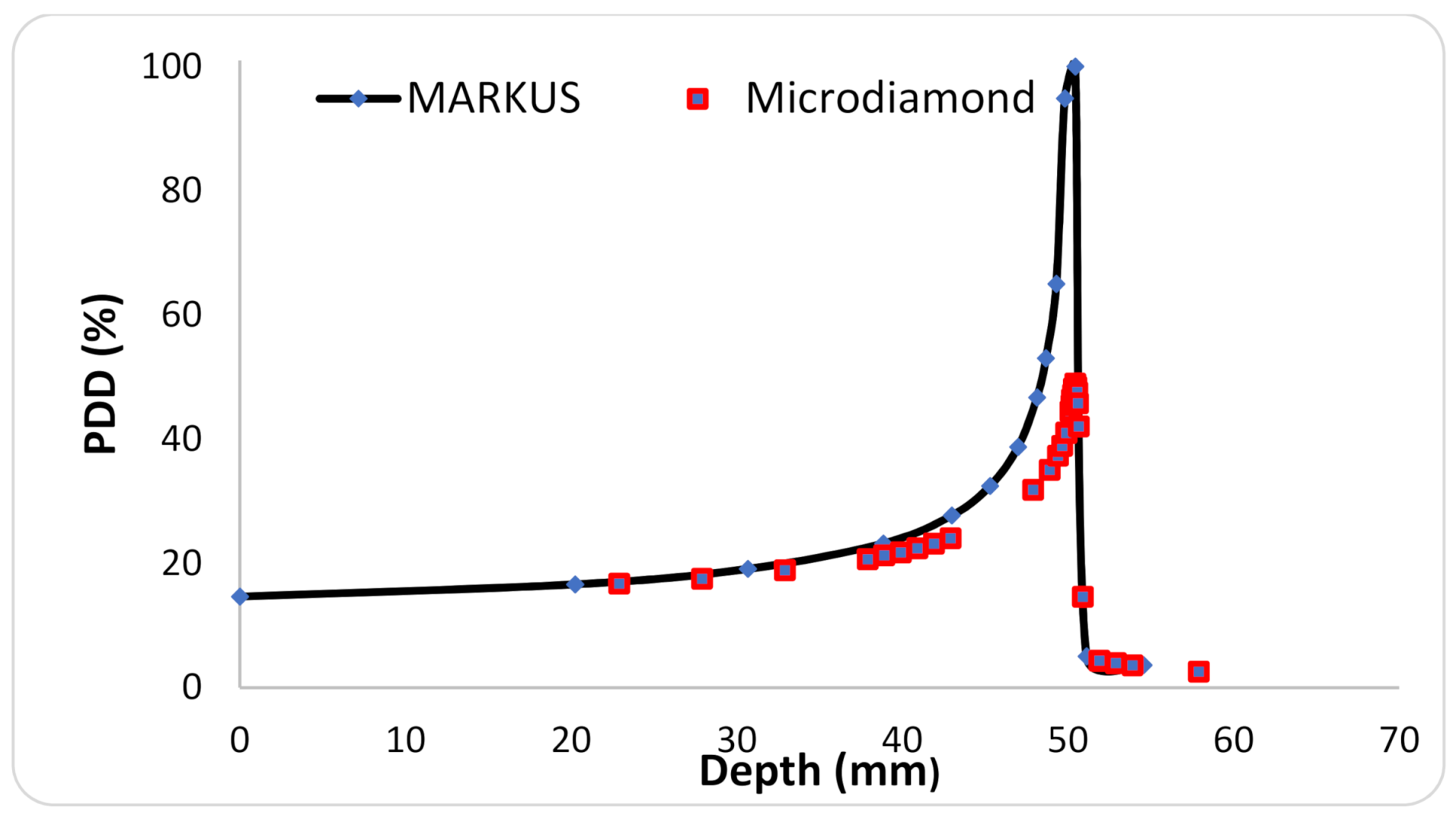
2.1. Irradiations
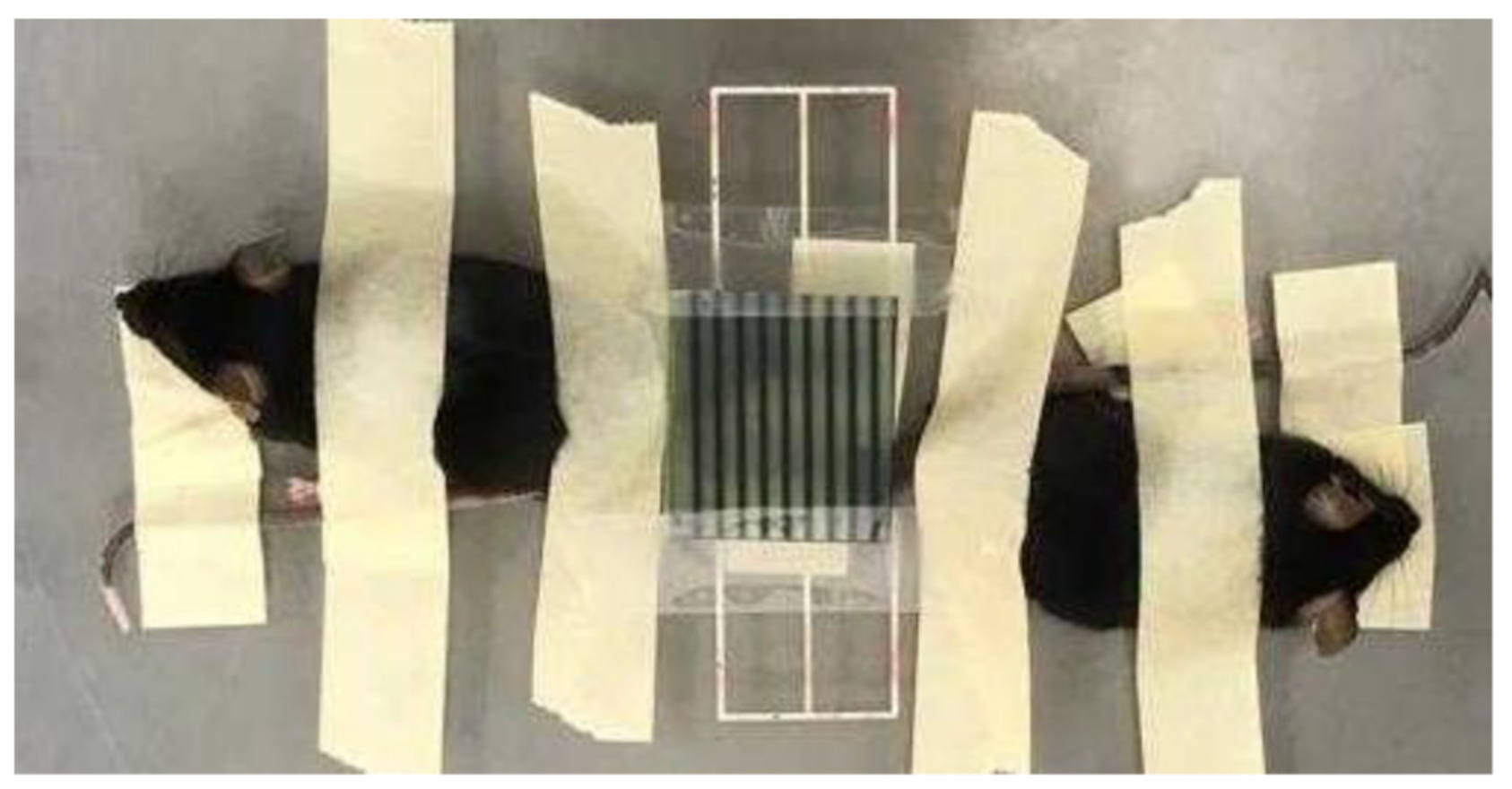
2.2. In Vivo Experiment
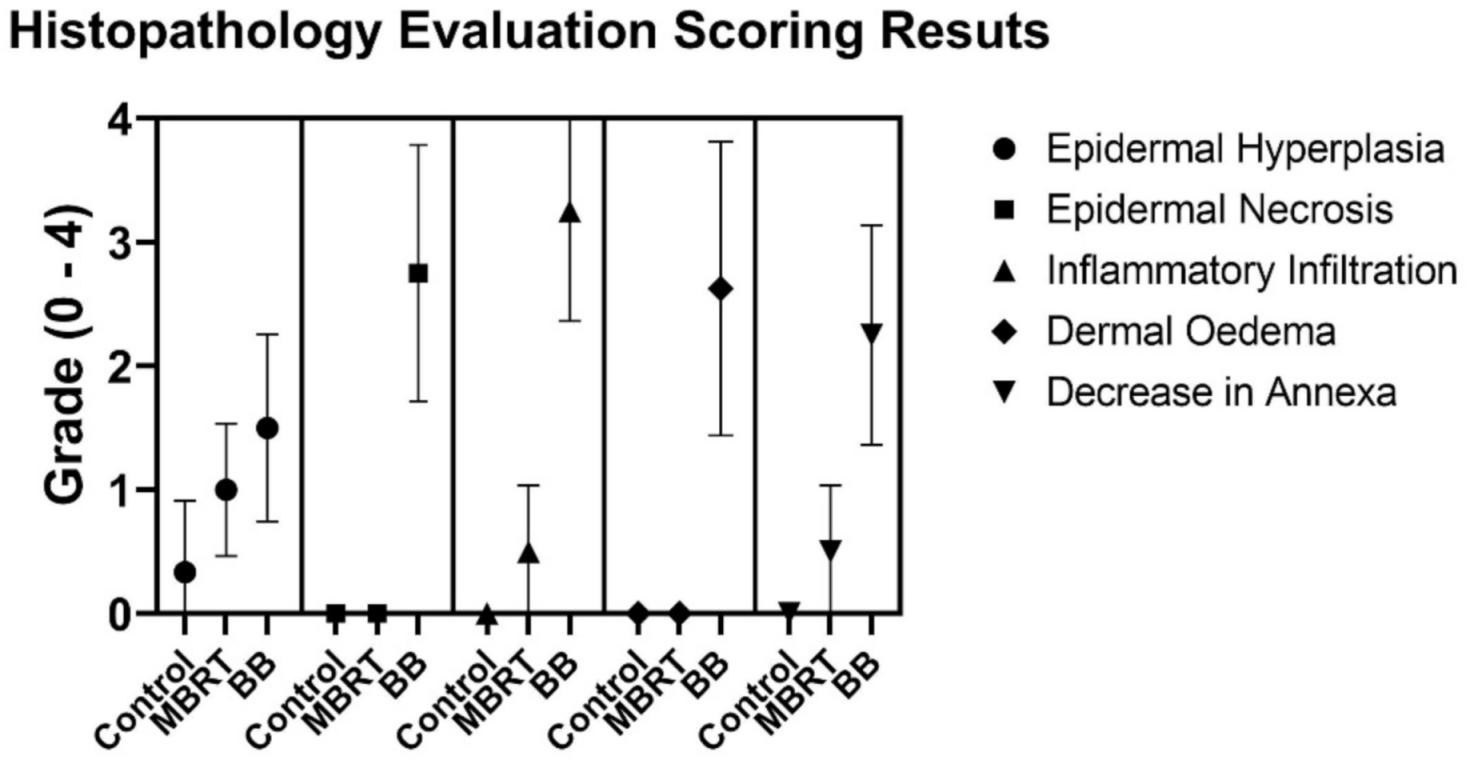
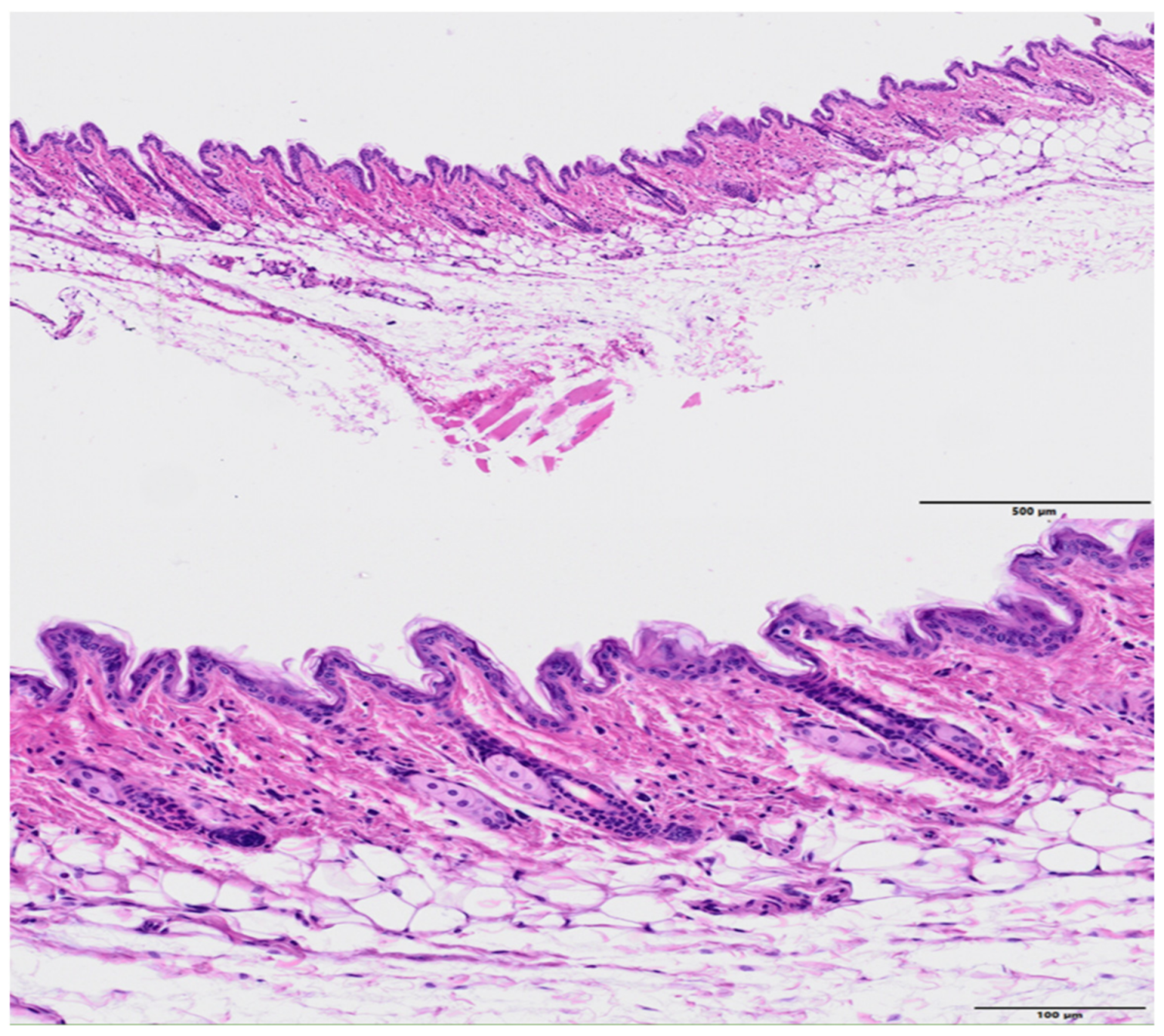
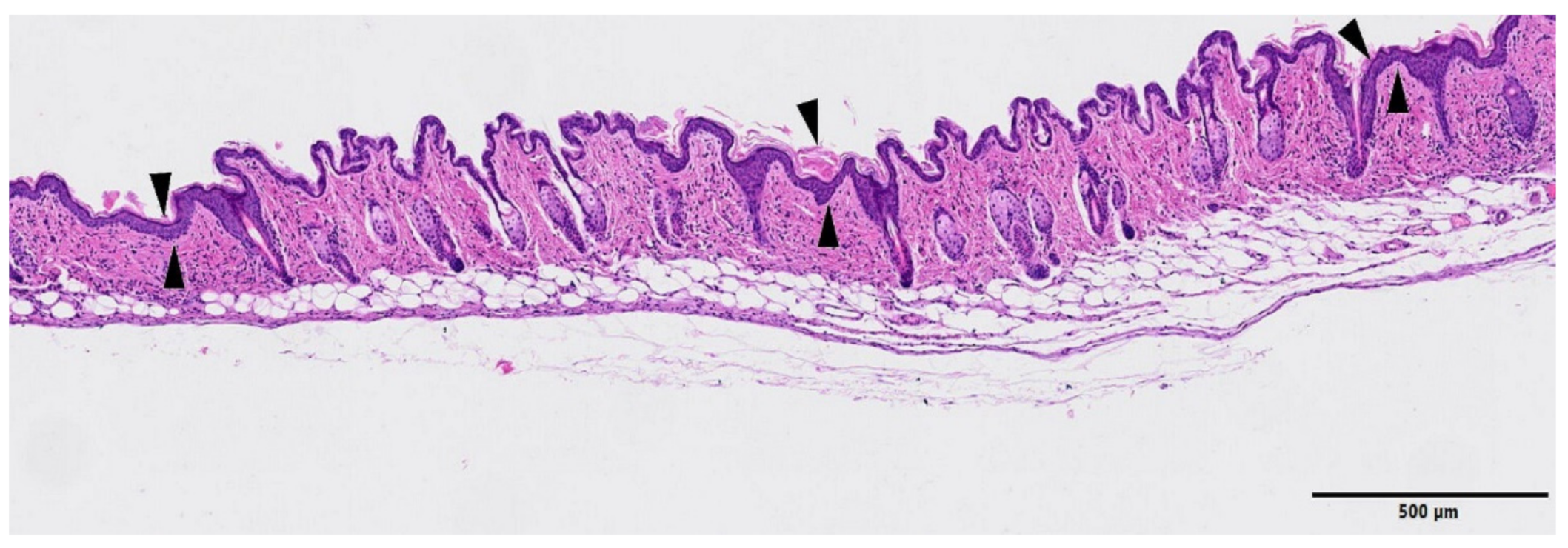
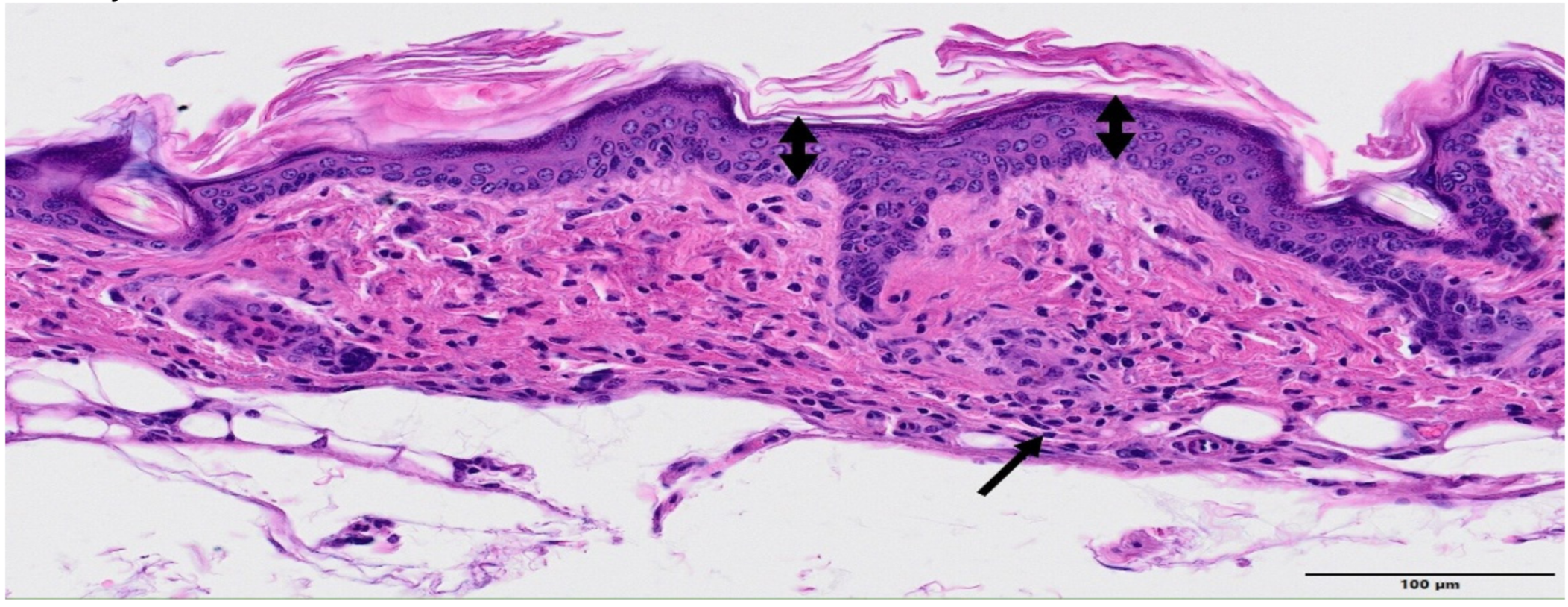
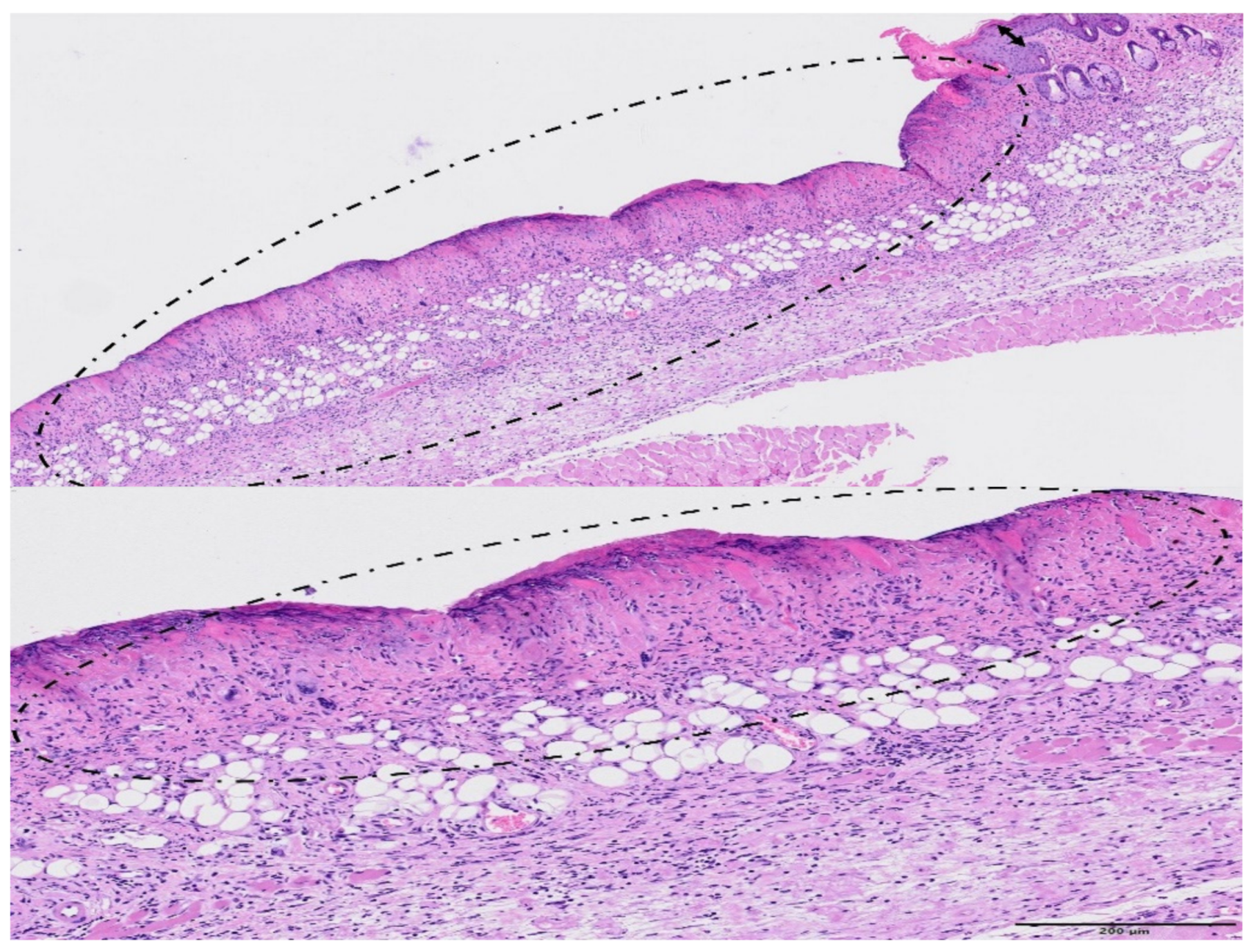
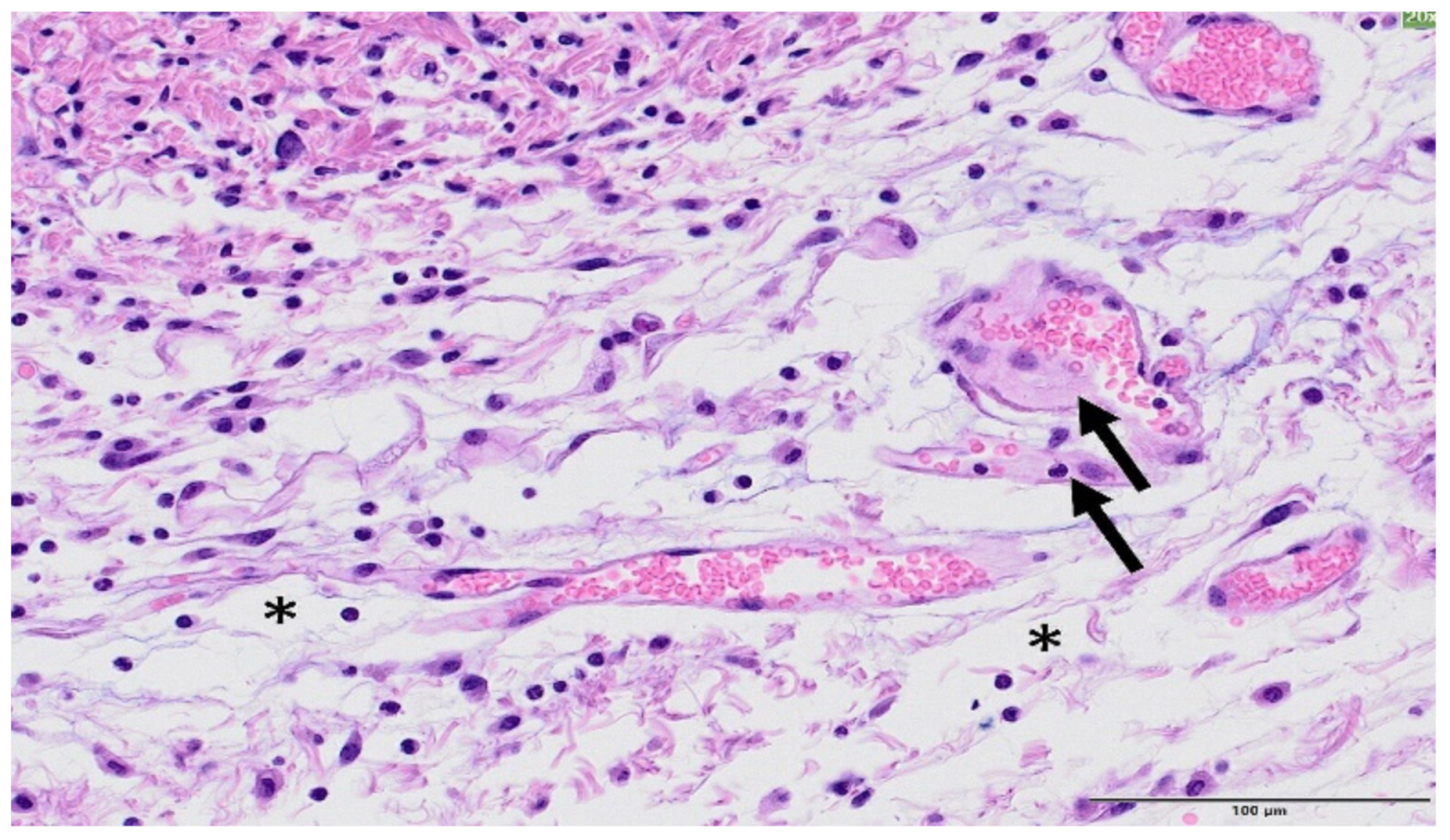
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Grade 1, Minimal | This corresponds to a histopathologic change ranging from inconspicuous to barely noticeable but so minor, small, or infrequent as to warrant no more than the least assignable grade. For multifocal or diffusely distributed lesions, this grade was used for processes where less than approximately 10% of the tissue in an average high-power field was involved. |
| Grade 2, Slight | This corresponds to a histopathologic change that is a noticeable but not a prominent feature of the tissue. For multifocal or diffusely distributed lesions, this grade was used for processes where between approximately 10% and 25% of the tissue in an average high-power field was involved. |
| Grade 3, Moderate | This corresponds to a histopathologic change that is a prominent but not a dominant feature of the tissue. For multifocal or diffusely distributed lesions, this grade was used for processes where between approximately 25% and 50% of the tissue in an average high-power field was involved. |
| Grade 4, Marked | This corresponds to a histopathologic change that is a dominant but not an overwhelming feature of the tissue. For multifocal or diffusely distributed lesions, this grade was used for processes where between approximately 50% and 95% of the tissue in an average high-power field was involved. |
| Grade 5, Severe | This corresponds to a histopathologic change that is an overwhelming feature of the tissue. For multifocal or diffusely distributed lesions, this grade was used for processes where greater than approximately 95% of the tissue in an average high-power field was involved. |
References
- Graham, K.; Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomed. 2018, 13, 6049–6058. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Q.; Yu, T.; Sun, S.; Wang, W.; Liu, G. Hypoxia promotes drug resistance in osteosarcoma cells via activating AMP-activated protein kinase (AMPK) signaling. J. Bone Oncol. 2016, 5, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Brizel, D.M.; Scully, S.P.; Harrelson, J.M.; Layfield, L.J.; Bean, J.M.; Prosnitz, L.R.; Dewhirst, M.W. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996, 56, 941–943. [Google Scholar] [PubMed]
- Krämer, M.; Weyrather, W.K.; Scholz, M. The increased biological effectiveness of heavy charged particles: From radiobiology to treatment planning. Technol. Cancer Res. Treat. 2003, 2, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Tinganelli, W.; Durante, M. Carbon ion radiobiology. Cancers 2020, 12, 3022. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.R.; Linstadt, D.E.; Bahary, J.P.; Petti, P.L.; Daftari, I.; Collier, J.M.; Gutin, P.H.; Gauget, G.; Phillips, T.L. Experience in charged particle irradiation of tumors of the skull base: 1977–1992. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 647–655. [Google Scholar] [CrossRef]
- Dilmanian, F.A.; Zhong, Z.; Bacarian, T.; Benveniste, H.; Romanelli, P.; Wang, R.; Welwart, J.; Yuasa, T.; Rosen, E.M.; Anschel, D.J. Interlaced X-ray microplanar beams: A radiosurgery approach with clinical potential. Proc. Natl. Acad. Sci. USA 2006, 103, 9709–9714. [Google Scholar] [CrossRef]
- Deman, P.; Vautrin, M.; Adam, J.-F.; Edouard, M.; Stupar, V.; Bobyk, L.; Farion, R.; Elleaume, H.; Rémy, C.; Barbier, E.L.; et al. Monochromatic minibeams radiotherapy: From healthy tissue-sparing effect studies toward first experimental glioma bearing rats therapy. Int. J. Radiat. Oncol. 2012, 82, e693–e700. [Google Scholar] [CrossRef]
- Prezado, Y.; Deman, P.; Varlet, P.; Jouvion, G.; Gil, S.; Le Clec’H, C.; Bernard, H.; Le Duc, G.; Sarun, S. Tolerance to dose escalation in minibeam radiation therapy applied to normal rat brain: Long-term clinical, radiological and histopathological analysis. Radiat. Res. 2015, 184, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Prezado, Y.; Dos Santos, M.; Pouzoulet, F.; Gonzalez, W.; Jouvion, G.; Guardiola, C.; Heinrich, S.; Labiod, D.; Juchaux, M.; Jourdain, L.; et al. Transfer of Minibeam Radiation Therapy into a cost-effective equipment for radiobiological studies: A proof of concept. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Prezado, Y.; Jouvion, G.; Dendale, R.; Jourdain, L.; Sebrie, C.; Pouzoulet, F.; Hardy, D.; Patriarca, A.; Nauraye, C.; Bergs, J.; et al. Proton minibeam radiation therapy spares normal rat brain: Long-term clinical, radiological and histopathological analysis. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Lamirault, C.; Doyère, V.; Juchaux, M.; Pouzoulet, F.; Labiod, D.; Dendale, R.; Patriarca, A.; Nauraye, C.; Le Dudal, M.; Jouvion, G.; et al. Short and long-term evaluation of the impact of proton minibeam radiation therapy on motor, emotional and cognitive functions. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Girst, S.; Greubel, C.; Multhoff, G.; Dollinger, G.; Schmid, T.E.; Reindl, J.; Siebenwirth, C.; Zlobinskaya, O.; Walsh, D.W.; Ilicic, K.; et al. Proton minibeam radiation therapy reduces side effects in an in vivo mouse ear model. Int. J. Radiat. Oncol. 2016, 95, 234–241. [Google Scholar] [CrossRef]
- Prezado, Y.; Martínez-Rovira, I.; Thengumpallil, S.; Deman, P. Dosimetry protocol for the preclinical trials in white-beam minibeam radiation therapy. Med. Phys. 2011, 38, 5012–5020. [Google Scholar] [CrossRef]
- Sotiropoulos, M.; Brisebard, E.; Le Dudal, M.; Jouvion, G.; Juchaux, M.; Crépin, D.; Sebrie, C.; Jourdain, L.; Labiod, D.; Lamirault, C.; et al. X-rays minibeam radiation therapy at a conventional irradiator: Pilot evaluation in F98-glioma bearing rats and dose calculations in a human phantom. Clin. Transl. Radiat. Oncol. 2021, 27, 44–49. [Google Scholar] [CrossRef]
- Prezado, Y.; Sarun, S.; Gil, S.; Deman, P.; Bouchet, A.; Le Duc, G. Increase of lifespan for glioma-bearing rats by using minibeam radiation therapy. J. Synchrotron Radiat. 2011, 19, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Prezado, Y.; Jouvion, G.; Patriarca, A.; Nauraye, C.; Guardiola, C.; Juchaux, M.; Lamirault, C.; Labiod, D.; Jourdain, L.; Sebrie, C.; et al. Proton minibeam radiation therapy widens the therapeutic index for high-grade gliomas. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Prezado, Y.; Jouvion, G.; Patriarca, A.; Dendale, R.; Guardiola, C.; Gonzalez, W.; Juchaux, M.; Bergs, J.; Nauraye, C.; Labiod, D.; et al. Tumor control in Rg2 glioma-bearing rats: A comparison between proton minibeam therapy and standard proton therapy. Int. J. Radiat. Oncol. 2019, 104, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Lamirault, C.; Brisebard, E.; Hardy, D.; De Marzi, L.; Dendale, R.; Jouvion, G.; Prezado, Y.; Patriarca, A.; Juchaux, M.; Crepin, D.; et al. Spatially modulated proton minibeams results in the same increase of lifespan as a uniform target dose coverage in F98-glioma-bearing rats. Radiat. Res. 2020, 194, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.N.; Kierski, T.M.; Kasoji, S.K.; Abrantes, A.S.; Dayton, P.A.; Chang, S.X. Conventional dose rate spatially-fractionated radiation therapy (SFRT) treatment response and its association with dosimetric parameters—A preclinical study in a Fischer 344 rat model. PLoS ONE 2020, 15, e0229053. [Google Scholar] [CrossRef]
- Peucelle, C.; Martínez-Rovira, I.; Prezado, Y. Spatial fractionation of the dose using neon and heavier ions: A Monte Carlo study. Med. Phys. 2015, 42, 5928–5936. [Google Scholar] [CrossRef]
- González, W.; Prezado, Y. Spatial fractionation of the dose in heavy ions therapy: An optimization study. Med. Phys. 2018, 45, 2620–2627. [Google Scholar] [CrossRef]
- Tsujii, H.; Mizoe, J.-E.; Kamada, T.; Baba, M.; Kato, S.; Kato, H.; Tsuji, H.; Yamada, S.; Yasuda, S.; Ohno, T.; et al. Overview of clinical experiences on carbon ion radiotherapy at NIRS. Radiother. Oncol. 2004, 73, S41–S49. [Google Scholar] [CrossRef]
- Yasuda, H.; Fujitaka, K. Responses of TLD-BeO:Na (UD-170A) to heavy ions and space radiation. Radiat. Prot. Dosim. 2001, 94, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Rossomme, S.; Hopfgartner, J.; Vynckier, S.; Palmans, H. Under-response of a PTW-60019 microDiamond detector in the Bragg peak of a 62 MeV/n carbon ion beam. Phys. Med. Biol. 2016, 61, 4551–4563. [Google Scholar] [CrossRef] [PubMed]
- Rossomme, S.; Marinelli, M.; Verona-Rinati, G.; Romano, F.; Cirrone, P.A.G.; Kacperek, A.; Vynckier, S.; Palmans, H. Response of synthetic diamond detectors in proton, carbon, and oxygen ion beams. Med. Phys. 2017, 44, 5445–5449. [Google Scholar] [CrossRef]
- Martisikova, M.; Jakel, O. Dosimetric properties of Gafchromic(R) EBT films in medical carbon ion beams. Phys. Med. Biol. 2010, 55, 5557–5567. [Google Scholar] [CrossRef] [PubMed]
- Prezado, Y.; Martínez-Rovira, I.; Sánchez, M. Scatter factors assessment in microbeam radiation therapy. Med. Phys. 2012, 39, 1234–1238. [Google Scholar] [CrossRef]
- Leith, J.T.; Woodruff, K.H.; Howard, J.; Lyman, J.T.; Smith, P.; Lewinsky, B.S. Early and late effects of accelerated charged particles on normal tissues. Int. J. Radiat. Oncol. 1977, 3, 103–108. [Google Scholar] [CrossRef]
- Hirayama, R.; Uzawa, A.; Obara, M.; Takase, N.; Koda, K.; Ozaki, M.; Noguchi, M.; Matsumoto, Y.; Li, H.; Yamashita, K.; et al. Determination of the relative biological effectiveness and oxygen enhancement ratio for micronuclei formation using high-LET radiation in solid tumor cells: An in vitro and in vivo study. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 793, 41–47. [Google Scholar] [CrossRef]
- Chaze, T.; Hornez, L.; Chambon, C.; Haddad, I.; Vinh, J.; Peyrat, J.-P.; Benderitter, M.; Guipaud, O. Serum proteome analysis for profiling predictive protein markers associated with the severity of skin lesions induced by ionizing radiation. Proteomes 2013, 1, 40–69. [Google Scholar] [CrossRef] [PubMed]
- Linstadt, D.E.; Castro, J.R.; Phillips, T.L. Neon ion radiotherapy: Results of the phase I/II clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 761–769. [Google Scholar] [CrossRef]
- Archambeau, J.O.; Pezner, R.; Wasserman, T. Pathophysiology of irradiated skin and breast. Int. J. Radiat. Oncol. 1995, 31, 1171–1185. [Google Scholar] [CrossRef]
- Tatsuzaki, H.; Arimoto, T.; Inada, T.; Uwamino, Y.; Nakamura, T.; Yaguchi, M.; Akisada, M. Early skin reaction following superficial proton irradiation. J. Radiat. Res. 1991, 32, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Koike, S.; Ohmachi, Y.; Ando, Y.; Kobashi, G. Tumor induction in mice after local irradiation with single doses of either carbon-ion beams or gamma rays. Int. J. Radiat. Biol. 2014, 90, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Uzawa, A.; Ando, K.; Kase, Y.; Hirayama, R.; Matsumoto, Y.; Matsufuji, N.; Koike, S.; Kobashi, G. Designing a ridge filter based on a mouse foot skin reaction to spread out Bragg-peaks for carbon-ion radiotherapy. Radiother. Oncol. 2015, 115, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Belyakov, O.V.; Mitchell, S.A.; Parikh, D.; Randers-Pehrson, G.; Marino, S.A.; Amundson, S.A.; Geard, C.R.; Brenner, D.J. From The Cover: Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc. Natl. Acad. Sci. USA 2005, 102, 14203–14208. [Google Scholar] [CrossRef]
- Sedelnikova, O.A.; Nakamura, A.; Kovalchuk, O.; Koturbash, I.; Mitchell, S.A.; Marino, S.A.; Brenner, D.J.; Bonner, W.M. DNA double-strand breaks form in bystander cells after microbeam irradiation of three-dimensional human tissue models. Cancer Res. 2007, 67, 4295–4302. [Google Scholar] [CrossRef]
- Dilmanian, F.A.; Qu, Y.; Feinendegen, L.E.; Peña, L.A.; Bacarian, T.; Henn, F.A.; Kalef-Ezra, J.; Liu, S.; Zhong, Z.; McDonald, J.W. Tissue-sparing effect of x-ray microplanar beams particularly in the CNS: Is a bystander effect involved? Exp. Hematol. 2007, 35, 69–77. [Google Scholar] [CrossRef]
- Aragona, M.; Dekoninck, S.; Rulands, S.; Lenglez, S.; Mascré, G.; Simons, B.D.; Blanpain, C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017, 8, 14684. [Google Scholar] [CrossRef]
- Serduc, R.; Bräuer-Krisch, E.; Brochard, T.; Rémy, C.; Barbier, E.L.; Bravin, A.; Le Duc, G.; Depaulis, A.; Estève, F.; Laissue, J.A.; et al. High-precision radiosurgical dose delivery by interlaced microbeam arrays of high-flux low-energy synchrotron X-rays. PLoS ONE 2010, 5, e9028. [Google Scholar] [CrossRef] [PubMed]
- Potez, M.; Fernandez-Palomo, C.; Bouchet, A.; Trappetti, V.; Donzelli, M.; Krisch, M.; Laissue, J.; Volarevic, V.; Djonov, V. Synchrotron microbeam radiation therapy as a new approach for the treatment of radioresistant melanoma: Potential underlying mechanisms. Int. J. Radiat. Oncol. 2019, 105, 1126–1136. [Google Scholar] [CrossRef] [PubMed]

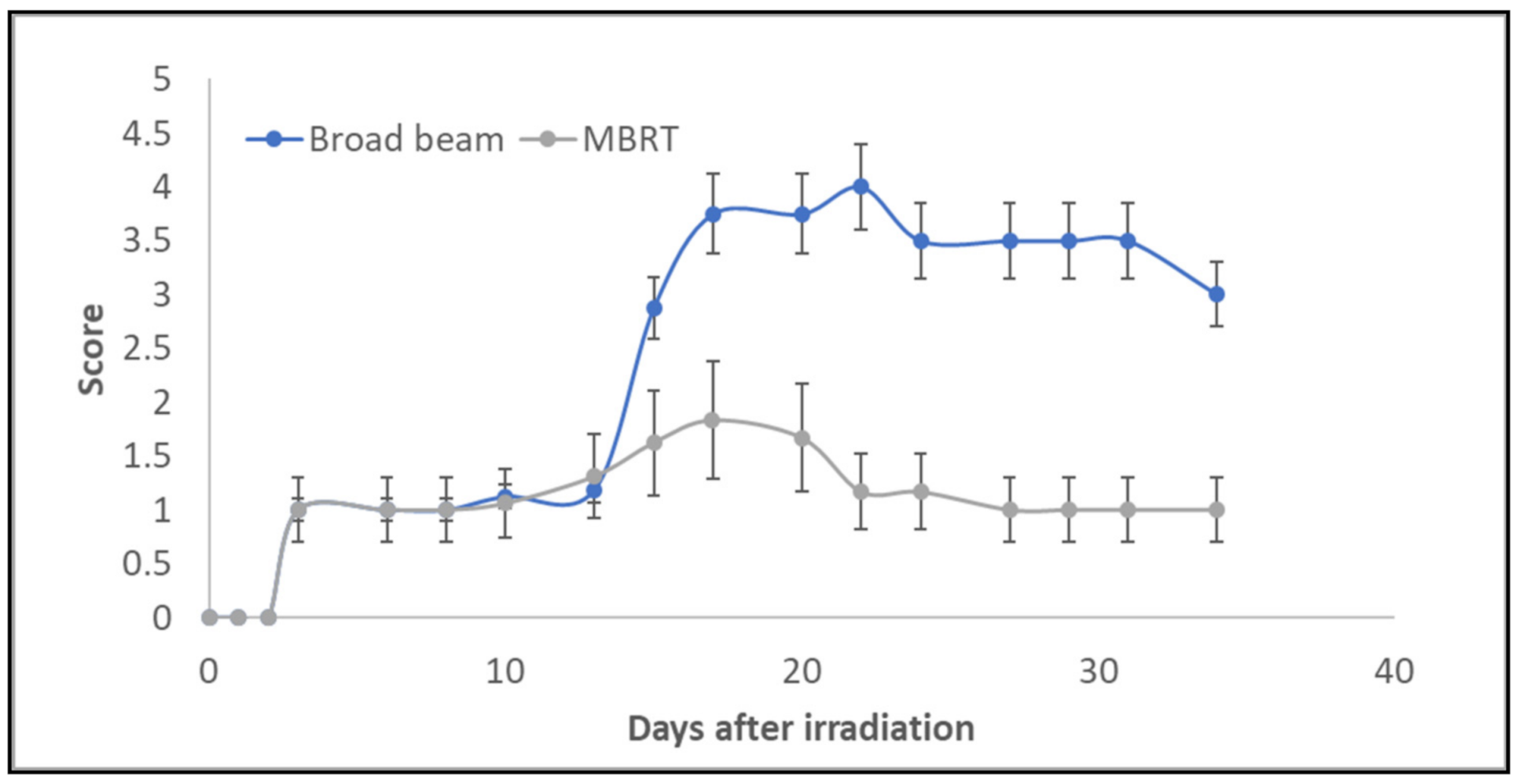
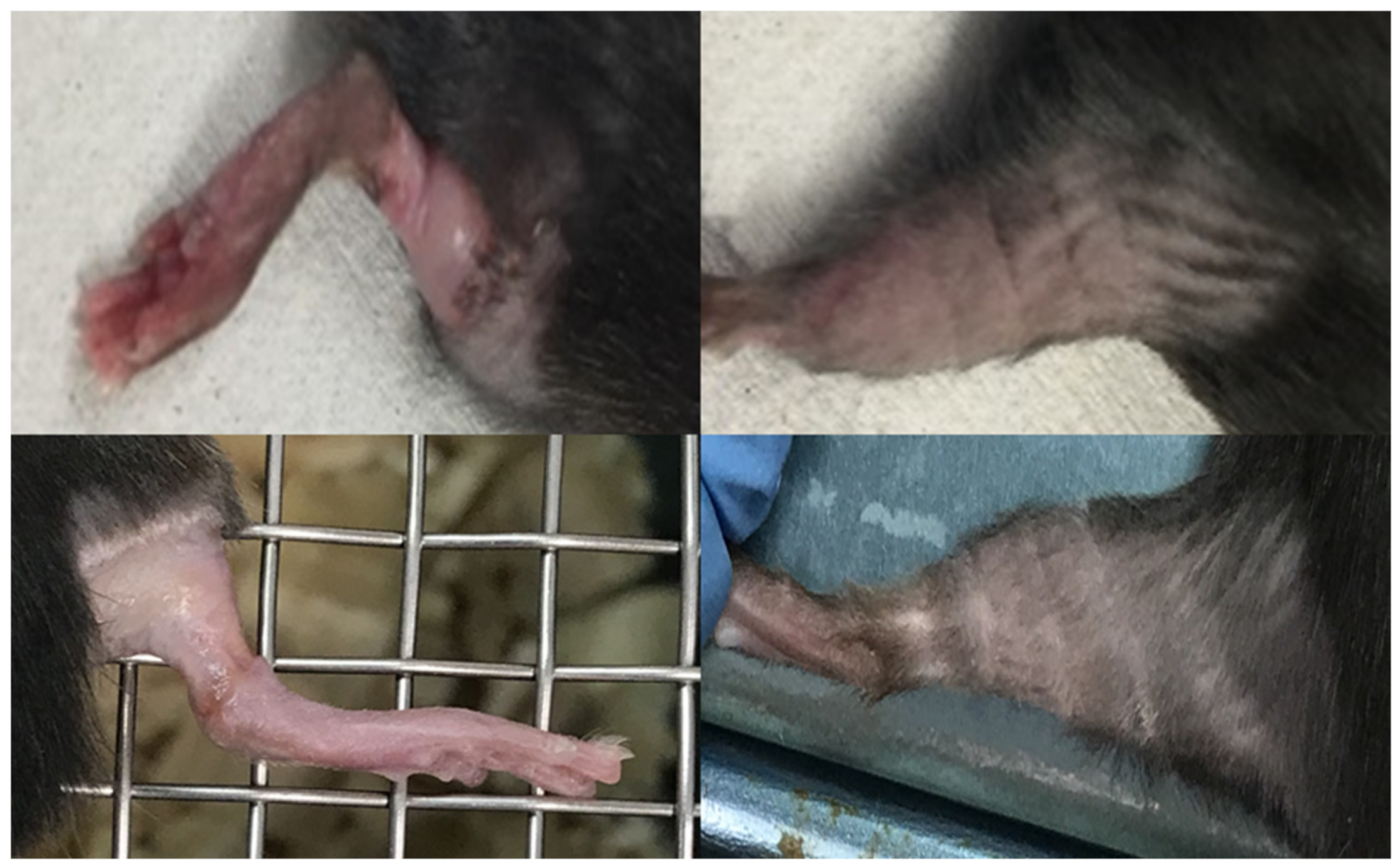
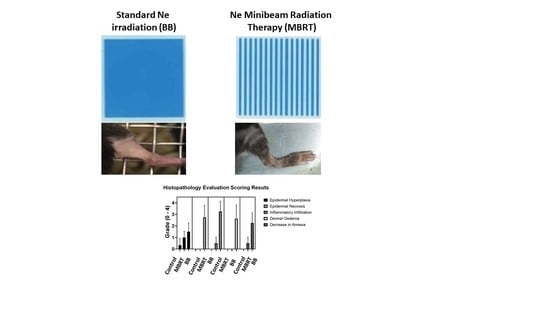
| Score | Observation |
|---|---|
| Normal | 0 |
| Erythema | 1 |
| Dry desquamation | 2 |
| Moist desquamation | 3 |
| Ulceration | 4 |
| Necrosis | 5 |
| GROUP | Epidermal Hyperplasia | Epidermal Necrosis | Inflammation Infiltration | Dermal Edema | Decrease in Annexa |
|---|---|---|---|---|---|
| Control | 0.3 ± 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| MBRT | 1.0 ± 0.5 | 0.0 | 0.5 ± 0.5 | 0.0 | 0.5 ± 0.5 |
| BB | 1.3 ± 0.7 | 2.8 ± 1.0 | 3.3 ± 0.9 | 2.6 ± 1.2 | 2.3 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prezado, Y.; Hirayama, R.; Matsufuji, N.; Inaniwa, T.; Martínez-Rovira, I.; Seksek, O.; Bertho, A.; Koike, S.; Labiod, D.; Pouzoulet, F.; et al. A Potential Renewed Use of Very Heavy Ions for Therapy: Neon Minibeam Radiation Therapy. Cancers 2021, 13, 1356. https://doi.org/10.3390/cancers13061356
Prezado Y, Hirayama R, Matsufuji N, Inaniwa T, Martínez-Rovira I, Seksek O, Bertho A, Koike S, Labiod D, Pouzoulet F, et al. A Potential Renewed Use of Very Heavy Ions for Therapy: Neon Minibeam Radiation Therapy. Cancers. 2021; 13(6):1356. https://doi.org/10.3390/cancers13061356
Chicago/Turabian StylePrezado, Yolanda, Ryochi Hirayama, Naruhiro Matsufuji, Taku Inaniwa, Immaculada Martínez-Rovira, Olivier Seksek, Annaïg Bertho, Sachiko Koike, Dalila Labiod, Frederic Pouzoulet, and et al. 2021. "A Potential Renewed Use of Very Heavy Ions for Therapy: Neon Minibeam Radiation Therapy" Cancers 13, no. 6: 1356. https://doi.org/10.3390/cancers13061356
APA StylePrezado, Y., Hirayama, R., Matsufuji, N., Inaniwa, T., Martínez-Rovira, I., Seksek, O., Bertho, A., Koike, S., Labiod, D., Pouzoulet, F., Polledo, L., Warfving, N., Liens, A., Bergs, J., & Shimokawa, T. (2021). A Potential Renewed Use of Very Heavy Ions for Therapy: Neon Minibeam Radiation Therapy. Cancers, 13(6), 1356. https://doi.org/10.3390/cancers13061356