Quantification of Differential Response of Tumour and Normal Cells to Microbeam Radiation in the Absence of FLASH Effects
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Clonogenic Assay
2.3. Irradiation Procedure
2.4. Dose Mixing
2.5. Cell Survival Analysis
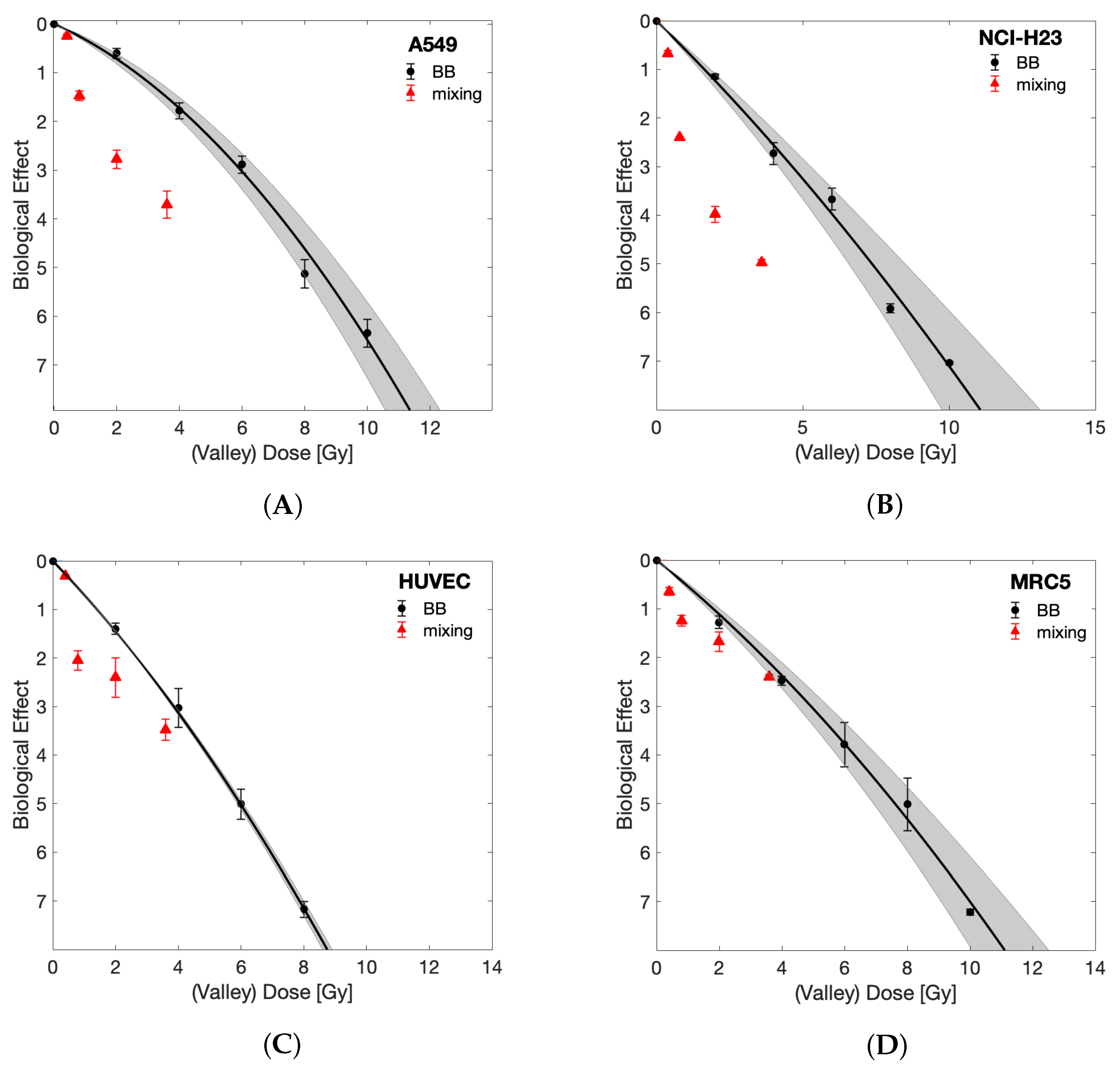
3. Results
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DVH | Dose rate volume histogram |
| RT | Radiation therapy |
| MRT | Microbeam radiation therapy |
| BB | Broad beam |
| OAR | Organ at risk |
| PVDR | Peak-to-valley dose ratio |
| LQ model | Linear quadratic model |
Appendix A. Tabulated Cell Survival Data
| Dose [Gy] | HUVEC | MRC5 | NCI-H23 | A549 |
|---|---|---|---|---|
| 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| 2 | 0.25 ± 0.03 | 0.28 ± 0.04 | 0.32 ± 0.02 | 0.55 ± 0.06 |
| 4 | 0.048 ± 0.019 | 0.084 ± 0.008 | 0.065 ± 0.014 | 0.17 ± 0.03 |
| 6 | 0.007 ± 0.002 | 0.023 ± 0.01 | 0.026 ± 0.006 | 0.056 ± 0.01 |
| 8 | 0.001 ± 0.0001 | 0.007 ± 0.004 | 0.003 ± 0.0002 | 0.006 ± 0.002 |
| 10 | 0.001 ± 0.00005 | 0.001 ± 0.00002 | 0.002 ± 0.001 |
| Mean Dose [Gy] | HUVEC | MRC5 | NCI-H23 | A549 |
|---|---|---|---|---|
| 0 | 1 ± 0 | 0.5 ± 0.25 | 1 ± 0 | 1 ± 0 |
| 2 | 0.57 ± 0.02 | 0.3 ± 0.14 | 0.32 ± 0.02 | 0.57 ± 0.08 |
| 6 | 0.13 ± 0.05 | 0.09 ± 0.02 | 0.059 ± 0.004 | 0.17 ± 0.03 |
| 10 | 0.06 ± 0.01 | 0.035 ± 0.011 | 0.009 ± 0.0004 | 0.049 ± 0.012 |
| 14 | 0.03 ± 0.01 | 0.021 ± 0.006 | 0.007 ± 0.0002 | 0.006 ± 0.004 |
| 18 | 0.017 ± 0.004 | 0.01 ± 0.003 | 0.003 ± 0.0002 | 0.004 ± 0.002 |
| 22 | 0.003 ± 0.001 | 0.002 ± 0.001 | ||
| 26 | 0.001 ± 0.0006 | 0.001 ± 0.0001 |
| Mean Dose [Gy] | HUVEC | MRC5 | NCI-H23 | A549 |
|---|---|---|---|---|
| 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| 2 | 0.59 ± 0.01 | 0.42 ± 0.03 | 0.41 ± 0.02 | 0.62 ± 0.02 |
| 4 | 0.1 ± 0.02 | 0.23 ± 0.024 | 0.073 ± 0.001 | 0.18 ± 0.02 |
| 10 | 0.072 ± 0.029 | 0.15 ± 0.029 | 0.015 ± 0.002 | 0.05 ± 0.009 |
| 18 | 0.025 ± 0.005 | 0.072 ± 0.004 | 0.006 ± 0.0004 | 0.02 ± 0.005 |
References
- Slatkin, D.N.; Spanne, P.; Dilmanian, F.; Sandborg, M. Microbeam radiation therapy. Med. Phys. 1992, 19, 1395–1400. [Google Scholar] [CrossRef]
- Slatkin, D.; Spanne, P.; Dilmanian, F.; Gebbers, J.; Laissue, J. Subacute neuropathological effects of microplanar beams of X-rays from a synchrotron wiggler. Proc. Natl. Acad. Sci. USA 1995, 92, 8783–8787. [Google Scholar] [CrossRef] [Green Version]
- Dilmanian, F.; Morris, G.; Le, G.D.; Huang, X.; Ren, B.; Bacarian, T.; Allen, J.; Kalef-Ezra, J.; Orion, I.; Rosen, E.; et al. Response of avian embryonic brain to spatially segmented X-ray microbeams. Cell. Mol. Biol. 2001, 47, 485–493. [Google Scholar] [PubMed]
- Laissue, J.A.; Bartzsch, S.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Dalléry, D.; Djonov, V.; Hanson, A.L.; Hopewell, J.W.; Kaser-Hotz, B.; et al. Response of the rat spinal cord to X-ray microbeams. Radiother. Oncol. 2013, 106, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, A.; Serduc, R.; Laissue, J.A.; Djonov, V. Effects of microbeam radiation therapy on normal and tumoral blood vessels. Phys. Med. Eur. J. Med. Phys. 2015, 31, 634–641. [Google Scholar] [CrossRef] [Green Version]
- Serduc, R.; Van de Looij, Y.; Francony, G.; Verdonck, O.; Van Der Sanden, B.; Laissue, J.; Farion, R.; Bräuer-Krisch, E.; Siegbahn, E.A.; Bravin, A.; et al. Characterization and quantification of cerebral edema induced by synchrotron X-ray microbeam radiation therapy. Phys. Med. Biol. 2008, 53, 1153. [Google Scholar] [CrossRef]
- Laissue, J.A.; Blattmann, H.; Di Michiel, M.; Slatkin, D.N.; Lyubimova, N.; Guzman, R.; Zimmermann, W.; Birrer, S.; Bley, T.; Kircher, P.; et al. Weanling piglet cerebellum: A surrogate for tolerance to MRT (microbeam radiation therapy) in pediatric neuro-oncology. In Penetrating Radiation Systems and Applications III; International Society for Optics and Photonics: San Diego, CA, USA, 2001; Volume 4508, pp. 65–74. [Google Scholar]
- Potez, M.; Bouchet, A.; Wagner, J.; Donzelli, M.; Bräuer-Krisch, E.; Hopewell, J.W.; Laissue, J.; Djonov, V. Effects of Synchrotron X-ray Micro-beam Irradiation on Normal Mouse Ear Pinnae. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 680–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laissue, J.A.; Geiser, G.; Spanne, P.O.; Dilmanian, F.A.; Gebbers, J.O.; Geiser, M.; Wu, X.Y.; Makar, M.S.; Micca, P.L.; Nawrocky, M.M.; et al. Neuropathology of ablation of rat gliosarcomas and contiguous brain tissues using a microplanar beam of synchrotron-wiggler-generated X rays. Int. J. Cancer 1998, 78, 654–660. [Google Scholar] [CrossRef]
- Bräuer-Krisch, E.; Serduc, R.; Siegbahn, E.; Le Duc, G.; Prezado, Y.; Bravin, A.; Blattmann, H.; Laissue, J. Effects of pulsed, spatially fractionated, microscopic synchrotron X-ray beams on normal and tumoral brain tissue. Mutat. Res. Rev. Mutat. Res. 2010, 704, 160–166. [Google Scholar] [CrossRef]
- Regnard, P.; Le Duc, G.; Bräuer-Krisch, E.; Tropres, I.; Siegbahn, E.A.; Kusak, A.; Clair, C.; Bernard, H.; Dallery, D.; Laissue, J.A.; et al. Irradiation of intracerebral 9L gliosarcoma by a single array of microplanar X-ray beams from a synchrotron: Balance between curing and sparing. Phys. Med. Biol. 2008, 53, 861. [Google Scholar] [CrossRef]
- Bouchet, A.; Lemasson, B.; Le Duc, G.; Maisin, C.; Bräuer-Krisch, E.; Siegbahn, E.A.; Renaud, L.; Khalil, E.; Rémy, C.; Poillot, C.; et al. Preferential effect of synchrotron microbeam radiation therapy on intracerebral 9L gliosarcoma vascular networks. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1503–1512. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Zhang, L.; Frank, J.E.; Inscoe, C.R.; Burk, L.M.; Hadsell, M.; Lee, Y.Z.; Lu, J.; Chang, S.; Zhou, O. Treating Brain Tumor with Microbeam Radiation Generated by a Compact Carbon-Nanotube-Based Irradiator: Initial Radiation Efficacy Study. Radiat. Res. 2015, 184, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Potez, M.; Bouchet, A.; Flaender, M.; Rome, C.; Collomb, N.; Grotzer, M.; Krisch, M.; Djonov, V.; Balosso, J.; Brun, E.; et al. Synchrotron X-ray Boost Delivered by Microbeam Radiation Therapy after Conventional X-ray Therapy Fractionated in Time Improves F98 Glioma Control. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Potez, M.; Fernandez-Palomo, C.; Bouchet, A.; Trappetti, V.; Donzelli, M.; Krisch, M.; Laissue, J.; Volarevic, V.; Djonov, V. Synchrotron Microbeam Radiation Therapy as a New Approach for the Treatment of Radioresistant Melanoma: Potential Underlying Mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1126–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatasso, S.; Laissue, J.A.; Hlushchuk, R.; Graber, W.; Bravin, A.; Bräuer-Krisch, E.; Corde, S.; Blattmann, H.; Gruber, G.; Djonov, V. Microbeam radiation-induced tissue damage depends on the stage of vascular maturation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1522–1532. [Google Scholar] [CrossRef]
- Blattmann, H.; Gebbers, J.O.; Bräuer-Krisch, E.; Bravin, A.; Le Duc, G.; Burkard, W.; Di Michiel, M.; Djonov, V.; Slatkin, D.; Stepanek, J.; et al. Applications of synchrotron X-rays to radiotherapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2005, 548, 17–22. [Google Scholar] [CrossRef]
- Van Der Sanden, B.; Bräuer-Krisch, E.; Siegbahn, E.A.; Ricard, C.; Vial, J.C.; Laissue, J. Tolerance of arteries to microplanar X-ray beams. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1545–1552. [Google Scholar] [CrossRef] [Green Version]
- Brönnimann, D.; Bouchet, A.; Schneider, C.; Potez, M.; Serduc, R.; Bräuer-Krisch, E.; Graber, W.; von Gunten, S.; Laissue, J.A.; Djonov, V. Synchrotron microbeam irradiation induces neutrophil infiltration, thrombocyte attachment and selective vascular damage in vivo. Sci. Rep. 2016, 6, 33601. [Google Scholar] [CrossRef] [Green Version]
- Bouchet, A.; Sakakini, N.; El Atifi, M.; Le Clec’h, C.; Brauer, E.; Moisan, A.; Deman, P.; Rihet, P.; Le Duc, G.; Pelletier, L. Early gene expression analysis in 9L orthotopic tumor-bearing rats identifies immune modulation in molecular response to synchrotron microbeam radiation therapy. PLoS ONE 2013, 8, e81874. [Google Scholar] [CrossRef] [Green Version]
- Ibahim, M.; Yang, Y.; Crosbie, J.; Stevenson, A.; Cann, L.; Paiva, P.; Rogers, P. Eosinophil-associated gene pathways but not eosinophil numbers are differentially regulated between synchrotron microbeam radiation treatment and synchrotron broad-beam treatment by 48 h postirradiation. Radiat. Res. 2015, 185, 60–68. [Google Scholar] [CrossRef]
- Yang, Y.; Crosbie, J.C.; Paiva, P.; Ibahim, M.; Stevenson, A.; Rogers, P.A. In vitro study of genes and molecular pathways differentially regulated by synchrotron microbeam radiotherapy. Radiat. Res. 2014, 182, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, H.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Di Michiel, M.; Gebbers, J.O.; Hanson, A.; Lyubimova, N.; Slatkin, D.; Stepanek, J.; et al. Synergy of gene-mediated immunoprophylaxis and microbeam radiation therapy for advanced intracerebral rat 9L gliosarcomas. J. Neuro-Oncol. 2006, 78, 135–143. [Google Scholar] [CrossRef]
- Bouchet, A.; Sakakini, N.; Atifi, M.E.; Le Clec’h, C.; Bräuer-Krisch, E.; Rogalev, L.; Laissue, J.A.; Rihet, P.; Le Duc, G.; Pelletier, L. Identification of AREG and PLK1 pathway modulation as a potential key of the response of intracranial 9L tumor to microbeam radiation therapy. Int. J. Cancer 2015, 136, 2705–2716. [Google Scholar] [CrossRef]
- Asur, R.S.; Sharma, S.; Chang, C.W.; Penagaricano, J.; Kommuru, I.M.; Moros, E.G.; Corry, P.M.; Griffin, R.J. Spatially fractionated radiation induces cytotoxicity and changes in gene expression in bystander and radiation adjacent murine carcinoma cells. Radiat. Res. 2012, 177, 751–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, V.; Suchowerska, N.; Rogers, L.; Claridge Mackonis, E.; Oakes, S.; McKenzie, D.R. Grid therapy using high definition multileaf collimators: Realizing benefits of the bystander effect. Acta Oncol. 2017, 56, 1048–1059. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, L.M.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.; Crosbie, J.C.; Rogers, P.A. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef]
- Ibahim, M.J.; Crosbie, J.C.; Yang, Y.; Zaitseva, M.; Stevenson, A.W.; Rogers, P.A.; Paiva, P. An evaluation of dose equivalence between synchrotron microbeam radiation therapy and conventional broadbeam radiation using clonogenic and cell impedance assays. PLoS ONE 2014, 9, e100547. [Google Scholar] [CrossRef]
- Bromley, R.; Oliver, L.; Davey, R.; Harvie, R.; Baldock, C. Predicting the clonogenic survival of A549 cells after modulated X-ray irradiation using the linear quadratic model. Phys. Med. Biol. 2008, 54, 187. [Google Scholar] [CrossRef]
- Burger, K.; Ilicic, K.; Dierolf, M.; Günther, B.; Walsh, D.W.M.; Schmid, E.; Eggl, E.; Achterhold, K.; Gleich, B.; Combs, S.E.; et al. Increased cell survival and cytogenetic integrity by spatial dose redistribution at a compact synchrotron X-ray source. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Bartzsch, S.; Cummings, C.; Eismann, S.; Oelfke, U. A preclinical microbeam facility with a conventional X-ray tube. Med. Phys. 2016, 43, 6301–6308. [Google Scholar] [CrossRef] [PubMed]
- Bartzsch, S.; Lott, J.; Welsch, K.; Bräuer-Krisch, E.; Oelfke, U. Micrometer-resolved film dosimetry using a microscope in microbeam radiation therapy. Med. Phys. 2015, 42, 4069–4079. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 1989, 62, 679–694. [Google Scholar] [CrossRef]
- Fernandez-Palomo, C.; Trappetti, V.; Potez, M.; Pellicioli, P.; Krisch, M.; Laissue, J.; Djonov, V. Complete Remission of Mouse Melanoma after Temporally Fractionated Microbeam Radiotherapy. Cancers 2020, 12, 2656. [Google Scholar] [CrossRef] [PubMed]
- Dilmanian, F.A.; Morris, G.M.; Zhong, N.; Bacarian, T.; Hainfeld, J.F.; Kalef-Ezra, J.; Brewington, L.J.; Tammam, J.; Rosen, E.M. Murine EMT-6 Carcinoma: High Therapeutic Efficacy of Microbeam Radiation Therapy. Radiat. Res. 2003, 159, 632–641. [Google Scholar] [CrossRef]
- Crosbie, J.C.; Anderson, R.L.; Rothkamm, K.; Restall, C.M.; Cann, L.; Ruwanpura, S.; Meachem, S.; Yagi, N.; Svalbe, I.; Lewis, R.A.; et al. Tumor cell response to synchrotron microbeam radiation therapy differs markedly from cells in normal tissues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 886–894. [Google Scholar] [CrossRef]
- Prise, K.M.; O’Sullivan, J.M. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer 2009, 9, 351–360. [Google Scholar] [CrossRef]
- Favaudon, V.; Fouillade, C.; Vozenin, M. Ultrahigh dose-rate, ”flash” irradiation minimizes the side-effects of radiotherapy. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2015, 19, 526–531. [Google Scholar]
- Schültke, E.; Fiedler, S.; Menk, R.H.; Jaekel, F.; Dreossi, D.; Casarin, K.; Tromba, G.; Bartzsch, S.; Kriesen, S.; Hildebrandt, G.; et al. Perspectives for microbeam irradiation at the SYRMEP beamline. J. Synchrotron Radiat. 2021, 28, 410–418. [Google Scholar] [CrossRef]
- Engels, E.; Li, N.; Davis, J.; Paino, J.; Cameron, M.; Dipuglia, A.; Vogel, S.; Valceski, M.; Khochaiche, A.; O’Keefe, A.; et al. Toward personalized synchrotron microbeam radiation therapy. Sci. Rep. 2020, 10, 8833. [Google Scholar] [CrossRef]
- Mothersill, C.; Rusin, A.; Fernandez-Palomo, C.; Seymour, C. History of bystander effects research 1905-present; what is in a name? Int. J. Radiat. Biol. 2018, 94, 696–707. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Radiation-induced bystander effects: Past history and future directions. Radiat. Res. 2001, 155, 759–767. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chakraborty, A. Radiation-induced bystander phenomenon: Insight and implications in radiotherapy. Int. J. Radiat. Biol. 2019, 95, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Dickey, J.S.; Baird, B.J.; Redon, C.E.; Avdoshina, V.; Palchik, G.; Wu, J.; Kondratyev, A.; Bonner, W.M.; Martin, O.A. Susceptibility to bystander DNA damage is influenced by replication and transcriptional activity. Nucleic Acids Res. 2012, 40, 10274–10286. [Google Scholar] [CrossRef]
- Megha Anilkumar, K.; Safa Abdul, S.B.; Sidonia Vallas, X.; Murugan, A.; Thayalan, K.; Perumal, V. Direct and Bystander Effect on Cervix Cancer Cells (SiHa) Exposed to High Dose-Rate Gamma Radiation Sourced from Ir 192 Used in Brachytherapy. Int. J. Radiol. Radiat. Oncol. 2015, 1, 007–013. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three- dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Brüningk, S.C.; Rivens, I.; Box, C.; Oelfke, U.; ter Haar, G. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci. Rep. 2020, 10, 1653. [Google Scholar] [CrossRef]
- Onozato, Y.; Kaida, A.; Harada, H.; Miura, M. Radiosensitivity of quiescent and proliferating cells grown as multicellular tumor spheroids. Cancer Sci. 2017, 108, 704–712. [Google Scholar] [CrossRef]
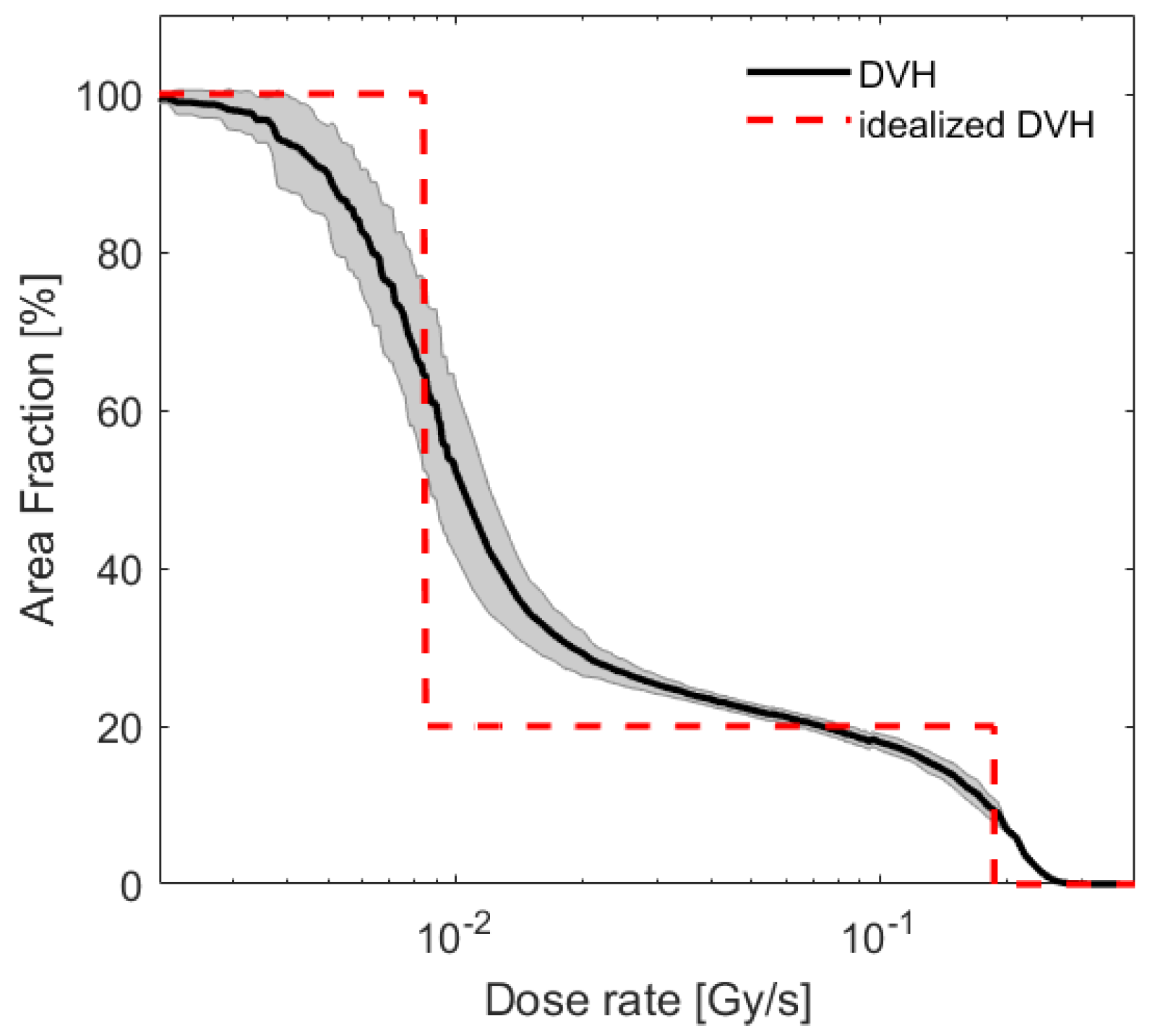
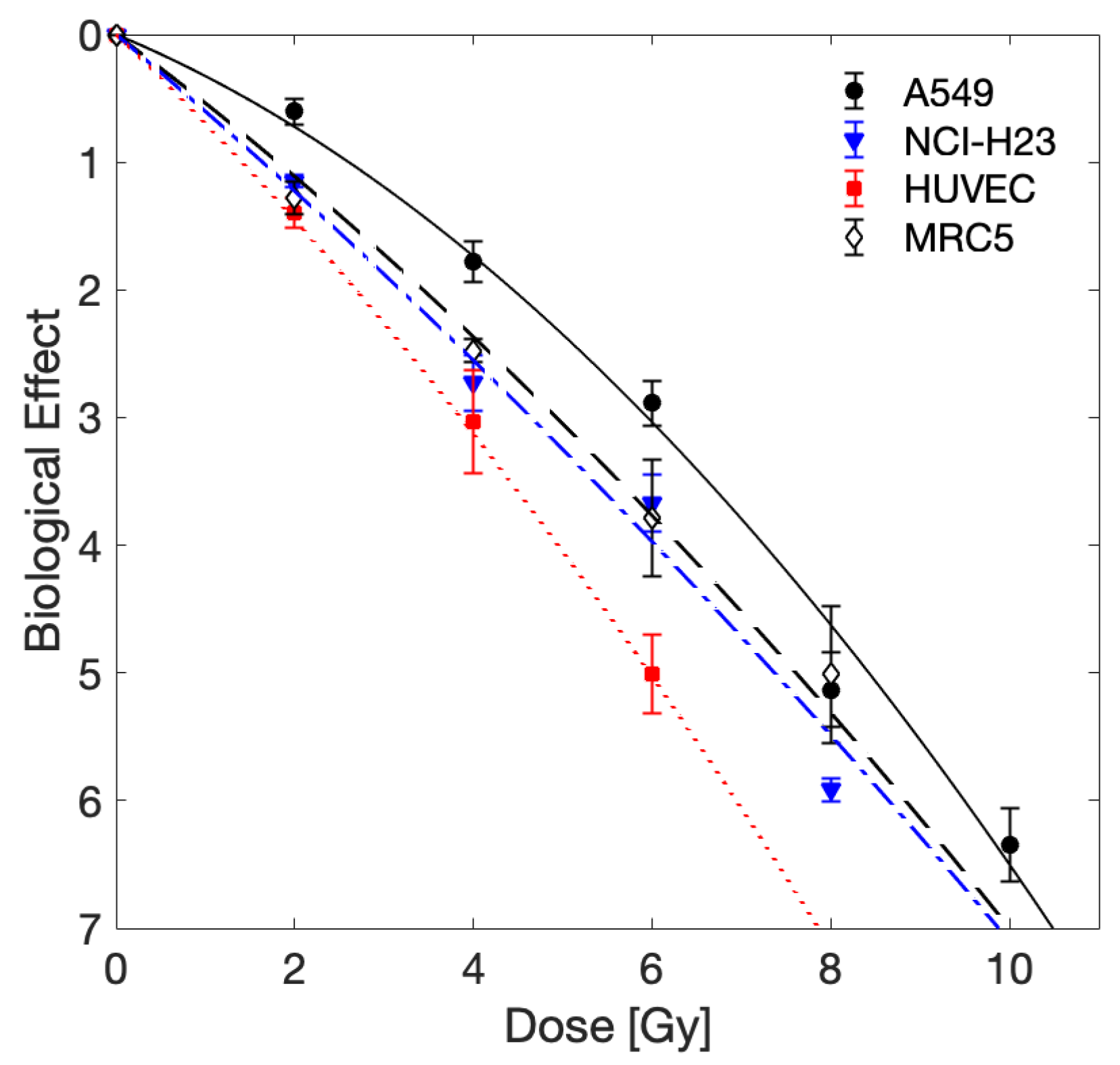
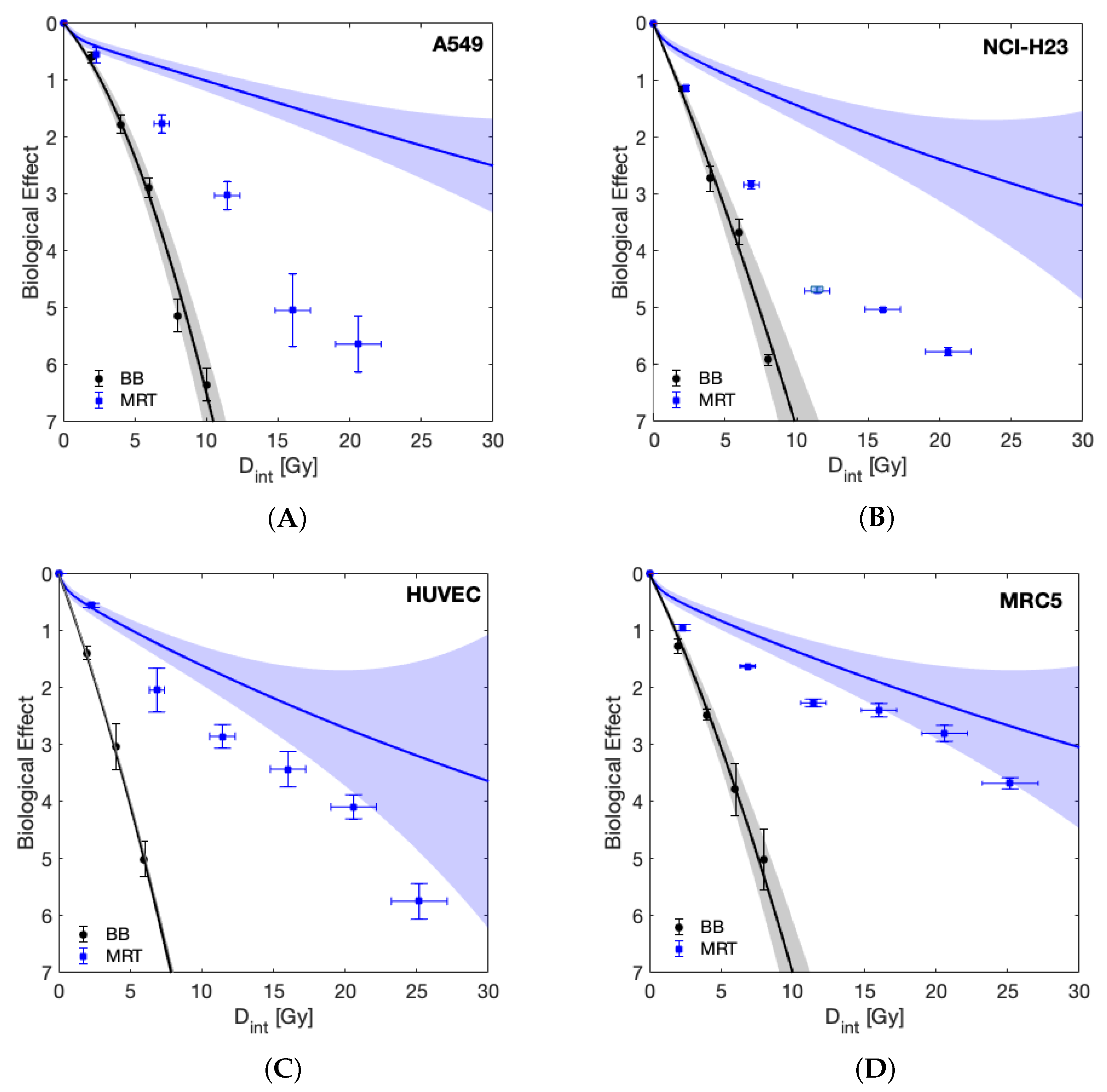
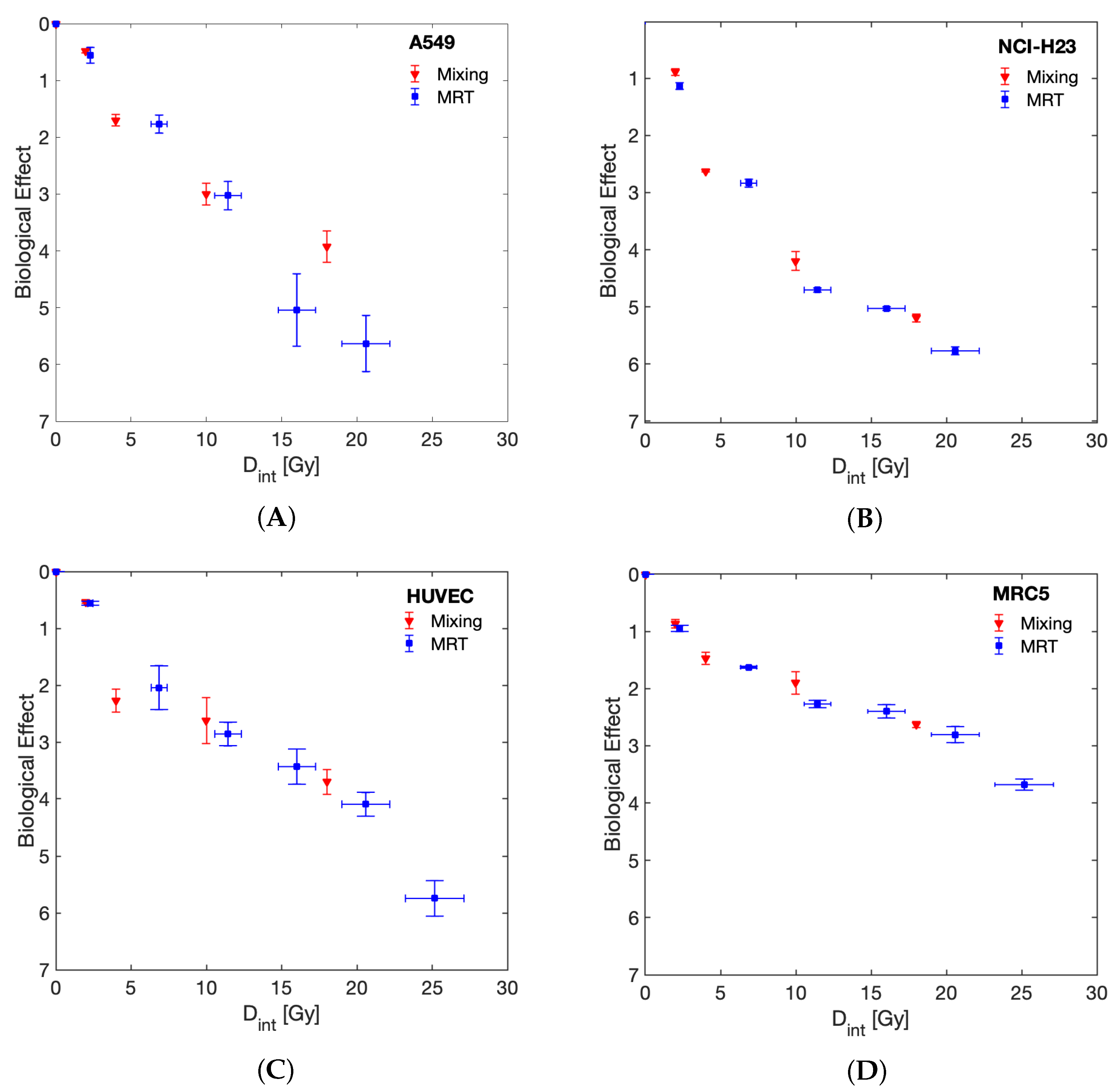
| Cell Line | ||
|---|---|---|
| MRC5 | ||
| NCI-H23 | ||
| A549 | ||
| HUVEC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steel, H.; Brüningk, S.C.; Box, C.; Oelfke, U.; Bartzsch, S.H. Quantification of Differential Response of Tumour and Normal Cells to Microbeam Radiation in the Absence of FLASH Effects. Cancers 2021, 13, 3238. https://doi.org/10.3390/cancers13133238
Steel H, Brüningk SC, Box C, Oelfke U, Bartzsch SH. Quantification of Differential Response of Tumour and Normal Cells to Microbeam Radiation in the Absence of FLASH Effects. Cancers. 2021; 13(13):3238. https://doi.org/10.3390/cancers13133238
Chicago/Turabian StyleSteel, Harriet, Sarah C. Brüningk, Carol Box, Uwe Oelfke, and Stefan H. Bartzsch. 2021. "Quantification of Differential Response of Tumour and Normal Cells to Microbeam Radiation in the Absence of FLASH Effects" Cancers 13, no. 13: 3238. https://doi.org/10.3390/cancers13133238
APA StyleSteel, H., Brüningk, S. C., Box, C., Oelfke, U., & Bartzsch, S. H. (2021). Quantification of Differential Response of Tumour and Normal Cells to Microbeam Radiation in the Absence of FLASH Effects. Cancers, 13(13), 3238. https://doi.org/10.3390/cancers13133238







