Surgical Outcome of Trigeminal Schwannomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Outcome Parameters
2.2. Statistics
2.3. Ethics Approval
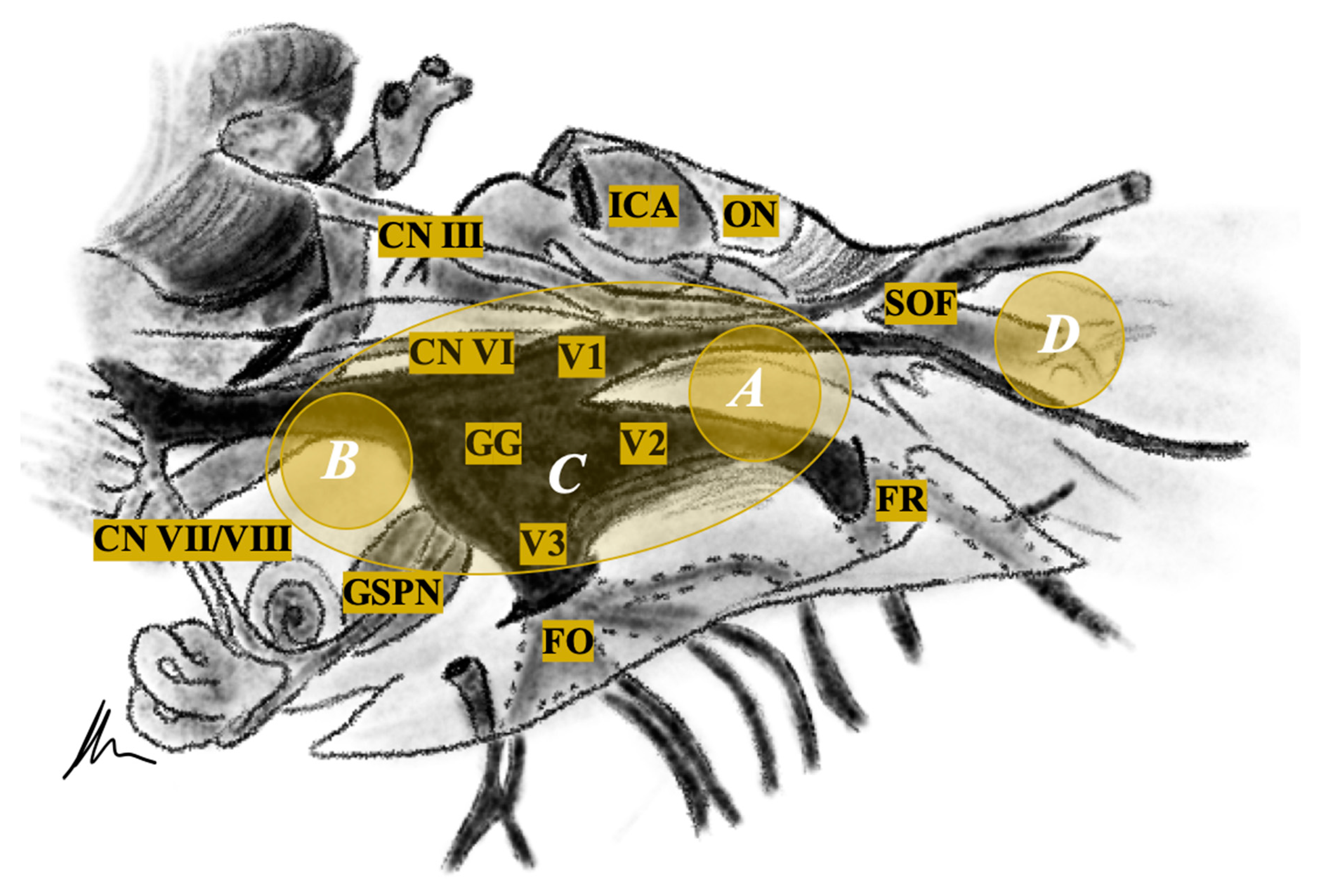
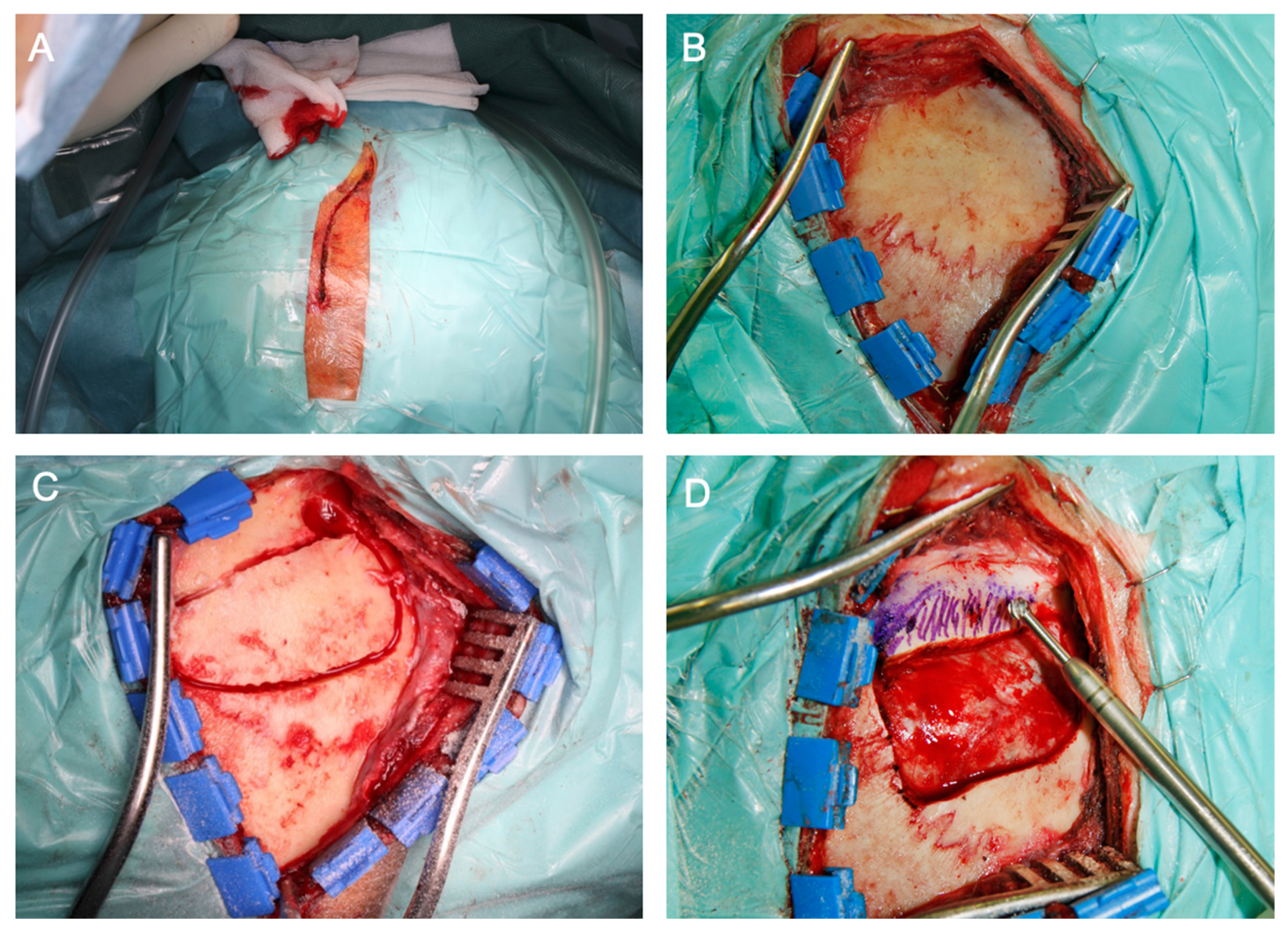
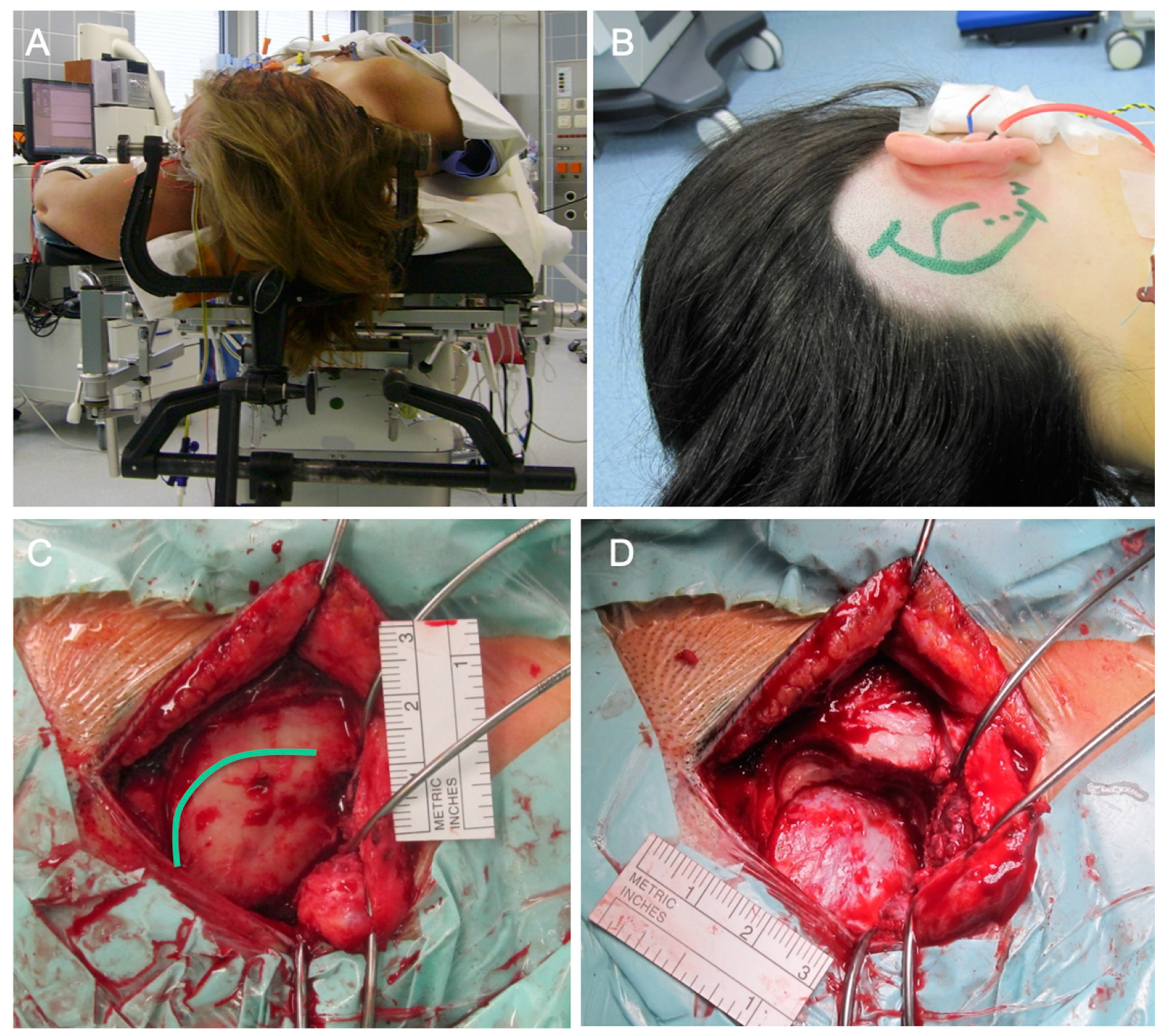
2.4. Surgical Approaches
2.4.1. Intra- or Extradural Subtemporal Middle Fossa Approach
2.4.2. Retrosigmoid Approach
2.4.3. Transnasal Endoscopic (Transpterygoid) Approach
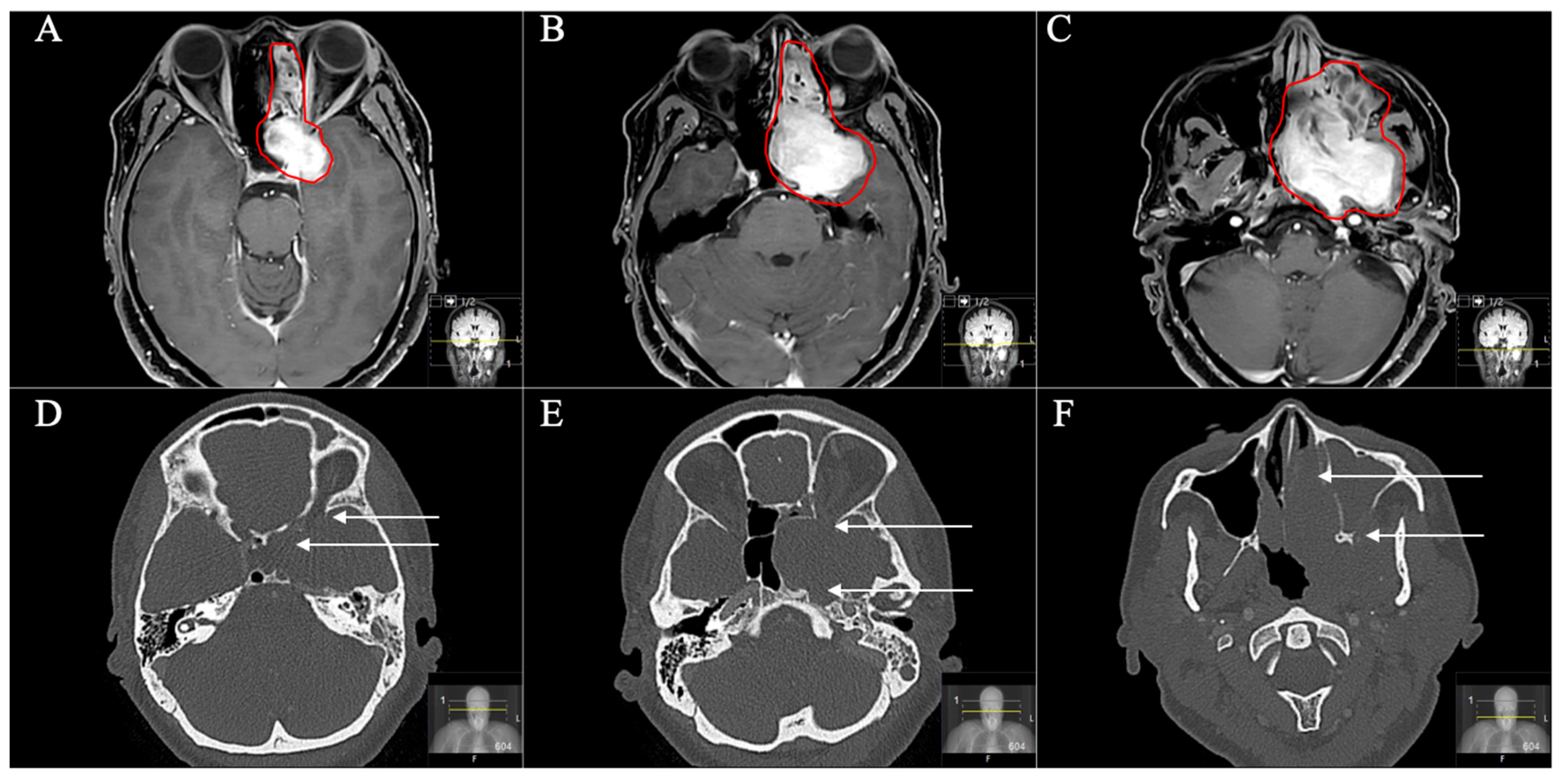
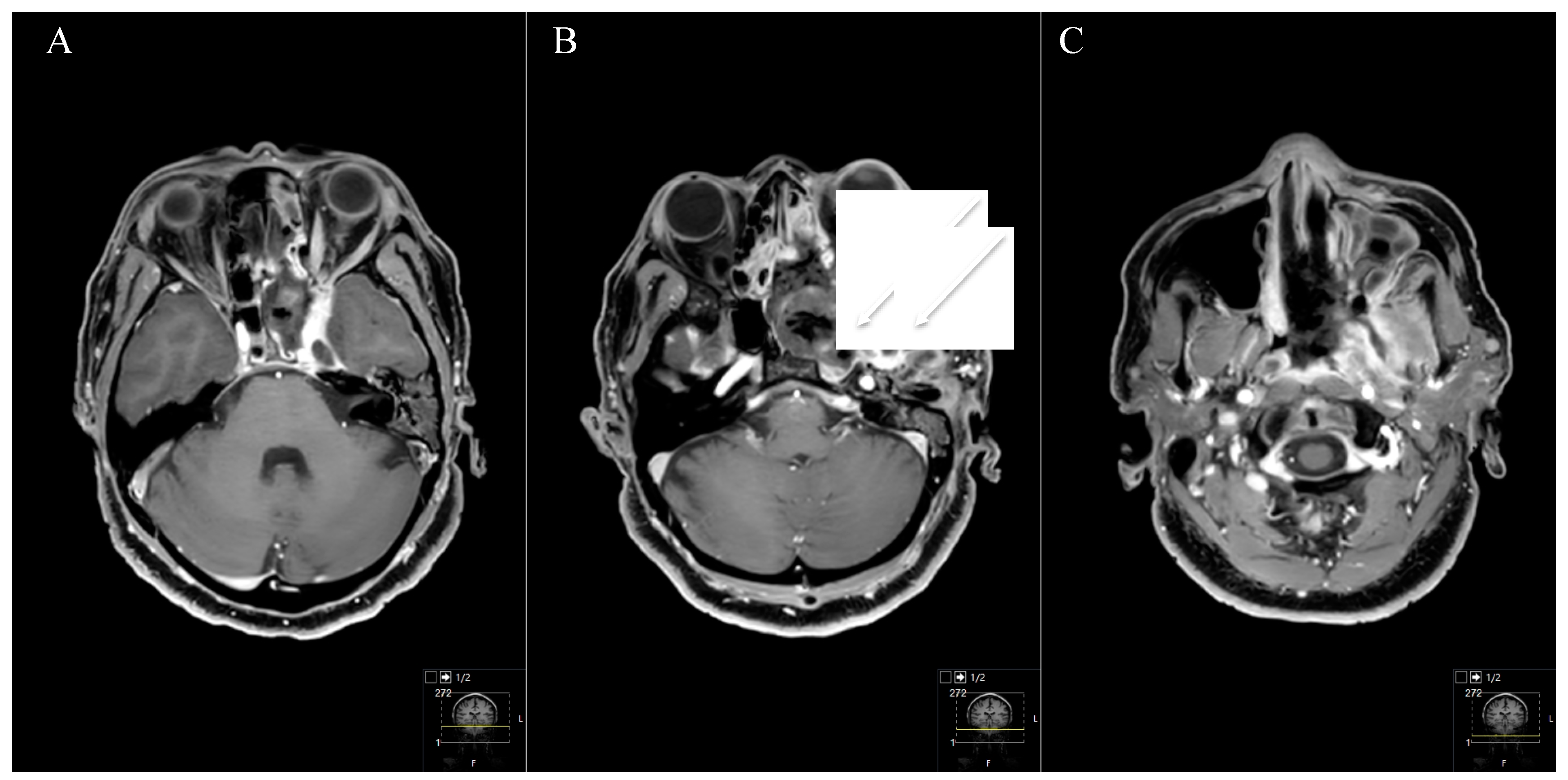
3. Results
3.1. Patient Population
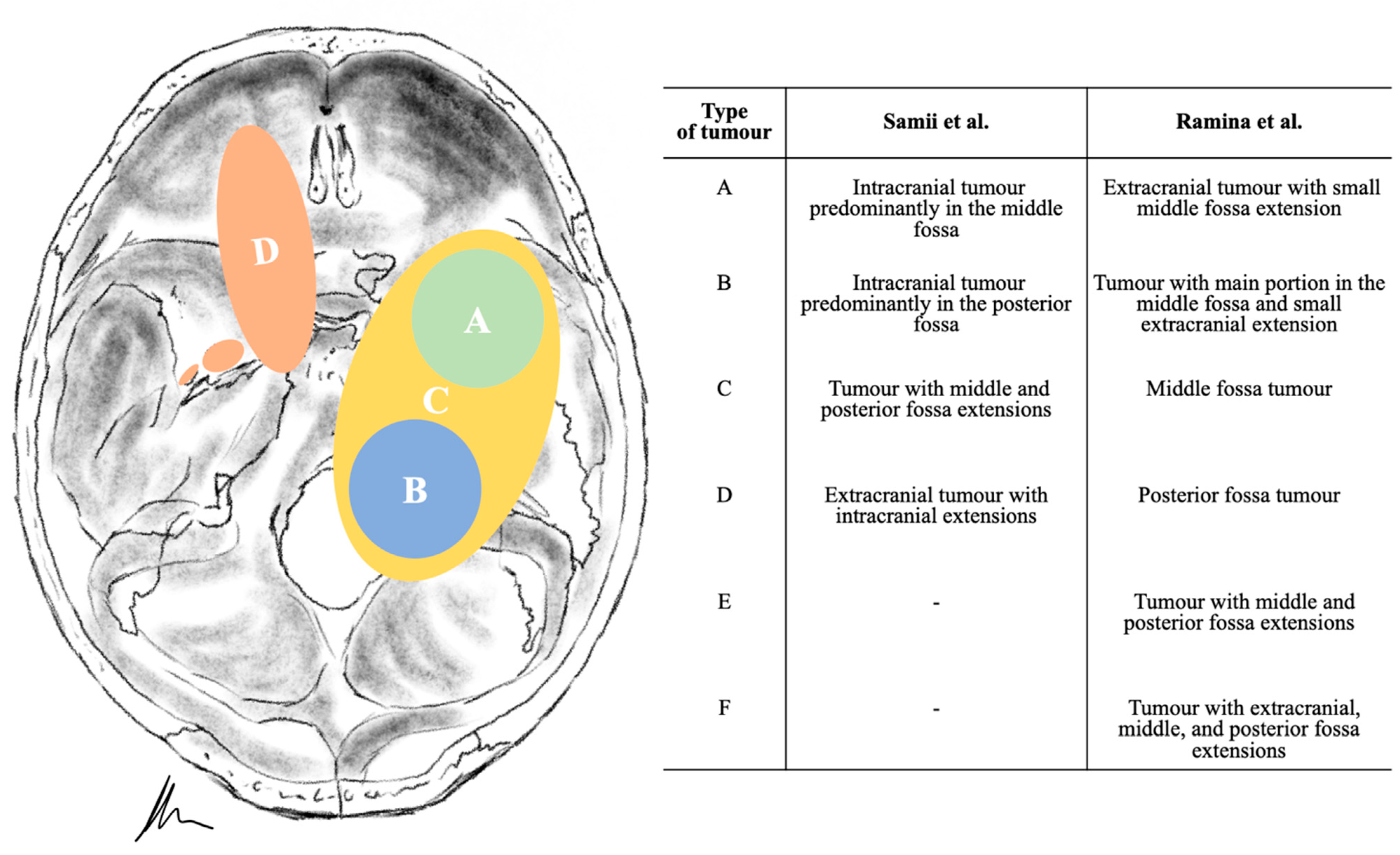
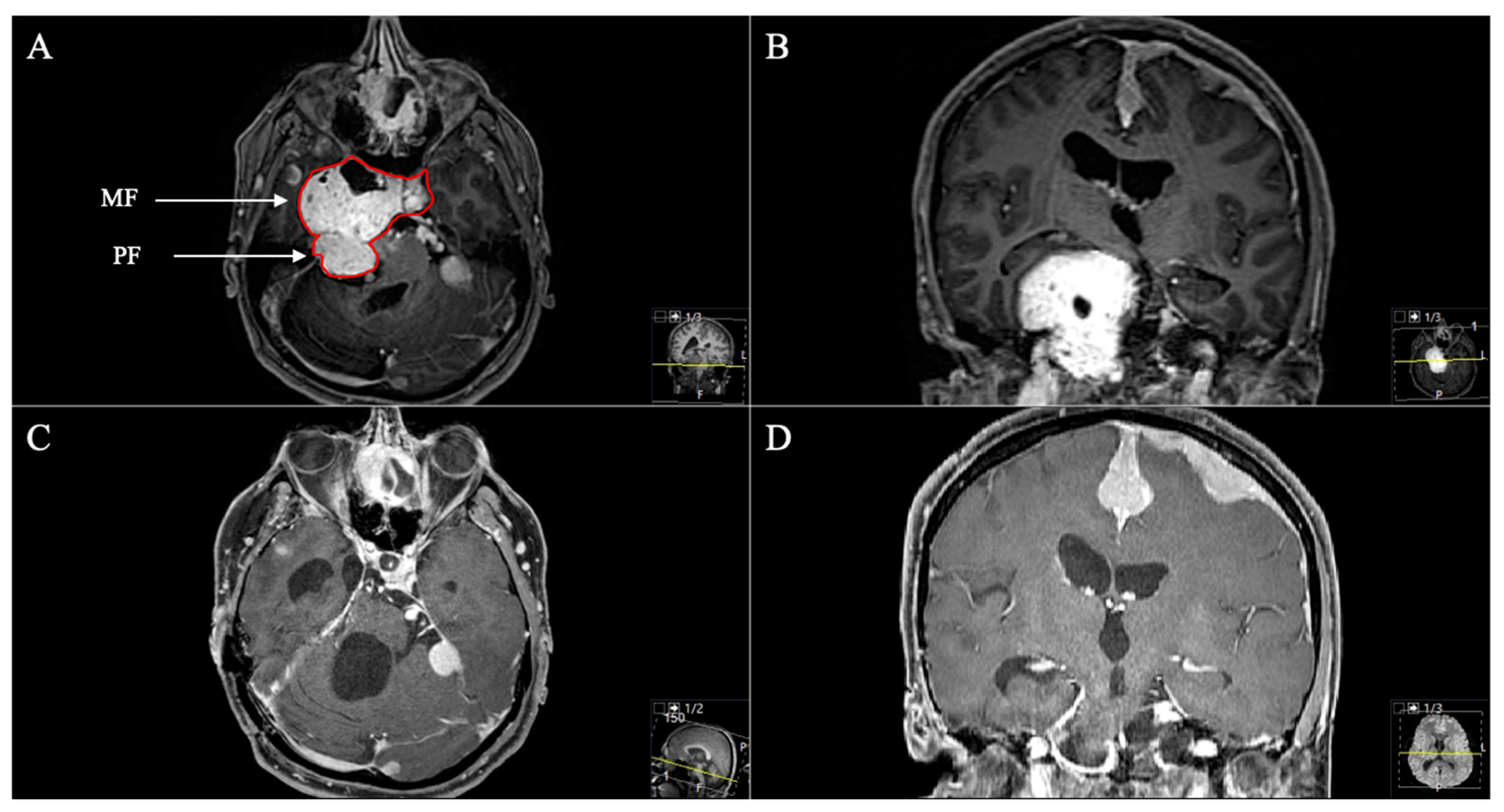
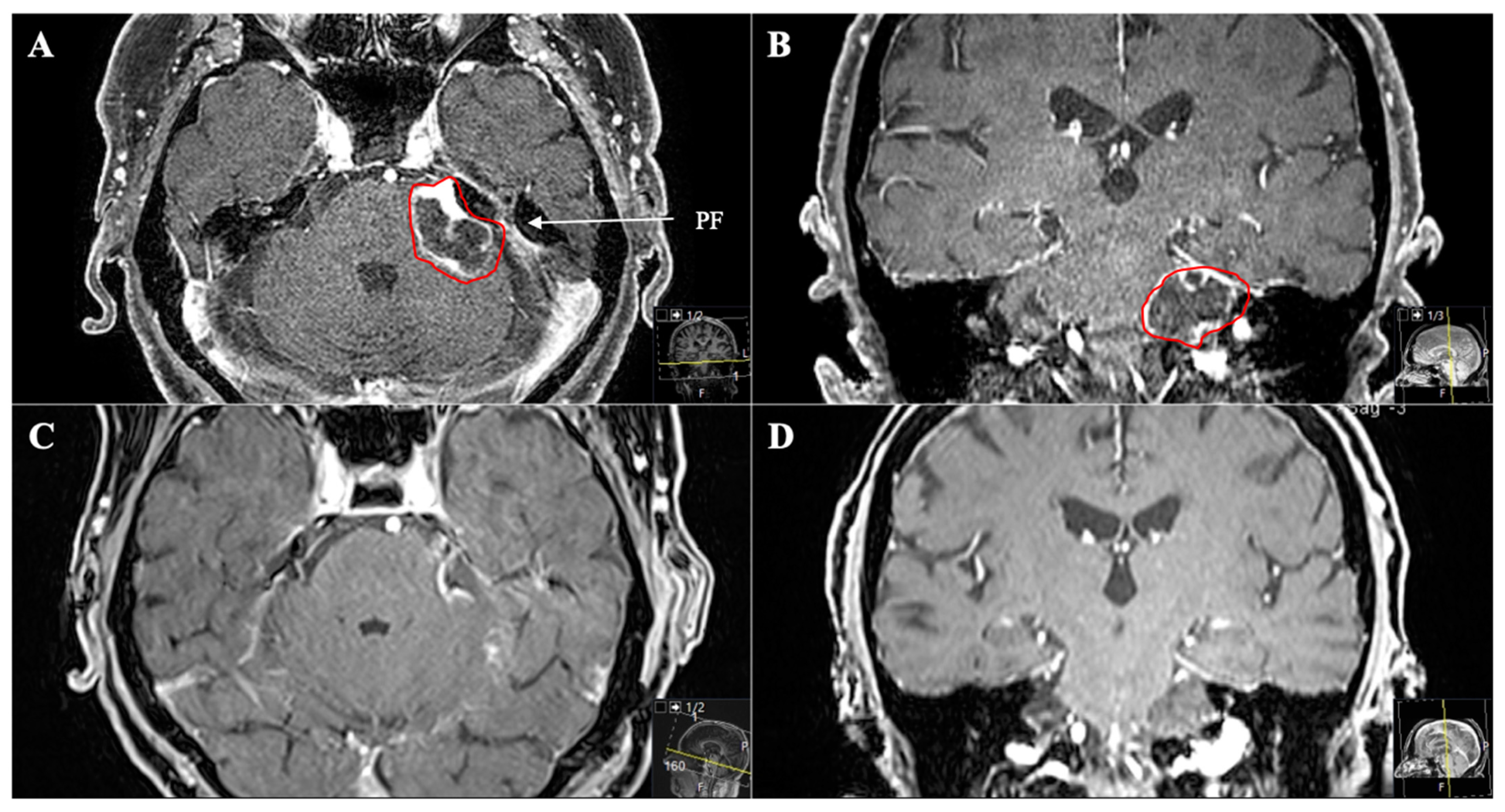
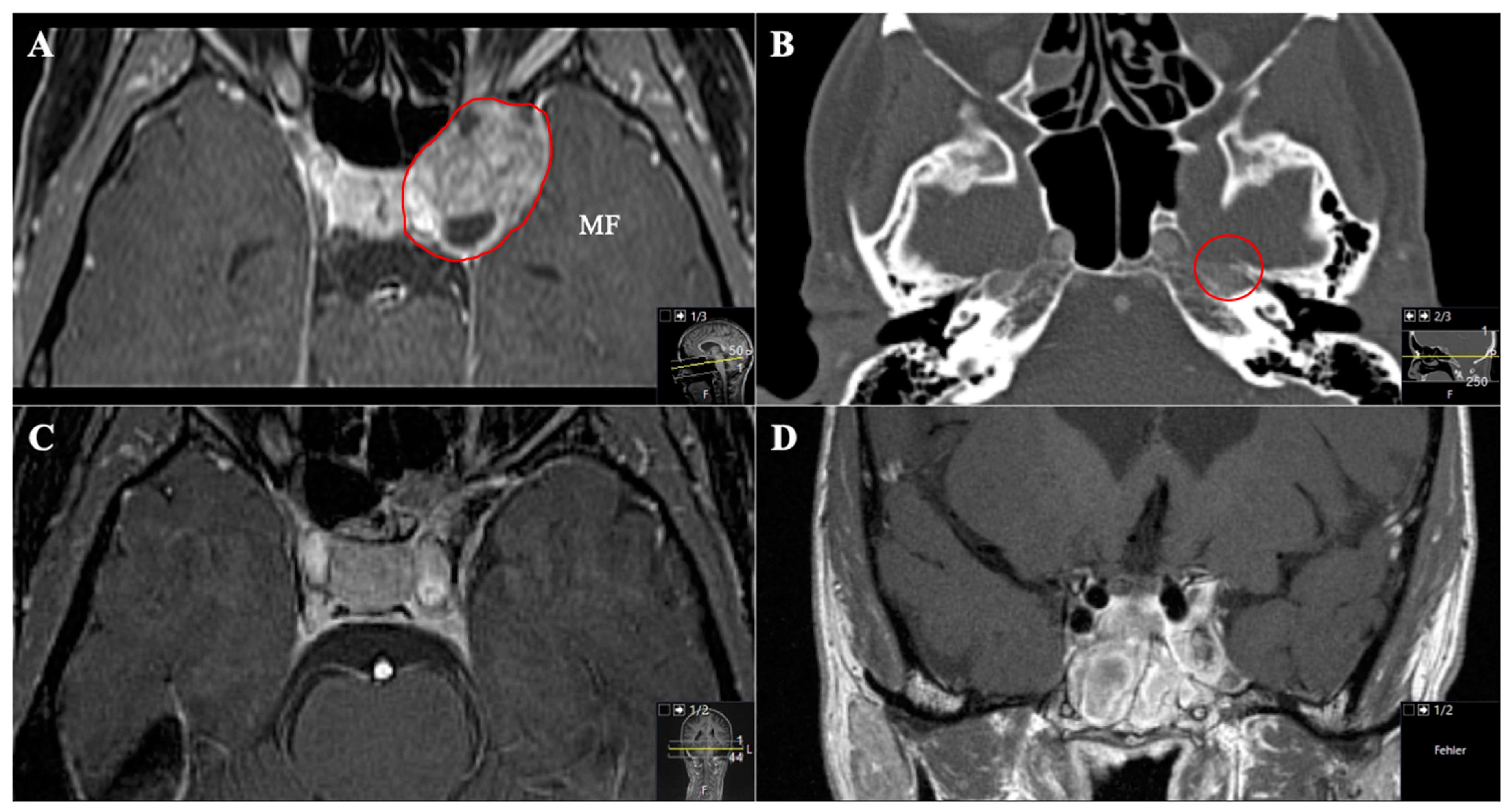
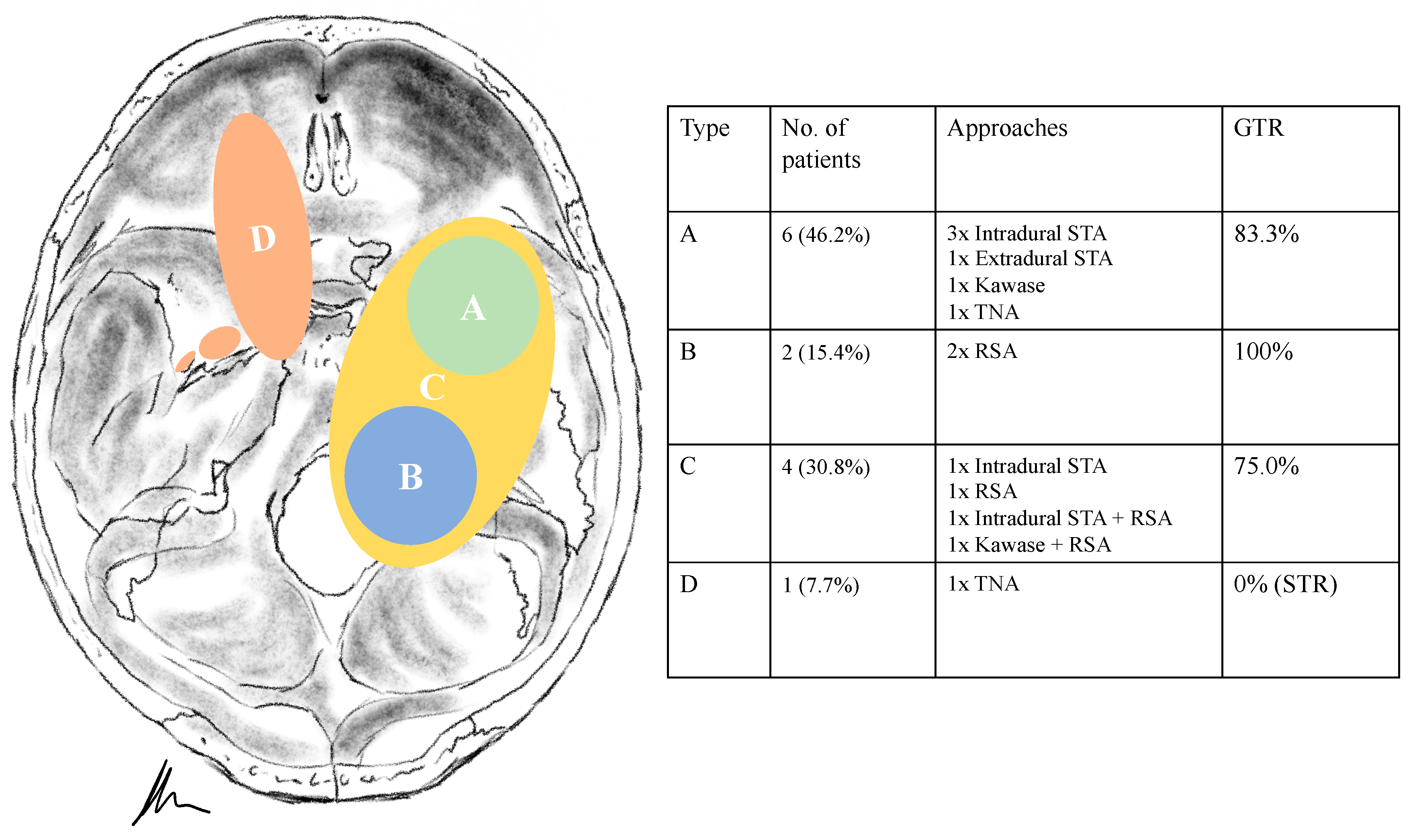
3.2. Tumour Location and Performed Approaches
3.3. Postoperative Outcome
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukaya, R.; Yoshida, K.; Ohira, T.; Kawase, T. Trigeminal schwannomas: Experience with 57 cases and a review of the literature. Neurosurg. Rev. 2010, 34, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, A.; Barnett, S.; Anand, V.; Agazzi, S. Multimodality Management of Trigeminal Schwannomas. J. Neurol. Surg. Part B Skull Base 2016, 77, 371–378. [Google Scholar] [CrossRef]
- Jefferson, G. The trigeminal neurinomas with some remarks on malignant invasion of the gasserian ganglion. Clin. Neurosurg. 1953, 1, 11–54. [Google Scholar] [CrossRef]
- Yoshida, K.; Kawase, T. Trigeminal neurinomas extending into multiple fossae: Surgical methods and review of the literature. J. Neurosurg. 1999, 91, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Migliori, M.M.; Tatagiba, M.; Babu, R. Surgical treatment of trigeminal schwannomas. J. Neurosurg. 1995, 82, 711–718. [Google Scholar] [CrossRef]
- Ramina, R.; Mattei, T.A.; Sória, M.G.; da Silva, E.B., Jr.; Leal, A.G.; Neto, M.C.; Fernandes, Y.B. Surgical management of trigeminal schwannomas. Neurosurg. Focus 2008, 25, E6. [Google Scholar] [CrossRef]
- Day, J.D.; Fukushima, T. The surgical management of trigeminal neuromas. Neurosurgery 1998, 42, 233–240; discussion 240–241. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.M.; Donaldson, A.M.; Mehta, A.; Tsiouris, A.J.; Anand, V.K.; Schwartz, T.H. Surgical management of trigeminal schwannomas: Defining the role for endoscopic endonasal approaches. Neurosurg. Focus 2014, 37, E17. [Google Scholar] [CrossRef]
- Taha, J.M.; Tew, J.M., Jr.; van Loveren, H.R.; Keller, J.T.; el-Kalliny, M. Comparison of conventional and skull base surgical approaches for the excision of trigeminal neurinomas. J. Neurosurg. 1995, 82, 719–725. [Google Scholar] [CrossRef]
- Park, H.H.; Hong, S.D.; Kim, Y.H.; Hong, C.K.; Woo, K.I.; Yun, I.S.; Kong, D.S. Endoscopic transorbital and endonasal approach for trigeminal schwannomas: A retrospective multicenter analysis (KOSEN-005). J. Neurosurg. 2020, 133, 467–476. [Google Scholar] [CrossRef]
- Goel, A.; Shah, A.; Muzumdar, D.; Nadkarni, T.; Chagla, A. Trigeminal neurinomas with extracranial extension: Analysis of 28 surgically treated cases. J. Neurosurg. 2010, 113, 1079–1084. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Ayoubi, S.; Gaber, E. Trigeminal schwannomas: Removal of dumbbell-shaped tumors through the expanded Meckel cave and outcomes of cranial nerve function. J. Neurosurg. 2002, 96, 453–463. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Kouyialis, A.T.; Stranjalis, G.; Papadogiorgakis, N.; Papavlassopoulos, F.; Ziaka, D.S.; Petsinis, V.; Sakas, D.E. Giant dumbbell-shaped middle cranial fossa trigeminal schwannoma with extension to the infratemporal and posterior fossae. Acta Neurochir. 2007, 149, 959–963; discussion 964. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, L.N.; Schramm, V.L.; Jones, N.F. Subtemporal-preauricular infratemporal fossa approach to large lateral and posterior cranial base neoplasms. J. Neurosurg. 1987, 67, 488. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.; Kim, E.-Y.; Aziz, K.M.A.; Hemida, S.; Keller, J.T.; van Loveren, H.R. The Subtemporal Interdural Approach to Dumbbell-Shaped Trigeminal Schwannomas: Cadaveric Prosection. Oper. Neurosurg. 2006, 59, ONS-270–ONS-278. [Google Scholar] [CrossRef]
- Tatagiba, M.; Acioly, M.A. Retrosigmoid Approach to the Posterior and Middle Fossae. In Samii’s Essentials in Neurosurgery; Springer: Berlin/Heidelberg, Germany, 2008; pp. 137–153. [Google Scholar]
- Elsmore, A.; Mendoza, N. The operative learning curve for vestibular schwannoma excision via the retrosigmoid approach. Br. J. Neurosurg. 2002, 16, 448–455. [Google Scholar] [CrossRef]
- Pamir, M.N.; Peker, S.; Bayraklı, F.; Kılıç, T.; Özek, M.M. Surgical treatment of trigeminal schwannomas. Neurosurg. Rev. 2007, 30, 329–337. [Google Scholar] [CrossRef]
- Guthikonda, B.; Theodosopoulos, P.V.; van Loveren, H.; Tew, J.M., Jr.; Pensak, M.L. Evolution in the assessment and management of trigeminal schwannoma. Laryngoscope 2008, 118, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Muzumdar, D.; Raman, C. Trigeminal neuroma: Analysis of surgical experience with 73 cases. Neurosurgery 2003, 52, 783–790; discussion 790. [Google Scholar] [CrossRef] [PubMed]
- Lesoin, F.; Rousseaux, M.; Villette, L.; Autricque, A.; Dhellemmes, P.; Pellerin, P.; Vaneecloo, J.M.; Leys, D.; Jomin, M. Neurinomas of the trigeminal nerve. Acta Neurochir. 1986, 82, 118–122. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Chen, G.; Liang, J.; Guo, H.; Song, G.; Bao, Y. Trigeminal schwannoma: A single-center experience with 43 cases and review of literature. Br. J. Neurosurg. 2021, 35, 49–56. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kida, Y.; Yoshimoto, M.; Koike, J. Trigeminal schwannomas: Results of Gamma Knife surgery in 37 cases. J. Neurosurg. JNS 2007, 106, 18. [Google Scholar] [CrossRef]
- Kuo, J.S.; Chen, J.C.; Yu, C.; Zelman, V.; Giannotta, S.L.; Petrovich, Z.; MacPherson, D.; Apuzzo, M.L. Gamma knife radiosurgery for benign cavernous sinus tumors: Quantitative analysis of treatment outcomes. Neurosurgery 2004, 54, 1385–1393; discussion 1393–1394. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.H.; Shepard, M.J.; Chen, C.-J.; Sheehan, J.P. Stereotactic Radiosurgery for Trigeminal Schwannomas: A 28-Year Single-Center Experience and Review of the Literature. World Neurosurg. 2018, 119, e874–e881. [Google Scholar] [CrossRef] [PubMed]
- Champ, C.E.; Mishra, M.V.; Shi, W.; Siglin, J.; Werner-Wasik, M.; Andrews, D.W.; Evans, J.J. Stereotactic Radiotherapy for Trigeminal Schwannomas. Neurosurgery 2012, 71, 270–277. [Google Scholar] [CrossRef]
- Combs, S.E.; Engelhard, C.; Kopp, C.; Wiedenmann, N.; Schramm, O.; Prokic, V.; Debus, J.; Molls, M.; Grosu, A.L. Long-term outcome after highly advanced single-dose or fractionated radiotherapy in patients with vestibular schwannomas-pooled results from 3 large German centers. Radiother. Oncol. 2015, 114, 378–383. [Google Scholar] [CrossRef]
- Combs, S.E.; Welzel, T.; Kessel, K.; Habermehl, D.; Rieken, S.; Schramm, O.; Debus, J. Hearing preservation after radiotherapy for vestibular schwannomas is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients’ self-reported outcome. Radiother. Oncol. 2013, 106, 175–180. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kato, T.; Iizuka, H.; Kida, Y. Long-Term Results for Trigeminal Schwannomas Treated With Gamma Knife Surgery. Int. J. Radiat. Oncol. 2013, 87, 1115–1121. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Yu, X.; Qi, S.; Du, Y.; Ni, W.; Hu, Y.; Tian, Z. Stereotactic Radiosurgery for Trigeminal Schwannoma: A Clinical Retrospective Study in 52 Cases. Ster. Funct. Neurosurg. 2013, 91, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Aboukais, R.; Bonne, N.X.; Baroncini, M.; Zairi, F.; Schapira, S.; Vincent, C.; Lejeune, J.P. Management of multiple tumors in neurofibromatosis type 2 patients. Neurochirurgie 2018, 64, 364–369. [Google Scholar] [CrossRef]
- Gugel, I.; Grimm, F.; Liebsch, M.; Zipfel, J.; Teuber, C.; Kluwe, L.; Mautner, V.F.; Tatagiba, M.; Schuhmann, M.U. Impact of Surgery on Long-Term Results of Hearing in Neurofibromatosis Type-2 Associated Vestibular Schwannomas. Cancers 2019, 11, 1376. [Google Scholar] [CrossRef]
- Yao, L.; Alahmari, M.; Temel, Y.; Hovinga, K. Therapy of sporadic and nf2-related vestibular schwannoma. Cancers 2020, 12, 835. [Google Scholar] [CrossRef]
- Golfinos, J.G.; Hill, T.C.; Rokosh, R.; Choudhry, O.; Shinseki, M.; Mansouri, A.; Friedmann, D.R.; Roland, J.T.; Kondziolka, D. A matched cohort comparison of clinical outcomes following microsurgical resection or stereotactic radiosurgery for patients with small- and medium-sized vestibular schwannomas. J. Neurosurg. JNS 2016, 125, 1472. [Google Scholar] [CrossRef] [PubMed]
- Wolbers, J.G.; Dallenga, A.H.; Mendez Romero, A.; van Linge, A. What intervention is best practice for vestibular schwannomas? A systematic review of controlled studies. BMJ Open 2013, 3, e001345. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, C.E.; Evans, D.G. Review of radiation therapy services for neurofibromatosis (NF2) patients in England. Br. J. Neurosurg. 2014, 28, 16–19. [Google Scholar] [CrossRef]
- Mathieu, D.; Kondziolka, D.; Flickinger, J.C.; Niranjan, A.; Williamson, R.; Martin, J.J.; Lunsford, L.D. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: An analysis of tumor control, complications, and hearing preservation rates. Neurosurgery 2007, 60, 460–468; discussion 468–470. [Google Scholar] [CrossRef]
- Phi, J.H.; Kim, D.G.; Chung, H.-T.; Lee, J.; Paek, S.H.; Jung, H.-W. Radiosurgical treatment of vestibular schwannomas in patients with neurofibromatosis type 2. Cancer 2009, 115, 390–398. [Google Scholar] [CrossRef]
- MacNally, S.P.; Rutherford, S.A.; Ramsden, R.T.; Evans, D.G.; King, A.T. Trigeminal schwannomas. Br. J. Neurosurg. 2008, 22, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Coy, S.; Rashid, R.; Stemmer-Rachamimov, A.; Santagata, S. An update on the CNS manifestations of neurofibromatosis type 2. Acta Neuropathol. 2020, 139, 643–665. [Google Scholar] [CrossRef]
- Fisher, L.M.; Doherty, J.K.; Lev, M.H.; Slattery, W.H., 3rd. Distribution of nonvestibular cranial nerve schwannomas in neurofibromatosis 2. Otol. Neurotol. 2007, 28, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Dolenc, V.V. Frontotemporal epidural approach to trigeminal neurinomas. Acta Neurochir. 1994, 130, 55–65. [Google Scholar] [CrossRef] [PubMed]
| Mdn. Age (years) | 57.5 | (36–83) | |
| Sex | Female | 7 | (53.8%) |
| Male | 6 | (46.2%) | |
| Preoperative Deficits | |||
| Decreased gustation | 2 | (15.4%) | |
| Cerebellar ataxia | 2 | (15.4%) | |
| Vertigo/Nausea | 2 | (15.4%) | |
| Headache | 3 | (23.1%) | |
| Visual impairment | 3 | (23.1%) | |
| Hypacusis | 4 | (30.8%) | |
| Facial pain | 7 | (53.8%) | |
| Trigeminal nerve hypesthesia | |||
| V1 | 6 | (46.2%) | |
| V2 | 8 | (61.5%) | |
| V3 | 7 | (53.8%) | |
| Mdn. duration of symptoms (months) | 6 | (0–288) | |
| Histopathology | |||
| Schwannoma WHO grade I | 13 | (100%) | |
| Mdn. Tumour Volume (cm3) | 8.05 | (2.7–81.8) | |
| Mdn. max. Tumour Diameter (cm) | 3.20 | (2.6–7.2) | |
| Mean max. Tumour Diameter (cm) | 3.44 | ||
| Type of Tumour | Samii et al. | Ramina et al. | No. of Patients | Approach | |
|---|---|---|---|---|---|
| A | C | 6 (46.2%) | Intradural STA Extradural STA Kawase TNA | 3 (23.1%) 1 (7.7%) 1 (7.7%) 1 (7.7%) | |
| B | D | 2 (15.4%) | RSA | 2 (15.4%) | |
| C | E | 4 (30.8%) | Intradural STA | 1 (7.7%) | |
| RSA Intradural STA + RSA | 2 (15.4%) 1 (7.7%) | ||||
| Kawase + RSA | 1 (7.7%) | ||||
| D | A | 1 (7.7%) | TNA | 1 (7.7%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aftahy, A.K.; Groll, M.; Barz, M.; Wagner, A.; Lange, N.; Butenschön, V.M.; Delbridge, C.; Bernhardt, D.; Meyer, B.; Negwer, C.; et al. Surgical Outcome of Trigeminal Schwannomas. Cancers 2021, 13, 1310. https://doi.org/10.3390/cancers13061310
Aftahy AK, Groll M, Barz M, Wagner A, Lange N, Butenschön VM, Delbridge C, Bernhardt D, Meyer B, Negwer C, et al. Surgical Outcome of Trigeminal Schwannomas. Cancers. 2021; 13(6):1310. https://doi.org/10.3390/cancers13061310
Chicago/Turabian StyleAftahy, Amir Kaywan, Maximilian Groll, Melanie Barz, Arthur Wagner, Nicole Lange, Vicki Marie Butenschön, Claire Delbridge, Denise Bernhardt, Bernhard Meyer, Chiara Negwer, and et al. 2021. "Surgical Outcome of Trigeminal Schwannomas" Cancers 13, no. 6: 1310. https://doi.org/10.3390/cancers13061310
APA StyleAftahy, A. K., Groll, M., Barz, M., Wagner, A., Lange, N., Butenschön, V. M., Delbridge, C., Bernhardt, D., Meyer, B., Negwer, C., & Gempt, J. (2021). Surgical Outcome of Trigeminal Schwannomas. Cancers, 13(6), 1310. https://doi.org/10.3390/cancers13061310








