HERC1 Regulates Breast Cancer Cells Migration and Invasion
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
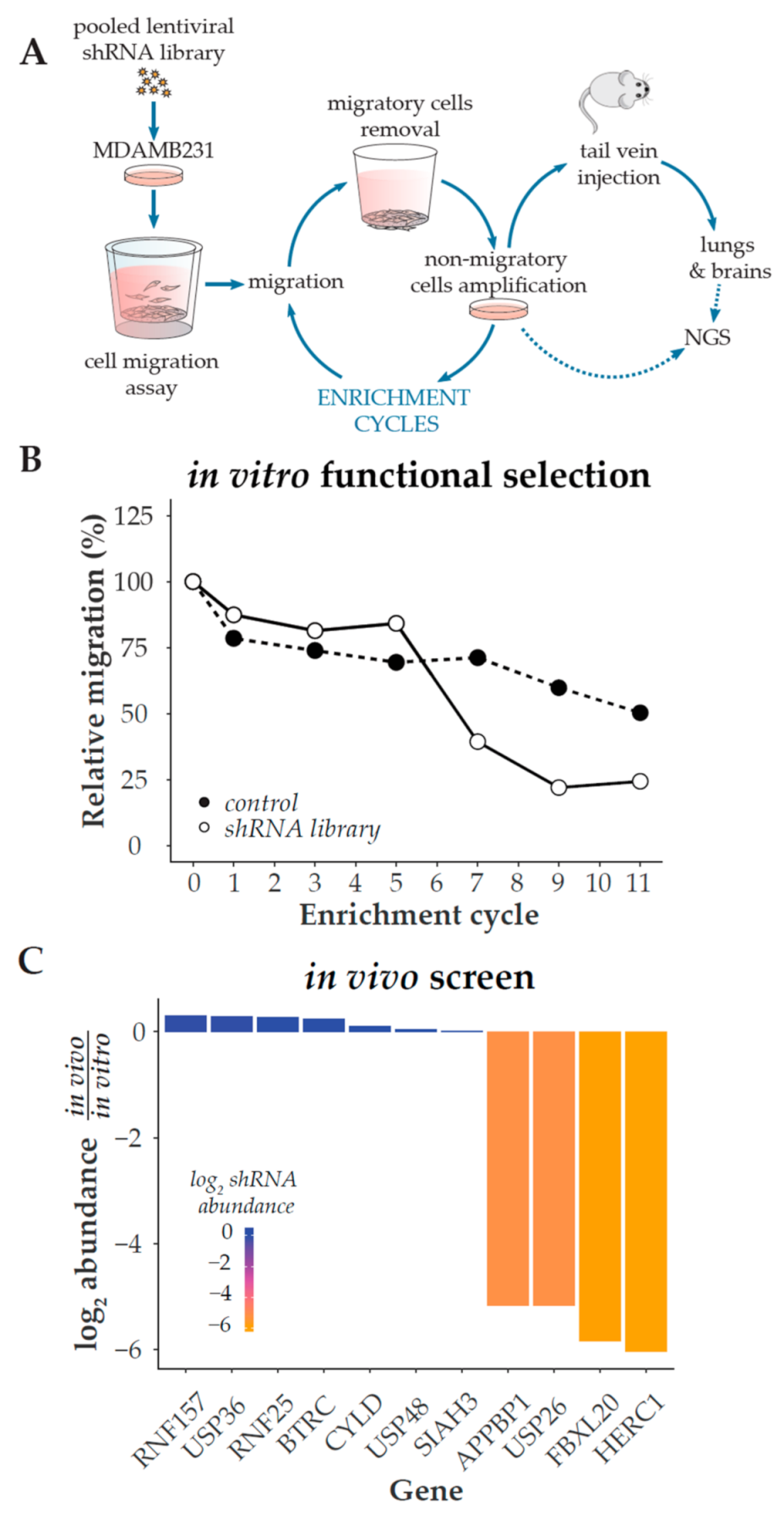
2.2. shRNA Screening
2.3. shRNA Library Preparation and Sequencing
2.4. shRNA Screen Sequencing Data Analysis
2.5. Boyden Chamber Migration Assay
2.6. Quantitative PCR
2.7. Area-Based Analysis of Proliferation Rate
2.8. 3D Clonogenic Assay
2.9. Wound Healing Assay
2.10. Noble Agar Invasion Assay
2.11. In Vivo Screen and Experimental Metastasis Model
2.12. Tumorigenesis Model
2.13. In Silico Analysis
2.14. Statistical Analysis
3. Results
3.1. Phenotypic Screening for Positive Regulators of Migration
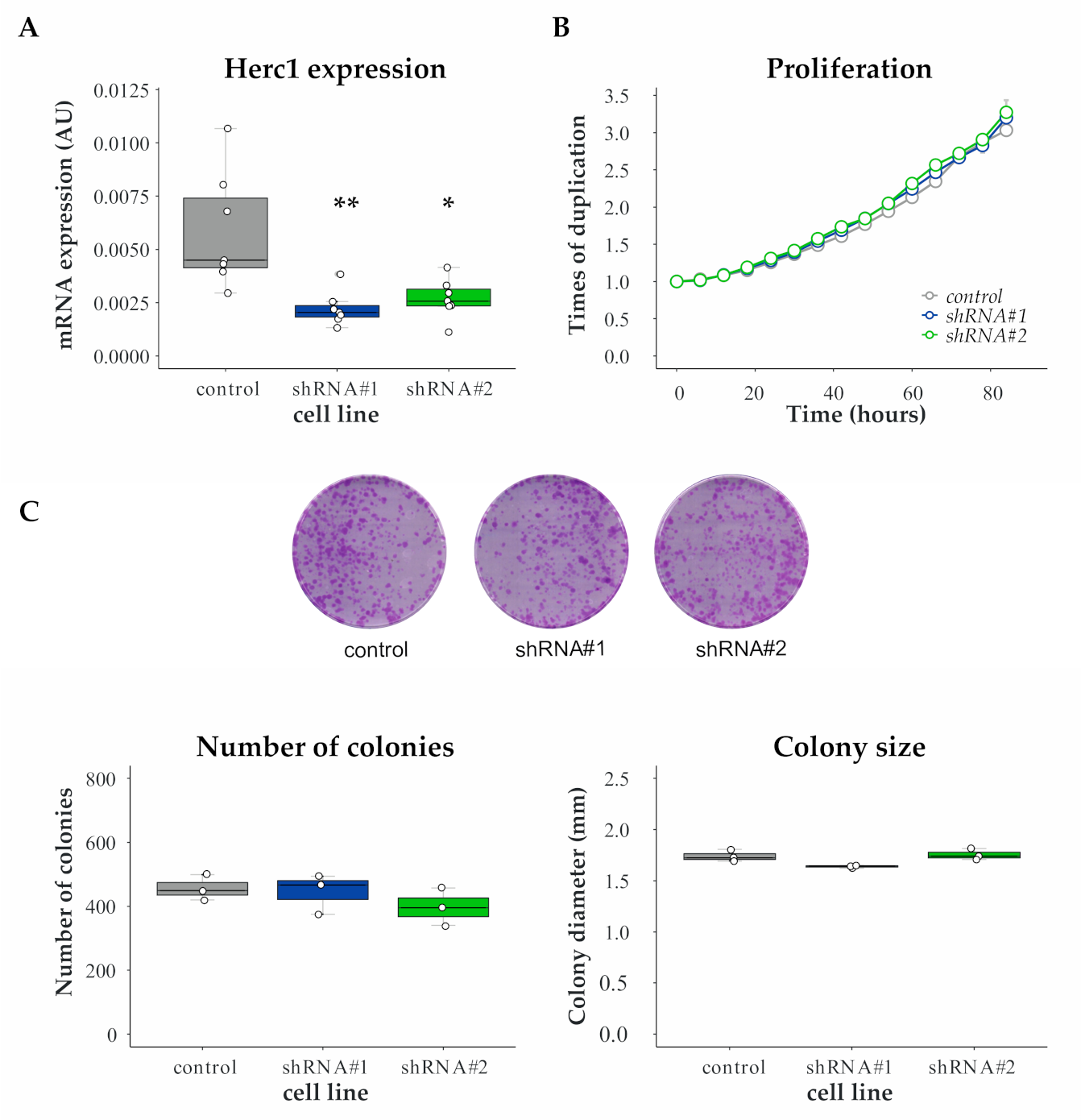
3.2. Proliferation Is Unaffected by HERC1 Silencing
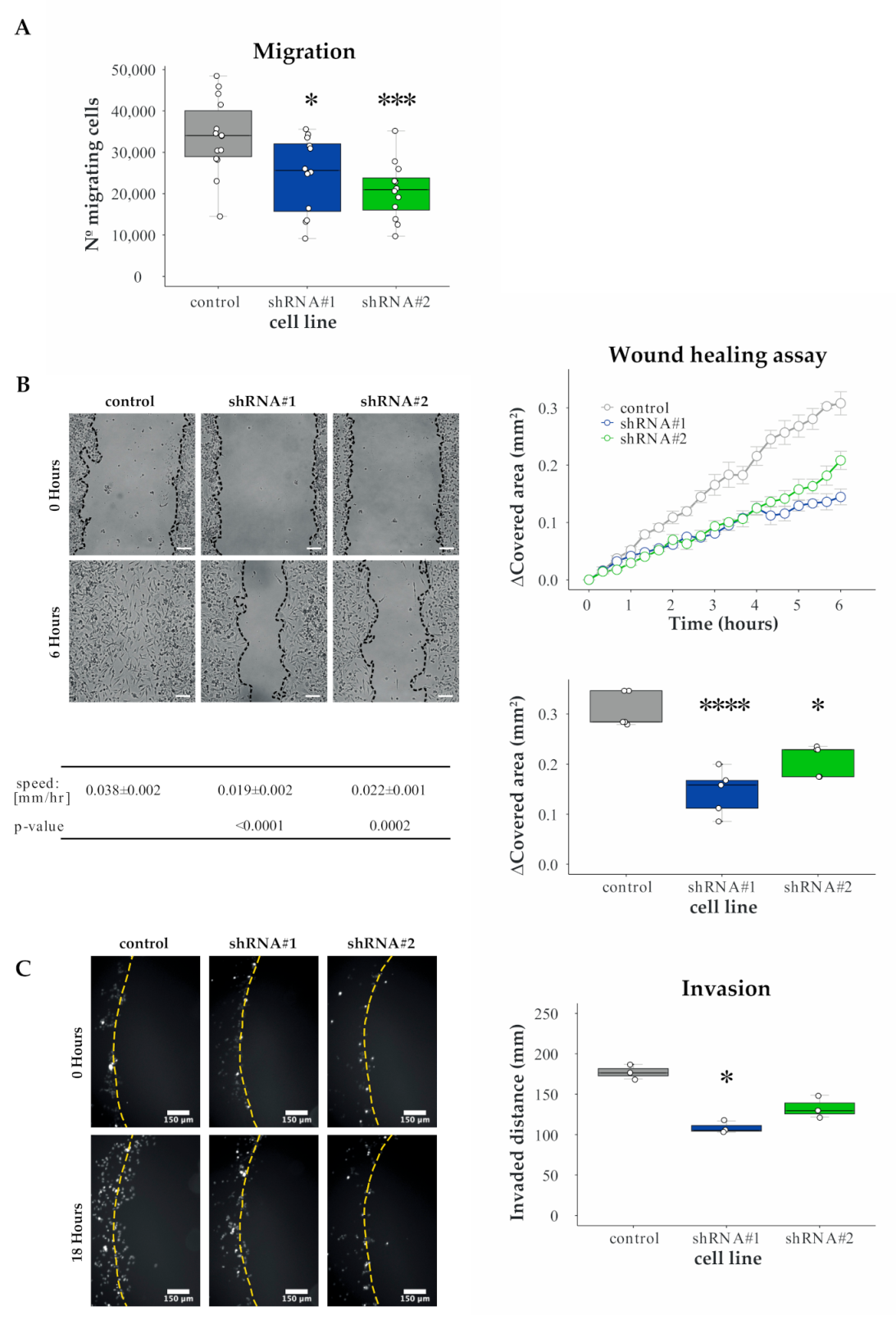
3.3. Validation of HERC1 as a Candidate Gene Regulating Migration
3.4. HERC1 Regulates Cell Invasion
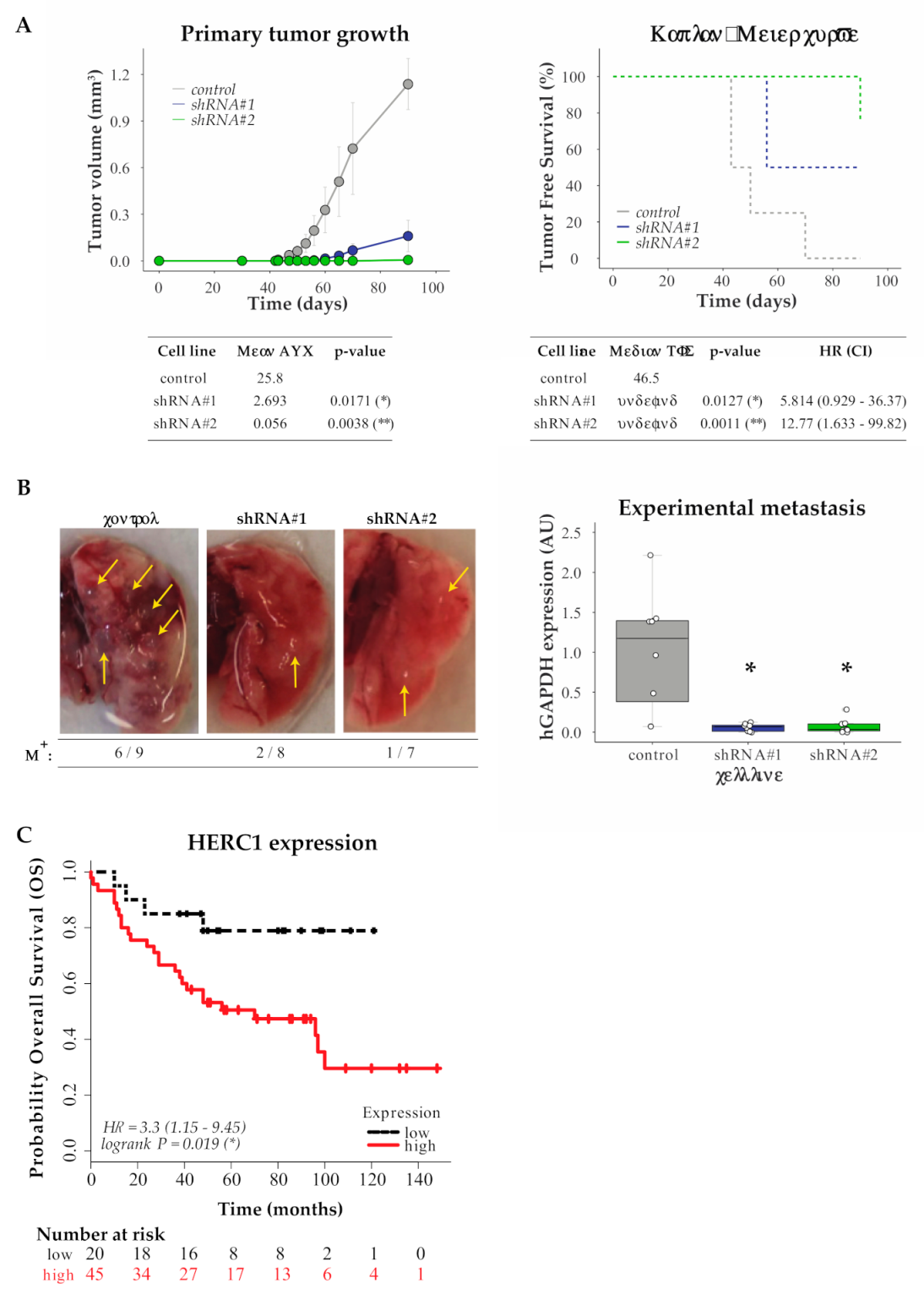
3.5. In Vivo Validation
3.6. In Silico Analysis for HERC1 Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Gallo, L.H.; Ko, J.; Donoghue, D.J. The importance of regulatory ubiquitination in cancer and metastasis. Cell Cycle 2017, 16, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Deshaies, R.J. Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 2014, 12, 94. [Google Scholar] [CrossRef]
- Ciechanover, A. The ubiquitin proteolytic system: From a vague idea, through basic mechanisms, and onto human diseases and drug targeting. Neurology 2005, 66, S7–S19. [Google Scholar] [CrossRef]
- Paul, S. Dysfunction of the ubiquitin-proteasome system in multiple disease conditions: Therapeutic approaches. BioEssays 2008, 30, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Petroski, M.D. The ubiquitin system, disease, and drug discovery. BMC Biochem. 2008, 9, S7. [Google Scholar] [CrossRef] [PubMed]
- Popovic, D.; Vucic, D.; Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014, 20, 1242–1253. [Google Scholar] [CrossRef]
- Reinstein, E.; Ciechanover, A. Narrative review: Protein degradation and human diseases: The ubiquitin connection. Ann. Intern. Med. 2006, 145, 676–684. [Google Scholar] [CrossRef]
- Hoeller, D.; Dikic, I. Targeting the ubiquitin system in cancer therapy. Nat. Cell Biol. 2009, 458, 438–444. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-L.; Kuo, Y.-C.; Ho, Y.-S.; Huang, Y.-H. Triple-Negative Breast Cancer: Current Understanding and Future Therapeutic Breakthrough Targeting Cancer Stemness. Cancers 2019, 11, 1334. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, J.; Haddad, F.G.; Eid, R.; Lambertini, M.; Kourie, H.R. Triple-negative breast cancer: Current perspective on the evolving therapeutic landscape. Int. J. Women’s Health 2019, 11, 431–437. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Bahnson, A.; Athanassiou, C.; Koebler, D.; Qian, L.; Shun, T.; Shields, D.; Yu, H.; Wang, H.; Goff, J.; Cheng, T.; et al. Automated measurement of cell motility and proliferation. BMC Cell Biol. 2005, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, Q. OpenCFU, a New Free and Open-Source Software to Count Cell Colonies and Other Circular Objects. PLoS ONE 2013, 8, e54072. [Google Scholar] [CrossRef]
- Ahmed, M.; Basheer, H.A.; Ayuso, J.M.; Ahmet, D.; Mazzini, M.; Patel, R.; Shnyder, S.D.; Vinader, V.; Afarinkia, K. Agarose Spot as a Comparative Method for in situ Analysis of Simultaneous Chemotactic Responses to Multiple Chemokines. Sci. Rep. 2017, 7, 1075. [Google Scholar] [CrossRef] [PubMed]
- Salvany, L.; Müller, J.; Guccione, E.; Rørth, P. The core and conserved role of MAL is homeostatic regulation of actin levels. Genes Dev. 2014, 28, 1048–1053. [Google Scholar] [CrossRef]
- Wiggins, H.; Rappoport, J. An agarose spot assay for chemotactic invasion. Biotechniques 2010, 48, 121–124. [Google Scholar] [CrossRef]
- Tinevez, J.-Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef]
- Llorens, M.C.; Rossi, F.A.; García, I.A.; Cooke, M.; Abba, M.C.; Lopez-Haber, C.; Barrio-Real, L.; Vaglienti, M.V.; Rossi, M.; Bocco, J.L.; et al. PKCα Modulates Epithelial-to-Mesenchymal Transition and Invasiveness of Breast Cancer Cells Through ZEB1. Front. Oncol. 2019, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Györffy, B.; Lanczky, A.; Eklund, A.C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res. Treat. 2009, 123, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhou, M.; Dorsey, T.H.; Prieto, D.A.; Wang, X.W.; Ruppin, E.; Veenstra, T.D.; Ambs, S. Integrated proteotranscriptomics of breast cancer reveals globally increased protein-mRNA concordance associated with subtypes and survival. Genome Med. 2018, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhang, C.; Chen, X.; Luo, J.; Wu, Q. FBXL20 promotes cell proliferation and metastasis through activating Wnt/β-catenin signaling pathway in esophageal cancer. Int. J. Clin. Exp. Med. 2017, 10, 7796–7805. [Google Scholar]
- Jin, Y.; Zhang, P.; Wang, Y.; Jin, B.; Zhou, J.; Zhang, J.; Pan, J. Neddylation Blockade Diminishes Hepatic Metastasis by Dampening Cancer Stem-Like Cells and Angiogenesis in Uveal Melanoma. Clin. Cancer Res. 2017, 24, 3741–3754. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, H.; Zhu, R.; Liu, Z. USP26 promotes esophageal squamous cell carcinoma metastasis through stabilizing Snail. Cancer Lett. 2019, 448, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Deng, S.; Duan, J.; Xie, X.; Xu, S.; Ran, M.; Dai, X.; Pu, Y.; Zhang, X. FBXL20 acts as an invasion inducer and mediates E-cadherin in colorectal adenocarcinoma. Oncol. Lett. 2014, 7, 2185–2191. [Google Scholar] [CrossRef]
- Nair, N.U.; Das, A.; Rogkoti, V.-M.; Fokkelman, M.; Marcotte, R.; De Jong, C.G.; Koedoot, E.; Lee, J.S.; Meilijson, I.; Hannenhalli, S.; et al. Migration rather than proliferation transcriptomic signatures are strongly associated with breast cancer patient survival. Sci. Rep. 2019, 9, 10989. [Google Scholar] [CrossRef]
- Rosa, J.L.; Casaroli-Marano, R.P.; Buckler, A.J.; Vilaro, S.; Barbacid, M. p619, a giant protein related to the chromosome condensation regulator RCC1, stimulates guanine nucleotide exchange on ARFi and Rab proteins. EMBO J. 1996, 15, 4262–4273. [Google Scholar] [CrossRef]
- Aggarwal, S.; Das Bhowmik, A.; Ramprasad, V.L.; Murugan, S.; Dalal, A. A splice site mutation inHERC1leads to syndromic intellectual disability with macrocephaly and facial dysmorphism: Further delineation of the phenotypic spectrum. Am. J. Med Genet. Part A 2016, 170, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Bachiller, S.; Roca-Ceballos, M.A.; García-Domínguez, I.; Pérez-Villegas, E.M.; Martos-Carmona, D.; Pérez-Castro, M.Á.; Real, L.M.; Rosa, J.L.; Tabares, L.; Venero, J.L.; et al. HERC1 Ubiquitin Ligase Is Required for Normal Axonal Myelination in the Peripheral Nervous System. Mol. Neurobiol. 2018, 55, 8856–8868. [Google Scholar] [CrossRef]
- Bachiller, S.; Rybkina, T.; Porras-García, E.; Pérez-Villegas, E.; Tabares, L.; Armengol, J.A.; Carrión, A.M.; Ruiz, R. The HERC1 E3 Ubiquitin Ligase is essential for normal development and for neurotransmission at the mouse neuromuscular junction. Cell. Mol. Life Sci. 2015, 72, 2961–2971. [Google Scholar] [CrossRef]
- Hashimoto, R.; Nakazawa, T.; Tsurusaki, Y.; Yasuda, Y.; Nagayasu, K.; Matsumura, K.; Kawashima, H.; Yamamori, H.; Fujimoto, M.; Ohi, K.; et al. Whole-exome sequencing and neurite outgrowth analysis in autism spectrum disorder. J. Hum. Genet. 2015, 61, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.; Bayam, E.; Wagner, C.; Rinaldi, B.; Kretz, P.F.; Tilly, P.; Roos, M.; McGillewie, L.; Bär, S.; Minocha, S.; et al. WD40-repeat 47, a microtubule-associated protein, is essential for brain development and autophagy. Proc. Natl. Acad. Sci. USA 2017, 114, E9308–E9317. [Google Scholar] [CrossRef]
- Lei, J.; Xu, H.; Liang, H.; Su, L.; Zhang, J.; Huang, X.; Song, Z.; Le, W.; Deng, H. Gene expression changes in peripheral blood from chinese han patients with tourette syndrome. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2012, 159B, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.S.; Schneider, T.; Rio, M.; Moutton, S.; Siquier-Pernet, K.; Verny, F.; Boddaert, N.; Desguerre, I.; Munich, A.; Rosa, J.L.; et al. A nonsense variant in HERC1 is associated with intellectual disability, megalencephaly, thick corpus callosum and cerebellar atrophy. Eur. J. Hum. Genet. 2016, 24, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, T.; Hadjebi, O.; Amair-Pinedo, F.; Tsurumi, T.; Langa, F.; Serikawa, T.; Sotelo, C.; Guénet, J.-L.; Rosa, J.L. Progressive Purkinje Cell Degeneration in tambaleante Mutant Mice Is a Consequence of a Missense Mutation in HERC1 E3 Ubiquitin Ligase. PLoS Genet. 2009, 5, e1000784. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Villegas, E.M.; Negrete-Díaz, J.V.; Porras-García, M.E.; Ruiz, R.; Carrión, A.M.; Rodríguez-Moreno, A.; Armengol, J.A.; De Mújica, J.; Ángel, A.B. Mutation of the HERC 1 Ubiquitin Ligase Impairs Associative Learning in the Lateral Amygdala. Mol. Neurobiol. 2017, 55, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Puffenberger, E.G.; Jinks, R.N.; Wang, H.; Xin, B.; Fiorentini, C.; Sherman, E.A.; Degrazio, D.; Shaw, C.; Sougnez, C.; Cibulskis, K.; et al. A homozygous missense mutation inHERC2associated with global developmental delay and autism spectrum disorder. Hum. Mutat. 2012, 33, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.; Hanneder, M.; Siegel, N.; Valli, A.; Hengstschläger, M. The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat. Res. Rev. Mutat. Res. 2008, 658, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Utine, G.E.; Taşkıran, E.Z.; Koşukcu, C.; Karaosmanoğlu, B.; Güleray, N.; Doğan, Ö.A.; Kiper, P.; Özlem, Ş.; Boduroğlu, K.; Alikaşifoğlu, M. HERC1 mutations in idiopathic intellectual disability. Eur. J. Med. Genet. 2017, 60, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Diouf, B.; Cheng, Q.; Krynetskaia, N.F.; Yang, W.; Cheok, M.; Pei, D.; Fan, Y.; Cheng, C.; Krynetskiy, E.Y.; Geng, H.; et al. Somatic deletions of genes regulating MSH2 protein stability cause DNA mismatch repair deficiency and drug resistance in human leukemia cells. Nat. Med. 2011, 17, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Holloway, A.; Simmonds, M.; Azad, A.; Fox, J.L.; Storey, A. Resistance to UV-induced apoptosis by β-HPV5 E6 involves targeting of activated BAK for proteolysis by recruitment of the HERC1 ubiquitin ligase. Int. J. Cancer 2014, 136, 2831–2843. [Google Scholar] [CrossRef]
- Pedrazza, L.; Schneider, T.; Bartrons, R.; Ventura, F.; Rosa, J.L. The ubiquitin ligase HERC1 regulates cell migration via RAF-dependent regulation of MKK3/p38 signaling. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, S.; Quarto, M.; Goisis, G.; Nuciforo, P.; Donzelli, M.; Jodice, G.; Pelosi, G.; Viale, G.; Pece, S.; Di Fiore, P.P. Alterations of ubiquitin ligases in human cancer and their association with the natural history of the tumor. Oncogene 2009, 28, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, F.A.; Calvo Roitberg, E.H.; Enriqué Steinberg, J.H.; Joshi, M.U.; Espinosa, J.M.; Rossi, M. HERC1 Regulates Breast Cancer Cells Migration and Invasion. Cancers 2021, 13, 1309. https://doi.org/10.3390/cancers13061309
Rossi FA, Calvo Roitberg EH, Enriqué Steinberg JH, Joshi MU, Espinosa JM, Rossi M. HERC1 Regulates Breast Cancer Cells Migration and Invasion. Cancers. 2021; 13(6):1309. https://doi.org/10.3390/cancers13061309
Chicago/Turabian StyleRossi, Fabiana Alejandra, Ezequiel Hernán Calvo Roitberg, Juliana Haydeé Enriqué Steinberg, Molishree Umesh Joshi, Joaquín Maximiliano Espinosa, and Mario Rossi. 2021. "HERC1 Regulates Breast Cancer Cells Migration and Invasion" Cancers 13, no. 6: 1309. https://doi.org/10.3390/cancers13061309
APA StyleRossi, F. A., Calvo Roitberg, E. H., Enriqué Steinberg, J. H., Joshi, M. U., Espinosa, J. M., & Rossi, M. (2021). HERC1 Regulates Breast Cancer Cells Migration and Invasion. Cancers, 13(6), 1309. https://doi.org/10.3390/cancers13061309






