TERT Promoter Alterations in Glioblastoma: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
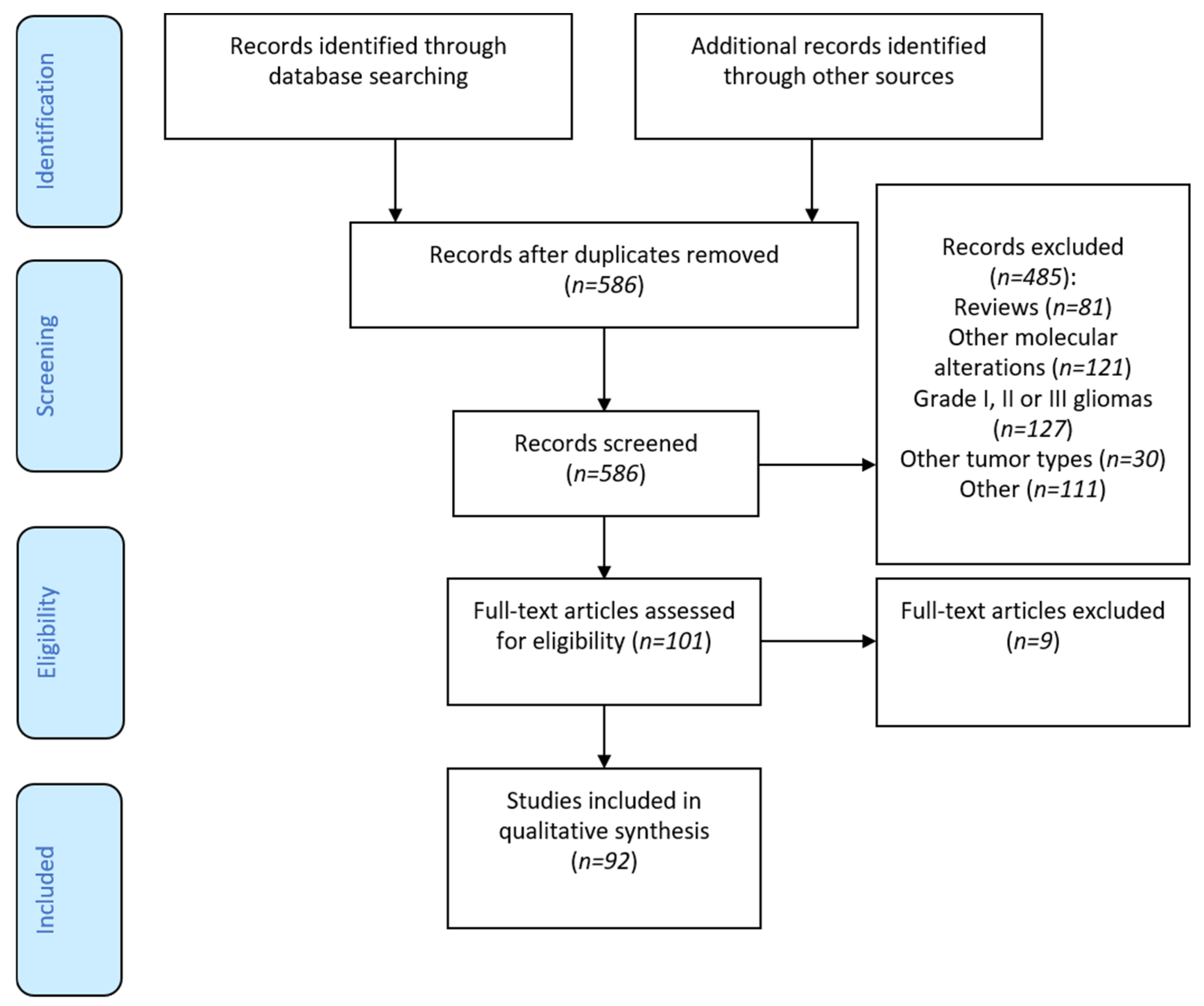
3.1. Flow-Chart
3.2. TERT Genomic Alterations in Glioblastoma
3.2.1. Incidence
3.2.2. Diagnosis
3.3. Physiopathology
3.3.1. Telomerase Activity: Overcoming Replicative Senescence
3.3.2. Association of TERTp Mutations and Other Molecular Alterations
3.3.3. TERTp Mutation Status: An Independent Prognostic Factor?
3.3.4. Pediatric Glioblastoma
3.3.5. Rare Tumors Subtypes
3.4. Therapeutic Implications and Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alternative Lengthening of Telomeres |
| ATRX | α-thalassemia/mental retardation syndrome X-linked |
| ctDNA | Circulating Tumor Deoxyribonucleic Acid |
| CNS | Central Nervous System |
| CSF | CerebroSpinal Fluid |
| DAXX | Death-associated protein 6 |
| ddPCR | Droplet digital Polymerase Chain Reaction |
| DC | Dentritic Cells |
| DCE-MRI | Dynamic Contrast Enhanced—Magnetic Resonance Imaging |
| DNA | Deoxyribonucleic Acid |
| EGFR | Epidermal Growth Factor Receptor |
| Erbb2 | v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 |
| ETS | E26 transformation-specific family transcription factor |
| IDH | Isocitrate Dehydrogenase |
| IDH-wt | Isocitrate Dehydrogenase—wild-type |
| IDH-mut | Isocitrate Dehydrogenase—mutated |
| hTERT | Human Telomerase Reverse Transcriptase |
| NGS | New Generation Sequencing |
| MGMT | O6-methylguanine-DNA methyltransferase |
| MRI | Magnetic Resonance Imaging |
| NOS | Not Otherwise Specified |
| OS | Overall Survival |
| pHGG | Pediatric High Grade Gliomas |
| PI3KR1 | Phosphatidylinositol 3-kinase regulatory subunit alpha |
| RNA | Ribonucleic Acid |
| RdRP | RNA-dependent RNA polymerase |
| TCGA | The Cancer Genome Atlas |
| TERC | Telomerase RNA template |
| TERTp | TElomerase Reverse Transcriptase Promoter |
| TERTp-mut | TElomerase Reverse Transcriptase Promoter mutated |
| TERTp-wt | TElomerase Reverse Transcriptase Promoter wild-type |
| TP53 | Tumor suppressor Protein 53 |
| UTSS | Upstream of the Transcription Start Site |
References
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary Brain Tumours in Adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2010–2014. Neuro-oncology 2017, 19, v1–v88. [Google Scholar] [CrossRef]
- Stupp, R.; Brada, M.; van den Bent, M.J.; Tonn, J.-C.; Pentheroudakis, G.; On behalf of the ESMO Guidelines Working Group. High-Grade Glioma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2014, 25, iii93–iii101. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant Gliomas in Adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Bergo, E.; Lombardi, G.; Guglieri, I.; Capovilla, E.; Pambuku, A.; Zagone, V. Neurocognitive Functions and Health-Related Quality of Life in Glioblastoma Patients: A Concise Review of the Literature. Eur. J. Cancer Care 2019, 28, e12410. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.-M.; Gallia, G.L.; et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT Promoter Mutations Occur Frequently in Gliomas and a Subset of Tumors Derived from Cells with Low Rates of Self-Renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Piatyszek, M.; Prowse, K.; Harley, C.; West, M.; Ho, P.; Coviello, G.; Wright, W.; Weinrich, S.; Shay, J. Specific Association of Human Telomerase Activity with Immortal Cells and Cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly Recurrent TERT Promoter Mutations in Human Melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Yamasaki, K.; Matsushita, Y.; Nakamura, T.; Shimokawa, A.; Takami, H.; Tanaka, S.; Mukasa, A.; Shirahata, M.; Shimizu, S.; et al. A Combination of TERT Promoter Mutation and MGMT Methylation Status Predicts Clinically Relevant Subgroups of Newly Diagnosed Glioblastomas. Acta Neuropathol. Commun. 2016, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Meyerson, M.; Counter, C.M.; Eaton, E.N.; Ellisen, L.W.; Steiner, P.; Caddle, S.D.; Ziaugra, L.; Beijersbergen, R.L.; Davidoff, M.J.; Liu, Q.; et al. HEST2, the Putative Human Telomerase Catalytic Subunit Gene, Is Up-Regulated in Tumor Cells and during Immortalization. Cell 1997, 90, 785–795. [Google Scholar] [CrossRef]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic Analysis of Telomere Length and Somatic Alterations in 31 Cancer Types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef]
- Mosrati, M.A.; Malmström, A.; Lysiak, M.; Krysztofiak, A.; Hallbeck, M.; Milos, P.; Hallbeck, A.-L.; Bratthäll, C.; Strandéus, M.; Stenmark-Askmalm, M.; et al. TERT Promoter Mutations and Polymorphisms as Prognostic Factors in Primary Glioblastoma. Oncotarget 2015, 6, 16663–16673. [Google Scholar] [CrossRef]
- Yuan, Y.; Qi, C.; Maling, G.; Xiang, W.; Yanhui, L.; Ruofei, L.; Yunhe, M.; Jiewen, L.; Qing, M. TERT Mutation in Glioma: Frequency, Prognosis and Risk. J. Clin. Neurosci. 2016, 26, 57–62. [Google Scholar] [CrossRef]
- Spiegl-Kreinecker, S.; Lötsch, D.; Ghanim, B.; Pirker, C.; Mohr, T.; Laaber, M.; Weis, S.; Olschowski, A.; Webersinke, G.; Pichler, J.; et al. Prognostic Quality of Activating TERT Promoter Mutations in Glioblastoma: Interaction with the Rs2853669 Polymorphism and Patient Age at Diagnosis. Neuro-oncology 2015, 17, 1231–1240. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, M.J.; Song, J.H.; Kim, E.S.; Kim, H.Y.; Min, K.-W. Clinical Implications of TERT Promoter Mutation on IDH Mutation and MGMT Promoter Methylation in Diffuse Gliomas. Pathol. Res. Pract. 2018, 214, 881–888. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Liu, Y.; Liu, X.; Zhang, C.; Wang, L.; Li, S.; Ma, J.; Jiang, T. Brain Regions Associated with Telomerase Reverse Transcriptase Promoter Mutations in Primary Glioblastomas. J. Neurooncol. 2016, 128, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Pierini, T.; Nardelli, C.; Lema Fernandez, A.G.; Pierini, V.; Pellanera, F.; Nofrini, V.; Gorello, P.; Moretti, M.; Arniani, S.; Roti, G.; et al. New Somatic TERT Promoter Variants Enhance the Telomerase Activity in Glioblastoma. Acta Neuropathol. Commun. 2020, 8, 145. [Google Scholar] [CrossRef]
- Labussiere, M.; Boisselier, B.; Mokhtari, K.; Di Stefano, A.-L.; Rahimian, A.; Rossetto, M.; Ciccarino, P.; Saulnier, O.; Paterra, R.; Marie, Y.; et al. Combined Analysis of TERT, EGFR, and IDH Status Defines Distinct Prognostic Glioblastoma Classes. Neurology 2014, 83, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Fukai, J.; Kodama, Y.; Hirose, T.; Okita, Y.; Moriuchi, S.; Nonaka, M.; Tsuyuguchi, N.; Terakawa, Y.; Uda, T.; et al. Characteristics and Outcomes of Elderly Patients with Diffuse Gliomas: A Multi-Institutional Cohort Study by Kansai Molecular Diagnosis Network for CNS Tumors. J. Neurooncol. 2018, 140, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, J.; Almeida, A.; Pópulo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT Promoter Mutations in Human Cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef]
- You, H.; Wu, Y.; Chang, K.; Shi, X.; Chen, X.-D.; Yan, W.; Li, R. Paradoxical Prognostic Impact of TERT Promoter Mutations in Gliomas Depends on Different Histological and Genetic Backgrounds. CNS Neurosci. 2017, 23, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Pesenti, C.; Paganini, L.; Fontana, L.; Veniani, E.; Runza, L.; Ferrero, S.; Bosari, S.; Menghi, M.; Marfia, G.; Caroli, M.; et al. Mass Spectrometry-Based Assay for the Molecular Diagnosis of Glioma: Concomitant Detection of Chromosome 1p/19q Codeletion, and IDH1, IDH2, and TERT Mutation Status. Oncotarget 2017, 8, 57134–57148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diplas, B.H.; Liu, H.; Yang, R.; Hansen, L.J.; Zachem, A.L.; Zhao, F.; Bigner, D.D.; McLendon, R.E.; Jiao, Y.; He, Y.; et al. Sensitive and Rapid Detection of TERT Promoter and IDH Mutations in Diffuse Gliomas. Neuro-oncology 2019, 21, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, M.Y.; Li, L.; Deng, Q.; Liu, F.; Luo, Y.; Wang, L.; Yao, G.; Zhu, D.; Lu, H.; et al. Detection of IDH1 and TERT Promoter Mutations with Droplet Digital PCR in Diffuse Gliomas. Int. J. Clin. Exp. Pathol. 2020, 13, 230–238. [Google Scholar]
- Fontanilles, M.; Marguet, F.; Beaussire, L.; Magne, N.; Pépin, L.-F.; Alexandru, C.; Tennevet, I.; Hanzen, C.; Langlois, O.; Jardin, F.; et al. Cell-Free DNA and Circulating TERT Promoter Mutation for Disease Monitoring in Newly-Diagnosed Glioblastoma. Acta Neuropathol. Commun. 2020, 8, 179. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Wald, A.I.; Melan, M.A.; Roy, S.; Zhong, S.; Hamilton, R.L.; Lieberman, F.S.; Drappatz, J.; Amankulor, N.M.; Pollack, I.F.; et al. Targeted Next-Generation Sequencing Panel (GlioSeq) Provides Comprehensive Genetic Profiling of Central Nervous System Tumors. Neuro-oncology 2016, 18, 379–387. [Google Scholar] [CrossRef]
- Higa, N.; Akahane, T.; Yokoyama, S.; Yonezawa, H.; Uchida, H.; Takajo, T.; Kirishima, M.; Hamada, T.; Matsuo, K.; Fujio, S.; et al. A Tailored Next-generation Sequencing Panel Identified Distinct Subtypes of Wildtype IDH and TERT Promoter Glioblastomas. Cancer Sci. 2020, 111, 3902. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Schrimpf, D.; Jones, D.T.W.; Meyer, J.; Kratz, A.; Reuss, D.; Capper, D.; Koelsche, C.; Korshunov, A.; Wiestler, B.; et al. Next-Generation Sequencing in Routine Brain Tumor Diagnostics Enables an Integrated Diagnosis and Identifies Actionable Targets. Acta Neuropathol. 2016, 131, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Synhaeve, N.E.; van den Bent, M.J.; French, P.J.; Dinjens, W.N.M.; Atmodimedjo, P.N.; Kros, J.M.; Verdijk, R.; Dirven, C.M.F.; Dubbink, H.J. Clinical Evaluation of a Dedicated next Generation Sequencing Panel for Routine Glioma Diagnostics. Acta Neuropathol. Commun. 2018, 6, 126. [Google Scholar] [CrossRef]
- Zacher, A.; Kaulich, K.; Stepanow, S.; Wolter, M.; Köhrer, K.; Felsberg, J.; Malzkorn, B.; Reifenberger, G. Molecular Diagnostics of Gliomas Using Next Generation Sequencing of a Glioma-Tailored Gene Panel: Next Generation Molecular Diagnostics of Gliomas. Brain Pathol. 2017, 27, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Euskirchen, P.; Bielle, F.; Labreche, K.; Kloosterman, W.P.; Rosenberg, S.; Daniau, M.; Schmitt, C.; Masliah-Planchon, J.; Bourdeaut, F.; Dehais, C.; et al. Same-Day Genomic and Epigenomic Diagnosis of Brain Tumors Using Real-Time Nanopore Sequencing. Acta Neuropathol. 2017, 134, 691–703. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.K.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. CIMPACT-NOW Update 3: Recommended Diagnostic Criteria for “Diffuse Astrocytic Glioma, IDH-Wildtype, with Molecular Features of Glioblastoma, WHO Grade IV. ” Acta Neuropathol. 2018, 136, 805–810. [Google Scholar] [CrossRef]
- Barritault, M.; Picart, T.; Poncet, D.; Fenouil, T.; d’Hombres, A.; Gabut, M.; Guyotat, J.; Jouanneau, E.; Ameli, R.; Joubert, B.; et al. Avoiding New Biopsies by Identification of IDH1 and TERT Promoter Mutation in Nondiagnostic Biopsies From Glioma Patients. Neurosurgery 2020. [Google Scholar] [CrossRef]
- Yamashita, K.; Hatae, R.; Hiwatashi, A.; Togao, O.; Kikuchi, K.; Momosaka, D.; Yamashita, Y.; Kuga, D.; Hata, N.; Yoshimoto, K.; et al. Predicting TERT Promoter Mutation Using MR Images in Patients with Wild-Type IDH1 Glioblastoma. Diagn. Interv. Imaging 2019, 100, 411–419. [Google Scholar] [CrossRef]
- Ozturk-Isik, E.; Cengiz, S.; Ozcan, A.; Yakicier, C.; Ersen Danyeli, A.; Pamir, M.N.; Özduman, K.; Dincer, A. Identification of IDH and TERTp Mutation Status Using 1H-MRS in 112 Hemispheric Diffuse Gliomas. J. Magn Reson Imaging 2020, 51, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; lyu, G.; He, W.; Lei, Y.; Lin, F.; Wang, M.; Zhang, H.; Liang, L.; Feng, Y.; Yang, J. DSC and DCE Histogram Analyses of Glioma Biomarkers, Including IDH, MGMT, and TERT, on Differentiation and Survival. Acad. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Deniel, A.; Marguet, F.; Beaussire, L.; Tobenas-Dujardin, A.-C.; Peillon, C.; Gambirasio, M.-A.; Veresezan, O.; Magne, N.; Di Fiore, F.; Laquerrière, A.; et al. TERTp Mutation Detection in Plasma by Droplet-Digital Polymerase Chain Reaction in Spinal Myxopapillary Ependymoma with Lung Metastases. World Neurosurg. 2019, 130, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Juratli, T.A.; Stasik, S.; Zolal, A.; Schuster, C.; Richter, S.; Daubner, D.; Juratli, M.A.; Thowe, R.; Hennig, S.; Makina, M.; et al. TERT Promoter Mutation Detection in Cell-Free Tumor-Derived DNA in Patients with IDH Wild-Type Glioblastomas: A Pilot Prospective Study. Clin. Cancer Res. 2018, 24, 5282–5291. [Google Scholar] [CrossRef]
- Mouliere, F.; Chandrananda, D.; Piskorz, A.M.; Moore, E.K.; Morris, J.; Ahlborn, L.B.; Mair, R.; Goranova, T.; Marass, F.; Heider, K.; et al. Enhanced Detection of Circulating Tumor DNA by Fragment Size Analysis. Sci. Transl. Med. 2018, 10, eaat4921. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, T.F.; Keil, V.C.; Hadizadeh, D.R.; Gielen, G.H.; Fimmers, R.; Waha, A.; Heidenreich, B.; Kumar, R.; Schild, H.H.; Simon, M. New Prognostic Factor Telomerase Reverse Transcriptase Promotor Mutation Presents without MR Imaging Biomarkers in Primary Glioblastoma. Neuroradiology 2017, 59, 1223–1231. [Google Scholar] [CrossRef]
- Tian, H.; Wu, H.; Wu, G.; Xu, G. Noninvasive Prediction of TERT Promoter Mutations in High-Grade Glioma by Radiomics Analysis Based on Multiparameter MRI. BioMed Res. Int. 2020, 2020, 3872314. [Google Scholar] [CrossRef]
- Ivanidze, J.; Lum, M.; Pisapia, D.; Magge, R.; Ramakrishna, R.; Kovanlikaya, I.; Fine, H.A.; Chiang, G.C. MRI Features Associated with TERT Promoter Mutation Status in Glioblastoma. J. Neuroimaging 2019, 29, 357–363. [Google Scholar] [CrossRef]
- de Lange, T. How Telomeres Solve the End-Protection Problem. Science 2009, 326, 948–952. [Google Scholar] [CrossRef]
- Masutomi, K.; Hahn, W.C. Telomerase and Tumorigenesis. Cancer Lett. 2003, 194, 163–172. [Google Scholar] [CrossRef]
- Arita, H.; Narita, Y.; Fukushima, S.; Tateishi, K.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Collins, V.P.; Kawahara, N.; et al. Upregulating Mutations in the TERT Promoter Commonly Occur in Adult Malignant Gliomas and Are Strongly Associated with Total 1p19q Loss. Acta Neuropathol. 2013, 126, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.J.A.; Rube, H.T.; Kreig, A.; Mancini, A.; Fouse, S.D.; Nagarajan, R.P.; Choi, S.; Hong, C.; He, D.; Pekmezci, M.; et al. The Transcription Factor GABP Selectively Binds and Activates the Mutant TERT Promoter in Cancer. Science 2015, 348, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Law, M.J.; Lower, K.M.; Voon, H.P.J.; Hughes, J.R.; Garrick, D.; Viprakasit, V.; Mitson, M.; De Gobbi, M.; Marra, M.; Morris, A.; et al. ATR-X Syndrome Protein Targets Tandem Repeats and Influences Allele-Specific Expression in a Size-Dependent Manner. Cell 2010, 143, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Heaphy, C.M.; de Wilde, R.F.; Jiao, Y.; Klein, A.P.; Edil, B.H.; Shi, C.; Bettegowda, C.; Rodriguez, F.J.; Eberhart, C.G.; Hebbar, S.; et al. Altered Telomeres in Tumors with ATRX and DAXX Mutations. Science 2011, 333, 425. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, B.; Rachakonda, P.S.; Hosen, I.; Volz, F.; Hemminki, K.; Weyerbrock, A.; Kumar, R. TERT Promoter Mutations and Telomere Length in Adult Malignant Gliomas and Recurrences. Oncotarget 2015, 6, 10617–10633. [Google Scholar] [CrossRef]
- Abou-El-Ardat, K.; Seifert, M.; Becker, K.; Eisenreich, S.; Lehmann, M.; Hackmann, K.; Rump, A.; Meijer, G.; Carvalho, B.; Temme, A.; et al. Comprehensive Molecular Characterization of Multifocal Glioblastoma Proves Its Monoclonal Origin and Reveals Novel Insights into Clonal Evolution and Heterogeneity of Glioblastomas. Neuro-oncology 2017, 19, 546–557. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.-Y.; Kim, W.K.; Lee, J.-K.; Park, J.; et al. Human Glioblastoma Arises from Subventricular Zone Cells with Low-Level Driver Mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Körber, V.; Yang, J.; Barah, P.; Wu, Y.; Stichel, D.; Gu, Z.; Fletcher, M.N.C.; Jones, D.; Hentschel, B.; Lamszus, K.; et al. Evolutionary Trajectories of IDHWT Glioblastomas Reveal a Common Path of Early Tumorigenesis Instigated Years Ahead of Initial Diagnosis. Cancer Cell 2019, 35, 692–704. [Google Scholar] [CrossRef]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef]
- Lee, Y.; Koh, J.; Kim, S.-I.; Won, J.K.; Park, C.-K.; Choi, S.H.; Park, S.-H. The Frequency and Prognostic Effect of TERT Promoter Mutation in Diffuse Gliomas. Acta Neuropathol. Commun. 2017, 5, 62. [Google Scholar] [CrossRef]
- Killela, P.J.; Pirozzi, C.J.; Healy, P.; Reitman, Z.J.; Lipp, E.; Rasheed, B.A.; Yang, R.; Diplas, B.H.; Wang, Z.; Greer, P.K.; et al. Mutations in IDH1, IDH2, and in the TERT Promoter Define Clinically Distinct Subgroups of Adult Malignant Gliomas. Oncotarget 2014, 5, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Azevedo, A.; Esteves, S.; Marques, A.R.; Martins, C.; Costa, I.; Mafra, M.; Bravo Marques, J.M.; Roque, L.; Pojo, M. Clinical Insights Gained by Refining the 2016 WHO Classification of Diffuse Gliomas with: EGFR Amplification, TERT Mutations, PTEN Deletion and MGMT Methylation. BMC Cancer 2019, 19, 968. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Lie, A.; Li, T.; Chowdhury, R.; Liu, F.; Ozer, B.; Wei, B.; Green, R.M.; Ellingson, B.M.; Wang, H.; et al. Human TERT Promoter Mutation Enables Survival Advantage from MGMT Promoter Methylation in IDH1 Wild-Type Primary Glioblastoma Treated by Standard Chemoradiotherapy. Neuonc 2016, now189. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Wang, Q.; Yan, X.; Wang, J. The TERT Promoter Mutation Status and MGMT Promoter Methylation Status, Combined with Dichotomized MRI-Derived and Clinical Features, Predict Adult Primary Glioblastoma Survival. Cancer Med. 2018, 7, 3704–3712. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cai, J.; Yan, W.; Zhang, W.; Wang, Y.; Chen, B.; Li, G.; Li, S.; Wu, C.; Yao, K.; et al. Classification Based on Mutations of TERT Promoter and IDH Characterizes Subtypes in Grade II/III Gliomas. Neuro-oncology 2016, 18, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Razis, E.; Kotoula, V.; Koliou, G.-A.; Papadopoulou, K.; Vrettou, E.; Giannoulatou, E.; Tikas, I.; Labropoulos, S.V.; Rigakos, G.; Papaemmanoyil, S.; et al. Is There an Independent Role of TERT and NF1 in High Grade Gliomas? Transl. Oncol. 2020, 13, 346–354. [Google Scholar] [CrossRef]
- Gao, K.; Li, G.; Qu, Y.; Wang, M.; Cui, B.; Ji, M.; Shi, B.; Hou, P. TERT Promoter Mutations and Long Telomere Length Predict Poor Survival and Radiotherapy Resistance in Gliomas. Oncotarget 2016, 7, 8712–8725. [Google Scholar] [CrossRef]
- Geng, P.; Zhao, X.; Ou, J.; Li, J.; Sa, R.; Liang, H. TERT Genetic Mutations as Prognostic Marker in Glioma. Mol. Neurobiol. 2017, 54, 3665–3669. [Google Scholar] [CrossRef]
- Chen, C.; Han, S.; Meng, L.; Li, Z.; Zhang, X.; Wu, A. TERT Promoter Mutations Lead to High Transcriptional Activity under Hypoxia and Temozolomide Treatment and Predict Poor Prognosis in Gliomas. PLoS ONE 2014, 9, e100297. [Google Scholar] [CrossRef]
- Simon, M.; Hosen, I.; Gousias, K.; Rachakonda, S.; Heidenreich, B.; Gessi, M.; Schramm, J.; Hemminki, K.; Waha, A.; Kumar, R. TERT Promoter Mutations: A Novel Independent Prognostic Factor in Primary Glioblastomas. Neuro-oncology 2015, 17, 45–52. [Google Scholar] [CrossRef]
- Batista, R.; Cruvinel-Carloni, A.; Vinagre, J.; Peixoto, J.; Catarino, T.A.; Campanella, N.C.; Menezes, W.; Becker, A.P.; de Almeida, G.C.; Matsushita, M.M.; et al. The Prognostic Impact of TERT Promoter Mutations in Glioblastomas Is Modified by the Rs2853669 Single Nucleotide Polymorphism: TERTP Mutations and SNP in GBM Survival. Int. J. Cancer 2016, 139, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Hewer, E.; Prebil, N.; Berezowska, S.; Gutt-Will, M.; Schucht, P.; Dettmer, M.S.; Vassella, E. Diagnostic Implications of TERT Promoter Mutation Status in Diffuse Gliomas in a Routine Clinical Setting. Virchows Arch. 2017, 471, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Nonoguchi, N.; Ohta, T.; Oh, J.-E.; Kim, Y.-H.; Kleihues, P.; Ohgaki, H. TERT Promoter Mutations in Primary and Secondary Glioblastomas. Acta Neuropathol. 2013, 126, 931–937. [Google Scholar] [CrossRef]
- Pekmezci, M.; Rice, T.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Hansen, H.; Sicotte, H.; Kollmeyer, T.M.; McCoy, L.S.; Sarkar, G.; et al. Adult Infiltrating Gliomas with WHO 2016 Integrated Diagnosis: Additional Prognostic Roles of ATRX and TERT. Acta Neuropathol. 2017, 133, 1001–1016. [Google Scholar] [CrossRef]
- Wick, W.; Hartmann, C.; Engel, C.; Stoffels, M.; Felsberg, J.; Stockhammer, F.; Sabel, M.C.; Koeppen, S.; Ketter, R.; Meyermann, R.; et al. NOA-04 Randomized Phase III Trial of Sequential Radiochemotherapy of Anaplastic Glioma With Procarbazine, Lomustine, and Vincristine or Temozolomide. J. Clin. Oncol. 2009, 27, 5874–5880. [Google Scholar] [CrossRef]
- Sturm, D.; Pfister, S.M.; Jones, D.T.W. Pediatric Gliomas: Current Concepts on Diagnosis, Biology, and Clinical Management. JCO 2017, 35, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.; Nuñez, F.M.; Gauss, J.C.; Thompson, S.; Brumley, E.; Lowenstein, P.; Castro, M.G. Hemispherical Pediatric High-Grade Glioma: Molecular Basis and Therapeutic Opportunities. IJMS 2020, 21, 9654. [Google Scholar] [CrossRef]
- Blionas, A.; Giakoumettis, D.; Klonou, A.; Neromyliotis, E.; Karydakis, P.; Themistocleous, M.S. Paediatric Gliomas: Diagnosis, Molecular Biology and Management. Ann. Transl. Med. 2018, 6, 251. [Google Scholar] [CrossRef] [PubMed]
- Salloum, R.; McConechy, M.K.; Mikael, L.G.; Fuller, C.; Drissi, R.; DeWire, M.; Nikbakht, H.; De Jay, N.; Yang, X.; Boue, D.; et al. Characterizing Temporal Genomic Heterogeneity in Pediatric High-Grade Gliomas. Acta Neuropathol. Commun. 2017, 5, 78. [Google Scholar] [CrossRef]
- Paugh, B.S.; Qu, C.; Jones, C.; Liu, Z.; Adamowicz-Brice, M.; Zhang, J.; Bax, D.A.; Coyle, B.; Barrow, J.; Hargrave, D.; et al. Integrated Molecular Genetic Profiling of Pediatric High-Grade Gliomas Reveals Key Differences With the Adult Disease. JCO 2010, 28, 3061–3068. [Google Scholar] [CrossRef]
- Wu, G.; Diaz, A.K.; Paugh, B.S.; Rankin, S.L.; Ju, B.; Li, Y.; Zhu, X.; Qu, C.; Chen, X.; Zhang, J.; et al. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project The Genomic Landscape of Diffuse Intrinsic Pontine Glioma and Pediatric Non-Brainstem High-Grade Glioma. Nat. Genet. 2014, 46, 444–450. [Google Scholar] [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.-Y.; Jones, D.T.W.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.-A.K.; Tönjes, M.; et al. Driver Mutations in Histone H3.3 and Chromatin Remodelling Genes in Paediatric Glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Broniscer, A.; McEachron, T.A.; Lu, C.; Paugh, B.S.; Becksfort, J.; Qu, C.; Ding, L.; Huether, R.; Parker, M.; et al. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project Somatic Histone H3 Alterations in Pediatric Diffuse Intrinsic Pontine Gliomas and Non-Brainstem Glioblastomas. Nat. Genet. 2012, 44, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Sturm, D.; Witt, H.; Hovestadt, V.; Khuong-Quang, D.-A.; Jones, D.T.W.; Konermann, C.; Pfaff, E.; Tönjes, M.; Sill, M.; Bender, S.; et al. Hotspot Mutations in H3F3A and IDH1 Define Distinct Epigenetic and Biological Subgroups of Glioblastoma. Cancer Cell 2012, 22, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.; Garcia-Fabiani, M.B.; Carney, S.; Altshuler, D.; Núñez, F.J.; Méndez, F.M.; Núñez, F.; Lowenstein, P.R.; Castro, M.G. Mutant ATRX: Uncovering a New Therapeutic Target for Glioma. Expert Opin. Ther. Targets 2018, 22, 599–613. [Google Scholar] [CrossRef]
- Kaley, T.; Touat, M.; Subbiah, V.; Hollebecque, A.; Rodon, J.; Lockhart, A.C.; Keedy, V.; Bielle, F.; Hofheinz, R.-D.; Joly, F.; et al. BRAF Inhibition in BRAF V600 -Mutant Gliomas: Results From the VE-BASKET Study. JCO 2018, 36, 3477–3484. [Google Scholar] [CrossRef]
- Mayr, L.; Guntner, A.S.; Madlener, S.; Schmook, M.T.; Peyrl, A.; Azizi, A.A.; Dieckmann, K.; Reisinger, D.; Stepien, N.M.; Schramm, K.; et al. Cerebrospinal Fluid Penetration and Combination Therapy of Entrectinib for Disseminated ROS1/NTRK-Fusion Positive Pediatric High-Grade Glioma. JPM 2020, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Koelsche, C.; Sahm, F.; Capper, D.; Reuss, D.; Sturm, D.; Jones, D.T.W.; Kool, M.; Northcott, P.A.; Wiestler, B.; Böhmer, K.; et al. Distribution of TERT Promoter Mutations in Pediatric and Adult Tumors of the Nervous System. Acta Neuropathol. 2013, 126, 907–915. [Google Scholar] [CrossRef]
- Castelo-Branco, P.; Choufani, S.; Mack, S.; Gallagher, D.; Zhang, C.; Lipman, T.; Zhukova, N.; Walker, E.J.; Martin, D.; Merino, D.; et al. Methylation of the TERT Promoter and Risk Stratification of Childhood Brain Tumours: An Integrative Genomic and Molecular Study. Lancet Oncol. 2013, 14, 534–542. [Google Scholar] [CrossRef]
- Dorris, K.; Sobo, M.; Onar-Thomas, A.; Panditharatna, E.; Stevenson, C.B.; Gardner, S.L.; DeWire, M.D.; Pierson, C.R.; Olshefski, R.; Rempel, S.A.; et al. Prognostic Significance of Telomere Maintenance Mechanisms in Pediatric High-Grade Gliomas. J. Neurooncol. 2014, 117, 67–76. [Google Scholar] [CrossRef]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 2017, 14, 284–297. [Google Scholar] [CrossRef]
- Reis, R.M.; Könü-Lebleblicioglu, D.; Lopes, J.M.; Kleihues, P.; Ohgaki, H. Genetic Profile of Gliosarcomas. Am. J. Pathol. 2000, 156, 425–432. [Google Scholar] [CrossRef]
- Actor, B.; Cobbers, J.M.J.L.; Büschges, R.; Wolter, M.; Knobbe, C.B.; Reifenberger, G.; Weber, R.G. Comprehensive Analysis of Genomic Alterations in Gliosarcoma and Its Two Tissue Components: Genomic Alterations in Gliosarcoma. Genes Chromosom. Cancer 2002, 34, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Ohta, T.; Nonoguchi, N.; Satomi, K.; Capper, D.; Pierscianek, D.; Sure, U.; Vital, A.; Paulus, W.; Mittelbronn, M.; et al. Genetic Alterations in Gliosarcoma and Giant Cell Glioblastoma: Gliosarcoma and Giant Cell Glioblastoma. Brain Pathol. 2016, 26, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Lowder, L.; Hauenstein, J.; Woods, A.; Chen, H.-R.; Rupji, M.; Kowalski, J.; Olson, J.J.; Saxe, D.; Schniederjan, M.; Neill, S.; et al. Gliosarcoma: Distinct Molecular Pathways and Genomic Alterations Identified by DNA Copy Number/SNP Microarray Analysis. J. Neurooncol. 2019, 143, 381–392. [Google Scholar] [CrossRef]
- Smith, D.R.; Wu, C.-C.; Saadatmand, H.J.; Isaacson, S.R.; Cheng, S.K.; Sisti, M.B.; Bruce, J.N.; Sheth, S.A.; Lassman, A.B.; Iwamoto, F.M.; et al. Clinical and Molecular Characteristics of Gliosarcoma and Modern Prognostic Significance Relative to Conventional Glioblastoma. J. Neurooncol. 2018, 137, 303–311. [Google Scholar] [CrossRef]
- Kozak, K.R.; Moody, J.S. Giant Cell Glioblastoma: A Glioblastoma Subtype with Distinct Epidemiology and Superior Prognosis. Neuro-oncology 2009, 11, 833–841. [Google Scholar] [CrossRef]
- Martinez, R.; Roggendorf, W.; Baretton, G.; Klein, R.; Toedt, G.; Lichter, P.; Schackert, G.; Joos, S. Cytogenetic and Molecular Genetic Analyses of Giant Cell Glioblastoma Multiforme Reveal Distinct Profiles in Giant Cell and Non-Giant Cell Subpopulations. Cancer Genet. Cytogenet. 2007, 175, 26–34. [Google Scholar] [CrossRef]
- Cantero, D.; Mollejo, M.; Sepúlveda, J.M.; D’Haene, N.; Gutiérrez-Guamán, M.J.; Rodríguez de Lope, Á.; Fiaño, C.; Castresana, J.S.; Lebrun, L.; Rey, J.A.; et al. TP53, ATRX Alterations, and Low Tumor Mutation Load Feature IDH-Wildtype Giant Cell Glioblastoma despite Exceptional Ultra-Mutated Tumors. Neuro Oncol. Adv. 2020, 2, vdz059. [Google Scholar] [CrossRef] [PubMed]
- Broniscer, A.; Tatevossian, R.G.; Sabin, N.D.; Klimo, P.; Dalton, J.; Lee, R.; Gajjar, A.; Ellison, D.W. Clinical, Radiological, Histological and Molecular Characteristics of Paediatric Epithelioid Glioblastoma: Epithelioid Glioblastoma in Children. Neuropathol. Appl. Neurobiol. 2014, 40, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt-DeMasters, B.K.; Aisner, D.L.; Birks, D.K.; Foreman, N.K. Epithelioid GBMs Show a High Percentage of BRAF V600E Mutation: Am. J. Surg. Pathol. 2013, 37, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, S.; Korshunov, A.; Lai, S.H.; Dabiri, S.; Patil, S.; Li, R.; Shih, C.-S.; Bonnin, J.M.; Baker, J.A.; Du, E.; et al. Epithelioid Glioblastomas and Anaplastic Epithelioid Pleomorphic Xanthoastrocytomas-Same Entity or First Cousins?: Epithelioid GBM and Anaplastic Epithelioid PXA. Brain Pathol. 2016, 26, 215–223. [Google Scholar] [CrossRef]
- Matsumura, N.; Nakajima, N.; Yamazaki, T.; Nagano, T.; Kagoshima, K.; Nobusawa, S.; Ikota, H.; Yokoo, H. Concurrent TERT Promoter and BRAF V600E Mutation in Epithelioid Glioblastoma and Concomitant Low-Grade Astrocytoma: TERT Promoter and BRAF Mutation in E-GBM. Neuropathology 2017, 37, 58–63. [Google Scholar] [CrossRef]
- Korshunov, A.; Chavez, L.; Sharma, T.; Ryzhova, M.; Schrimpf, D.; Stichel, D.; Capper, D.; Sturm, D.; Kool, M.; Habel, A.; et al. Epithelioid Glioblastomas Stratify into Established Diagnostic Subsets upon Integrated Molecular Analysis. Brain Pathol. 2018, 28, 656–662. [Google Scholar] [CrossRef]
- Tesileanu, C.M.S.; Dirven, L.; Wijnenga, M.M.J.; Koekkoek, J.A.F.; Vincent, A.J.P.E.; Dubbink, H.J.; Atmodimedjo, P.N.; Kros, J.M.; van Duinen, S.G.; Smits, M.; et al. Survival of Diffuse Astrocytic Glioma, IDH1/2 Wildtype, with Molecular Features of Glioblastoma, WHO Grade IV: A Confirmation of the CIMPACT-NOW Criteria. Neuro-oncology 2020, 22, 515–523. [Google Scholar] [CrossRef]
- Bajaj, S.; Kumar, M.S.; Peters, G.; Mayur, Y. Targeting Telomerase for Its Advent in Cancer Therapeutics. Med. Res. Rev. 2020, 40, 1871–1919. [Google Scholar] [CrossRef]
- Takahashi, M.; Miki, S.; Fujimoto, K.; Fukuoka, K.; Matsushita, Y.; Maida, Y.; Yasukawa, M.; Hayashi, M.; Shinkyo, R.; Kikuchi, K.; et al. Eribulin Penetrates Brain Tumor Tissue and Prolongs Survival of Mice Harboring Intracerebral Glioblastoma Xenografts. Cancer Sci. 2019, 110, 2247–2257. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Maida, Y.; Yasukawa, M.; Kato, T.; Yoshida, M.; Masutomi, K. Eribulin Mesylate Targets Human Telomerase Reverse Transcriptase in Ovarian Cancer Cells. PLoS ONE 2014, 9, e112438. [Google Scholar] [CrossRef]
- Baerlocher, G.M.; Oppliger Leibundgut, E.; Ottmann, O.G.; Spitzer, G.; Odenike, O.; McDevitt, M.A.; Röth, A.; Daskalakis, M.; Burington, B.; Stuart, M.; et al. Telomerase Inhibitor Imetelstat in Patients with Essential Thrombocythemia. N. Engl. J. Med. 2015, 373, 920–928. [Google Scholar] [CrossRef]
- Marian, C.O.; Cho, S.K.; Mcellin, B.M.; Maher, E.A.; Hatanpaa, K.J.; Madden, C.J.; Mickey, B.E.; Wright, W.E.; Shay, J.W.; Bachoo, R.M. The Telomerase Antagonist, Imetelstat, Efficiently Targets Glioblastoma Tumor-Initiating Cells Leading to Decreased Proliferation and Tumor Growth. Clin. Cancer Res. 2010, 16, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Salloum, R.; Hummel, T.R.; Kumar, S.S.; Dorris, K.; Li, S.; Lin, T.; Daryani, V.M.; Stewart, C.F.; Miles, L.; Poussaint, T.Y.; et al. A Molecular Biology and Phase II Study of Imetelstat (GRN163L) in Children with Recurrent or Refractory Central Nervous System Malignancies: A Pediatric Brain Tumor Consortium Study. J. Neurooncol. 2016, 129, 443–451. [Google Scholar] [CrossRef]
- Mancini, A.; Xavier-Magalhães, A.; Woods, W.S.; Nguyen, K.-T.; Amen, A.M.; Hayes, J.L.; Fellmann, C.; Gapinske, M.; McKinney, A.M.; Hong, C.; et al. Disruption of the Β1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner. Cancer Cell 2018, 34, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Biray Avci, C.; Dogan, F.; Ozates Ay, N.P.; Goker Bagca, B.; Abbaszadeh, Z.; Gunduz, C. Effects of Telomerase Inhibitor on Epigenetic Chromatin Modification Enzymes in Malignancies. J. Cell Biochem. 2018, 119, 9817–9824. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, C.; Venkataswamy, M.M.; Sibin, M.K.; Srinivas Bharath, M.M.; Chetan, G.K. Down Regulation of Human Telomerase Reverse Transcriptase (HTERT) Expression by BIBR1532 in Human Glioblastoma LN18 Cells. Cytotechnology 2018, 70, 1143–1154. [Google Scholar] [CrossRef]
- Negrini, S.; De Palma, R.; Filaci, G. Anti-Cancer Immunotherapies Targeting Telomerase. Cancers 2020, 12, 2260. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Kaneko, S. Telomerase-Targeted Cancer Immunotherapy. IJMS 2019, 20, 1823. [Google Scholar] [CrossRef]
- Vik-Mo, E.O.; Nyakas, M.; Mikkelsen, B.V.; Moe, M.C.; Due-Tønnesen, P.; Suso, E.M.I.; Sæbøe-Larssen, S.; Sandberg, C.; Brinchmann, J.E.; Helseth, E.; et al. Therapeutic Vaccination against Autologous Cancer Stem Cells with MRNA-Transfected Dendritic Cells in Patients with Glioblastoma. Cancer Immunol. Immunother. 2013, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Luo, F.; Tang, C.; Chen, D.; Qin, Z.; Hua, W.; Xu, M.; Zhong, P.; Yu, S.; Chen, D.; et al. Molecular Subgroups and B7-H4 Expression Levels Predict Responses to Dendritic Cell Vaccines in Glioblastoma: An Exploratory Randomized Phase II Clinical Trial. Cancer Immunol. Immunother. 2018, 67, 1777–1788. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, X.; Jin, K.; Zhu, J.; Wang, Y.; Xiong, S.; Mao, Y.; Zhou, L. B7-H4 Is Preferentially Expressed in Non-Dividing Brain Tumor Cells and in a Subset of Brain Tumor Stem-like Cells. J. Neurooncol. 2008, 89, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ye, H.; Qi, Z.; Mo, L.; Yue, Q.; Baral, A.; Hoon, D.S.B.; Vera, J.C.; Heiss, J.D.; Chen, C.C.; et al. B7-H4(B7x)–Mediated Cross-Talk between Glioma-Initiating Cells and Macrophages via the IL6/JAK/STAT3 Pathway Lead to Poor Prognosis in Glioma Patients. Clin. Cancer Res. 2016, 22, 2778–2790. [Google Scholar] [CrossRef] [PubMed]
- Adotévi, O.; Dosset, M.; Galaine, J.; Beziaud, L.; Godet, Y.; Borg, C. Targeting Antitumor CD4 Helper T Cells with Universal Tumor-Reactive Helper Peptides Derived from Telomerase for Cancer Vaccine. Hum. Vaccines Immunother. 2013, 9, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
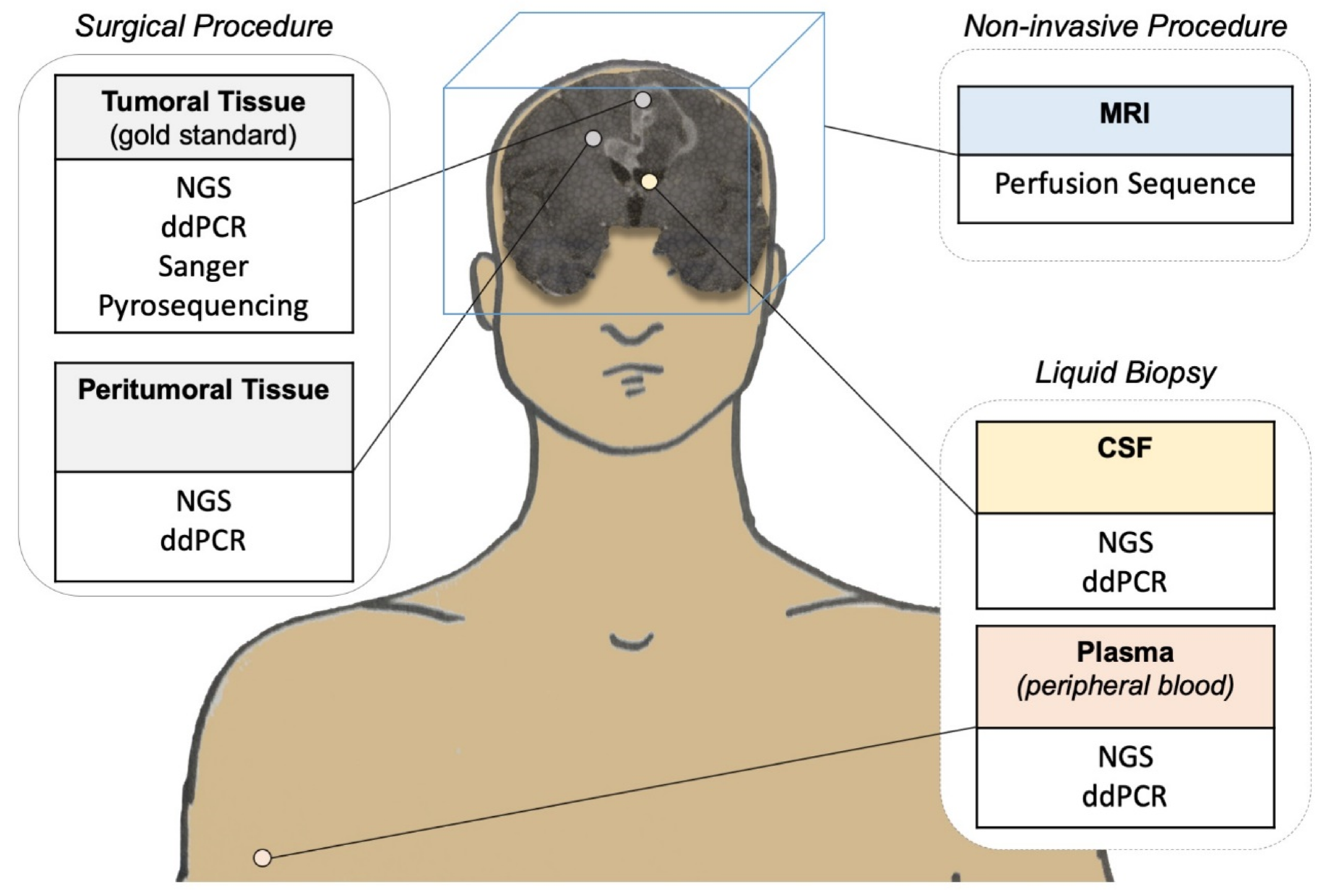

| Method | Population (Number of Patients) | Reference Method | Accuracy | Reference |
|---|---|---|---|---|
| Molecular biology techniques on the tumor | ||||
| NGS targeted panel | 18 glioblastomas | - | 7 TERTp mut/18 (38.9%) | [32] |
| NGS targeted panel | 47 glioblastomas | Sanger sequencing | 30 TERTp mut/47 (64%) Se 99%, Spe 100% | [34] |
| NGS targeted panel | 121 gliomas | Sanger sequencing | 66 TERTp mut/121 Se 100%, Spe 100% | [36] |
| Nanopore | 16 glioblastomas | NGS | Se 100%, Spe 60% | [37] |
| Droplet digital PCR | 52 grade IV gliomas | Sanger sequencing | Se 100%, Spe 100% | [31] |
| Molecular biology techniques on the periphery of the tumor | ||||
| Snapshot | 22 gliomas | NGS | Se 87.5%, Spe 100% | [39] |
| MRI parameters | ||||
| Support Vector Machine | 112 gliomas | Tumor sequencing | Se 85.7%, Spe 54.8% | [40] |
| Spectroscopy | 112 gliomas | Tumor sequencing | Se 83.3%, Spe 95.2% | [41] |
| Dynamic susceptibility contrast- and dynamic contrast-enhanced- MRI | 60 gliomas | Tumor sequencing | Se 56–84%, Spe 53.6%–83.3% | [42] |
| Population (Number of Patients) | TERTp-mut vs. TERTp-wt Glioblastoma (Median Overall Survival, Months) | Independent Factor? | Reference |
|---|---|---|---|
| 453 IDH-wt glioblastomas | 14.6 vs. 18.8 | Uncertain Confounding factor with MGMTp methylation | [14] |
| 303 IDH-wt glioblastomas | 18.5 vs. 17.8, p = 0.3845 | No | [64] |
| 358 glioblastomas (322 [89.9%] IDH-wt) | 9.6 vs. 9.3, p = 0.22 | No Association with IDH mutation | [75] |
| 395 IDH-wt glioblastomas | 13.7 vs. 17.5, p = 0.006 | Uncertain Confounding factor with EGFR amplification | [24] |
| 178 IDH-wt glioblastomas | 11 vs. 16, p = 0.038 | Uncertain Association with tumor resection and exposition to temozolomide | [72] |
| 243 IDH-unknown glioblastomas | 10 vs. 21, p < 0.001 | Uncertain No stratification on IDH statusAssociation with TERT polymorphism rs2853669 | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olympios, N.; Gilard, V.; Marguet, F.; Clatot, F.; Di Fiore, F.; Fontanilles, M. TERT Promoter Alterations in Glioblastoma: A Systematic Review. Cancers 2021, 13, 1147. https://doi.org/10.3390/cancers13051147
Olympios N, Gilard V, Marguet F, Clatot F, Di Fiore F, Fontanilles M. TERT Promoter Alterations in Glioblastoma: A Systematic Review. Cancers. 2021; 13(5):1147. https://doi.org/10.3390/cancers13051147
Chicago/Turabian StyleOlympios, Nathalie, Vianney Gilard, Florent Marguet, Florian Clatot, Frédéric Di Fiore, and Maxime Fontanilles. 2021. "TERT Promoter Alterations in Glioblastoma: A Systematic Review" Cancers 13, no. 5: 1147. https://doi.org/10.3390/cancers13051147
APA StyleOlympios, N., Gilard, V., Marguet, F., Clatot, F., Di Fiore, F., & Fontanilles, M. (2021). TERT Promoter Alterations in Glioblastoma: A Systematic Review. Cancers, 13(5), 1147. https://doi.org/10.3390/cancers13051147






