Simple Summary
Cutaneous squamous cell carcinoma (cSCC) is the second most prevalent skin cancer globally. Immunosuppression raises cSCC incidence rates, while high immunogenicity of the cutaneous tissue enables topical immunotherapy. Intriguingly, expanded applications of programmed death-1 (PD-1) blockade therapies have revealed cSCC to be one of the most amenable targets. These clinical observations prompted us to redefine cSCC biology and review current knowledge about cSCC from multiple viewpoints that involve epidemiology, clinicopathology, molecular genetics, molecular immunology, and developmental biology. This synthesis reinforces the following hypothesis: PD-1 blockade effectively restores the immunity specially allowed to exist within the fully cornified squamous epithelium, that is, the epidermis.
Abstract
Cutaneous squamous cell carcinoma (cSCC) is the second most prevalent skin cancer globally. Because most cSCC cases are manageable by local excision/radiotherapy and hardly become life-threatening, they are often excluded from cancer registries in most countries. Compared with cutaneous melanoma that originates from the melanin-producing, neural crest-derived epidermal resident, keratinocyte (KC)-derived cancers are influenced by the immune system with regards to their pathogenetic behaviour. Congenital or acquired immunosurveillance impairments compromise tumoricidal activity and raises cSCC incidence rates. Intriguingly, expanded applications of programmed death-1 (PD-1) blockade therapies have revealed cSCC to be one of the most amenable targets, particularly when compared with the mucosal counterparts arisen in the esophagus or the cervix. The clinical observation reminds us that cutaneous tissue has a peculiarly high immunogenicity that can evoke tumoricidal recall responses topically. Here we attempt to redefine cSCC biology and review current knowledge about cSCC from multiple viewpoints that involve epidemiology, clinicopathology, molecular genetics, molecular immunology, and developmental biology. This synthesis not only underscores the primal importance of the immune system, rather than just a mere accumulation of ultraviolet-induced mutations but also reinforces the following hypothesis: PD-1 blockade effectively restores the immunity specially allowed to exist within the fully cornified squamous epithelium, that is, the epidermis.
1. Introduction and Overview
Cutaneous Squamous Cell Carcinoma (cSCC) in the Age of Immunotherapy
Cutaneous neoplasms, benign or malign, are defined as aberrantly accumulated patches of mutated or altered cells [1,2]. Key genomic features of malignancy are readily identified in cSCC precursor actinic keratosis (AK) [3], benign squamous neoplasms [4] or even normal aged skin [5]. Clinical observations suggest that host immune responses serve as selective pressure and ultimately shape the outcome of malignant progression [6]. A prime clinical example is demonstrated in organ transplant recipients [7]. Both long-term immunosuppression and the graft-versus-host reaction (GVHR) [7,8]/lichenoid tissue reaction [9,10], which attacks the germinal layer of the epidermis, fuel the development of SCCs. Keratoacanthomas (KAs), which are self-involuting squamous cell epitheliomas, develop in individuals with transforming growth factor-beta (TGF-β) signaling haploinsufficiency [11]. Topical immunotherapy for cSCC precursors is about to become a mechanism-based regimen [12]. These clinical observations clearly delineate the profound impact of the immune system on the cSCC pathology.
Although most cSCC cases are generally nonlethal and manageable with surgical excision or radiotherapy [13], no systemic therapies have been approved for unresectable, life-threatening advanced diseases. Before the emergence of receptor tyrosine kinase inhibitors (RTKIs), classical, platinum-based treatment regimens were a common practice [14]. An epidermal growth factor receptor (EGFR) blockade offers a legitimate, biology-based option but does not warrant durable responses [14]. Cancer immunotherapy had only been implemented in the past decade, beginning with the introduction of immune checkpoint inhibitors (ICIs) into the clinical practice. In the realm of dermatology, ICIs achieve durable responses in advanced cutaneous malignant melanomas, turning the deadly disease into a manageable ailment [15]. Dermatologists have also experienced the consequences of aberrantly activated immune responses, i.e., immune-related adverse events (irAEs) [16], whose cutaneous involvement is frequent [17]. Only recently, the extended application of ICIs has finally made us realize that cSCC could be an amenable target [18,19], providing another reliable treatment option for advanced, potentially lethal cSCCs [18,20]. Because ICIs reactivate immune responses in the peripheral tissue, we try to address outstanding questions, “what makes cSCC an amenable target?,” or “why the skin is highly immunogenic?” This manuscript reviews current knowledge about cSCC from multiple points of view and discuss rational management strategies for this common neoplasm in the age of immunotherapy.
2. Epidemiology of cSCC
Basal cell carcinoma (BCC) and cSCC, which are often referred to as keratinocyte (KC) cancer in aggregation [21], are the most common skin cancers globally, and cSCC is the second most common skin cancer [22]. Because most cSCC cases tend to be metachronous but nonlethal [13,23], this entity is often excluded from cancer registries, and most statistical data are based on surveys or treatment data from subsets of national populations [24]. cSCC commonly arises from its precursor actinic keratosis in sun-damaged skin. The highest incidence is observed in fair-skinned people who have fair eye, skin and hair color, as well as people with inborn errors in melanin synthesis, i.e., oculocutaneous albinism [25]. Based on the cSCC incidence in dark-skinned people (African and Asian heritage), high ambient ultraviolet (UV) radiation (UVR) levels confer greater risk [24]. An Australian systematic review suggested an estimated cSCC incidence of as high as 2% in 2002, with the highest rates recorded in Queensland [26]. A recent registry-based data in the Netherlands revealed incidence rates of 107.6 per 100,000 people–year (PY) for men and 68.7 per 100,000 PY for women, which correspond to US-standardized rates [27]. Given that increasing population ageing is expected to further increase the incidence of cSCC, there is a need for mechanism-based, legitimate management strategies that can accommodate the elderly [22].
3. Clinicopathological Stratification of cSCC
3.1. Clinical and Histopathologic Stratification
The low differentiation capacity of epithelial cells corresponds to the loss of cell polarity and cell–cell adhesion and the gain of invasive and metastatic potential, often accompanied by epithelial–mesenchymal transition (EMT) [28]. At the molecular level, poorly differentiated cSCC (desmoplastic/spindle cell/sarcomatoid variant) acquires expression of the mesenchymal intermediate filament vimentin [29,30] and typically loses the adhesion molecule E-cadherin [29,31,32]. Skin cancer tissue comprises tumor cells and stromal responses, and malignant biological behaviors of skin neoplasms can be differentiated on the basis of appearance [33]. The best example is likely KA, which is also known as molluscum sebaceum [34], or Sabouraud’s ‘button epithelioma’ [35]. KA is a well-differentiated cSCC subtype that exhibits a symmetric, crateriform appearance and a large central keratin plug with pronounced, well-differentiated squamous cell proliferation, and it often displays spontaneous regression [34,35]. Well-differentiated verrucous carcinoma harbors low metastatic potential, whereas highly infiltrative desmoplastic cSCC possesses higher risks of recurrence and metastasis [13].
3.1.1. Factors Associated with Local Recurrence and Metastasis
In general, cSCC carries an excellent prognosis, but a subset of tumors has a high risk of poor outcomes, including metastasis and mortality rates of 3.7 and 2.8%, respectively [36]. Because 70% of deaths are attributable to unresectable locoregional disease rather than distant organ metastases [37], clinicopathological risk stratification and the early detection of lymph node metastases are mandatory [13].
Tumor diameter >2.0 cm is the risk factor most highly associated with disease-specific death, as it confers a 19-fold higher risk of death from cSCC than tumor diameter <2 cm [13]. Tumor depth is also associated with local recurrence and metastasis. Specifically, Breslow thickness >2 mm and invasion into deep tissues (fascia, muscle, perichondrium or periosteum) are known factors [13]. Although the perineural involvement of cSCC is uncommon, large-caliber nerve (>0.1 mm) involvement is associated with nodal metastasis and mortality [13]. Recurrent cSCCs carry a much worse prognosis and a higher risk of spread to regional lymph nodes and distant metastases, as indicated by rates of 45% for ear cSCC and 32% for lip cSCC [13]. cSCCs that arose on particular anatomic sites, such as the ears and lips, have been reported to have local recurrence rates exceeding 10% in the absence of non-Mohs modalities [13]. Scar tissue caused by chronic inflammation, such as leg ulcers, burn scar, radiation dermatitis and discoid lupus, can reportedly elevate the rate of metastasis to 26% [13]. Immunosuppression compromises immunosurveillance and increase the risks of local recurrence and metastasis [13].
3.1.2. Staging Systems for cSCC
Currently, cSCC staging systems from the American Joint Committee on Cancer (AJCC) [38]/Union for International Cancer Control (UICC) [39] staging manual and Brigham and Women’s Hospital (BWH) [40] are available. Because cSCCs have been excluded from the Surveillance, Epidemiology, and End Results tracking program and cancer registries, the AJCC/UICC classification does not have access to such population-based data [36,40]. Moreover, the eighth edition of the AJCC staging system, which entered clinical use in January 2018, includes an updated classification on only head and neck cases [38]. From a practical point of view, stratifying the high-risk group that require further checkup is essential [40]. To address this issue, the BWH system was proposed as an alternative tumuor staging system [40]. Studies on risk stratification performance between the BWH and AJCC systems revealed that the former offers superior distinctiveness, homogeneity and monotonicity [41]. Therefore, the BWH system could avoid the inappropriate upstaging of low-risk cases [36]. Overall, the BWH system appears to be a practical risk-stratification system that offers a legitimate follow-up strategy at the initial time of diagnosis (Table 1).

Table 1.
Summary of the BWH and AJCC eighth edition classification systems for cutaneous squamous cell carcinoma.
4. Etiology of cSCC: Exogenous Factors
As mentioned previously, UVR (both UVB and UVA) is a bona fide major risk factor for cSCC [22,42]. The use of a tanning bed increases the risk of KC cancer in a UV dose-dependent manner. Incremented or occupational sun exposure is more directly related to the incidence of cSCC than to that of BCC [22]. Immunosuppression is another important risk factor, and long-term immunosuppressive therapy in solid-organ transplant recipients increases the risk of cSCC by a factor of 100 compared with that in the general population [42]. Infection by oncogenic human papillomavirus (HPV), which is believed to inhibit DNA repair, is also associated with cSCC [24,43]. Human immunodeficiency virus (HIV) infection decreases peripheral blood CD4 counts and elevates the incidence of KC cancer [44]. Exposure to polyaromatic hydrocarbons or arsenic represents a classic environmental/occupational risk factor [24].
5. Etiology of cSCC: Endogenous Factors
In addition to UVR sensitivities determined by skin types, genetic factors predispose individuals to cSCC. Identified links between genotype and phenotype allow us to understand the pathomechanism of cSCC development. In principle, inborn errors in DNA repairing or immune signaling pathways are predisposing factors for cSCC (Table 2).

Table 2.
Genetic predisposing factors of cutaneous squamous cell carcinoma. Genetically defined conditions are listed.
5.1. Defective DNA Repair
Defective DNA repair impairs genome maintenance and increase the mutational load (Table 2) [45,46]. The classic example is xeroderma pigmentosum, which is caused by the failure of nucleotide excision repair following UV damage [47]. Deficiencies in DNA interstrand cross-link repair cause the bone marrow (BM) failure syndrome Fanconi anemia (FA) [48]. Compromised telomerase function leads to dyskeratosis congenita, which is accompanied by dysplastic nails and oral leukoplakia, and typical progeria features [45,49]. Germline DNA mismatch repair mutations cause Muir–Torre syndrome, which is associated with KAs and sebaceous neoplasia/internal malignancies [50].
5.2. Primary Immunodeficiency
The immune system is the site of various genotoxic stresses that occur during immune system maturation and immune responses [51]; DNA-altering mechanisms are important in the development of T and B cells, as observed in V(D)J recombination, immunoglobulin class switch recombination and the generation of somatic hypermutations [52,53]. Therefore, it is not surprising that primary immunodeficiencies lead to autoimmunity and increased susceptibility to infections or malignancies [51]. Regarding the susceptibility to cSCC, impaired surveillance against oncogenic viruses or mutated KC is considered responsible [51,54].
5.2.1. Epidermodysplasia Verruciformis (EV)
EV is characterized by increased susceptibility to cutaneous beta HPV infection and cSCC [55] in association with the global suppression of adaptive cell-mediated immune responses in the skin [56,57,58,59,60]. Loss-of-function (LOF) mutations in evolutionary conserved transmembrane protein channel-like gene family members EVER1/EVER2 represent the classic predisposing factor [61] that accounts for 75% of cases [55]. In addition to genetic factors [55], patients with severe combined immune deficiency (SCID) [62] or HIV infection [63] can also display EV-like phenotypes. Moreover, EV develops in patients who have undergone BM transplantation, suggesting that non-BM-dependent, innate immune components could be the disease driver. This raises the possibility that an impaired KC-intrinsic innate immune response is responsible for the phenotype [62], as suggested previously [56,57,64]. Accordingly, it was recently uncovered that LOF mutations in calcium and integrin binding 1, which forms a complex with EVER1/EVER2 and inhibits intracellular HPV expansion in KCs, underlie EV phenotypes [65].
5.2.2. GATA Binding Protein 2 (GATA2) Deficiency/Monocytopenia and Mycobacterial infection (MonoMAC) Syndrome
The interleukin-12 (IL-12)/IL-23p40/interferon-gamma axis control adaptive cell-mediated immune responses. Defective functioning of monocyte/B cell/natural killer cell in GATA2 deficiency [66,67] is considered responsible for sporadic MonoMAC syndrome [68].
5.2.3. WHIM Syndrome
Germline gain-of-function (GOF) mutations in C-X-C chemokine receptor type 4 cause warts, hypogammaglobulinemia, infections and myelocathexis (WHIM) syndrome [69]. C-X-C chemokine ligand 8 (CXCL8)/CXCR2-mediated and CXCL12/CXCR4-mediated signaling controls BM release of polymorphonuclear neutrophils (PMNs). GOF mutations in CXCR4 are considered to inhibit the PMN release from BM and compromise primary immune responses [70].
5.2.4. Hyper-IgE Recurrent Infection Syndrome (HIES)
The pathologic characteristics of atopic dermatitis (AD) involve elevated serum IgE levels and impaired cell-mediated immune responses [71], and HIES phenotypes somewhat resemble AD. As recurrent staphylococcal infections in AD implies, in most cases defects in T helper 17 (Th17) cell differentiation [72] underlie HIES pathology. IL-6 signaling [73], its downstream target signal transducer and activator of transcription 3 (STAT3) [74] and retinoic acid receptor-orphan receptor-γt [75,76] promote Th17 cell differentiation. LOF mutations in STAT3 [77], IL-6 receptor [78] and IL-6 signal transducer [79] result in HIES.
5.3. Impaired TGF-β Signalling Pathway
The TGF-β signaling pathway is a fundamental biological pathway that regulates several cellular processes in the skin, including epidermal differentiation [80,81] and carcinogenesis [31,82,83]. TGF-β signaling inhibits DNA synthesis [84], mediates DNA damage responses [85] and suppresses genomic instability [86,87]. The hypomorphic allele of the type I TGF-β receptor TGFBR1*6A is known as a low-penetrance allele that predisposes individuals to breast cancer, ovarian cancer, hematologic malignancies [88] and colorectal cancer [89]. Likewise, LOF mutations in TGFBR1 cause self-healing cSCC-like lesions that resemble KAs in Ferguson–Smith disease (FSD) [90], similarly as the pan-TGF-β–blocking antibody fresolimumab (GC1008) [91,92]. Germline [93]/somatic [94,95] LOF TGFBR2 mutations are associated with tumorigenesis, and advanced cSCCs exhibit low TGFBRII expression levels [31,96]. In principle, TGF-β confers cancer resistance.
FSD in the Context of Cancer Immunoediting
Self-healing multiple squamous epitheliomas in patients with FSD [11], or alternatively TGF-β blockade [91,92], could give us interesting insights. Adaptive immunity restrains cancer cells in a state of dormancy [6]. However, some clones overcome the selective pressure, and resistant clones evolve. This theoretical framework represents cancer immunoediting [6,97]. We try to illustrate FSD in the context of cancer immunoediting.
Germline haploinsufficiency in TGFBR1 (FSD) likely affects cutaneous cancer immunosurveillance. Epidermal Langerhans cells (LCs) present tissue antigens to draining lymph nodes in steady state [98], and the TGF-β signaling pathway maintains epidermal LC networks in mice [99]. Therefore, the first possibility is that patients with FSD exhibit impaired epithelial antigen priming.
TGF-β signaling primarily suppresses cytotoxic T lymphocytes (CTLs)-mediated tumoricidal activity [100]. Conversely, tumor cell-derived TGF-β augments metastatic potential of cSCC [31,82,101], possibly producing an immnosuppressive microenvironment [31]. Therefore, the TGF-β1–rich microenvironment primarily leads to the CD4+ regulatory T cell (Treg)-mediated suppression of tumoricidal activities [100]. Possible mechanisms in FSD are as follows: (i) impaired epidermal LC network maintenance compromises tumor surveillance [102], promotes KC proliferation [80,81] and gives rise to squamous epitheliomas [103]; (ii) impaired Treg effector function augments tumoricidal activity [11]; and (iii) impairment of the autocrine or paracrine loop of TGF-β signaling inhibits EMT or immunoevasion, respectively [31,82,96,101] (Figure 1).
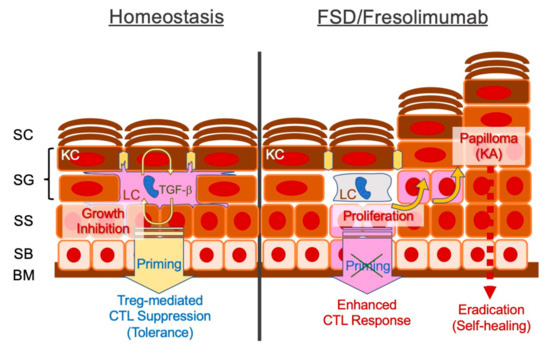
Figure 1.
Schematic representation of defective transforming growth factor-beta (TGF-β) signaling in the epidermis and possible pathomechanisms of self-healing squamous epithelioma observed in Ferguson–Smith disease (FSD) or after treatment with the TGF-β antibody fresolimumab. Epidermal Langerhans cells (LCs) require an autocrine/paracrine loop of TGF-β. LCs establish an intercellular network of differentiated epidermal layers, induce tolerance in steady state and protect against potentially harmful cytotoxic T cell (CTL)-mediated immune responses. Disrupted TGF-β signaling may augment keratinocyte DNA synthesis, facilitating the formation of epitheliomas (papillomas), whereas unleashed CTLs eradicate the tumor. BM, basement membrane; SB, stratum basale; SS, stratum spinosum; SG, stratum granulosum; SC, stratum corneum.
The spontaneous regression of cancers, which is likely mediated through natural resistance [104], is a common phenomenon that has been described for almost a century [104,105,106,107]. However, applying the cancer immunoediting concept [6,97] to TGF-β signaling may provide fascinating insights for clinical cancer research.
6. Beyond Targeted Therapy
6.1. Any Druggable Targets in cSCCs?
Until recently, there had been approved systematic therapy for patients with cSCC. Although EGFR inhibition remains a legitimate, biology-based option with substantial treatment efficacy, a relatively short progression-free survival (<8 months) in a phase 2 trial suggests drug resistance [14].
The personalized medicine concept has been developed in recent decades, and the advent of massive parallel sequencing combined with single-cell technology has accelerated the comprehensive understanding of tumor microenvironments from multiple biological aspects [108,109]. Moreover, multi-omic analyses started to uncover the trajectory of clonal events during cancer evolution [110]. These data-driven biomedical methods may pinpoint a yet-to-be-defined Achilles’ heel of cSCC, in line with the effects of trastuzumab (human epidermal growth factor receptor 2 inhibitor) in breast cancer [111], cetuximab (EGFR inhibitor) in colorectal cancer [112], imatinib (RTKI) in dermatofibrosarcoma protuberans [113] and dabrafenib (BRAF inhibitor)/trametinib (MEK inhibitor) in malignant melanoma [114]. However, investigations to date have revealed that recurrently altered genes [3,115,116,117] (Table 3), as well as the clonal selection, are conserved features of human malignancies. In this chapter, we would like to review the history of melanoma research that has yielded successful therapeutic measures targeting against the mitogen-activated protein kinase (MAPK) signalling cascade [15,114,118,119]. This comparison may facilitate an in-depth understanding of cSCC in the age of immunotherapy.

Table 3.
Recurrently mutated genes in cSCCs.
6.2. Genetic Component of Malignant Melanoma
The melanocyte, uniquely located in the basal epidermal layer, constitutes an important part of protection against UVR by supplying neighboring basal KCs with melanosomes, which are melanin-laden organelles [120]. Melanoma, a neoplasm of transformed melanocytes, has been the subject of intensive research because of its high lethality [121,122]. Inborn errors in genes that control the G1 checkpoint, such as cyclin-dependent kinase inhibitor 2A (CDKN2A), enhance cellular proliferation and result in familial melanomas [123,124,125]. Because high proliferation rates represent a hallmark feature of cancers, this type of germline variant also gives rise to higher incidence rates of non-melanoma malignancies, such as pancreatic cancer (Table 4) [123].

Table 4.
Genetic predisposition factors for cutaneous melanoma. Genetically defined conditions are listed.
6.2.1. MAPK Signaling Cascade and RASopathy
The MAPK signaling cascade regulates a wide range of cellular responses, including cell cycle regulation [126]. Various external stimuli, particularly ligand binding with membrane-bound growth factor receptors, activate MAPK signaling [126]. The RAS oncogene, a GTPase [127], is the first messenger of this intracellular signaling cascade [128]. Upon binding with GTP, RAS then recruits the RAF kinase to the plasma membrane [129], triggering a series of downstream intracellular phosphorylation events [126,127]. It is noteworthy that compared with cancer-prone LOF G1 checkpoint mutations [123,125,130,131], the MAPK signaling cascade is indispensable for normal embryonic development in mice [132,133]. This is in accordance with multi-system developmental anomalies termed RASopathies, which are caused by germline MAPK-activating mutations in humans (Table 5) [134].

Table 5.
The RASopathies. Note that individual germline mutations in the mitogen-activated protein kinase (MAPK) pathway can cause distinctive disease manifestations.
6.2.2. Germline BRAF Mutations and Cardio-Facio-Cutaneous (CFC) Syndrome
CFC syndrome is a RASopathy associated with germline mutations in KRAS [135], BRAF (non-V600E) [135,136] and MAP2K1 (MEK1)/MAP2K2 (MEK2) [136]. Despite the presence of multiple melanocytic nevi (MNs) [134,137], CFC-associated BRAF variants [135,136] do not increase the incidence of melanoma or cSCCs (Table 5). Moreover, CFC-associated germline BRAF variants do not necessarily lead to MAPK activation [136], meaning that the clinical phenotypes do not necessarily reflect the degrees of MAPK signaling cascade activation [136]. This is in stark contrast with cancer-associated constitutively active somatic BRAFV600E mutations [138]. Thus, it is worthwhile to clarify why BRAFV600E is the selfish gene [139] that drives the clonal evolution of transformed melanocytes [140].
6.2.3. Evolutionary Trajectory of Melanocytic Neoplasms
The MN, commonly known as the pigmented mole, is a benign melanocytic neoplasm. Because MNs present heterogeneous histopathologic features, the premise that all MNs are pre-malignant has been a subject of debate among dermatologists and dermatopathologists [141,142,143,144]. In particular, the term “dysplastic nevus (DN),” which was initially given for MNs in patients with familial melanoma [143], has been the subject of debate and the source of confusion for clinical practitioners [141,142]. This dispute finally led to the National Institutes of Health recommendation that DN should no longer be used for histopathological diagnosis in 1992 [145]. However, a molecular genetics study provided important evidence that could reconcile the controversy. Specifically, MNs harbor BRAFV600E mutations with a similar frequency as melanomas [146], suggesting that the accumulation of activating mutation is the early neoplastic event of MN development [147]. Evidence from animal studies further support this notion. For instance, a study using a zebrafish model revealed that the BRAFV600E mutation is sufficient to promote MN formation [148]. It was also demonstrated that the early clonal events (acquisition of BRAFV600E), with concomitant loss of tumor suppressors such as tumor protein 53 (TP53) [148,149] or phosphatase and tensin homolog (PTEN) [150], drive clonal evolution in cooperation with UVR [149]. Recent human studies essentially confirmed these findings. Unequivocally benign MN lesions exclusively harbored the BRAFV600E mutation, whereas the majority of MNs categorized as intermediate were enriched with mutations in NRAS, CDKN2A or telomerase reverse transcriptase [151]. Despite the clonal selection at the earlier stage of progression, PTEN/TP53 mutations were found only in advanced primary melanomas, and copy-number alterations became prevalent in invasive melanomas [151], all of which are the universal features of malignant progression. The comparison between the RASopathies and the trajectory analysis of melanoma reminded us of the antagonistic pleiotropy or the cancer field [104] theories. In short, the tissue homeostasis is the product of well-designed gene expression program. The reverse is also true; the cancer tissue (or developmental disorders) can result either from aberrations in designing (gene sequences) or execution (gene expression).
6.3. Genetic Mosaicism and the Gene Expression Programme
Chromatin regulators are frequently mutated in cancers. These mutations could modify chromatin and thus reprogram gene expression [152]. These adaptive, plastic but heritable cellular responses are indispensable for the development of organs [153], senescence [154] and immune responses [155], as well as cancers [156]. In this light, it was experimentally demonstrated that the neural crest progenitor transcription factor sex-determining region Y-box 10 converts BRAFV600E-expressing melanocytes (MNs) into melanomas [157]. Melanoma with BRAFV600E/K (or other mutations) are sensitive to the targeted therapy because of these specific activating mutations that are not typically found in cSCC [158]; while they can be found in colon cancer [159], it is true that the activation of other pathways such as EGFR are responsible for lack of activity for single agent BRAF targeted therapies.
A previous report showed that the BRAF inhibitor vemurafenib provokes the development of cSCCs/KAs because of the paradoxical activation of MAPK located distally [158]. Mutations in RAS oncogene promote cellular transformation fueled by the acquisition of cell cycle-altering passenger mutations [158]. Because up to 60% of the squamous epitheliomas are considered to harbor MAPK-activating HRASQ61L mutations [158], pr-eexisting KC mutations could determine the outcome (Figure 2). Mechanistically, when EGFR-RAS signaling is activated, inhibitor binding induces conformational changes in the RAF kinase domain, which in turn causes the wild-type RAF isoform to dimerize, translocalize to the membrane, and interact with RAS-GTP [160]. Although these observations clearly illustrate the dependence on specific oncogenes in specific epidermal cell-lineages, caution should be exercised to avoid the oversimplification. Let us take developmental disorders caused by the genetic mosaicism of oncogenes, as with germline mutations in the RASopathy, somatic mutations in the developmental pathway can render very different consequences. Postzygotic HRAS/KRAS mutations produce the organoid nevus (benign hamartoma) called nevus sebaceous, and more severe consequences can manifest as the developmental anomaly termed Schimmelpenning syndrome, in which aberrations in the ectodermal development are thought to cause cerebral, ocular and skeletal defects [161]. By analogy, somatic mutations in oncogenic fibroblast growth factor receptor 3 (FGFR3) or phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) result in seborrheic keratosis (senile wart) [4] or epidermal nevi [162]. Recurrent somatic mutations in the PIK3-AKT signaling pathway that affect the cortical development result in hemimegalencephaly [163].
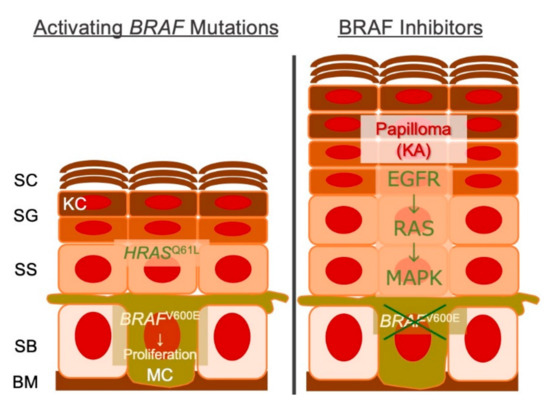
Figure 2.
Schematic representation cSCC/KAs secondary to BRAF inhibition. Melanocytes with BRAFV600E/K (or other mutations) are sensitive to the targeted therapy, whereas KC with HRASQ61L (or other mutations) are not. BM, basement membrane; SB, stratum basale; SS, stratum spinosum; SG, stratum granulosum; SC, stratum corneum; KC, keratinocyte; MC, melanocyte; KA, keratoacanthoma.
Lineage tracing studies have clearly illustrated the importance of the cell lineage of KC cancers. Although both cSCC and BCC are neoplasms of KC origin [21], their biological behaviors significantly differ from each other. BCC depends on the Sonic Hedgehog (SHH) pathway for its emergence [164,165], whereas cSCCs are dependent on EGFR-RAS signaling [158]. Forced overexpression of KRasG12D in the interfollicular epidermis or hair follicle (HF) bulge stem cells produce papillomas, whereas that in the SHH-secreting HF matrix cells does not [166]. Discrete KC cancer lineages thus influence treatment efficacy for BCC, which makes a less amenable ICI target than cSCCs [167,168,169]. Therefore, as successful cross-talk between stem cells and the microenvironment (niche) determines the outcome of organoid structure development [170], the outcome of malignant progression [171] requires such gene–microenvironment interactions [139].
7. Immune Checkpoint Inhibition for cSCC
7.1. PD-1 Blockade For cSCC
The introduction of ICIs targeting the programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway or cytotoxic T-lymphocyte antigen 4 (CTLA-4) launched a revolution in anti-cancer therapy [16]. This strategy embodies the concept that the host’s exhausted pre-existing anti-tumor immunity can be reactivated. In particular, PD-1/PD-L1 blockade is considered to activate CTL-mediated tumoricidal responses at the effector phase and exhibit efficacy against myriad malignancies [172,173].
The first clinical report describing the efficacy of ICIs involved a case of metastatic cSCC in the lungs in an allogeneic renal transplant recipient [174]. Following the administration of the anti-PD-1 antibody (PD-1Ab) nivolumab, the patient experienced an 85% reduction in the tumor burden at the expense of steroid-refractory severe allograft rejection [174]. Subsequently, a case of locally advanced cSCC with nearly complete tumor regression after four cycles of treatment with the PD-1Ab pembrolizumab was reported [175]. This remarkable efficacy was reproducible in a case series in which five of six patients (83%) experienced a clinical response [176]. A phase 2 study of the PD-1Ab cemiplimab in a cohort of patients with locally advanced or metastatic cSCC revealed objective response (OR) rates of 44% [18] and 34.3–47% [19,177], respectively. A recently published case series has also proven similar findings, with OR rates of 34% [178]. Eastern cooperative oncology group performance status, rather than concurrent immunosuppression, affected the efficacy [178]. Overall, ICIs could offer promising therapeutic efficacy for advanced cSCC compared with the effects of conventional systemic therapy [179].
7.2. What Makes cSCC an Amenable Target for PD-1 Blockade?
UV exposure results in a high tumor mutational burden (TMB) and likely increases the susceptibility of cSCCs to ICI, as suggested by numerous studies of various malignancies [180,181,182,183,184,185]. Nevertheless, the efficacy of PD-1 blockade for cSCCs is unusually high compared to the OR rates of phase 2/3 studies of non-cutaneous SCCs, including cancers of the head and neck (13.3%) [186], esophagus (17–19%) [187,188], cervix (12.2%) [189] and lungs (20%) [190] (Table 6). This difference becomes more evident in primary studies in which ICI-treated cSCCs were analyzed in sub-clusters. The OR rate for cSCC lesions goes down from 50 to 56.7% to 24%, when the same regional sites have undergone two or more surgical procedures [18,191].

Table 6.
The outcomes of clinical trials of programmed death-1 (PD-1) blockade for squamous cell carcinomas (SCCs).
Clinical observations suggest that multiple cSCC recurrence episodes are associated with poorer clinical outcomes [13,192,193], including ICI resistance [18]. This presumably is closely associated with EMT [28] or immunoevasion [31,82]. Therefore, the presence of the so-called immunologically cold microenvironment [194,195] could compromise the host’s optimal tumoricidal activity, which needs to be reactivated in situ [16,196]. Contrarily, epidermal KCs, from which cSCC develop [166], could induce high immunogenicity.
7.2.1. Immune–Anatomical Principle of the Squamous Epithelium
The skin epidermis is a stratified squamous epithelium facing an arid, harsh terrestrial environment. Terrestrial amniotes are armed with a specialized barrier, namely the stratum corneum (SC), to cope with such an essential requirement [197]. The major threat to the human epidermis is desiccation. We develop dry, scaly skin during the dry winter season, and skincare moisturizers alleviate such symptoms. Analogously, epidermal KCs produce lipids that prevent desiccation, and congenital defects in this machinery can sometimes manifest as severe, plate-like ichthyotic hyperkeratosis. The other important but less conspicuous feature compared to desiccation tolerance is structural integrity, which is maintained by the sulfur-rich proteinous deposition formed at the cell periphery, termed cornified cell envelopes (CEs) [198]. CEs stabilize the cytoskeleton and protect against myriad noxious and genotoxic stimuli, such as UVR [199]. Thus, akin to the mortar and the brick model, terminal differentiation of the epidermis, i.e., cornification, leads to a functional dichotomy in the SC [200]. Because defective epidermal differentiation is a hallmark feature of malignant progression [13] that reduces the response to PD-1 blockade [18], we started to suspect that the superior outcome of PD-1 blockade in patients with cSCCs may be attributable to the primary location of the tumor, namely the skin, which is covered by the SC [201]. Clinical observations could corroborate this notion, as the skin is often a target of GVHR, [7] drug toxicity [202] and irAEs [16,17,203], all of which are associated with extremely strong CTL-mediated immune responses.
7.2.2. Contact Allergy and Topical Immunotherapy
Perhaps the epitome of such cutaneous CTL responses is contact hypersensitivity (CHS), which models the allergic contact dermatitis and utilizes both perforin/granzyme and Fas/Fas-ligand apoptotic pathways as effectors [204]. The mucosal tissue does not exhibit CHS but rather induces tolerance in a well-known immune–anatomical principle [205]. Given that the oral cavity, esophagus, vagina, rectum, anterior chamber of the eyes and epidermis are all covered by stratified squamous epithelium, it may not necessarily be illogical to infer that the unusually high immunogenicity of the dry-surfaced squamous epithelium could be attributable to the presence or absence of the SC [201]. An important fact is that the SC expresses the primary cytokine IL-1α [206]. This idea initially stemmed from clinical observations that the SC causes sterile inflammation [207], as observed in ruptured epidermal cysts or cystic acne [208]. Subsequently, it was demonstrated that SC extract exhibits high co-stimulatory activity and induces pyrexia/neutrophilia when intravenously injected into mice [209]. Therefore, epidermal differentiation (cornification) [200] appears to confer immunogenicity in the earliest afferent phase of local inflammatory responses [209], including CHS [210,211]. This may be an important explanation why topical therapy is feasible for cSCC (precursors) [12]. Alternatively, recent evidence regarding LC ontogeny may provide additional insight. Although LCs reside in squamous epithelia and exhibit similar transcriptomic signatures and functions [212], their ontogenic trajectories substantially differ depending on the niches (the epidermis or the squamous mucosa) [212] or the context (UV-damaged vs. steady-state epidermis) [213]. Therefore, it could be inferred that the epidermal differentiation program yields a fully cornified stratified squamous epithelium and renders superior immunogenicity through taking advantage of mononuclear phagocyte system’s plasticity [201,214].
8. Overcoming Immune Resistance
8.1. Microenvironmental Factors for Efficient Immune Checkpoint Blockade
It is inarguable that the pleiotropic cytokine TGF-β is one of the most important microenvironment-derived soluble factors in almost every aspect of cSCC pathology in that it initiates DNA damage responses [85], promotes EMT, [31,82,215], causes immunoevasion [31,216] and confers resistance to ICIs [18], as discussed previously.
The immunoedited [6,97] or immunologically cold microenvironment [194,195] could compromise the host’s optimal tumoricidal activity, which must be reactivated in situ [16,196]. Because successful PD-1 blockade requires a pre-existing immunologically hot microenvironment, cSCCs with multiple local recurrences are associated with poorer outcomes [18,191]. We conclude that the malignant behavior of tumor cells highly depends on the surrounding microenvironment or niche, which is the embodiment of the gene expression program [153,217,218,219]. Epigenomic changes could ultimately lead to the accumulation of ‘selfish’ genes [29,139], such as HRAS/KRAS [220] or BRAFV600E [138], through altering gene expression program.
8.2. TGF-β Signalling Blockade
Despite their low TMB, cSCCs arise in sites of chronic inflammation, such as burn scars (Marjolin’s ulcer) or autosomal recessive dystrophic epidermolysis bullosa (RDEB) lesions, and these cancers are often invasive and metastatic [221]. In patients with RDEB, the absence of epidermal–dermal adhesion causes repeated episodes of scarring inflammation, which leads to epithelial migration/proliferation, fibrosis and extracellular matrix (ECM) remodeling while promoting the evolution of clones distinct from UV-associated cSCC [222]. In particular, the TGF-β signaling pathway plays a significant role in RDEB pathology by modulating ECM remodeling through cell–cell contact [223]. RDEB–cSCC cells are dependent on this intracellular signaling [224]. Despite the presence of EMT/immunoevasion in RDEB–cSCC cells [31,82], recent clinical observations suggest that PD-1 blockade holds promise [20,225]. However, this might not be the case if the microenvironment (niche) allows RDEB-cSCC cells to lose lineage commitment.
Recent clinical observations suggest that high mutational loads do not necessarily define the likelihood of response to PD-1 blockade in locally advanced/unresectable cSCCs [18,191] or metastatic melanomas [226,227]. This is in line with the fact that successful PD-1 blockade significantly alters gene expression programs [219,228,229] in the microenvironment (niche), in which immune responses arise [172,173]. Therefore, oncologists need to overcome the immunologically cold CTL-excluding microenvironment [228,229]. It is also known that suboptimal responses to PD-1 blockade are associated with TGF-β signaling signatures [100,228,230], supporting the legitimacy of manipulating the tissue factor [231,232]. Because most patients with cSCC die from poorly controlled local disease, rather than systemic metastatic spread [37], this approach will be of substantial benefit to such patients. Indeed, the angiotensin II type 1 receptor antagonist losartan counteracts the TGF-β signaling pathway, reduces ECM remodeling/fibrosis and ameliorates RDEB-associated cutaneous symptoms in mice [223]. However, systemic blockade of TGF-β could run the risk of severe autoimmune episodes given the phenotype of TGF-β–deficient mice [233].
TGF-β is stored as a pro–TGF-β precursor, and multiple post-translational modifications activates the TGF-b signaling [100]. Because immunosuppressive Tregs characteristically produce TGF-β upon T cell receptor stimulation [234], blockade of this immunosuppressive circuit [100] represents a legitimate approach to overcome the immune resistance program [219,228,229]. A promising approach is targeting a receptor for latent TGF-β, the biologically inactive form of TGF-β [100]. Antibodies raised against the membrane protein glycoprotein A repetitions predominant (GARP) inhibited intratumoral the Treg production of TGF-β and successfully eradicated PD-1 blockade-resistant tumors in mice [235]. A clinical trial of anti-GARP antibodies is currently underway [235].
9. Conclusions
Recent translational evidence revealed that dysregulated gene expression programs [152], rather than the mutational landscape per se [1,236], could define cancer tissue and immune responses [219,228,229]. Cell lineages/fates determine the development of a given structure [218] that subsequently tailors immune responses [231,232]. Analogously, cSCC, which arises from KCs upon lineage commitment for the fully cornified epidermis [166], is a more amenable target for PD-1 blockade than mucosal SCC or BCC [186,187,188,189]. By extension, the differentiation treatment of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid) [237] or acute myeloid leukemia with inhibitors of FMS-like tyrosine kinase 3 [238] reprograms gene expression. However, this sometimes provokes the differentiation syndrome. Excessive blood neutrophil production predicts a poorer clinical outcome both in differentiation therapies [239] and PD-1 blockade [240]. Therefore, targeted therapeutic measures in the next generation need to effectively divert the cancer gene expression program, as has been proved in PD-1 blockade [219,228,229].
DNA/histone modifications, which are located distally to the genome sequences, highly influence gene expression programs. Since the discovery of DNA methylation in 1980 [241], the epigenetics has been the subject of intensive investigations and potentially makes an attractive potential therapeutic target [242]. Extensive global reprogramming of epigenetic patterns, such as gain/loss in DNA methylation or changes to histone marks (acetylation/phosphorylation), characterize malignancies [243]. At the DNA level, hypermethylation of GC-rich promoter sequences can downregulate tumor suppressor genes. At the histone level, hyperacetylation/hypomethylation loosens the chromatin structure, leading to the chromosomal instability. Although hypermethylation in cancer genomes alter histone modifications and thus gene expression programs, the efficacy of DNA methyltransferase inhibitors was not as striking as expected in myeloproliferative disorders [242]. Whether this is also the case with solid tumors remains unclear to date [244]. Epigenetic modification of T cell, as well as tumor cell could another attractive way to overcome the immune resistance [245]. It is already known that PD1 gene promoter demethylation is imprinted during the effector phase of CTL exhaustion in mice [246], and the chromatin accessibility of circulating CD8+ T cells [247] or CTLA4 methylation [248] determines the outcome of ICI in humans. Preclinical studies have shown that low-dose administration of the demethylating agent decitabine rejuvenates the cytotoxic activity and overcomes immunosuppression associated with chronic viral infection [249]. Although these pieces of evidence hold promise for epigenetic therapies in combination with ICIs, the key for successful intervention would depend on the timing and the circumstances [218]. The successful clinical application of this legitimate, mechanism-based disease control strategy may await additional measures, such as efficient and reproducible biomonitoring or accurate drug delivery.
By reviewing the current knowledge about cSCC from multiple perspectives, we realize that cellular immune responses are the key to effective cancer immunosurveillance. As HIES denotes, AD is characterized by broad defects in the epidermal differentiation program (cornification) [250], which potentially fails to imprint the innate immunological memory (CHS) [155,201,210,214,251]. Although this hypothesis is largely based on inference at the moment [201,214], future translational studies based on the important lessons from the bedside may uncover the exact mechanism involving the epimmunome [252] or epidermal immune microenvironment [253].
Funding
This research was supported in part by the following JSPS KAKENHI Grant, Early-Career Scientists (18K16018 to YI). The authors state no conflict of interest.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| AD | atopic dermatitis |
| AJCC | American Joint Committee on Cancer |
| AJCC8 | 8th Edition of AJCC staging system |
| AK | actinic keratosis |
| BCC | Basal cell carcinoma |
| BM | bone marrow |
| BWH | Brigham and Women’s Hospital |
| CDKN2A | cyclin-dependent kinase inhibitor 2A |
| CE | cornified cell envelope |
| CFC | Cardio-Facio-Cutaneous |
| CHS | contact hypersensitivity |
| CIB1 | calcium and integrin binding 1 |
| cSCC | cutaneous squamous cell carcinoma |
| CTL | cytotoxic T lymphocytes |
| CTLA-4 | cytotoxic T-lymphocyte antigen 4 |
| CXCR | C-X-C chemokine receptor |
| DN | dysplastic nevus |
| ECM | extracellular matrix |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial-mesenchymal transition |
| EV | epidermodysplasia verruciformis |
| FA | Fanconi anaemia |
| FLT3 | FMS-like tyrosine kinase 3 |
| FSD | Ferguson-Smith disease |
| GARP | glycoprotein A repetitions predominant |
| GATA2 | GATA binding protein 2 |
| GOF | gain-of-function |
| GTP | guanosine triphosphate |
| GVHR | graft-versus host reaction |
| HER2 | human epidermal growth factor receptor 2 |
| HIES | hyper-IgE recurrent infection syndrome |
| HIV | human immunodeficiency virus |
| HPV | human papilloma virus |
| ICI | immune checkpoint inhibitor |
| interleukin | IL |
| irAE | immune-related adverse event |
| KA | keratoacanthoma |
| KC | keratinocyte |
| LC | Langerhans cell |
| LOF | Loss-of-function |
| MAPK | mitogen-activated protein kinase |
| MEK1 | MAP2K1 |
| MEK2 | MAP2K2 |
| MN | melanocytic nevus |
| MonoMAC | monocytopenia and mycobacterial infection |
| NOTCH1 | notch receptor 1 |
| OCA | oculocutaneous albinism |
| OR | objective responses |
| PD-1 | programmed death-1 |
| PD-1Ab | anti-PD-1 antibody |
| PD-L1 | programmed death-ligand 1 |
| PID | primary immunodeficiency |
| PMN | polymorphonuclear neutrophil |
| PTEN | phosphatase and tensin homolog |
| PY | people/year |
| R | receptor |
| RDEB | recessive dystrophic epidermolysis bullosa |
| RTKI | receptor tyrosine kinase inhibitors |
| SC | stratum corneum |
| SCID | severe combined immune deficiency |
| SEER | Surveillance, Epidemiology, and End Results |
| SHH | Sonic Hedgehog |
| SOX10 | sex determining region Y-box 10 |
| ST | signal transducer |
| STAT | signal transducer and activator of transcription |
| TERT | telomerase reverse transcriptase |
| TGF-b | transforming growth factor beta |
| Th | T helper |
| TMB | tumour mutational burden |
| TP53 | tumour protein 53 |
| TYK2 | tyrosine kinase 2 |
| UICC | Union for International Cancer Control |
| UV | ultraviolet |
| UVR | UV radiation |
| WHIM | Warts, Hypogammaglobulinemia, Infections, Myelocathexis |
References
- Tomasetti, C. Mutated clones are the new normal. Science 2019, 364, 938–939. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.C.; Torres, M.; Real, F.X. Somatic mosaicism: On the road to cancer. Nat. Rev. Cancer 2016, 16, 43–55. [Google Scholar] [CrossRef]
- Chitsazzadeh, V.; Coarfa, C.; Drummond, J.A.; Nguyen, T.; Joseph, A.; Chilukuri, S.; Charpiot, E.; Adelmann, C.H.; Ching, G.; Nguyen, T.N.; et al. Cross-species identification of genomic drivers of squamous cell carcinoma development across preneoplastic intermediates. Nat. Commun. 2016, 7, 1–17. [Google Scholar] [CrossRef]
- Hafner, C.; López-Knowles, E.; Luis, N.M.; Toll, A.; Baselga, E.; Fernández-Casado, A.; Hernández, S.; Ribé, A.; Mentzel, T.; Stoehr, R.; et al. Oncogenic PIK3CA mutations occur in epidermal nevi and seborrheic keratoses with a characteristic mutation pattern. Proc. Natl. Acad. Sci. USA 2007, 104, 13450–13454. [Google Scholar] [CrossRef] [PubMed]
- Yizhak, K.; Aguet, F.; Kim, J.; Hess, J.M.; Kübler, K.; Grimsby, J.; Frazer, R.; Zhang, H.; Haradhvala, N.J.; Rosebrock, D.; et al. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science 2019, 364, eaaw0726. [Google Scholar] [CrossRef] [PubMed]
- Koebel, C.M.; Vermi, W.; Swann, J.B.; Zerafa, N.; Rodig, S.J.; Old, L.J.; Smyth, M.J.; Schreiber, R.D. Adaptive immunity maintains occult cancer in an equilibrium state. Nat. Cell Biol. 2007, 450, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Sarantopoulos, S.; Cardones, A.R.; Sullivan, K.M. How I treat refractory chronic graft-versus-host disease. Blood 2019, 133, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.E.; Metayer, C.; Rizzo, J.D.; Socié, G.; Sobocinski, K.A.; Flowers, M.E.D.; Travis, W.D.; Travis, L.B.; Horowitz, M.M.; Deeg, H.J. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: An international case-control study. Blood 2005, 105, 3802–3811. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer, R.D. Lichenoid Tissue Reaction/Interface Dermatitis: Clinical and Histological Perspectives. J. Investig. Dermatol. 2009, 129, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Regauer, S.; Reich, O.; Eberz, B. Vulvar cancers in women with vulvar lichen planus: A clinicopathological study. J. Am. Acad. Dermatol. 2014, 71, 698–707. [Google Scholar] [CrossRef]
- Goudie, D.R.; D’Alessandro, M.; Merriman, B.; Lee, H.; Szeverényi, I.; Avery, S.; O’Connor, B.D.; Nelson, S.F.; Coats, S.E.; Stewart, A.; et al. Multiple self-healing squamous epithelioma is caused by a disease-specific spectrum of mutations in TGFBR. Nat. Genet. 2011, 43, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Tabacchi, M.; Eliane, J.-P.; Tuchayi, S.M.; Manivasagam, S.; Mirzaalian, H.; Turkoz, A.; Kopan, R.; Schaffer, A.; Saavedra, A.P.; et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J. Clin. Investig. 2016, 127, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Petrow, P.; Scheer-Senyarich, I.; Duvillard, P.; Lacroix, L.; Gelly, J.; Certain, A.; Duval, X.; Crickx, B.; Buffard, V.; et al. Phase II Study of Cetuximab as First-Line Single-Drug Therapy in Patients With Unresectable Squamous Cell Carcinoma of the Skin. J. Clin. Oncol. 2011, 29, 3419–3426. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Postow, M.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Ogawa, T.; Ishitsuka, Y.; Saito, A.; Nakamura, Y.; Watanabe, R.; Okiyama, N.; Fujisawa, Y.; Fujimoto, M. Immune microenvironment controls the outcome of PD-1 blockade in cutaneous immune response. Allergy 2019, 74, 2257–2261. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Grob, J.-J.; Gonzalez, R.; Basset-Seguin, N.; Vornicova, O.; Schachter, J.; Joshi, A.; Meyer, N.; Grange, F.; Piulats, J.M.; Bauman, J.R.; et al. Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629). J. Clin. Oncol. 2020, 38, JCO1903054. [Google Scholar] [CrossRef] [PubMed]
- Piccerillo, A.; El Hachem, M.; De Vito, R.; De Luca, E.V.; Peris, K. Pembrolizumab for Treatment of a Patient with Multiple Cutaneous Squamous Cell Carcinomas and Recessive Dystrophic Epidermolysis Bullosa. JAMA Dermatol. 2020, 156, 708. [Google Scholar] [CrossRef]
- Karimkhani, C.; Boyers, L.N.; Dellavalle, R.P.; Weinstock, M.A. It’s time for “keratinocyte carcinoma” to replace the term “nonmelanoma skin cancer”. J. Am. Acad. Dermatol. 2015, 72, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Nehal, K.S.; Bichakjian, C.K. Update on Keratinocyte Carcinomas. N. Engl. J. Med. 2018, 379, 363–374. [Google Scholar] [CrossRef]
- Niijima, Y.; Ishitsuka, Y.; Inoue, S.; Maruyama, H.; Nakamura, Y.; Fujisawa, Y.; Fujimoto, M. Reconstruction using a vertical “sagging cheek” advancement flap for defects following full-thickness excision of non-melanoma skin cancer in the elderly: A case series. Eur. J. Dermatol. EJD 2019, 29, 654–656. [Google Scholar] [CrossRef]
- Green, A.; Olsen, C. Cutaneous squamous cell carcinoma: An epidemiological review. Br. J. Dermatol. 2017, 177, 373–381. [Google Scholar] [CrossRef]
- Nikolaou, V.; Stratigos, A.J.; Tsao, H. Hereditary Nonmelanoma Skin Cancer. Semin. Cutan. Med. Surg. 2012, 31, 204–210. [Google Scholar] [CrossRef]
- Perera, E.; Gnaneswaran, N.; Staines, C.; Win, A.K.; Sinclair, R. Incidence and prevalence of non-melanoma skin cancer in Australia: A systematic review. Australas. J. Dermatol. 2015, 56, 258–267. [Google Scholar] [CrossRef]
- Tokez, S.; Hollestein, L.; Louwman, M.; Nijsten, T.; Wakkee, M. Incidence of Multiple vs First Cutaneous Squamous Cell Carcinoma on a Nationwide Scale and Estimation of Future Incidences of Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2020, 156, 1300. [Google Scholar] [CrossRef] [PubMed]
- Tarin, D. The Fallacy of Epithelial Mesenchymal Transition in Neoplasia. Cancer Res. 2005, 65, 5996–6001. [Google Scholar] [CrossRef]
- Nassar, D.; Latil, M.; Boeckx, B.; Lambrechts, D.; Blanpain, C. Genomic landscape of carcinogen-induced and genetically induced mouse skin squamous cell carcinoma. Nat. Med. 2015, 21, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, S.; Saida, T.; Takizawa, Y.; Tokuda, Y.; Ito, T.; Fujioka, F.; Sakaki, T.; Uchida, N.; Arase, S.; Takeda, K. Vimentin-positive squamous cell carcinoma arising in a burn scar. A highly malignant neoplasm composed of acantholytic round keratinocytes. Arch. Dermatol. 1989, 125, 1672–1676. [Google Scholar] [CrossRef]
- Han, G.; Lu, S.-L.; Li, A.G.; He, W.; Corless, C.L.; Kulesz-Martin, M.; Wang, X.-J. Distinct mechanisms of TGF- 1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J. Clin. Investig. 2005, 115, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Fuller, L.C.; Allen, M.H.; Montesu, M.; Barker, J.N.; Macdonald, D.M. Expression of E-cadherin in human epidermal non-melanoma cutaneous tumours. Br. J. Dermatol. 1996, 134, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.B. A Philosophy of Practice of Surgical Pathology: Dermatopathology as Model; Ardor Scribendi: New York, NY, USA, 1999. [Google Scholar]
- MacCormac, H.; Scarff, R.W. Molluscum Sebaceum. Br. J. Dermatol. 1936, 48, 624–626. [Google Scholar] [CrossRef]
- Rook, A.; Whimster, I. Keratoacanthoma—A thirty year retrospect. Br. J. Dermatol. 1979, 100, 41–47. [Google Scholar] [CrossRef]
- Ruiz, E.S.; Karia, P.S.; Besaw, R.; Schmults, C.D. Performance of the American Joint Committee on Cancer Staging Manual, 8th Edition vs the Brigham and Women’s Hospital Tumor Classification System for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2019, 155, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Eigentler, T.K.; Leiter, U.; Häfner, H.-M.; Garbe, C.; Röcken, M.; Breuninger, H. Survival of Patients with Cutaneous Squamous Cell Carcinoma: Results of a Prospective Cohort Study. J. Investig. Dermatol. 2017, 137, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Edge, S.B. AJCC Cancer Staging Manual, 8th ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM classification of malignant tumours—towards common understanding and reasonable expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef]
- Jambusaria-Pahlajani, A.; Kanetsky, P.A.; Karia, P.S.; Hwang, W.-T.; Gelfand, J.M.; Whalen, F.M.; Elenitsas, R.; Xu, X.; Schmults, C.D. Evaluation of AJCC Tumor Staging for Cutaneous Squamous Cell Carcinoma and a Proposed Alternative Tumor Staging System. JAMA Dermatol. 2013, 149, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Karia, P.S.; Jambusaria-Pahlajani, A.; Harrington, D.P.; Murphy, G.F.; Qureshi, A.A.; Schmults, C.D. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital Tumor Staging for Cutaneous Squamous Cell Carcinoma. J. Clin. Oncol. 2014, 32, 327–334. [Google Scholar] [CrossRef]
- Madeleine, M.; Patel, N.; Plasmeijer, E.; Engels, E.; Bavinck, J.B.; Toland, A.; Green, A.; the Keratinocyte Carcinoma Consortium (KeraCon) Immunosuppression Working Group. Epidemiology of keratinocyte carcinomas after organ transplantation. Br. J. Dermatol. 2017, 177, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Arron, S.T.; Ruby, J.G.; Dybbro, E.; Ganem, D.; DeRisi, J.L. Transcriptome Sequencing Demonstrates that Human Papillomavirus Is Not Active in Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2011, 131, 1745–1753. [Google Scholar] [CrossRef]
- Asgari, M.M.; Ray, G.T.; Quesenberry, C.P.; Katz, K.A.; Silverberg, M.J. Association of Multiple Primary Skin Cancers with Human Immunodeficiency Virus Infection, CD4 Count, and Viral Load. JAMA Dermatol. 2017, 153, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.L.; Cau, P.; Lévy, N. Molecular bases of progeroid syndromes. Hum. Mol. Genet. 2006, 15, R151–R161. [Google Scholar] [CrossRef] [PubMed]
- Hasty, P.; Campisi, J.; Hoeijmakers, J.; Van Steeg, H.; Vijg, J. Aging and Genome Maintenance: Lessons from the Mouse? Science 2003, 299, 1355–1359. [Google Scholar] [CrossRef]
- Cleaver, J.E. Defective Repair Replication of DNA in Xeroderma Pigmentosum. Nat. Cell Biol. 1968, 218, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Deans, A.J.; West, S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer 2011, 11, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P.; Baerlocher, G.M.; Savage, S.A.; Chanock, S.J.; Weksler, B.B.; Willner, J.P.; Peters, J.A.; Giri, N.; Lansdorp, P.M. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood 2007, 110, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Kruse, R.; Ruzicka, T. DNA mismatch repair and the significance of a sebaceous skin tumor for visceral cancer prevention. Trends Mol. Med. 2004, 10, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, N.; Hedayat, M.; Aghamohammadi, A.; Nichols, K.E. Primary immunodeficiency diseases associated with increased susceptibility to viral infections and malignancies. J. Allergy Clin. Immunol. 2011, 127, 1329–1341.e2. [Google Scholar] [CrossRef] [PubMed]
- Guidos, C.J.; Williams, C.J.; Grandal, I.; Knowles, G.; Huang, M.T.; Danska, J.S. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocyte precursors. Genes Dev. 1996, 10, 2038–2054. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Bassing, C.H. V(D)J Recombination Exploits DNA Damage Responses to Promote Immunity. Trends Genet. 2017, 33, 479–489. [Google Scholar] [CrossRef]
- Alter, B.P. Inherited bone marrow failure syndromes: Considerations pre- and posttransplant. Hematology 2017, 2017, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Przybyszewska, J.; Zlotogorski, A.; Ramot, Y. Re-evaluation of epidermodysplasia verruciformis: Reconciling more than 90 years of debate. J. Am. Acad. Dermatol. 2017, 76, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Pyrhönen, S.; Jablonska, S.; Obałek, S.; Kuismanen, E. Immune reactions in epidermodysplasia verruciformis. Br. J. Dermatol. 1980, 102, 247–254. [Google Scholar] [CrossRef]
- Majewski, S.; Malejczyk, J.; Jablonska, S.; Misiewicz, J.; Rudnicka, L.; Obalek, S.; Orth, G. Natural cell-mediated cytotoxicity against various target cells in patients with epidermodysplasia verruciformis. J. Am. Acad. Dermatol. 1990, 22, 423–427. [Google Scholar] [CrossRef]
- De Oliveira, W.R.P.; Carrasco, S.; Neto, C.F.; Rady, P.; Tyring, S.K. Nonspecific cell-mediated immunity in patients with epidermodysplasia verruciformis. J. Dermatol. 2003, 30, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Crequer, A.; Troeger, A.; Patin, E.; Ma, C.S.; Picard, C.; Pedergnana, V.; Fieschi, C.; Lim, A.; Abhyankar, A.; Gineau, L.; et al. Human RHOH deficiency causes T cell defects and susceptibility to EV-HPV infections. J. Clin. Investig. 2012, 122, 3239–3247. [Google Scholar] [CrossRef] [PubMed]
- Horev, L.; Unger, S.; Molho-Pessach, V.; Meir, T.; Maly, A.; Stepensky, P.; Zamir, M.; Keller, B.; Babay, S.; Warnatz, K.; et al. Generalized verrucosis and HPV-3 susceptibility associated with CD4 T-cell lymphopenia caused by inherited human interleukin-7 deficiency. J. Am. Acad. Dermatol. 2015, 72, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Ramoz, N.; Rueda, L.-A.; Bouadjar, B.; Montoya, L.-S.; Orth, G.; Favre, M. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat. Genet. 2002, 32, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Laffort, C.; Le Deist, F.; Favre, M.; Caillat-Zucman, S.; Radford-Weiss, I.; Fraitag, S.; Blanche, S.; Cavazzana-Calvo, M.; Basile, G.D.S.; De Villartay, J.P.; et al. Severe cutaneous papillomavirus disease after haemopoietic stem-cell transplantation in patients with severe combined immune deficiency caused by common γc cytokine receptor subunit or JAK-3 deficiency. Lancet 2004, 363, 2051–2054. [Google Scholar] [CrossRef]
- Rogers, H.D.; MacGregor, J.L.; Nord, K.M.; Tyring, S.; Rady, P.; Engler, D.E.; Grossman, M.E. Acquired epidermodysplasia verruciformis. J. Am. Acad. Dermatol. 2009, 60, 315–320. [Google Scholar] [CrossRef]
- Cooper, K.D.; Androphy, E.J.; Lowy, D.; Katz, S.I. Antigen Presentation and T-Cell Activation in Epidermodysplasia Verruciformis. J. Investig. Dermatol. 1990, 94, 769–776. [Google Scholar] [CrossRef] [PubMed]
- De Jong, S.J.; Créquer, A.; Matos, I.; Hum, D.; Gunasekharan, V.; Lorenzo, L.; Jabot-Hanin, F.; Imahorn, E.; Arias, A.A.; Vahidnezhad, H.; et al. The human CIB1–EVER1–EVER2 complex governs keratinocyte-intrinsic immunity to β-papillomaviruses. J. Exp. Med. 2018, 215, 2289–2310. [Google Scholar] [CrossRef]
- Vinh, D.C.; Patel, S.Y.; Uzel, G.; Anderson, V.L.; Freeman, A.F.; Olivier, K.N.; Spalding, C.; Hughes, S.; Pittaluga, S.; Raffeld, M.; et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood 2010, 115, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Bigley, V.; Haniffa, M.; Doulatov, S.; Wang, X.-N.; Dickinson, R.; McGovern, N.; Jardine, L.; Pagan, S.; Dimmick, I.; Chua, I.; et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J. Exp. Med. 2011, 208, 227–234. [Google Scholar] [CrossRef]
- Hsu, A.P.; Sampaio, E.P.; Khan, J.; Calvo, K.R.; Lemieux, J.E.; Patel, S.Y.; Frucht, D.M.; Vinh, D.C.; Auth, R.D.; Freeman, A.F.; et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 2011, 118, 2653–2655. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, P.A.; Gorlin, R.J.; Lukens, J.N.; Taniuchi, S.; Bohinjec, J.; Francois, F.; Klotman, M.E.; Diaz, G.A. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat. Genet. 2003, 34, 70–74. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.H.; Murphy, P.M. WHIM syndrome: Immunopathogenesis, treatment and cure strategies. Immunol. Rev. 2018, 287, 91–102. [Google Scholar] [CrossRef]
- Ong, P.Y.; Leung, D.Y.M. Bacterial and Viral Infections in Atopic Dermatitis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 329–337. [Google Scholar] [CrossRef]
- McKenzie, B.S.; Kastelein, R.A.; Cua, D.J. Understanding the IL-23–IL-17 immune pathway. Trends Immunol. 2006, 27, 17–23. [Google Scholar] [CrossRef]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.A.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nat. Cell Biol. 2006, 445, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.O.; Panopoulos, A.D.; Nurieva, R.; Chang, S.H.; Wang, D.; Watowich, S.S.; Dong, C. STAT3 Regulates Cytokine-mediated Generation of Inflammatory Helper T Cells. J. Biol. Chem. 2007, 282, 9358–9363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lopes, J.E.; Chong, M.M.W.; Ivanov, I.I.; Min, R.; Victora, G.D.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nat. Cell Biol. 2008, 453, 236–240. [Google Scholar] [CrossRef]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef]
- Minegishi, Y.; Saito, M.; Tsuchiya, S.; Tsuge, I.; Takada, H.; Hara, T.; Kawamura, N.; Ariga, T.; Pasic, S.; Stojkovic, O.; et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nat. Cell Biol. 2007, 448, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.; Bal, S.K.; Egner, W.; Allen, H.L.; Raza, S.I.; Ma, C.A.; Gürel, M.; Zhang, Y.; Sun, G.; Sabroe, R.A.; et al. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J. Exp. Med. 2019, 216, 1986–1998. [Google Scholar] [CrossRef]
- Schwerd, T.; Twigg, S.R.; Aschenbrenner, D.; Manrique, S.; Miller, K.A.; Taylor, I.B.; Capitani, M.; McGowan, S.J.; Sweeney, E.; Weber, A.; et al. A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis. J. Exp. Med. 2017, 214, 2547–2562. [Google Scholar] [CrossRef]
- Sellheyer, K.; Bickenbach, J.R.; Rothnagel, J.A.; Bundman, D.; Longley, M.A.; Krieg, T.; Roche, N.S.; Roberts, A.B.; Roop, D.R. Inhibition of skin development by overexpression of transforming growth factor beta 1 in the epidermis of transgenic mice. Proc. Natl. Acad. Sci. USA 1993, 90, 5237–5241. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Greenhalgh, D.A.; Bickenbach, J.R.; Jiang, A.; Bundman, D.S.; Krieg, T.; Derynck, R.; Roop, D.R. Expression of a dominant-negative type II transforming growth factor (TGF-β) receptor in the epidermis of transgenic mice blocks TGF-β-mediated growth inhibition. Proc. Natl. Acad. Sci. USA 1997, 94, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Fowlis, D.J.; Bryson, S.; Duffie, E.; Ireland, H.; Balmain, A.; Akhurst, R.J. TGFβ1 Inhibits the Formation of Benign Skin Tumors, but Enhances Progression to Invasive Spindle Carcinomas in Transgenic Mice. Cell 1996, 86, 531–542. [Google Scholar] [CrossRef]
- Go, C.; Li, P.; Wang, X.J. Blocking transforming growth factor beta signaling in transgenic epidermis accelerates chemical carcinogenesis: A mechanism associated with increased angiogenesis. Cancer Res. 1999, 59, 2861–2868. [Google Scholar] [PubMed]
- Koff, A.; Ohtsuki, M.; Polyak, K.; Roberts, J.M.; Massague, J. Negative regulation of G1 in mammalian cells: Inhibition of cyclin E-dependent kinase by TGF-beta. Science 1993, 260, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Kirshner, J.; Jobling, M.F.; Pajares, M.J.; Ravani, S.A.; Glick, A.B.; Lavin, M.J.; Koslov, S.; Shiloh, Y.; Barcellos-Hoff, M.H. Inhibition of Transforming Growth Factor-β1 Signaling Attenuates Ataxia Telangiectasia Mutated Activity in Response to Genotoxic Stress. Cancer Res. 2006, 66, 10861–10869. [Google Scholar] [CrossRef] [PubMed]
- Glick, A.B.; Weinberg, W.C.; Wu, I.H.; Quan, W.; Yuspa, S.H. Transforming growth factor beta 1 suppresses genomic instability independent of a G1 arrest, p53, and Rb. Cancer Res. 1996, 56, 3645–3650. [Google Scholar] [PubMed]
- Markowitz, S.; Wang, J.; Myeroff, L.; Parsons, R.; Sun, L.; Lutterbaugh, J.; Fan, R.S.; Zborowska, E.; Kinzler, K.W.; Vogelstein, B.; et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 1995, 268, 1336–1338. [Google Scholar] [CrossRef] [PubMed]
- Kaklamani, V.G.; Hou, N.; Bian, Y.; Reich, J.; Offit, K.; Michel, L.S.; Rubinstein, W.; Rademaker, A.; Pasche, B. TGFBR1*6A and Cancer Risk: A Meta-Analysis of Seven Case-Control Studies. J. Clin. Oncol. 2003, 21, 3236–3243. [Google Scholar] [CrossRef]
- Valle, L.; Serena-Acedo, T.; Liyanarachchi, S.; Hampel, H.; Comeras, I.; Li, Z.; Zeng, Q.; Zhang, H.-T.; Pennison, M.J.; Sadim, M.; et al. Germline Allele-Specific Expression of TGFBR1 Confers an Increased Risk of Colorectal Cancer. Science 2008, 321, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.F. A case of multiple primary squamous-celled carcinomata of the skin in a young man, with spontaneous healing. Br. J. Dermatol. 1934, 46, 267–272. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Morris, J.C.; Lawrence, D.P.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Berzofsky, J.A.; Hsu, F.J.; Guitart, J. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor β by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol. Immunother. 2015, 64, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Reiss, M.; Hsu, F.J.; Berzofsky, J.A.; Lawrence, D.P. Phase I Study of GC1008 (Fresolimumab): A Human Anti-Transforming Growth Factor-Beta (TGFβ) Monoclonal Antibody in Patients with Advanced Malignant Melanoma or Renal Cell Carcinoma. PLoS ONE 2014, 9, e90353. [Google Scholar] [CrossRef]
- Lu, S.-L.; Kawabata, M.; Imamura, T.; Akiyama, Y.; Nomizu, T.; Miyazono, K.; Yuasa, Y. HNPCC associated with germline mutation in the TGF-β type II receptor gene. Nat. Genet. 1998, 19, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.A.; Tang, G.; Bindal, N.; Bamford, S.; Dawson, E.; Cole, C.; Kok, C.Y.; Jia, M.; Ewing, R.; Menzies, A.; et al. COSMIC (the Catalogue of Somatic Mutations in Cancer): A resource to investigate acquired mutations in human cancer. Nucleic Acids Res. 2009, 38, D652–D657. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Wang, X.-F.; Ng-Eaton, E.; Weinberg, R.A.; Lodish, H.F. Expression cloning of the TGF-β type II receptor, a functional transmembrane serine/threonine kinase. Cell 1992, 68, 775–785. [Google Scholar] [CrossRef]
- Guasch, G.; Schober, M.; Pasolli, H.A.; Conn, E.B.; Polak, L.; Fuchs, E. Loss of TGFβ Signaling Destabilizes Homeostasis and Promotes Squamous Cell Carcinomas in Stratified Epithelia. Cancer Cell 2007, 12, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Yoshino, M.; Yamazaki, H.; Naito, M.; Iyoda, T.; Omatsu, Y.; Shimoyama, S.; Letterio, J.J.; Nakabayashi, T.; Tagaya, H.; et al. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-β1-dependent cells. Int. Immunol. 2001, 13, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Doebel, T.; Voisin, B.; Nagao, K. Langerhans Cells—The Macrophage in Dendritic Cell Clothing. Trends Immunol. 2017, 38, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Travis, M.A.; Sheppard, D. TGF-β Activation and Function in Immunity. Annu. Rev. Immunol. 2014, 32, 51–82. [Google Scholar] [CrossRef]
- Weeks, B.H.; He, W.; Olson, K.L.; Wang, X.J. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res. 2001, 61, 7435–7443. [Google Scholar] [PubMed]
- Kel, J.M.; Girard-Madoux, M.J.H.; Reizis, B.; Clausen, B.E.; Van De Laar, L.; Buitenhuis, M.; Wensveen, F.M.; Janssen, H.L.; Coffer, P.J.; Woltman, A.M. TGF-β Is Required to Maintain the Pool of Immature Langerhans Cells in the Epidermis. J. Immunol. 2010, 185, 3248–3255. [Google Scholar] [CrossRef] [PubMed]
- Modi, B.G.; Neustadter, J.; Binda, E.; Lewis, J.; Filler, R.B.; Roberts, S.J.; Kwong, B.Y.; Reddy, S.; Overton, J.D.; Galan, A.; et al. Langerhans Cells Facilitate Epithelial DNA Damage and Squamous Cell Carcinoma. Science 2012, 335, 104–108. [Google Scholar] [CrossRef]
- Dunphy, J.E. Some Observations on the Natural Behavior of Cancer in Man. N. Engl. J. Med. 1950, 242, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Everson, T.C.; Cole, W.H. Spontaneous Regression of Cancer. Ann. Surg. 1956, 144, 366–383. [Google Scholar] [CrossRef]
- Penner, D.W. Spontaneous regression of a case of myosarcoma. Cancer 1953, 6, 776–779. [Google Scholar] [CrossRef]
- Cushing, H.; Wolbach, S.B. The Transformation of a Malignant Paravertebral Sympathicoblastoma into a Benign Ganglioneuroma. Am. J. Pathol. 1927, 3, 203–216.7. [Google Scholar] [PubMed]
- Nam, A.S.; Chaligne, R.; Landau, D.A. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat. Rev. Genet. 2021, 22, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Saadatpour, A.; Lai, S.; Guo, G.; Yuan, G.-C. Single-Cell Analysis in Cancer Genomics. Trends Genet. 2015, 31, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Leshchiner, I.; Livitz, D.; Gainor, J.F.; Rosebrock, D.; Spiro, O.; Martinez, A.; Mroz, E.; Lin, J.J.; Stewart, C.; Kim, J.; et al. Comprehensive analysis of tumour initiation, spatial and temporal progression under multiple lines of treatment. bioRxiv 2018. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Jonker, D.J.; O’Callaghan, C.J.; Karapetis, C.S.; Zalcberg, J.R.; Tu, D.; Au, H.-J.; Berry, S.R.; Krahn, M.; Price, T.; Simes, R.J.; et al. Cetuximab for the Treatment of Colorectal Cancer. N. Engl. J. Med. 2007, 357, 2040–2048. [Google Scholar] [CrossRef]
- McArthur, G.A.; Demetri, G.D.; Van Oosterom, A.; Heinrich, M.C.; Debiec-Rychter, M.; Corless, C.L.; Nikolova, Z.; Dimitrijevic, S.; Fletcher, J.A. Molecular and Clinical Analysis of Locally Advanced Dermatofibrosarcoma Protuberans Treated with Imatinib: Imatinib Target Exploration Consortium Study B. J. Clin. Oncol. 2005, 23, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; De Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.R.; Zhou, J.H.; Lee, J.J.; Drummond, J.A.; Peng, S.A.; Saade, R.E.; Tsai, K.Y.; Curry, J.L.; Tetzlaff, M.T.; Lai, S.Y.; et al. Mutational Landscape of Aggressive Cutaneous Squamous Cell Carcinoma. Clin. Cancer Res. 2014, 20, 6582–6592. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Hanna, G.J.; Laga, A.C.; Haddad, R.I.; Lorch, J.H.; Hammerman, P.S. Genomic Analysis of Metastatic Cutaneous Squamous Cell Carcinoma. Clin. Cancer Res. 2015, 21, 1447–1456. [Google Scholar] [CrossRef]
- Inman, G.J.; Wang, J.; Nagano, A.; Alexandrov, L.B.; Purdie, K.J.; Taylor, R.G.; Sherwood, V.; Thomson, J.; Hogan, S.; Spender, L.C.; et al. The genomic landscape of cutaneous SCC reveals drivers and a novel azathioprine associated mutational signature. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Carvajal, R.D. KIT as a Therapeutic Target in Metastatic Melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma withoutBRAFMutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Wasmeier, C.; Hume, A.N.; Bolasco, G.; Seabra, M. Melanosomes at a glance. J. Cell Sci. 2008, 121, 3995–3999. [Google Scholar] [CrossRef]
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Bastian, B.C. The Molecular Pathology of Melanoma: An Integrated Taxonomy of Melanocytic Neoplasia. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 239–271. [Google Scholar] [CrossRef]
- Goldstein, A.M.; Fraser, M.C.; Struewing, J.P.; Hussussian, C.J.; Ranade, K.; Zametkin, D.P.; Fontaine, L.S.; Organic, S.M.; Dracopoli, N.C.; Clark, W.H.; et al. Increased Risk of Pancreatic Cancer in Melanoma-Prone Kindreds withp16INK4Mutations. N. Engl. J. Med. 1995, 333, 970–975. [Google Scholar] [CrossRef]
- Hussussian, C.J.; Struewing, J.P.; Goldstein, A.M.; Higgins, P.A.T.; Ally, D.S.; Sheahan, M.D.; Clark, W.H.; Tucker, M.A.; Dracopoli, N.C. Germline p16 mutations in familial melanoma. Nat. Genet. 1994, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Weger, J.; Yang, Q.; Goldstein, A.M.; Tucker, M.A.; Walker, G.J.; Hayward, N.K.; Dracopoli, N.C. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nat. Genet. 1996, 12, 97–99. [Google Scholar] [CrossRef]
- Schaeffer, H.J.; Weber, M.J. Mitogen-Activated Protein Kinases: Specific Messages from Ubiquitous Messengers. Mol. Cell. Biol. 1999, 19, 2435–2444. [Google Scholar] [CrossRef]
- Crews, C.M.; Erikson, R.L. Extracellular signals and reversible protein phosphorylation: What to Mek of it all. Cell 1993, 74, 215–217. [Google Scholar] [CrossRef]
- Reddy, E.P.; Reynolds, R.K.; Santos, E.; Barbacid, M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nat. Cell Biol. 1982, 300, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Stokoe, D.; Macdonald, S.G.; Cadwallader, K.; Symons, M.; Hancock, J.F. Activation of Raf as a result of recruitment to the plasma membrane. Science 1994, 264, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Zindy, F.; Quelle, D.E.; Roussel, M.F.; Sherr, C.J. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene 1997, 15, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, T.; Zindy, F.; Roussel, M.F.; Quelle, D.E.; Downing, J.R.; Ashmun, R.A.; Grosveld, G.; Sherr, C.J. Tumor Suppression at the Mouse INK4a Locus Mediated by the Alternative Reading Frame Product p19 ARF. Cell 1997, 91, 649–659. [Google Scholar] [CrossRef]
- Wojnowski, L.; Zimmer, A.M.; Beck, T.W.; Hahn, H.; Bernal, R.; Rapp, U.R.; Zimmer, A. Endothelial apoptosis in Braf-deficient mice. Nat. Genet. 1997, 16, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Greenbaum, D.; Cichowski, K.; Mercer, K.; Murphy, E.; Schmitt, E.; Bronson, R.T.; Umanoff, H.; Edelmann, W.; Kucherlapati, R.; et al. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997, 11, 2468–2481. [Google Scholar] [CrossRef]
- Rauen, K.A. The RASopathies. Annu. Rev. Genom. Hum. Genet. 2013, 14, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Niihori, T.; Aoki, Y.; Narumi, Y.; Neri, G.; Cavé, H.; Verloes, A.; Okamoto, N.; Hennekam, R.C.M.; Gillessen-Kaesbach, G.; Wieczorek, D.; et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat. Genet. 2006, 38, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Viciana, P.; Tetsu, O.; Tidyman, W.E.; Estep, A.L.; Conger, B.A.; Cruz, M.S.; McCormick, F.; Rauen, K.A. Germline Mutations in Genes Within the MAPK Pathway Cause Cardio-facio-cutaneous Syndrome. Science 2006, 311, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; McKenzie, J.; Frieden, I.; Rauen, K. Dermatological findings in 61 mutation-positive individuals with cardiofaciocutaneous syndrome. Br. J. Dermatol. 2010, 164, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Boaz, N.T.; Dawkins, R. The Selfish Gene. Anthr. Q. 1979, 52, 124. [Google Scholar] [CrossRef]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef]
- Rosendahl, C.O.; Grant-Kels, J.M.; Que, S.K.T. Dysplastic nevus: Fact and fiction. J. Am. Acad. Dermatol. 2015, 73, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Lyle, S. Dysplastic Nevus: Atypical Mole or Typical Myth; Ackerman, D.A.B., Massi, T.N., Eds.; Ardor Scribendi, Ltd.: Philadelphia, PA, USA, 2000. [Google Scholar]
- Elder, D.E.; Goldman, L.I.; Goldman, S.C.; Greene, M.H.; Clark, W.H. Dysplastic nevus syndrome: A phenotypic association of sporadic cutaneous melanoma. Cancer 1980, 46, 1787–1794. [Google Scholar] [CrossRef]
- Clark, W.H.; Reimer, R.R.; Greene, M.; Ainsworth, A.M.; Mastrangelo, M.J. Origin of familial malignant melanomas from heritable melanocytic lesions. ‘The B-K mole syndrome’. Arch. Dermatol. 1978, 114, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, L.A.; Askin, F.B.; Chang, A.E.; Cohen, C.; Dutcher, J.P.; Gilgor, R.S.; Green, S.; Harris, E.L.; Havas, S.; Robinson, J.K.; et al. Diagnosis and Treatment of Early Melanoma. JAMA 1992, 268, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Pollock, P.M.; Harper, U.L.; Hansen, K.S.; Yudt, L.M.; Stark, M.S.; Robbins, C.M.; Moses, T.Y.; Hostetter, G.; Wagner, U.; Kakareka, J.W.; et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2003, 33, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; Von Deimling, A.; Bastian, B.C. Clonal BRAF Mutations in Melanocytic Nevi and Initiating Role of BRAF in Melanocytic Neoplasia. J. Natl. Cancer Inst. 2013, 105, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.; Widlund, H.R.; Kutok, J.L.; Kopani, K.R.; Amatruda, J.F.; Murphey, R.D.; Berghmans, S.; Mayhall, E.A.; Traver, D.; Fletcher, C.D.; et al. BRAF Mutations Are Sufficient to Promote Nevi Formation and Cooperate with p53 in the Genesis of Melanoma. Curr. Biol. 2005, 15, 249–254. [Google Scholar] [CrossRef]
- Viros, A.; Sanchez-Laorde, B.L.; Pedersen, M.; Furney, S.J.; Rae, J.; Hogan, K.; Ejiama, S.; Girotti, M.R.; Cook, M.; Dhomen, N.; et al. Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TPNat. Cell Biol. 2014, 511, 478–482. [Google Scholar] [CrossRef]
- Dankort, D.; Curley, D.P.; Cartlidge, R.A.; Nelson, B.; Karnezis, A.N.; Damsky, W.E., Jr.; You, M.J.; Depinho, R.A.; McMahon, M.; Bosenberg, M. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 2009, 41, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Yeh, I.; Kovalyshyn, I.; Sriharan, A.; Talevich, E.; Gagnon, A.; Dummer, R.; North, J.P.; Pincus, L.B.; Ruben, B.S.; et al. The Genetic Evolution of Melanoma from Precursor Lesions. N. Engl. J. Med. 2015, 373, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Flavahan, W.A.; Gaskell, E.; Bernstein, B.E. Epigenetic plasticity and the hallmarks of cancer. Science 2017, 357, eaal2380. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qu, J.; Liu, G.-H.; Belmonte, J.C.I. The ageing epigenome and its rejuvenation. Nat. Rev. Mol. Cell Biol. 2020, 21, 137–150. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Jones, P.A.; Issa, J.-P.J.; Baylin, P.A.J.S. Targeting the cancer epigenome for therapy. Nat. Rev. Genet. 2016, 17, 630–641. [Google Scholar] [CrossRef]
- Kaufman, C.K.; Mosimann, C.; Fan, Z.P.; Yang, S.; Thomas, A.J.; Ablain, J.; Tan, J.L.; Fogley, R.D.; Van Rooijen, E.; Hagedorn, E.J.; et al. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science 2016, 351, aad2197. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Viros, A.; Milagre, C.; Trunzer, K.; Bollag, G.; Spleiss, O.; Reis-Filho, J.S.; Kong, X.; Koya, R.C.; Flaherty, K.T.; et al. RASMutations in Cutaneous Squamous-Cell Carcinomas in Patients Treated with BRAF Inhibitors. N. Engl. J. Med. 2012, 366, 207–215. [Google Scholar] [CrossRef]
- Kopetz, S.; Desai, J.; Chan, E.; Hecht, J.R.; O’Dwyer, P.J.; Maru, D.M.; Morris, V.; Janku, F.; Dasari, A.; Chung, W.; et al. Phase II Pilot Study of Vemurafenib in Patients with Metastatic BRAF-Mutated Colorectal Cancer. J. Clin. Oncol. 2015, 33, 4032–4038. [Google Scholar] [CrossRef]
- Hatzivassiliou, G.; Song, K.; Yen, I.; Brandhuber, B.J.; Anderson, D.J.; Alvarado, R.; Ludlam, M.J.C.; Stokoe, D.; Gloor, S.L.; Vigers, G.; et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nat. Cell Biol. 2010, 464, 431–435. [Google Scholar] [CrossRef]
- Groesser, L.; Herschberger, E.; Ruetten, A.; Ruivenkamp, C.; Lopriore, E.; Zutt, M.; Langmann, T.; Singer, S.; Klingseisen, L.; Schneider-Brachert, W.; et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat. Genet. 2012, 44, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Häfner, C.; Toll, A.; Real, F.X. HRASMutation Mosaicism Causing Urothelial Cancer and Epidermal Nevus. N. Engl. J. Med. 2011, 365, 1940–1942. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Huynh, M.; Silhavy, J.L.; Kim, S.; Dixon-Salazar, T.; Heiberg, A.; Scott, E.; Bafna, V.; Hill, K.J.; Collazo, A.; et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat. Genet. 2012, 44, 941–945. [Google Scholar] [CrossRef]
- Fan, H.; Oro, A.E.; Scott, M.P.; Khavari, P.A. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat. Med. 1997, 3, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Oro, A.E.; Higgins, K.M.; Hu, Z.; Bonifas, J.M.; Epstein, E.H.; Scott, M.P. Basal Cell Carcinomas in Mice Overexpressing Sonic Hedgehog. Science 1997, 276, 817–821. [Google Scholar] [CrossRef]
- Lapouge, G.; Youssef, K.K.; Vokaer, B.; Achouri, Y.; Michaux, C.; Sotiropoulou, P.A.; Blanpain, C. Identifying the cellular origin of squamous skin tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 7431–7436. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Leidner, R.; Stankevich, E.; Piening, B.; Bifulco, C.; Lowy, I.; Fury, M.G. Responses of metastatic basal cell and cutaneous squamous cell carcinomas to anti-PD1 monoclonal antibody REGN J. Immunother. Cancer 2016, 4, 70. [Google Scholar] [CrossRef]
- Ikeda, S.; Goodman, A.M.; Cohen, P.R.; Jensen, T.J.; Ellison, C.K.; Frampton, G.; Miller, V.; Patel, S.P.; Kurzrock, R. Metastatic basal cell carcinoma with amplification of PD-L1: Exceptional response to anti-PD1 therapy. NPJ Genom. Med. 2016, 1, 16037. [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Lilo, M.T.; Ogurtsova, A.; Esandrio, J.; Xu, H.; Brothers, P.; Schollenberger, M.; Sharfman, W.H.; Taube, J.M. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J. Immunother. Cancer 2017, 5, 23. [Google Scholar] [CrossRef]
- Lay, K.; Yuan, S.; Gur-Cohen, S.; Miao, Y.; Han, T.; Naik, S.; Pasolli, H.A.; Larsen, S.B.; Fuchs, E. Stem cells repurpose proliferation to contain a breach in their niche barrier. eLife 2018, 7. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Nishimura, H.; Honjo, T. PD-1: An inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001, 22, 265–268. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Lipson, E.J.; Bagnasco, S.M.; Moore, J.; Jang, S.; Patel, M.J.; Zachary, A.A.; Pardoll, D.M.; Taube, J.M.; Drake, C.G. Tumor Regression and Allograft Rejection after Administration of Anti–PD-. N. Engl. J. Med. 2016, 374, 896–898. [Google Scholar] [CrossRef]
- Stevenson, M.L.; Wang, C.Q.F.; Abikhair, M.; Roudiani, N.; Felsen, D.; Krueger, J.G.; Pavlick, A.C.; Carucci, J.A. Expression of Programmed Cell Death Ligand in Cutaneous Squamous Cell Carcinoma and Treatment of Locally Advanced Disease with Pembrolizumab. JAMA Dermatol. 2017, 153, 299–303. [Google Scholar] [CrossRef]
- Tran, D.C.; Colevas, A.D.; Chang, A.L.S. Follow-up on Programmed Cell Death 1 Inhibitor for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2017, 153, 92–94. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Park, J.C.; Emerick, K.S.; Sullivan, R.J.; Kaufman, H.L.; Miller, D.M. Real-world assessment of response to anti-PD-1 therapy in advanced cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2021. [Google Scholar] [CrossRef]
- Jarkowski, A.; Hare, R.; Loud, P.; Skitzki, J.J.; Kane, J.M.; May, K.S.; Zeitouni, N.C.; Nestico, J.; Vona, K.L.; Groman, A.; et al. Systemic Therapy in Advanced Cutaneous Squamous Cell Carcinoma (CSCC). Am. J. Clin. Oncol. 2016, 39, 545–548. [Google Scholar] [CrossRef]
- Chan, T.A.; Wolchok, J.D.; Snyder, A. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2015, 373, 1984. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Callahan, M.K.; Awad, M.M.; Calvo, E.; Ascierto, P.A.; Atmaca, A.; Rizvi, N.A.; Hirsch, F.R.; Selvaggi, G.; Szustakowski, J.D.; et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2019, 35, 329. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Kudo, T.; Hamamoto, Y.; Kato, K.; Ura, T.; Kojima, T.; Tsushima, T.; Hironaka, S.; Hara, H.; Satoh, T.; Iwasa, S.; et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: An open-label, multicentre, phase 2 trial. Lancet Oncol. 2017, 18, 631–639. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.-Y.; Chin, K.; Kadowaki, S.; Ahn, M.-J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Chung, H.C.; Ros, W.; Delord, J.-P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Rischin, D.; Schmults, C.D.; Hernandez-Aya, L.F.; Meier, F.E.; Schadendorf, D.; Guminski, A.D.; Hauschild, A.; et al. Primary analysis of phase 2 results of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with locally advanced cutaneous squamous cell carcinoma (laCSCC). J. Clin. Oncol. 2019, 37, 6015. [Google Scholar] [CrossRef]
- Dzubow, L.M.; Rigel, D.S.; Robins, P. Risk factors for local recurrence of primary cutaneous squamous cell carcinomas. Treatment by microscopically controlled excision. Arch. Dermatol. 1982, 118, 900–902. [Google Scholar] [CrossRef]
- Harris, B.N.; Bayoumi, A.; Rao, S.; Moore, M.G.; Farwell, D.G.; Bewley, A.F. Factors Associated with Recurrence and Regional Adenopathy for Head and Neck Cutaneous Squamous Cell Carcinoma. Otolaryngol. Neck Surg. 2017, 156, 863–869. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Camus, M.; Tosolini, M.; Mlecnik, B.; Pagès, F.; Kirilovsky, A.; Berger, A.; Costes, A.; Bindea, G.; Charoentong, P.; Bruneval, P.; et al. Coordination of Intratumoral Immune Reaction and Human Colorectal Cancer Recurrence. Cancer Res. 2009, 69, 2685–2693. [Google Scholar] [CrossRef]
- Michot, J.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Nemes, Z.; Steinert, P.M. Bricks and mortar of the epidermal barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Mehrel, T.; Hohl, D.; Rothnagel, J.A.; Longley, M.A.; Bundman, D.; Cheng, C.; Lichti, U.; Bisher, M.E.; Steven, A.C.; Steinert, P.M.; et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell 1990, 61, 1103–1112. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Roop, D.R. Loricrin Confers Photoprotective Function against UVB in Corneocytes. J. Investig. Dermatol. 2018, 138, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Lippens, S.; Tschachler, E.; Declercq, W. Cell death by cornification. Biochim. et Biophys. Acta (BBA) Bioenerg. 2013, 1833, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, Y.; Roop, D.R.; Ogawa, T. “Structural imprinting” of the cutaneous immune effector function. Tissue Barriers 2020, 1851561. [Google Scholar] [CrossRef]
- Brockow, K.; Ardern-Jones, M.R.; Mockenhaupt, M.; Aberer, W.; Barbaud, A.; Caubet, J.; Spiewak, R.; Torres, M.J.; Mortz, C.G. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy 2019, 74, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ishitsuka, Y.; Iwamoto, K.; Koguchi-Yoshioka, H.; Tanaka, R.; Watanabe, R.; Fujisawa, Y.; Fujimoto, M. Programmed cell death 1 blockade-induced cutaneous sarcoid-like epithelioid granulomas in advanced melanoma: A case report. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e260–e261. [Google Scholar] [CrossRef] [PubMed]
- Kehren, J.; Desvignes, C.; Krasteva, M.; Ducluzeau, M.-T.; Assossou, O.; Horand, F.; Hahne, M.; Kägi, D.; Kaiserlian, D.; Nicolas, J.-F. Cytotoxicity Is Mandatory for CD8+ T Cell–mediated Contact Hypersensitivity. J. Exp. Med. 1999, 189, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Novak, N.; Haberstok, J.; Bieber, T.; Allam, J.-P. The immune privilege of the oral mucosa. Trends Mol. Med. 2008, 14, 191–198. [Google Scholar] [CrossRef]
- Luger, T.A.; Stadler, B.M.; Luger, B.M.; Mathieson, B.J.; Mage, M.; Schmidt, J.A.; Oppenheim, J.J. Murine epidermal cell-derived thymocyte-activating factor resembles murine interleukin J. Immunol. 1982, 128, 2147–2152. [Google Scholar]
- Diven, D.G.; Dozier, S.E.; Meyer, D.J.; Smith, E.B. Bacteriology of inflamed and uninflamed epidermal inclusion cysts. Arch. Dermatol. 1998, 134, 49–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dalziel, K.; Dykes, P.J.; Marks, R. Inflammation due to intra-cutaneous implantation of stratum corneum. Br. J. Exp. Pathol. 1984, 65, 107–115. [Google Scholar] [PubMed]
- Gahring, L.C.; Buckley, A.; Daynes, R.A. Presence of epidermal-derived thymocyte activating factor/interleukin 1 in normal human stratum corneum. J. Clin. Investig. 1985, 76, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ishitsuka, Y.; Nakamura, Y.; Kubota, N.; Saito, A.; Fujisawa, Y.; Watanabe, R.; Okiyama, N.; Suga, Y.; Roop, D.R.; et al. NRF2 Augments Epidermal Antioxidant Defenses and Promotes Atopy. J. Immunol. 2020, 205, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Nakae, S.; Naruse-Nakajima, C.; Sudo, K.; Horai, R.; Asano, M.; Iwakura, Y. IL-1α, but not IL-1β, is required for contact-allergen-specific T cell activation during the sensitization phase in contact hypersensitivity. Int. Immunol. 2001, 13, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Capucha, T.; Mizraji, G.; Segev, H.; Blecher-Gonen, R.; Winter, D.; Khalaileh, A.; Tabib, Y.; Attal, T.; Nassar, M.; Zelentsova, K.; et al. Distinct Murine Mucosal Langerhans Cell Subsets Develop from Pre-dendritic Cells and Monocytes. Nat. Immunol. 2015, 43, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Tacke, F.; Angeli, V.; Bogunovic, M.; Loubeau, M.; Dai, X.-M.; Stanley, E.R.; Randolph, G.J.; Merad, M. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006, 7, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, Y.; Roop, D.R. Loricrin: Past, Present, and Future. Int. J. Mol. Sci. 2020, 21, 2271. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Huang, Y.-H.; Chen, M.; Su, J.; Zou, Y.; Bardeesy, N.; Iacobuzio-Donahue, C.A.; Massagué, J. TGF-β Tumor Suppression through a Lethal EMT. Cell 2016, 164, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Sakai, E.; Nakayama, M.; Oshima, H.; Kouyama, Y.; Niida, A.; Fujii, S.; Ochiai, A.; Nakayama, K.I.; Mimori, K.; Suzuki, Y.; et al. Combined Mutation of Apc, Kras, and Tgfbr2 Effectively Drives Metastasis of Intestinal Cancer. Cancer Res. 2017, 78, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Doran, T.I.; Vidrich, A.; Sun, T.-T. Intrinsic and extrinsic regulation of the differentiation of skin, corneal and esophageal epithelial cells. Cell 1980, 22, 17–25. [Google Scholar] [CrossRef]
- Waddington, C. The Strategy of the Genes; Routledge: London, UK, 2014. [Google Scholar]
- Jerby-Arnon, L.; Shah, P.; Cuoco, M.S.; Rodman, C.; Su, M.-J.; Melms, J.C.; Leeson, R.; Kanodia, A.; Mei, S.; Lin, J.-R.; et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018, 175, 984–997.e24. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.S.; Toole, J.J.; Cunningham, J.M.; Chang, E.H.; Lowy, U.R.; Weinberg, R.A. Characterization of a human colon/lung carcinoma oncogene. Nat. Cell Biol. 1983, 302, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.M. Inflammation in epithelial skin tumours: Old stories and new ideas. Eur. J. Cancer 2006, 42, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.J.; Alexandrov, L.B.; Breems, N.Y.D.; Atanasova, V.S.; Farshchian, M.; Purdom, E.; Nguyen, T.N.; Coarfa, C.; Rajapakshe, K.; Prisco, M.; et al. APOBEC mutation drives early-onset squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. Sci. Transl. Med. 2018, 10, eaas9668. [Google Scholar] [CrossRef]
- Nyström, A.; Thriene, K.; Mittapalli, V.; Kern, J.S.; Kiritsi, D.; Dengjel, J.; Bruckner-Tuderman, L. Losartan ameliorates dystrophic epidermolysis bullosa and uncovers new disease mechanisms. EMBO Mol. Med. 2015, 7, 1211–1228. [Google Scholar] [CrossRef]
- Dayal, J.; Mason, S.; Salas-Alanis, J.; McGrath, J.; Taylor, R.; Mellerio, J.; Blyth, K.; South, A.; Inman, G. Heterogeneous addiction to transforming growth factor-beta signalling in recessive dystrophic epidermolysis bullosa-associated cutaneous squamous cell carcinoma. Br. J. Dermatol. 2020. [Google Scholar] [CrossRef]
- Khaddour, K.; Gorell, E.S.; Dehdashti, F.; Tang, J.Y.; Ansstas, G. Induced Remission of Metastatic Squamous Cell Carcinoma with an Immune Checkpoint Inhibitor in a Patient with Recessive Dystrophic Epidermolysis Bullosa. Case Rep. Oncol. 2020, 13, 911–915. [Google Scholar] [CrossRef]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016, 165, 35–44. [Google Scholar] [CrossRef]
- Hugo, W.; Shi, H.; Sun, L.; Piva, M.; Song, C.; Kong, X.; Moriceau, G.; Hong, A.; Dahlman, K.B.; Johnson, D.B.; et al. Non-genomic and Immune Evolution of Melanoma Acquiring MAPKi Resistance. Cell 2015, 162, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nat. Cell Biol. 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P.; Kamala, T. Tissue-based class control: The other side of tolerance. Nat. Rev. Immunol. 2011, 11, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.B.; Huh, C.G.; Becker, D.; Geiser, A.; Lyght, M.; Flanders, K.C.; Roberts, A.B.; Sporn, M.B.; Ward, J.M.; Karlsson, S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 1993, 90, 770–774. [Google Scholar] [CrossRef]
- Stockis, J.; Colau, D.; Coulie, P.G.; Lucas, S. Membrane protein GARP is a receptor for latent TGF-β on the surface of activated human Treg. Eur. J. Immunol. 2009, 39, 3315–3322. [Google Scholar] [CrossRef]
- De Streel, G.; Bertrand, C.; Chalon, N.; Liénart, S.; Bricard, O.; LeComte, S.; Devreux, J.; Gaignage, M.; De Boeck, G.; Mariën, L.; et al. Selective inhibition of TGF-β1 produced by GARP-expressing Tregs overcomes resistance to PD-1/PD-L1 blockade in cancer. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Martincorena, I.; Fowler, J.C.; Wabik, A.; Lawson, A.R.J.; Abascal, F.; Hall, M.W.J.; Cagan, A.; Murai, K.; Mahbubani, K.; Stratton, M.R.; et al. Somatic mutant clones colonize the human esophagus with age. Science 2018, 362, 911–917. [Google Scholar] [CrossRef]
- Warrell, R.P.; Frankel, S.R.; Miller, W.H.; Scheinberg, D.A.; Itri, L.M.; Hittelman, W.N.; Vyas, R.; Andreeff, M.; Tafuri, A.; Jakubowski, A.; et al. Differentiation Therapy of Acute Promyelocytic Leukemia with Tretinoin (All-trans-Retinoic Acid). N. Engl. J. Med. 1991, 324, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.P.; Schmidt-Arras, D. Novel Approaches to Target Mutant FLT3 Leukaemia. Cancers 2020, 12, 2806. [Google Scholar] [CrossRef]
- Breccia, M.; Mazzarella, L.; Bagnardi, V.; Disalvatore, D.; Loglisci, G.; Cimino, G.; Testi, A.M.; Avvisati, G.; Petti, M.C.; Minotti, C.; et al. Increased BMI correlates with higher risk of disease relapse and differentiation syndrome in patients with acute promyelocytic leukemia treated with the AIDA protocols. Blood 2012, 119, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, E.K.; Ms, J.R.F.; Panageas, K.S.; Ferraro, R.A.; Ba, J.M.S.; Postow, M.A.; Coit, D.G.; Ariyan, C.E. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer 2020, 126, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Taylor, S.M. Cellular differentiation, cytidine analogs and DNA methylation. Cell 1980, 20, 85–93. [Google Scholar] [CrossRef]
- Bates, S.E. Epigenetic Therapies for Cancer. N. Engl. J. Med. 2020, 383, 650–663. [Google Scholar] [CrossRef]
- Plass, C.; Pfister, S.M.; Lindroth, A.M.; Bogatyrova, O.; Claus, R.; Lichter, P. Mutations in regulators of the epigenome and their connections to global chromatin patterns in cancer. Nat. Rev. Genet. 2013, 14, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Burris, H.A.; Janku, F.; Shroff, R.T.; Cleary, J.M.; Azad, N.S.; Goyal, L.; Maher, E.A.; Gore, L.; Hollebecque, A.; et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: A phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 711–720. [Google Scholar] [CrossRef]
- Jones, P.A.; Ohtani, H.; Chakravarthy, A.; De Carvalho, D.D. Epigenetic therapy in immune-oncology. Nat. Rev. Cancer 2019, 19, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Youngblood, B.; Lee, J.; Sarkar, S.; Ahmed, R. Demethylation of the PD-1 Promoter Is Imprinted during the Effector Phase of CD8 T Cell Exhaustion. J. Virol. 2016, 90, 8934–8946. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.M.; Kim, G.; Kim, S.; Sim, J.H.; Choi, J.; Kim, M.; Kwon, M.; Ye, S.-K.; Lee, D.-S.; Cho, S.W.; et al. Chromatin accessibility of circulating CD8+ T cells predicts treatment response to PD-1 blockade in patients with gastric cancer. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Goltz, D.; Gevensleben, H.; Vogt, T.J.; Dietrich, J.; Golletz, C.; Bootz, F.; Kristiansen, G.; Landsberg, J.; Dietrich, D. CTLA4 methylation predicts response to anti–PD-1 and anti–CTLA-4 immunotherapy in melanoma patients. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, H.E.; Fan, Y.; Moustaki, A.; Abdelsamed, H.A.; Dash, P.; Dogra, P.; Carter, R.; Awad, W.; Neale, G.; Thomas, P.G.; et al. De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell 2017, 170, 142–157.e19. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Suárez-Fariñas, M.; Chiricozzi, A.; Nograles, K.E.; Shemer, A.; Fuentes-Duculan, J.; Cardinale, I.; Lin, P.; Bergman, R.; Bowcock, A.M.; et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J. Allergy Clin. Immunol. 2009, 124, 1235–1244.e58. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.G.; Goodarzi, M.; Drayton, D.L.; Von Andrian, U.H. T cell– and B cell–independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006, 7, 507–516. [Google Scholar] [CrossRef]
- Swamy, M.; Jamora, C.; Havran, W.L.; Hayday, A.C. Epithelial decision makers: In search of the ‘epimmunome’. Nat. Immunol. 2010, 11, 656–665. [Google Scholar] [CrossRef]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).